Abstract
Background
Although knees that have undergone anterior cruciate ligament reconstruction (ACLR) often exhibit normal laxity on clinical examination, abnormal kinematic patterns have been observed when the joint is dynamically loaded during whole body activity. This study investigated whether abnormal knee kinematics arise with loading under isolated dynamic movements.
Hypothesis
Tibiofemoral and patellofemoral kinematics of ACLR knees will be similar to those of the contralateral uninjured control knee during passive flexion-extension, with bilateral differences emerging when an inertial load is applied.
Study Design
Controlled laboratory study.
Methods
The bilateral knees of 18 subjects who had undergone unilateral ACLR within the past 4 years were imaged by use of magnetic resonance imaging (MRI). Their knees were cyclically (0.5 Hz) flexed passively. Subjects then actively flexed and extended their knees against an inertial load that induced stretch-shortening quadriceps contractions, as seen during the load acceptance phase of gait. A dynamic, volumetric, MRI sequence was used to track tibiofemoral and patellofemoral kinematics through 6 degrees of freedom. A repeated-measures analysis of variance was used to compare secondary tibiofemoral and patellofemoral kinematics between ACLR and healthy contralateral knees during the passive and active extension phases of the cyclic motion.
Results
Relative to the passive motion, inertial loading induced significant shifts in anterior and superior tibial translation, internal tibial rotation, and all patellofemoral degrees of freedom. As hypothesized, tibiofemoral and patellofemoral kinematics were bilaterally symmetric during the passive condition. However, inertial loading induced bilateral differences, with the ACLR knees exhibiting a significant shift toward external tibial rotation. A trend toward greater medial and anterior tibial translation was seen in the ACLR knees.
Conclusion
This study demonstrates that abnormal knee kinematic patterns in ACLR knees emerge during a simple, active knee flexion-extension task that can be performed in an MRI scanner.
Clinical Relevance
It is hypothesized that abnormal knee kinematics may alter cartilage loading patterns and thereby contribute to increased risk for osteoarthritis. Recent advances in quantitative MRI can be used to detect early cartilage degeneration in ACLR knees. This study demonstrates the feasibility of identifying abnormal ACLR kinematics by use of dynamic MRI, supporting the combined use of dynamic and quantitative MRI to investigate the proposed link between knee motion, cartilage contact, and early biomarkers of cartilage degeneration.
Keywords: knee kinematics, ACL reconstruction, biomechanics, MRI
Substantial interest has arisen in the ability of anterior cruciate ligament reconstruction (ACLR) to restore normal functional knee mechanics; as well, it is of interest whether residual abnormalities after surgery may contribute to the high rates of anterior knee pain2,31 and early-onset osteoarthritis seen in ACLR knees.32,37 While conventional anterior-posterior laxity tests often fail to detect significant differences in ACLR knees,8,40 numerous studies have found evidence of abnormal tibiofemoral kinematics during whole body movement such as gait,10,49 downhill running,55 and quasi-static lunges.57 In particular, a number of publications report a shift toward external tibial rotation and medial translation in ACLR knees compared with healthy knees.23,26
However, determining the underlying coordination and musculoskeletal factors giving rise to altered knee kinematics during complex, whole body movement can be challenging. Altered ground-reaction forces,20 neuromuscular coordination,16,20 and contralateral compensations23 have been observed in individuals with unilateral ACLR and thus contribute to knee mechanics observed in whole body movement. If altered knee kinematics can be induced during an isolated movement, this may represent a simpler paradigm to identify the effects of controllable surgical factors, such as graft type and tunnel position. Further, simpler isolated movements could be tested within small confines, such as a magnetic resonance imaging (MRI) bore, enabling the use of dynamic MRI sequences to capture tibiofemoral4,28,51 and patellofemoral13,14 kinematics with a high degree of accuracy. Dynamic MRI can also be coupled with anatomic and quantitative MRI to assess cartilage morphologic features and biomarkers of tissue composition18,19,46 to explore the potential link of abnormal joint mechanics to early cartilage degradation.
MRI assessment of tibiofemoral alignment in ACLR knees has previously been limited to quasi-static conditions,15,36,50 with mixed observations of increased anterior tibial translation36 or external tibial rotation.15 We recently introduced a 3D dynamic imaging sequence to accurately reconstruct 6 degrees of freedom tibiofemoral and patellofemoral kinematics during dynamic motion.28,29 This sequence improves upon previous MRI efforts by allowing full volumetric co-registration for tracking skeletal motion, minimizing kinematic errors due to out-of-plane motion in planar images, and reducing numerical integration drift in phase contrast images.28,38,42 However, it remains unclear whether abnormal ACLR knee motions seen in complex tasks23,55 can be induced in an isolated task, particularly given the physical range of motion and loading constraints associated with motion in a standard MRI scanner bore.
The goal of this work was to use dynamic MRI to investigate kinematic behavior of normal and ACLR knees during passive and active, isolated knee flexion-extension tasks. We hypothesized that ACLR knees would exhibit similar kinematics compared with the healthy contralateral knee when passively extended but would exhibit an increase in external tibial rotation when the joint is actively loaded. The potential insights gained from this study are important for establishing the utility of MRI-based techniques for assessing potential links between abnormal knee mechanics and the development of patellofemoral pain or early-onset osteoarthritis after reconstructive surgery.
METHODS
Subjects
Potential subjects were contacted after they were retrospectively identified from a voluntary database established by the University of Wisconsin Health Sports Medicine Outcomes Program. Patients were considered eligible if they had undergone a unilateral, primary ACLR within the past 1 to 4 years, had no concurrent ligament damage, and had no postoperative complications. To increase subject recruitment and reflect the variability across all treated subjects, we did not restrict the graft type used in potential subjects. The contralateral knee of potential subjects had no history of pain, injury, or surgery and no history of inflammatory or crystalline-induced arthritis. A letter was sent to all identified patients to introduce the study and was followed up with a phone call from the study coordinator. The database consisted of 451 patients from which 84 potential subjects were identified and contacted. Eighteen subjects (9 male, 9 female; age, 24.8 ± 5.7 [mean ± sd] years; body mass, 77.9 ± 16.7 kg; time after surgery, 1.6 ± 0.7 years; 9 subjects with bone–patellar tendon–bone grafts; 2 subjects with partial lateral meniscectomies) agreed to participate and enroll in the study after giving informed consent according to an institutional review board–approved protocol.
MRI Testing
Subjects were first placed supine within a clinical 3.0-T magnetic resonance scanner (MR750, General Electric Healthcare) with an 8-channel phased array extremity coil (Precision Eight TX/TR High Resolution Knee Array; InVivo) centered over their knee. A 3D IDEAL-SPGR (3-dimensional spoiled gradient recall-echo sequence with iterative decomposition of water and fat with echo asymmetry and least squares estimation fat-water separation); (resolution, 0.37 × 0.37 × 0.9 mm; repetition time, 10 ms; echo times, 4.5/5.5/6.1 ms; flip angle, 14°; receiver bandwidth, 41.7 kHz; field of view, 14 cm; matrix, 384 × 384; slice thickness, 0.9 mm; scan time, 6 minutes) was acquired in the axial plane to obtain high-resolution static images of the patients’ knees.
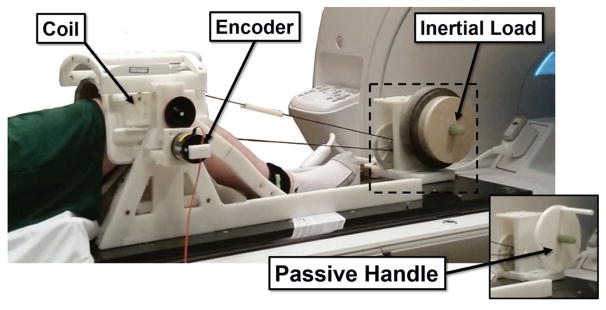
Subjects were then placed supine on an MRI-compatible loading device with their lower leg secured to a lever arm that rotated about the nominal knee flexion axis (Figure 1). Subjects performed 2 open chain tasks, one active and one passive, in a randomized order. In the passive task, a researcher cyclically (0.5 Hz) rotated the lever, moving the subject’s leg through the available flexion range of motion. Subjects were instructed to relax and neither aid nor impede the movement. In the active task, the lever was coupled by a belt and gears (net gear ratio, 10:1) to a shaft with inertial disks. Subjects were asked to actively flex and extend their knee through their available range of motion at a cyclic rate (0.5 Hz) guided by a metronome. The device applied an inertial load on the lower leg, inducing active stretch-shortening quadriceps contraction during One or more of the authors has declared the following potential conflict of interest or source of funding: Funding for research was provided by NIH grant AR062733 and EB015410. Funding for personal salaries was provided by the National Science Foundation Graduate Research Fellowship Program (M.F.V.) under Grant No. DGE-1256259 and the Robert W. Bolz Distinguished Graduate Fellowship Program (J.M.K.). the latter half of knee flexion and the first half of knee extension. The active task was designed to emulate the quadriceps loading that is seen during the early stance phase of gait when the quadriceps brake knee flexion and then induce knee extension.28
Figure 1.
An MRI-compatible loading device with inertial disks was used for the active loading task. A handle (see insert) replaced the inertial disks in the passive task and was used to cyclically move the limb.
A 3D SPGR sequence with VIPR (vastly undersampled isotropic projections); (1.5-mm isotropic resolution; repetition time, 4 ms; echo time, 1.4 ms; flip angle, 8°; receiver bandwidth, 32.5 kHz; unique radial lines, 93,922; field of view, 48 cm) continuously collected volumetric data for 5 minutes during both flexion tasks. An MRI-compatible rotary encoder (Micronor) was used to track the lever angle throughout the passive and active motion tasks. Lever angle was then used to retrospectively sort SPGR-VIPR projections into 60 equally spaced bins over the motion cycle. Image reconstruction with the sorted projections was then used to create 60 volumetric image sets over each motion cycle.28
Kinematic Measures
Femoral, tibial, and patellar bones were manually segmented (MIMICS; Materialise Group) from the 3D IDEAL-SPGR images to produce subject-specific bone models. Bone models were smoothed and then meshed to 7000, 7000, and 2000 triangles for the femoral, tibial, and patellar bones, respectively (Geomagic; and MeshLab, Visual Computing Lab-ISTI-CNR). Anatomic coordinate systems were independently defined for each bone by use of an automatic algorithm that places the axes based on the bone’s inertial and geometric properties.39,45 The origins of the coordinate systems were placed at the centroid of a best-fit cylinder to the femoral condyles, the center of mass of the tibial plateau, and the centroid of the patella.
Bone models were then manually placed in the first frame of the dynamic images. Kinematic trajectories were automatically tracked via Powell’s method for optimization,44 to minimize the sum of squared values of the dynamic images at the location of bone model vertices. The solution for a frame was used as the initial guess for the position and orientation in the following frame, until kinematic trajectories for each bone were determined over the full motion cycle. We have previously shown that this technique for model-based tracking of SPGR-VIPR images provides kinematics with precisions within 0.8° and 0.5 mm.29
Tibiofemoral and patellofemoral kinematics were defined at each frame as the position and orientation of the tibia and patella relative to the femur, respectively.21 Kinematics were low-pass filtered at a cutoff frequency of 5 Hz. Secondary kinematics (ie, degrees of freedom other than tibiofemoral flexion) were then interpolated to every 2.5° of tibiofemoral flexion through the extension phase of the kinematic cycle. A knee flexion angle change of less than 2.5° was observed between frames in 90% of all collected data. Only kinematics during extension were considered, as this is the primary loading period during the active task.
Statistics
Repeated-measures analysis of variance (ANOVA) was used to test the effects of flexion angle (repeated within-subject measures at every 2.5° of extension) and either load (active vs passive for both knee conditions) or surgery (reconstructed vs healthy for both loading conditions separately) on differences in secondary tibiofemoral and patellofemoral kinematics. If a crossover interaction between the main effects (ie, leg-by-angle or load-by-angle) was detected in the ANOVA (P < .10, adjusted for reduced power for detection), then a Bonferroni test was used to determine group-based differences in limb status or loading while correcting for multiple comparisons. If no crossover interaction effect was detected, the main effects were examined for differences. Significance was set to P <.05 for all tests but the interaction effects.
RESULTS
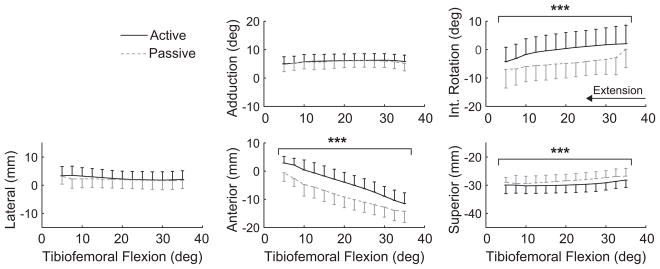
During passive knee extension, the tibia translated anteriorly and rotated externally in all knees, consistent with the screw-home mechanism (Figure 2). Relative to the passive task, inertial loading induced significant increases in internal tibial rotation and anterior and inferior tibial translation (Figure 2, Table 1).
Figure 2.
Load-dependent changes in secondary tibiofemoral kinematics of healthy contralateral knees during knee extension. Active loading induced a significant increase in internal tibial rotation as well as anterior and inferior translation (***P <.001). Kinematic curves are plotted as the mean ± 1 standard deviation.
TABLE 1.
Changes in Tibiofemoral and Patellofemoral Kinematics Due to the Addition of Inertial Loading in Healthy and ACLR Kneesa
| Healthy Knees | ACLR Knees | |||
|---|---|---|---|---|
|
|
|
|||
| Mean (95% CI) | P Value | Mean (95% CI) | P Value | |
| Tibiofemoral | ||||
| Adduction, deg | 0.3 (−0.1 to 0.6) | .328 | 0.4 (−0.4 to 1.1) | .145 |
| Internal rotation, deg | 4.9 (3.8 to 5.9) | <.001 | 3.5 (2.2 to 4.8) | <.001 |
| Lateral translation, mm | 0.4 (−0.2 to 1.1) | .321 | −0.1 (−0.7 to 0.4) | .378 |
| Anterior translation, mm | 4.5 (4.1 to 5.0) | <.001 | 5.3 (3.9 to 6.7) | <.001 |
| Superior translation, mm | −1.3 (−1.7 to −0.9) | <.001 | −1.5 (−2.3 to −0.8) | <.001 |
| Patellofemoral | ||||
| Flexion, deg | −2.6 (−3.5 to −1.6) | <.001 | −3.2 (−4.8 to −1.5) | <.001 |
| Lateral rotation, deg | 1.8 (1.1 to 2.4) | <.001 | 1.7 (0.6 to 2.8) | .005 |
| Medial tilt, deg | 1.2 (0.6 to 1.9) | <.001 | 0.9 (0.2 to 1.6) | <.001 |
| Lateral translation, mm | 1.0 (0.5 to 1.5) | <.001 | 0.8 (0.2 to 1.5) | <.001 |
| Anterior translation, mm | 0.7 (0.2 to 1.2) | .002 | 1.0 (0.1 to 1.9) | .020 |
| Superior translation, mm | 4.5 (3.8 to 5.2) | <.001 | 5.1 (3.5 to 6.6) | <.001 |
Values are expressed as mean and 95% confidence interval (CI) of the difference between the active and passive loading conditions. Significant differences associated with loading are boldface. ACLR, anterior cruciate ligament reconstruction.
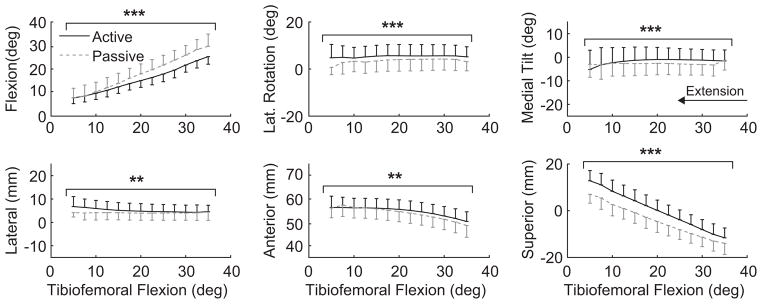
Relative to the femur, the patella translated superiorly, anteriorly, and slightly laterally with passive knee extension (Figure 3). All 6 degrees of freedom in patellofemoral kinematics were altered with inertial loading (Table 1). Significant increases were found in anterior, superior, and lateral patellar translations relative to the passive condition. The patella was also more extended, medially tilted, and laterally rotated with the addition of inertial loading.
Figure 3.
Load-dependent changes in patellofemoral kinematics of healthy contralateral knees during knee extension. Active loading produced a significant change in the kinematics of all 6 degrees of freedom (**P <.01, ***P <.001). Kinematic curves are plotted as the mean ± 1 standard deviation.
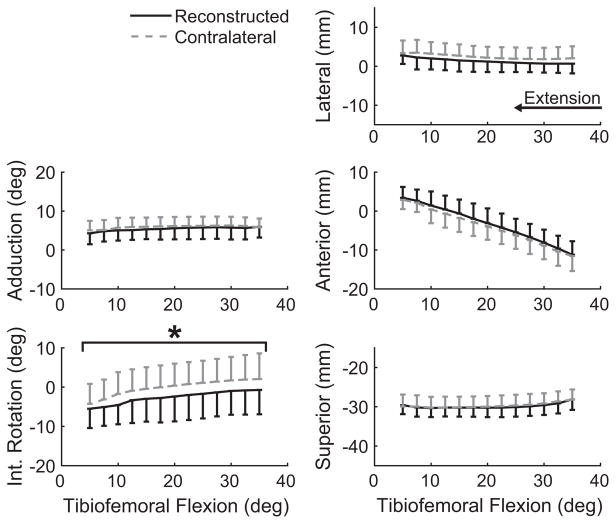
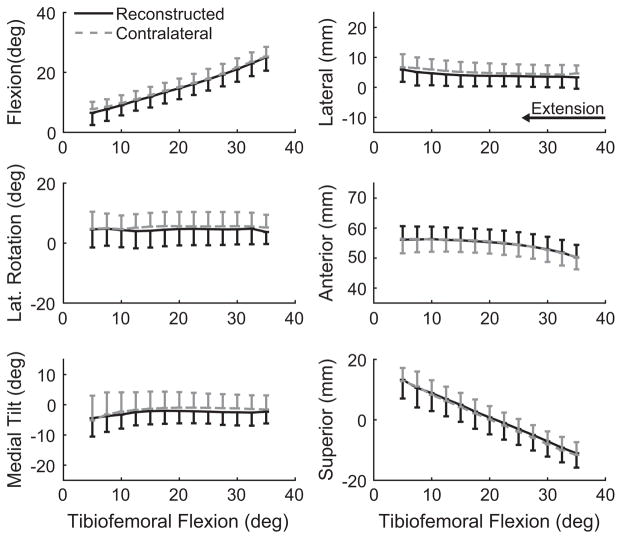
ACLR knees exhibited statistically equivalent, passive tibiofemoral and patellofemoral kinematics compared with the intact contralateral knees, although there was a tendency toward more tibial adduction in the ACLR knees (Table 2). Active motion against an inertial load, however, induced bilateral differences in knee kinematics. In particular, ACLR knees displayed a more externally rotated tibia (Figure 4) compared with the contralateral knee. The magnitudes of this shift averaged 2.4° (Table 2). While not significant, ACLR knees also exhibited a shift of 1.1 mm toward greater medial tibial translation relative to the contralateral knee (P = .066). No significant bilateral differences were noted in the patellofemoral joint, although the ACLR knees tended to exhibit more medial rotation (P = .183), lateral tilt (P = .138), and medial translation (P = .195) of the patella (Table 2, Figure 5).
TABLE 2.
Effect of Anterior Cruciate Ligament Reconstruction on Bilateral Kinematic Differences During Passive and Active Loadinga
| Passive Loading | Active Loading | |||
|---|---|---|---|---|
|
|
|
|||
| Mean (95% CI) | P Value | Mean (95% CI) | P Value | |
| Tibiofemoral | ||||
| Abduction, deg | −0.8 (−2.0 to 0.5) | .107 | −0.6 (−1.8 to 0.6) | .374 |
| Internal rotation, deg | −1.1 (−3.9 to 1.6) | .477 | −2.4 (−4.8 to 0.0) | .021 |
| Lateral translation, mm | −0.5 (−1.8 to 0.8) | .564 | −1.1 (−2.2 to 0.0) | .066 |
| Anterior translation, mm | 0.0 (−1.1 to 1.2) | .355 | 0.8 (−0.3 to 1.8) | .112 |
| Superior translation, mm | 0.0 (−0.8 to 0.8) | .686 | −0.2 (−0.8 to 0.3) | .366 |
| Patellofemoral | ||||
| Flexion, deg | 0.1 (−1.6 to 1.8) | .816 | −0.5 (−1.4 to −0.4) | .436 |
| Lateral rotation, deg | −0.9 (−2.6 to 0.9) | .230 | −0.9 (−2.6 to 0.7) | .183 |
| Medial tilt, deg | −0.7 (−2.0 to 0.5) | .210 | −0.9 (−2.3 to 0.5) | .138 |
| Lateral translation, mm | −0.8 (−2.1 to 0.5) | .199 | −1.0 (−2.6 to 0.5) | .195 |
| Anterior translation, mm | −0.4 (−1.2 to 0.5) | .391 | −0.1 (−0.7 to 0.5) | .572 |
| Superior translation, mm | 0.2 (−0.9 to 1.3) | .550 | 0.6 (−0.6 to 1.9) | .251 |
Values are expressed as mean and 95% confidence interval (CI) of the measurements from the anterior cruciate ligament reconstructed (ACLR) legs minus the measurements from the healthy legs. Significant differences associated with ACLR are boldface.
Figure 4.
Secondary tibiofemoral kinematics during active extension. Knees with anterior cruciate ligament reconstruction exhibited significantly (*P <.05) greater external rotation (P = .021) and a nonsignificant bias toward medial and anterior tibial translation. Kinematic curves are plotted as the mean ± 1 standard deviation.
Figure 5.
Patellofemoral kinematics during active extension against an inertial load exhibited no significant bilateral differences. Kinematic curves are plotted as the mean ± 1 standard deviation.
DISCUSSION
An understanding of the causes and implications of abnormal kinematics in ACLR knees is potentially important for reducing the high prevalence of anterior knee pain2,31 and early onset osteoarthritis32,37 in this population. In this study, we have shown that abnormal tibiofemoral kinematics can be elicited and measured in ACLR knees during active loaded movements within an MRI bore. Specifically, ACLR knees exhibit a more externally rotated tibia relative to the intact contralateral knee at a magnitude consistent with measurements during whole-body tasks, such as downhill running.23 Hence, it seems viable to use widely available MRI scanners to track physiologically relevant changes in knee mechanics after ACLR.
Dynamic imaging is crucial for identifying the relatively small changes in kinematics that can be present in surgically reconstructed knees. Motion analysis studies have identified some changes in ACLR knee kinetics during gait12,17,59 but generally lack the precision to track small kinematic shifts in nonsagittal rotations.5,48 Using biplane fluoroscopy, Tashman54 demonstrated that ACLR patients at 4 to 12 months after surgery exhibited a bias toward external tibial rotation during downhill running. Follow-up studies discovered a medial shift of the tibia in ACLR knees and a longitudinal tendency for increased anterior tibial translation, potentially due to graft relaxation over time.23 While we did not observe a statistically significant difference in ACLR tibial translation, we noted a bias toward a medial and anterior shift of similar magnitude as previously observed (Table 2).
The most salient result of this study was that loading could induce an external rotation shift in ACLR knees during an isolated knee flexion-extension task. The simple task used an inertial loading paradigm, which induced a lengthening quadriceps contraction with knee flexion and a subsequent shortening quadriceps contraction with knee extension.28 Similar active stretch-shortening quadriceps contractions are seen during the load acceptance phase of gait.6,60 While our device did not impose a compressive load to the foot as during gait, there is no difference in native ACL strain between open chain and closed chain knee flexion,7 indicating that the kinematic sensitivity to ACLR may be similar between locomotor and loaded flexion-extension tasks. Further, open chain exercise reduces hamstring co-contraction33 and anterior knee stability,61 potentially heightening the sensitivity of knee kinematics to the effect of the ACL graft.
While numerous studies have used dynamic imaging approaches to measure altered tibiofemoral kinematics in ACLR subjects, measures of patellofemoral kinematics have been limited to sequential static MRI under partial weightbearing52 or quasi-static fluoroscopy images of single-leg lunges.57 Patellofemoral joint behavior after ACLR is important to consider, given clinical reports of quadriceps weakness, knee flexion contractures, patellofem-oral pain, and osteoarthritis.27,41,47,53 Prior studies of altered patellofemoral kinematics after ACLR are mixed, providing no evidence of changes after ACLR in cadaveric25 and static in vivo imaging studies.52 However, a biplane fluoroscopy study of a quasi-static single-leg lunge found greater lateral patellar rotation, tilt, and translation in subjects who had undergone ACLR with a patellar tendon autograft.57 In the current study, we did not observe statistically significant kinematic differences in the patellofemoral joint of ACLR knees during motion, although we did see a small bias toward medial patellar rotation, lateral tilt, and medial translation (Table 2). Our task loaded the quadriceps in a flexed knee posture where the patella is confined within the trochlear groove. Thus, the effects of ACLR on patellofemoral kinematics in the loaded task may be relatively subtle, although the implications for cartilage tissue loading could still be pronounced.34
Our study population had mixed clinical presentations (eg, meniscal health) and had undergone varied surgical techniques (eg, graft selection3). Previous reports suggest different functional62 and clinical outcomes43 between knees reconstructed with patellar and hamstring tendon autografts, although potential differences in knee kinematics between knees treated with different grafts are less conclusive.58,59 Other variations in surgical parameters, such as tunnel position,11,22 initial tension,10 and fixation method,1 are also known to influence knee biomechanical behavior. This mixed presentation could contribute to the kinematic variability across our subjects and obscure smaller bilateral differences that may be clinically relevant. We could not test for the effect of graft type or surgical parameters due to a lack of statistical power, although we plan to explore these effects in future studies with increased enrollment.
The ability to image kinematic differences in ACLR knees within an MRI bore is attractive due to the modality’s ability to image soft tissue morphologic features and to measure quantitative biomarkers of composition. Models of cartilage morphologic characteristics derived from high-resolution static images can be coupled with accurate kinematics to determine the effects of abnormal kinematics on cartilage contact.9,24,30,56 We recently showed that the subtle changes in kinematics after ACLR, as seen in this study, can shift contact to more posterior regions of the tibial plateau.30 Further, recent advances in quantitative imaging have allowed indirect assessments of cartilage health via measures of cartilage proteoglycan content19 and collagen integrity,35 which are quantified using T1ρ and T2 relaxation rates, respectively. These tools have been used to find signs of early cartilage degeneration within 1 year after surgery, before the onset of morphological changes to the cartilage.35 By combining kinematics and cartilage contact with these morphological and compositional cartilage maps, MRI provides the opportunity of directly linking altered cartilage mechanics after ACLR with signs of early cartilage degeneration and the eventual onset of osteoarthritis.30
In summary, we used a novel dynamic MRI protocol to explore in vivo tibiofemoral and patellofemoral differences between healthy and ACLR knees under different loading conditions. We found that active knee extension against an inertial load induced significant shifts in tibiofemoral kinematics that were consistent with kinematic differences observed during complex, whole body movement. This study supports the use of coupled use of static, quantitative and dynamic MRI to explore potential links between abnormal knee mechanics and early osteoarthritis after ACLR.
Acknowledgments
The authors gratefully acknowledge the contributions of Rachel Lenhart, Colin Smith, Arezu Monawer, James Hermus, Kelli Hellenbrand, Sara John, and Christopher Westphal.
References
- 1.Anderson CJ, Westerhaus BD, Pietrini SD, et al. Kinematic impact of anteromedial and posterolateral bundle graft fixation angles on double-bundle anterior cruciate ligament reconstructions. Am J Sports Med. 2010;38(8):1575–1583. doi: 10.1177/0363546510364841. [DOI] [PubMed] [Google Scholar]
- 2.Bach BR, Jones GT, Sweet FA, Hager CA. Arthroscopy-assisted anterior cruciate ligament reconstruction using patellar tendon substitution two- to four-year follow-up results. Am J Sports Med. 1994;22(6):758–767. doi: 10.1177/036354659402200606. [DOI] [PubMed] [Google Scholar]
- 3.Barenius B, Nordlander M, Ponzer S, Tidermark J, Eriksson K. Quality of life and clinical outcome after anterior cruciate ligament reconstruction using patellar tendon graft or quadrupled semitendinosus graft: an 8-year follow-up of a randomized controlled trial. Am J Sports Med. 2010;38(8):1533–1541. doi: 10.1177/0363546510369549. [DOI] [PubMed] [Google Scholar]
- 4.Barrance PJ, Williams GN, Novotny JE, Buchanan TS. A method for measurement of joint kinematics in vivo by registration of 3-D geometric models with cine phase contrast magnetic resonance imaging data. J Biomech Eng. 2005;127(5):829. doi: 10.1115/1.1992524. [DOI] [PubMed] [Google Scholar]
- 5.Benoit DL, Ramsey DK, Lamontagne M, Xu L, Wretenberg P, Renström P. Effect of skin movement artifact on knee kinematics during gait and cutting motions measured in vivo. Gait Posture. 2006;24(2):152–164. doi: 10.1016/j.gaitpost.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Besier TF, Fredericson M, Gold GE, Beaupre GS, Delp SL. Knee muscle forces during walking and running in patellofemoral pain patients and pain-free controls. J Biomech. 2009;42(7):8. doi: 10.1016/j.jbiomech.2009.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beynnon BD, Johnson RJ, Fleming BC, Stankewich CJ, Renström PA, Nichols CE. The strain behavior of the anterior cruciate ligament during squatting and active flexion-extension: a comparison of an open and a closed kinetic chain exercise. Am J Sports Med. 1997;25(6):823–829. doi: 10.1177/036354659702500616. [DOI] [PubMed] [Google Scholar]
- 8.Bignozzi S, Zaffagnini S, Lopomo N, Fu FH, Irrgang JJ, Marcacci M. Clinical relevance of static and dynamic tests after anatomical double-bundle ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2009;18(1):37–42. doi: 10.1007/s00167-009-0853-6. [DOI] [PubMed] [Google Scholar]
- 9.Borotikar BS, Sheehan FT. In vivo patellofemoral contact mechanics during active extension using a novel dynamic MRI-based methodology. Osteoarthritis Cartilage. 2013;21:1886–1894. doi: 10.1016/j.joca.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brady MF, Bradley MP, Fleming BC, Fadale PD, Hulstyn MJ, Banerjee R. Effects of initial graft tension on the tibiofemoral compressive forces and joint position after anterior cruciate ligament reconstruction. Am J Sports Med. 2006;35(3):395–403. doi: 10.1177/0363546506294363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brophy RH, Pearle AD. Single-bundle anterior cruciate ligament reconstruction: a comparison of conventional, central, and horizontal single-bundle virtual graft positions. Am J Sports Med. 2009;37(7):1317–1323. doi: 10.1177/0363546509333007. [DOI] [PubMed] [Google Scholar]
- 12.Butler RJ, Minick KI, Ferber R, Underwood F. Gait mechanics after ACL reconstruction: implications for the early onset of knee osteoarthritis. Br J Sports Med. 2009;43(5):366–370. doi: 10.1136/bjsm.2008.052522. [DOI] [PubMed] [Google Scholar]
- 13.Carlson VR, Boden BP, Sheehan FT. Patellofemoral kinematics and tibial tuberosity-trochlear groove distances in female adolescents with patellofemoral pain. Am J Sports Med. 2016;45(5):1102–1109. doi: 10.1177/0363546516679139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlson VR, Sheehan FT, Shen A, Yao L, Jackson JN, Boden BP. The relationship of static tibial tubercle-trochlear groove measurement and dynamic patellar tracking. Am J Sports Med. 2017;45(8):1856–1863. doi: 10.1177/0363546517700119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carpenter RD, Majumdar S, Ma CB. Magnetic resonance imaging of 3-dimensional in vivo tibiofemoral kinematics in anterior cruciate ligament-reconstructed knees. Arthroscopy. 2009;25(7):760–766. doi: 10.1016/j.arthro.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Ciccotti MG, Kerlan RK, Perry J, Pink M. An electromyographic analysis of the knee during functional activities, II: the anterior cruciate ligament-deficient and -reconstructed profiles. Am J Sports Med. 1994;22(5):651–658. doi: 10.1177/036354659402200513. [DOI] [PubMed] [Google Scholar]
- 17.DeVita P, Hortobagyi T, Barrier J. Gait biomechanics are not normal after anterior cruciate ligament reconstruction and accelerated rehabilitation. Med Sci Sports Exerc. 1998;30:1481–1488. doi: 10.1097/00005768-199810000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology. 2004;232(2):592–598. doi: 10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duvvuri U, Kudchodkar S, Reddy R, Leigh JS. T1ρ relaxation can assess longitudinal proteoglycan loss from articular cartilage in vitro. Osteoarthritis Cartilage. 2002;10(11):838–844. doi: 10.1053/joca.2002.0826. [DOI] [PubMed] [Google Scholar]
- 20.Gokeler A, Hof AL, Arnold MP, Dijkstra PU, Postema K, Otten E. Abnormal landing strategies after ACL reconstruction. Scand J Med Sci Sports. 2010;20(1):12–21. doi: 10.1111/j.1600-0838.2008.00873.x. [DOI] [PubMed] [Google Scholar]
- 21.Grood E, Suntay W. A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng. 1983;105(2):9. doi: 10.1115/1.3138397. [DOI] [PubMed] [Google Scholar]
- 22.Hefzy M, Grood E. Sensitivity of insertion locations on length patterns of anterior cruciate ligament fibers. J Biomech Eng. 1986;108(1):73–82. doi: 10.1115/1.3138583. [DOI] [PubMed] [Google Scholar]
- 23.Hofbauer M, Thorhauer ED, Abebe E, Bey M, Tashman S. Altered tibiofemoral kinematics in the affected knee and compensatory changes in the contralateral knee after anterior cruciate ligament reconstruction. Am J Sports Med. 2014;42(11):2715–2721. doi: 10.1177/0363546514549444. [DOI] [PubMed] [Google Scholar]
- 24.Hosseini A, Van de Velde S, Gill TJ, Li G. Tibiofemoral cartilage contact biomechanics in patients after reconstruction of a ruptured anterior cruciate ligament. J Orthop Res. 2012;30(11):1781–1788. doi: 10.1002/jor.22122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsieh YF, Draganich LF, Ho SH, Reider B. The effects of removal and reconstruction of the anterior cruciate ligament on patellofemoral kinematics. Am J Sports Med. 1998;26(2):201–209. doi: 10.1177/03635465980260020901. [DOI] [PubMed] [Google Scholar]
- 26.Imhauser C, Mauro C, Choi D, et al. Abnormal tibiofemoral contact stress and its association with altered kinematics after center-center anterior cruciate ligament reconstruction: an in vitro study. Am J Sports Med. 2013;41(4):815–825. doi: 10.1177/0363546512475205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Järvelä T, Paakkala T, Kannus P, Järvinen M. The incidence of patellofemoral osteoarthritis and associated findings 7 years after anterior cruciate ligament reconstruction with a bone-patellar tendon-bone autograft. Am J Sports Med. 2001;29(1):18–24. doi: 10.1177/03635465010290010701. [DOI] [PubMed] [Google Scholar]
- 28.Kaiser J, Bradford R, Johnson K, Wieben O, Thelen DG. Measurement of tibiofemoral kinematics using highly accelerated 3D radial sampling. Magn Reson Med. 2013;69(5):1310–1316. doi: 10.1002/mrm.24362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaiser J, Monawer A, Chaudhary R, et al. Accuracy of model-based tracking of knee kinematics and cartilage contact measured by dynamic volumetric MRI. Med Eng Physics. 2016;38(10):1131–1135. doi: 10.1016/j.medengphy.2016.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaiser J, Vignos MF, Liu F, Kijowski R, Thelen DG. American Society of Biomechanics Clinical Biomechanics Award 2015: MRI assessments of cartilage mechanics, morphology and composition following reconstruction of the anterior cruciate ligament. Clin Biomech. 2016;34:38–44. doi: 10.1016/j.clinbiomech.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kartus J, Magnusson L, Stener S, Brandsson S, Eriksson BI, Karlsson J. Complications following arthroscopic anterior cruciate ligament reconstruction: a 2–5-year follow-up of 604 patients with special emphasis on anterior knee pain. Knee Surg Sports Traumatol Arthrosc. 1999;7(1):2–8. doi: 10.1007/s001670050112. [DOI] [PubMed] [Google Scholar]
- 32.Kessler MA, Behrend H, Henz S, Stutz G, Rukavina A, Kuster MS. Function, osteoarthritis and activity after ACL-rupture: 11 years follow-up results of conservative versus reconstructive treatment. Knee Surg Sports Traumatol Arthrosc. 2008;16(5):442–448. doi: 10.1007/s00167-008-0498-x. [DOI] [PubMed] [Google Scholar]
- 33.Kvist J, Gillquist J. Sagittal plane knee translation and electromyographic activity during closed and open kinetic chain exercises in anterior cruciate ligament-deficient patients and control subjects. Am J Sports Med. 2001;29(1):72–82. doi: 10.1177/03635465010290011701. [DOI] [PubMed] [Google Scholar]
- 34.Lenhart RL, Smith CR, Vignos MF, Kaiser J, Heiderscheit BC, Thelen DG. Influence of step rate and quadriceps load distribution on patellofemoral cartilage contact pressures during running. J Biomech. 2015;48(11):2871–2878. doi: 10.1016/j.jbiomech.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Kuo D, Theologis A, et al. Cartilage in anterior cruciate ligament-reconstructed knees: MR T1rho and T2-initial experience with 1-year follow-up. Radiology. 2011;258(2):10. doi: 10.1148/radiol.10101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Logan MC, Williams A, Lavelle J, Gedroyc W, Freeman M. Tibiofemoral kinematics following successful anterior cruciate ligament reconstruction using dynamic multiple resonance imaging. Am J Sports Med. 2004;32(4):984–992. doi: 10.1177/0363546503261702. [DOI] [PubMed] [Google Scholar]
- 37.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 38.Meyer FG, Constable RT, Sinusas AJ, Duncan JS. Tracking myocardial deformation using phase contrast MR velocity fields: a stochastic approach. IEEE Trans Med Imaging. 1996;15(4):453–465. doi: 10.1109/42.511749. [DOI] [PubMed] [Google Scholar]
- 39.Miranda DL, Rainbow MJ, Leventhal EL, Crisco JJ, Fleming BC. Automatic determination of anatomical coordinate systems for three-dimensional bone models of the isolated human knee. J Biomech. 2010;43(8):4. doi: 10.1016/j.jbiomech.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nebelung S, Deitmer G, Gebing R, Reichwein F, Nebelung W. Improved outcomes after anterior cruciate ligament reconstruction with quadrupled hamstring autografts and additional bone plug augmentation at five year follow-up. Int Orthop. 2012;37(3):399–405. doi: 10.1007/s00264-012-1542-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neuman P, Kostogiannis I, Friden T, Roos H, Dahlberg LE, Englund M. Patellofemoral osteoarthritis 15 years after anterior cruciate ligament injury—a prospective cohort study. Osteoarthritis Cartilage. 2009;17(3):284–290. doi: 10.1016/j.joca.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Pelc NJ, Drangova M, Pelc LR, et al. Tracking of cyclic motion with phase-contrast cine MR velocity data. J Magn Reson Imaging. 1995;5(3):339–345. doi: 10.1002/jmri.1880050319. [DOI] [PubMed] [Google Scholar]
- 43.Pinczewski LA, Lyman J, Salmon LJ, Russell VJ, Roe J, Linklater J. A 10-year comparison of anterior cruciate ligament reconstructions with hamstring tendon and patellar tendon autograft. Am J Sports Med. 2007;35(4):564–574. doi: 10.1177/0363546506296042. [DOI] [PubMed] [Google Scholar]
- 44.Powell MJD. An efficient method for finding the minimum of a function of several variables without calculating derivatives. Comput J. 1964;7(2):155. [Google Scholar]
- 45.Rainbow MJ, Miranda DL, Cheung RT, et al. Automatic determination of an anatomical coordinate system for a three-dimensional model of the human patella. J Biomech. 2013;46(12):2093–2096. doi: 10.1016/j.jbiomech.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Regatte RR, Akella SV, Lonner JH, Kneeland JB, Reddy R. T1rho relaxation mapping in human osteoarthritis (OA) cartilage: comparison of T1rho with T2. J Magn Reson Imaging. 2006;23(4):547–553. doi: 10.1002/jmri.20536. [DOI] [PubMed] [Google Scholar]
- 47.Sachs RA, Daniel DM, Stone ML, Garfein RF. Patellofemoral problems after anterior cruciate ligament reconstruction. Am J Sports Med. 1989;17(6):760–765. doi: 10.1177/036354658901700606. [DOI] [PubMed] [Google Scholar]
- 48.Sati M, De Guise J, Larouche S, Drouin G. Quantitative assessment of skin-bone movement at the knee. Knee. 1996;3(3):121–138. [Google Scholar]
- 49.Scanlan SF, Chaudhari AM, Dyrby CO, Andriacchi TP. Differences in tibial rotation during walking in ACL reconstructed and healthy contralateral knees. J Biomech. 2010;43(9):1817–1822. doi: 10.1016/j.jbiomech.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scarvell J, Smith P, Refshauge K, Galloway H, Woods K. Does anterior cruciate ligament reconstruction restore normal knee kinematics? A prospective MRI analysis over two years. J Bone Joint Surg Br. 2006;88(3):324–330. doi: 10.1302/0301-620X.88B3.16787. [DOI] [PubMed] [Google Scholar]
- 51.Sheehan FT, Zajac FE, Drace JE. Using cine phase contrast magnetic resonance imaging to non-invasively study in vivo knee dynamics. J Biomech. 1998;31(1):6. doi: 10.1016/s0021-9290(97)00109-7. [DOI] [PubMed] [Google Scholar]
- 52.Shin CS, Carpenter RD, Majumdar S, Ma CB. Three-dimensional in vivo patellofemoral kinematics and contact area of anterior cruciate ligament-deficient and -reconstructed subjects using magnetic resonance imaging. Arthroscopy. 2009;25(11):1214–1223. doi: 10.1016/j.arthro.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 53.Shino K, Nakagawa S, Inoue M, Horibe S, Yoneda M. Deterioration of patellofemoral articular surfaces after anterior cruciate ligament reconstruction. Am J Sports Med. 1993;21(2):206–211. doi: 10.1177/036354659302100208. [DOI] [PubMed] [Google Scholar]
- 54.Tashman S. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32(4):975–983. doi: 10.1177/0363546503261709. [DOI] [PubMed] [Google Scholar]
- 55.Tashman S, Kolowich P, Collon D, Anderson K, Anderst W. Dynamic function of the ACL-reconstructed knee during running. Clin Orthop Relat Res. 2007;454:66–73. doi: 10.1097/BLO.0b013e31802bab3e. [DOI] [PubMed] [Google Scholar]
- 56.Thorhauer E, Tashman S. Validation of a method for combining biplanar radiography and magnetic resonance imaging to estimate knee cartilage contact. Med Eng Physics. 2015;37(10):937–947. doi: 10.1016/j.medengphy.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van de Velde SK, Gill TJ, DeFrate LE, Papannagari R, Li G. The effect of anterior cruciate ligament deficiency and reconstruction on the patellofemoral joint. Am J Sports Med. 2008;36(6):1150–1159. doi: 10.1177/0363546508314404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Webster KE, Feller JA. The knee adduction moment in hamstring and patellar tendon anterior cruciate ligament reconstructed knees. Knee Surg Sports Traumatol Arthrosc. 2012;20(11):2214–2219. doi: 10.1007/s00167-011-1835-z. [DOI] [PubMed] [Google Scholar]
- 59.Webster KE, Wittwer JE, O’Brien J, Feller JA. Gait patterns after anterior cruciate ligament reconstruction are related to graft type. Am J Sports Med. 2005;33(2):247–254. doi: 10.1177/0363546504266483. [DOI] [PubMed] [Google Scholar]
- 60.Whittington B, Silder A, Heiderscheit B, Thelen DG. The contribution of passive-elastic mechanisms to lower extremity joint kinetics during human walking. Gait Posture. 2008;27:7. doi: 10.1016/j.gaitpost.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yack HJ, Collins CE, Whieldon TJ. Comparison of closed and open kinetic chain exercise in the anterior cruciate ligament-deficient knee. Am J Sports Med. 1993;21(1):49–54. doi: 10.1177/036354659302100109. [DOI] [PubMed] [Google Scholar]
- 62.Zaffagnini S, Bruni D, Marcheggiani Muccioli GM, et al. Single-bundle patellar tendon versus non-anatomical double-bundle hamstrings ACL reconstruction: a prospective randomized study at 8-year minimum follow-up. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):390–397. doi: 10.1007/s00167-010-1225-y. [DOI] [PubMed] [Google Scholar]