Selection bias calls into question whether natural regeneration is really more successful than active tropical forest restoration.
Abstract
Several recent meta-analyses have aimed to determine whether natural regeneration is more effective at recovering tropical forests than active restoration (for example, tree planting). We reviewed this literature and found that comparisons between strategies are biased by positive site selection. Studies of natural forest regeneration are generally conducted at sites where a secondary forest was already present, whereas tree planting studies are done in a broad range of site conditions, including non-forested sites that may not have regenerated in the absence of planting. Thus, a level of success in forest regeneration is guaranteed for many studies representing natural regeneration, but not for those representing active restoration. The complexity of optimizing forest restoration is best addressed by paired experimentation at the same site, replicated across landscapes. Studies that have taken this approach reach different conclusions than those arising from meta-analyses; the results of paired experimental comparisons emphasize that natural regeneration is a highly variable process and that active restoration and natural regeneration are complementary strategies.
How and where to restore tropical forests have become topics of global importance for addressing the interrelated challenges of climate change, biodiversity loss, and desertification (1–3). A suite of recent meta-analyses have attempted to advance the state of the science by comparing active forest restoration or reforestation approaches (for example, tree planting) to natural regeneration (that is, forest regeneration with little or no intervention by humans) (4–6). On the basis of comparisons across many studies, these meta-analyses find little to no advantage in actively intervening to catalyze forest succession. For example, Crouzeilles et al. (6) determined that naturally regenerated tropical forests have higher plant diversity, tree density, and tree height than forests established by active restoration, and they concluded that tropical forest restoration is more successful using the former approach. However, this and other meta-analytical data sets contain an inherent, positive selection bias for naturally regenerated forests that casts doubt on the robustness of these conclusions.
The bias in these meta-analyses arises from a mismatch between the pool of studies that contain data about active forest restoration and the pool of studies that contain data about natural forest regeneration (Fig. 1). With few exceptions, studies of natural regeneration and active restoration have been conducted at separate locations. Most studies evaluating natural regeneration, for instance, occurred in sites where secondary forest was present at the start of the study [for example, (7–10)]. In contrast, active tree planting studies are typically initiated on entirely deforested sites [for example, (11–13)], including sites that likely would not have established at all without assistance (for example, strip mines). Hence, many natural regeneration studies focus on forests that have already passed through a filter that excludes less resilient, more degraded sites where forest succession would be slow or stalled. Active restoration studies are not exposed to the same site selection filter, so it is hardly surprising that active restoration should perform weakly in meta-analyses when compared to secondary forests that had already established.
Fig. 1. Positive selection bias in recent meta-analyses comparing active restoration to natural regeneration.
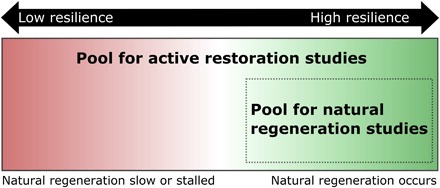
For example, take a tree plantation where seedlings were planted on degraded agricultural land, and compare that plantation to a 10-year-old secondary forest discovered on post-agricultural land. All else being equal, the plantation has the opportunity to fail to establish, but if the secondary forest had not already established it would never have been considered as a study site.
Documented cases of slow or arrested forest succession are widespread throughout the tropics [for example, (14–18)]. These and other studies attribute slow or stalled recovery to a suite of factors including the severity of prior land use, the distance to propagule sources, and the intrinsic resilience of species that make up a given community (19). Meta-analyses comparing active restoration and natural forest regeneration have sometimes attempted to statistically control for some of these factors, but what limits forest growth at a given site can be idiosyncratic and there may not be enough replication in the literature to conduct a rigorous meta-analysis. A few of these factors include soil quality (20, 21), fire (22), presence of seed-dispersing animals (23), competition from exotic grasses (24), and herbivory (25). Moreover, statistical controls still fail to establish a counterfactual; a rigorous meta-analysis would have to select only those natural regeneration studies in which failed regeneration was a possibility.
In some cases, meta-analyses are further complicated by questionable categorizations of actions that qualify as active restoration or natural regeneration. For instance, Parrota and Knowles (26) evaluated a restoration treatment at a bauxite mine in Brazil that included using heavy machinery to level the overburden and replace 15 cm of topsoil and woody debris from a stockpile. This treatment was considered to be “natural regeneration” by Crouzeilles et al. (6), but without this expensive human intervention, it is doubtful that forest would have regenerated on the bare, mined surface. Likewise, Crouzeilles et al. (6) considered plantations of exotic, agroforestry trees to be active restoration (27, 28) and compared these to the rapid, natural regeneration of selectively logged forests (29); neither treatment is widely representative of projects seeking to restore native tropical forests.
The complexity of conditions that can influence whether natural regeneration will be slow or stalled highlights the need for paired evaluations of active restoration and natural regeneration at the same site (30). The relatively small number of studies where this has been done highlights the fact that the process of natural regeneration can be highly variable, sometimes producing a secondary forest quickly (31) and sometimes not (32). Similar conclusions are reported from a large-scale analysis of >2000 Brazilian forest restoration projects spanning nearly 700,000 ha. Comparing passive and active restoration at sites that were all initially deforested, Brancalion et al. (33) found that, although active restoration and natural regeneration both had variable outcomes, tree planting generally increased canopy cover and tree and shrub diversity. Given the discrepancies between meta-analyses and site-based comparisons, definitive evaluation of the effects of tree planting on the pace of tropical forest recovery would benefit from a systematic review using formal quality assessment (34).
For restoration practitioners seeking evidence-based management strategies, a take-home message from the empirical literature is that it is often worthwhile to observe natural forest recovery for a year or two to assess if natural regeneration will accomplish management objectives before deciding whether some form of active intervention is needed (19). If an adaptive management process indicates that action is warranted, possible interventions range from low-intensity assisted natural regeneration to higher-intensity plantations (35). Despite the implication by recent meta-analyses that active restoration and natural regeneration are competitive and mutually exclusive strategies, in reality, human interventions to restore tropical forests fall on a continuous gradient of intensity and are often synergistic.
Acknowledgments
We thank K. Holl, R. Cole, A. Kulikowski, J. Aronson, E. Fernandez Barrancos, and I. Loza for the stimulating discussions and two anonymous reviewers for comments on an earlier version of the manuscript. Author contributions: J.L.R., M.E.F., and R.A.Z. conceived and wrote this manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper. Additional data related to this paper may be requested from the authors.
REFERENCES AND NOTES
- 1.United Nations Convention on Biological Diversity, Decision adopted by the conference of the parties to the Convention on Biological Diversity at its eleventh meeting. XI/16. Ecosystem restoration (United Nations Convention on Biological Diversity, 2012).
- 2.United Nations Convention to Combat Desertification, Land matters for climate. Reducing the gap and approaching the target (United Nations Convention to Combat Desertification, 2015).
- 3.United Nations Framework Convention on Climate Change, Adoption of the Paris Agreement (United Nations Framework Convention on Climate Change, 2015).
- 4.Meli P., Holl K. D., Benayas J. M. R., Jones H. P., Jones P. C., Montoya D., Mateos D. M., A global review of past land use, climate, and active vs. passive restoration effects on forest recovery. PLOS ONE 12, e0171368 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonner M. T. L., Schmidt S., Shoo L. P., A meta-analytical global comparison of aboveground biomass accumulation between tropical secondary forests and monoculture plantations. For. Ecol. Manage. 291, 73–86 (2013). [Google Scholar]
- 6.Crouzeilles R., Ferreira M. S., Chazdon R. L., Lindenmayer D. B., Sansevero J. B. B., Monteiro L., Iribarrem A., Latawiec A. E., Strassburg B. B. N., Ecological restoration success is higher for natural regeneration than for active restoration in tropical forests. Sci. Adv. 3, e1701345 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Letcher S. G., Chazdon R. L., Rapid recovery of biomass, species richness, and species composition in a forest chronosequence in northeastern Costa Rica. Biotropica 41, 608–617 (2009). [Google Scholar]
- 8.Marín-Spiotta E., Ostertag R., Silver W. L., Long-term patterns in tropical reforestation: Plant community composition and aboveground biomass accumulation. Ecol. Appl. 17, 828–839 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Hughes R. F., Kauffman J. B., Jaramillo V. J., Biomass, carbon, and nutrient dynamics of secondary forests in a humid tropical region of México. Ecology 80, 1892–1907 (1999). [Google Scholar]
- 10.Sierra C. A., del Valle J. I., Orrego S. A., Moreno F. H., Harmon M. E., Zapata M., Colorado G. J., Herrera M. A., Lara W., Restrepo D. E., Berrouet L. M., Loaiza L. M., Benjumea J. F., Total carbon stocks in a tropical forest landscape of the Porce region, Colombia. For. Ecol. Manage. 243, 299–309 (2007). [Google Scholar]
- 11.Dominguez-Haydar Y., Armbrecht I., Response of ants and their seed removal in rehabilitation areas and forests at El Cerrejón coal mine in Colombia. Restor. Ecol. 19, 178–184 (2011). [Google Scholar]
- 12.MacGregor-Fors I., Blanco-García A., Lindig-Cisneros R., Bird community shifts related to different forest restoration efforts: A case study from a managed habitat matrix in Mexico. Ecol. Eng. 36, 1492–1496 (2010). [Google Scholar]
- 13.Nakamura A., Proctor H., Catterall C. P., Using soil and litter arthropods to assess the state of rainforest restoration. Ecol. Manage. Restor. 4, S20–S28 (2003). [Google Scholar]
- 14.Lamb D., Erskine P. D., Parrotta J. A., Restoration of degraded tropical forest landscapes. Science 310, 1628–1632 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Arroyo-Rodríguez V., Melo F. P. L., Martínez-Ramos M., Bongers F., Chazdon R. L., Meave J. A., Norden N., Santos B. A., Leal I. R., Tabarelli M., Multiple successional pathways in human-modified tropical landscapes: New insights from forest succession, forest fragmentation and landscape ecology research. Biol. Rev. 92, 326–340 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Uhl C., Buschbacher R., Serrao E. A. S., Abandoned pastures in eastern Amazonia. I. Patterns of plant succession. J. Ecol. 76, 663–681 (1988). [Google Scholar]
- 17.Pareliussen I., Olsson E. G. A., Armbruster W. S., Factors limiting the survival of native tree seedlings used in conservation efforts at the edges of forest fragments in upland Madagascar. Restor. Ecol. 14, 196–203 (2006). [Google Scholar]
- 18.Blackham G. V., Webb E. L., Corlett R. T., Natural regeneration in a degraded tropical peatland, Central Kalimantan, Indonesia: Implications for forest restoration. For. Ecol. Manage. 324, 8–15 (2014). [Google Scholar]
- 19.Holl K. D., Aide T. M., When and where to actively restore ecosystems? For. Ecol. Manage. 261, 1558–1563 (2011). [Google Scholar]
- 20.Carpenter F. L., Mayorga S. P., Quintero E. G., Schroeder M., Land-use and erosion of a Costa Rican Ultisol affect soil chemistry, mycorrhizal fungi and early regeneration. For. Ecol. Manage. 144, 1–17 (2001). [Google Scholar]
- 21.Araújo A. S. F., Cesarz S., Leite L. F. C., Borges C. D., Tsai S. M., Eisenhauer N., Soil microbial properties and temporal stability in degraded and restored lands of Northeast Brazil. Soil Biol. Biochem. 66, 175–181 (2013). [Google Scholar]
- 22.Aide T. M., Zimmerman J. K., Pascarella J. B., Rivera L., Marcano-Vega H., Forest regeneration in a chronosequence of tropical abandoned pastures: Implications for restoration ecology. Restor. Ecol. 8, 328–338 (2000). [Google Scholar]
- 23.Caves E. M., Jennings S. B., HilleRisLambers J., Tewksbury J. J., Rogers H. S., Natural experiment demonstrates that bird loss leads to cessation of dispersal of native seeds from intact to degraded forests. PLOS ONE 8, e65618 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caughlin T. T., Elliott S., Lichstein J. W., When does seed limitation matter for scaling up reforestation from patches to landscapes? Ecol. Appl. 26, 2439–2450 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Cole R. J., Litton C. M., Vegetation response to removal of non-native feral pigs from Hawaiian tropical montane wet forest. Biol. Invasions 16, 125–140 (2014). [Google Scholar]
- 26.Parrotta J. A., Knowles O. H., Restoration of tropical moist forests on bauxite-mined lands in the Brazilian Amazon. Restor. Ecol. 7, 103–116 (1999). [Google Scholar]
- 27.Fimbel R. A., Fimbel C. C., The role of exotic conifer plantations in rehabilitating degraded tropical forest lands: A case study from the Kibale Forest in Uganda. For. Ecol. Manage. 81, 215–226 (1996). [Google Scholar]
- 28.Beukema H., van Noordwijk M., Terrestrial pteridophytes as indicators of a forest-like environment in rubber production systems in the lowlands of Jambi, Sumatra. Agric. Ecosyst. Environ. 104, 63–73 (2004). [Google Scholar]
- 29.Bicknell J., Peres C. A., Vertebrate population responses to reduced-impact logging in a neotropical forest. For. Ecol. Manage. 259, 2267–2275 (2010). [Google Scholar]
- 30.Shoo L. P., Catterall C. P., Stimulating natural regeneration of tropical forest on degraded land: Approaches, outcomes, and information gaps. Restor. Ecol. 21, 670–677 (2013). [Google Scholar]
- 31.Gilman A. C., Letcher S. G., Fincher R. M., Perez A. I., Madell T. W., Finkelstein A. L., Corrales-Araya F., Recovery of floristic diversity and basal area in natural forest regeneration and planted plots in a Costa Rican wet forest. Biotropica 48, 798–808 (2016). [Google Scholar]
- 32.Holl K. D., Zahawi R. A., Factors explaining variability in woody above-ground biomass accumulation in restored tropical forest. For. Ecol. Manage. 319, 36–43 (2014). [Google Scholar]
- 33.Brancalion P. H. S., Schweizer D., Gaudare U., Mangueira J. R., Lamonato F., Farah F. T., Nave A. G., Rodrigues R. R., Balancing economic costs and ecological outcomes of passive and active restoration in agricultural landscapes: The case of Brazil. Biotropica 48, 856–867 (2016). [Google Scholar]
- 34.Bilotta G. S., Milner A. M., Boyd I. L., Quality assessment tools for evidence from environmental science. Environ. Evid. 3, 14 (2014). [Google Scholar]
- 35.S. D. Elliott, D. Blakesley, K. Hardwick, Restoring Tropical Forests: A Practical Guide (Kew Publishing, 2013). [Google Scholar]


