Abstract
Cardiovascular disease has earned its place as one of the leading non-communicable diseases that has become a modern-day global epidemic. The rising incidence and prevalence of Chronic Kidney Disease (CKD) has added to this enormous burden, given that CKD is now recognized as an established risk factor for accelerated cardiovascular disease. In fact, cardiovascular disease remains the leading cause of death in the CKD population with significant prognostic implications. Alterations in vitamin D levels as renal function declines has been invariably linked to the development of cardiovascular disease beyond a mere epiphenomenon, and has become an important focus in recent years in our search for new therapies. Another compound, cinacalcet that belongs to the calcimimetic class of agents has also taken center stage over the past few years as a potential cardiovasculo-protective agent. However, given limited well-designed randomized trials to inform us, our clinical practice for the management of cardiovascular disease in CKD has not been refined adequately. This article considers the biological mechanisms, regulation and current experimental, clinical and trial data available to help guide the therapeutic use of vitamin D and calcimmetics in the setting of CKD and cardiovascular disease.
Keywords: Vitamin D, calcimimetics, calcium sensing receptors (CaSRs), cardiovascular, left ventricular function, vascular calcification, arterial stiffening, heart failure
Introduction
Over the course of the last century, the world has witnessed a striking epidemiological transition in the predominant causes of death, from infectious diseases and nutritional deficiencies to non-communicable diseases1. Among the non-communicable diseases, cardiovascular diseases (CVD) has become the modern-day global epidemic and a leading cause of death2. The staggering health and health-care economic burden of these non-communicable diseases taxed upon both the developing and developed nations alike became the focal point of the global heads of state assembly at the United Nations High-Level Meeting in 2011. This was the first time the United Nations tackled a health issue since the HIV/AIDS epidemic in 2001 and formally recognized the threat that non-communicable conditions such as CVD constitutes for the twenty-first century3.
Models that include population aging, increasing rates of urbanization and globalization, and health behaviors that raise the burden of cardiovascular risk factors for ischemic heart disease (IHD) partially account for this CVD epidemiological transition4. Other well-established modifiable risk factors for CVD include hyperlipidemia, smoking, hypertension and diabetes. However, in recent years, Chronic Kidney Disease (CKD) has emerged as a powerful risk factor for the development of accelerated CVD5. In fact, CVD is the leading cause of death in patients with CKD and renal function decline has significant prognostic implications.
End-stage renal disease (ESRD) patients on dialysis have a 10 to 30-fold higher cardiovascular mortality rate compared to the general population despite stratification for sex, gender and race5. Most cardiovascular deaths in ESRD are attributable to arrhythmia and congestive heart failure and ischemic heart disease that can lead to sudden cardiac death6. Among patients who experience an ACS, 40% of these patients have at least a moderate degree of decline in kidney function with an eGFR below 60 ml min−1 1.73 m−2 7. The risk of ACS is directly related to declining renal function3. Furthermore, these patients have a 1 year mortality rate of approximately 25% compared with 5% in patients with normal renal function8.
The development of accelerated CVD that occurs as renal function declines involves a multitude of highly complex pathogenic pathways, involving abnormal mineral and bone metabolism, uremic toxins, inflammation, anemia, sympathetic nerve activation, activation of the renin-angiotensin-aldosterone system (RAAS) and endothelial dysfunction9. Central to these pathways are complex interactions between several target organs that include the kidney, bone, vascular and cardiac systems. Interactions between these organ systems form critical homeostatic endocrine loops that help ensure calcium and phosphorus are highly regulated. The effect of this tight regulation is to maintain appropriate skeletal mineralization and avoid extraskeletal calcification10. Failure of this system therefore leads to two inter-related disease processes, overt mineral bone disease (MBD) and CVD. As kidney function declines, breakdown of the kidney-bone, kidney-vascular and kidney-heart axes can lead to high calcium and phosphate levels and increasing parathyroid hormone (PTH), fibroblast growth factor (FGF)-23 levels, low Klotho levels and reduced synthesis of active 1,25-dihydroxyvitamin D levels. Alterations in each of these component variables have been invariably linked to the development of progressive CVD and accelerated age-related changes of the cardiovascular system5,11.
Therapeutic strategies to prevent or treat accelerated CVD in renal failure have largely focused on targeting one or more of these endocrine or mineral alterations. Traditionally, dietary phosphate control or use of various phosphate binders in parallel with the control of hypertension and statin therapy have been the mainstay of CVD prevention in CKD. However, therapeutic strategies involving vitamin D and its analogues, and calcimimetics have garnished increasing attention over the past few years. Both vitamin D and calcimimetics are widely used for the treatment of secondary hyperparathyroidism in CKD. Many experimental and observational studies have linked vitamin D deficiency and the high PTH, high phosphate and high calcium milieu associated with advanced secondary hyperparathyroidism to various cardiovascular outcomes in CKD12,13. Therefore, it has made sense for nephrologist to target these changes with vitamin D and calcimimetics. Careful follow-up of these component alterations are therefore important in CKD-MBD management as outlined in the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines14.
However, alterations of the serum levels of these individual components are insufficient to guide therapy and studies that look beyond these changes to cardiovascular outcomes and hard clinical endpoints such as cardiovascular or all-cause mortality are demanded. Despite this, few well-designed randomized controlled trials are available to help guide vitamin D and calcimimetic therapy for the treatment of MBD and life-limiting CVD in the CKD population. As a result, clinicians are left with potentially equivocal recommendations that place a large reliance on serum PTH to help guide these therapies. This has left uncertainty in the field of nephrology. This article reviews the plausible biological mechanisms, human studies and available clinical trial data for the use of vitamin D and calcimimetics. Given the multiple redundant pathways that link the cardiac and renal systems, specifically we will focus on the application of vitamin D and calcimimetics for the management of CVD in patients with advanced CKD.
VITAMIN D THERAPY
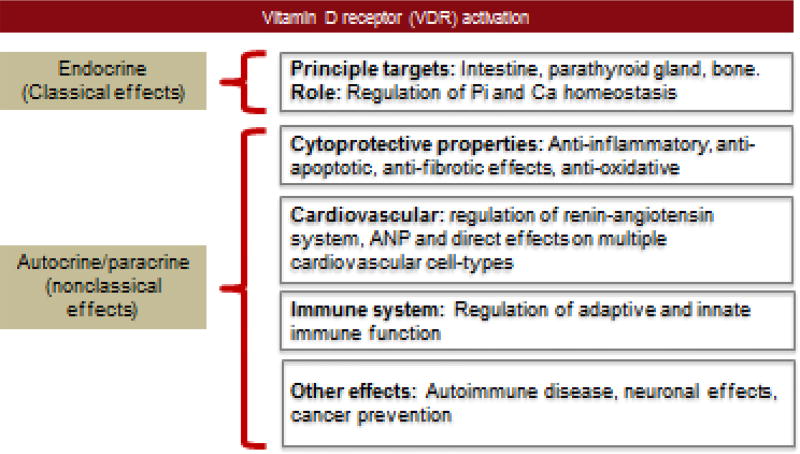
Vitamin D is indispensable to human health and plays a critical role in the integration and regulation of multiple physiological and metabolic systems. Vitamin D effects can be thought off as having two main functional arms: endocrine or “classical” functions mediated by circulating vitamin D produced by the kidney, and autocrine/paracrine “non-classical” functions that are exerted by locally produced extra-renal vitamin D (figure 1). Endocrine functions of vitamin D mediate a complex interplay between the kidney, bone, parathyroid gland and intestine that is largely involved in regulating mineral homeostasis. Given the widespread expression of 1α-hydroxylase and the vitamin D receptors (VDR) across many organ systems, both endocrine and locally produced autocrine/paracrine vitamin D have been found to exert pleiotropic effects in the regulation of normal organ physiology and exert cytoprotective functions (figure 1).
Figure 1. Functions of Vitamin D.
Vitamin D deficiency is a global public health problem and is a highly prevalent condition in CKD patients, with estimates as high as 70–80% in some studies15. Despite the introduction of international and European guidelines to supplement vitamin D in the dialysis population, reports over the past decade consistently show vitamin D deficiency in this population. Both active 1,25-dihydroxyvitamin D and nutritional 25-hydroxyvitamin D, from here on referred to as calcitriol and calcidiol are deficient in the majority of patients with CKD. Vitamin D metabolism is ubiquitously altered in CKD and concentrations decrease early before PTH levels begin to rise as GFR declines. Plasma calcidiol levels decline when the glomerular filtration rate falls below 45 ml/min/1.73m2 16. Active calcitriol levels decline to the lower limits of normal when patients reach the advanced stages of CKD stage G2, with evidently low levels by CKD stage G4.
Many clinicians define vitamin D deficiency as < 20 ng/ml, insufficiency as 20–29.9 ng/ml and ≥ 30 ng/ml as sufficient (table 1). Various mechanisms have been implicated in the development of vitamin D deficiency in CKD, including loss of renal mass, hyperparathyroidism, hyperphosphatemia, metabolic acidosis, reduced megalin expression, urinary loss of vitamin D binding protein (DBP), accumulation of uremic toxins, and high FGF-23 levels leading to suppressed 1α-hydroxylase enzyme activity and increased catabolism by promoting 24-hydroxylase activity17,18. Renal 1α-hydroxylase is also highly dependent on the concentration of its substrate and deficiency of calcidiol in CKD is an important determinant of calcitriol levels19. Not surprisingly, substrate availability is an important determinant of circulating calcitriol levels.
Table 1.
Vitamin D Status
| Active vitamin D analogues | ||
|---|---|---|
| Nmol/L | Ng/ml | |
| Deficiency | <50 | <20 |
| Insufficiency | 50–74 | 20–30 |
| Optimal range | 75–100 | 20–40 |
| Sufficiency | 75–250 | 20–100 |
| Intoxication | >375 | >150 |
Vitamin D analogues
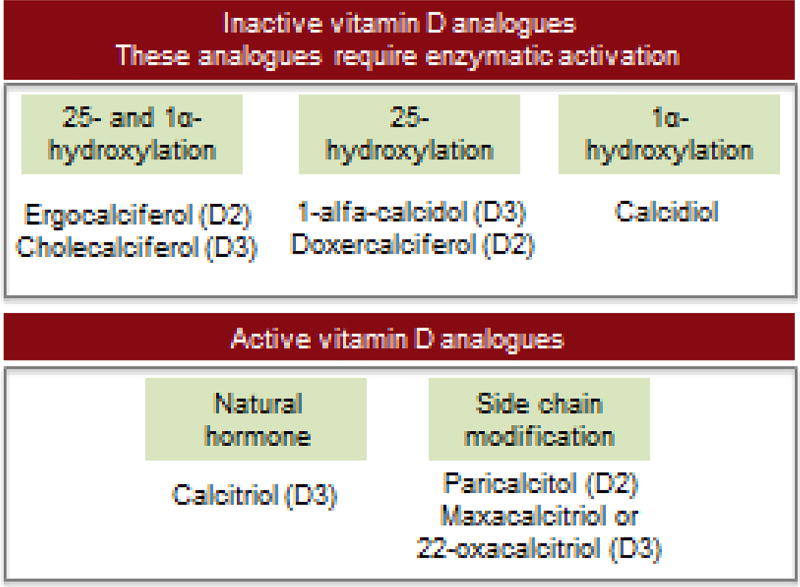
Vitamin D compounds in CKD have been commercially available since the 1950s. These compounds include calcitriol (1,25-dihydroxyvitamin D3), its prodrug alfacalcidol (1α-hydroxyvitamin D3) and calcidiol (25-hydroxyvitamin D3) (figure 2). Subsequent vitamin D analogues currently commercially available include paricalcitol, 22-oxacalcitriol or maxacalcitriol and doxercalciferol. Paricalcitol and 22-oxacalcitriol are active vitamin D analogues that bind directly to the VDR. Doxercalciferol is analogous to alfacalcidol, a prodrug for 1,25-hydroxyvitamin D that requires enzymatic activation by 25-hydroxylation in the liver.
Figure 2. Active and inactive vitamin D formulations.
VDR-activating compounds can be broadly divided into two main groups: The first group constitute those that are inactive vitamin D analogues which are prodrugs. These compounds require specific hydroxylation after intake as shown. The second group includes the already active vitamin D anagloues.
An increasing body of evidence suggests variations between the vitamin D compounds and analogues. Despite their structural differences, for many years it was thought that native vitamin D2 and vitamin D3 are equipotent given that they were found to exert identical sets of biological responses through the VDR20. In one meta-analysis that included 10 randomized controlled trials, the authors found that vitamin D3 was more efficacious at raising calcidiol concentrations compared to vitamin D2 20. Additionally, studies have noted significant variations in the PTH-lowering effects of vitamin D compounds21,22. Meta-analyses have noted that newer vitamin D analogues (doxercalciferol, maxacalcitol, paricalcitol and falecalcitriol) significantly lowered PTH (mean reduction, 98 pg/mL) compared with placebo (3 studies including 163 patients). However, no significant PTH reduction was noted with established vitamin D compounds such as calcitriol and alfacalcidol (6 studies including 187 patients), rather they were found to be associated with increased serum calcium and phosphorous levels. Many of the studies were however inadequately powered and ambiguity remains regarding their differential PTH-lower effects.
The central role of vitamin D in regulating cardiovascular health
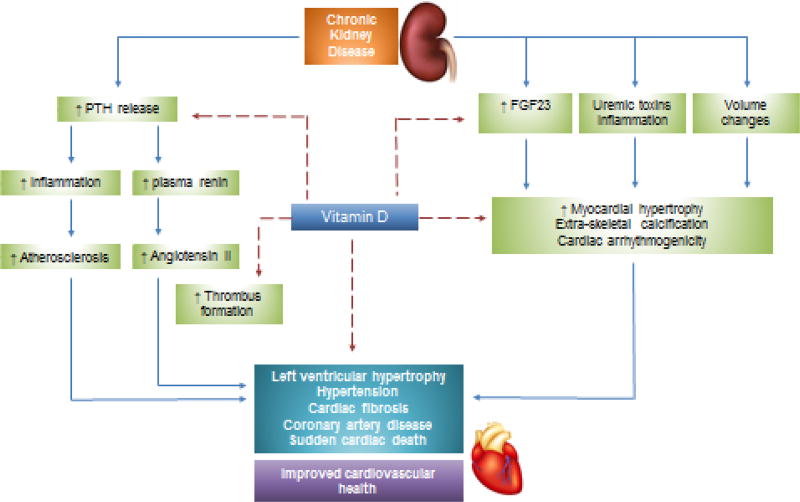
Robust experimental, clinical and epidemiologic evidence have revealed potential links between vitamin D deficiency and the pathogenesis of CVD (figure 3). Signaling components of the vitamin D hormonal system are widely expressed across the various cell types of the cardiovascular system, including myocardial cells, endothelial cells, vascular smooth muscle, fibroblasts and pericytes. Increasing evidence points toward a therapeutic role of vitamin D in the regulation of cardiovascular health and the reduction of cardiovascular disease burden. The VDR knockout mice develop abnormal blood mineral levels, hyperparathyroidism, upregulation of the RAAS, hypertension, left ventricular hypertrophy, heart failure as well as other features resembling premature aging 23,24. Conversely, supplementation with vitamin D has been shown to abrogate these effects25. Calcitriol directly suppresses renin expression, and regulates the proliferation of vascular smooth muscle cells (VSMC) and cardiac myocytes, in vitro. In addition to decreasing vascular tone, activated vitamin D therapy has been shown to decrease cyclooxygenase-1 expression and production of reactive oxygen species radicals in spontaneously hypertensive rats 26.
Figure 3. Multi-system interaction pathways critical to the regulation of cardiovascular health.
Vitamin D deficiency is a major risk factor for cardiovascular morbidity and mortality owing to its pleiotropic cardiovasculo-protective properties. Potential multi-modal protective effects of vitamin D include anti-inflammatory and anti-calcific effects, regulation of FGF-23 and PTH, anti-thrombogenic properties and regulation of vascular tone.
Many observational clinical studies have also linked vitamin D deficiency to cardiovascular disease. The Framingham Offspring Study included 1739 Caucasian subjects and showed that participants who had calcidiol levels below 15 ng/ml had a multivariable-adjusted hazard ratio of 1.62 (95% CI 1.11–2.36) for incident cardiovascular events (including myocardial infarction, coronary insufficiency, angina, stroke, transient ischemic attacks, peripheral claudication or heart failure) compared to participants whose calcidiol levels were ≥ 15ng/ml. In fact, each 10ng/ml increment in serum 25-hydroxyvitamin D has been shown to be associated with a 14% reduction in mortality (relative risk, 0.86; 95% CI 0.82–0.91)27. In the prospective cohort of incident dialysis patients in the Netherlands (the NECOSAD study), the investigators reported a significantly higher short-term (Hazard Ratio, HR 2.0, CI 1.0–3.8) and long-term (HR 1.5, CI 1.0–2.1) cardiovascular mortality in patients with severe calcidiol deficiency (defined as ≤ 10ng/ml) when compared with patients with calcidiol levels above 10 ng/ml.
In a retrospective cohort study that examined baseline data from the National Health and Nutrition Examination Survey (NHANES) III (1998–1994) and cause-specific mortality through 2001 using the National Death Index, the investigators found that participants with calcidiol levels in the lowest quartile (mean 13.9 ng/mL) had a higher adjusted risk of cardiovascular death compared with those in the higher three quartiles (incident rate ratio [IRR] = 1.40; 95% CI, 1.16–1.70). Moreover, there was a significant excess age- and sex- adjusted cardiovascular mortality observed in blacks vs white (IRR = 1.38, 95% CI 1.13–1.70) which was attenuated by adjustment for calcidiol levels28. These experimental and clinical data places vitamin D at a critical nodal point in our search for novel therapies beyond a mere epiphenomenon, whereby vitamin D status is not solely reflective as a secondary effect or a single surrogate marker.
Vitamin D and arterial disease
Vascular calcification is a highly prevalent condition in CKD that contributes to arterial hardening, cardiac strain and sudden cardiac death. Observational studies have demonstrated that serum calcitriol levels are inversely correlated with coronary artery calcification in the general population29,30. In experimental animal models, administration of pharmacological doses of calcitriol resulted in increased aortic calcification, however this was not seen in animals treated with paricalcitol31. In other experimental models, both calcitriol and paricalcitol analogues were protective against vascular calcification at dosages sufficient to correct secondary hyperparathyroidism32. Higher dosages of both these analogues – as expected with any active vitamin D compound – were however found to induce aortic calcification.
These results suggest that the choice of vitamin D analogues is critical given their differential effects on the arterial wall, that vasculo-protective properties may be seen at clinically relevant dosages and vasculo-toxic effects exist in a dose-dependent relationship. At a molecular level, a number of mechanistic studies suggest that vitamin D can stimulate the expression of endogenous calcification inhibitors such as Klotho33 and Osteopontin34 in the vascular wall. Vitamin D may render arteries susceptible to anti-calcific effects of FGF-23 by reversing arterial Klotho deficiency in CKD33. Other properties such as anti-inflammatory effects, inhibition of pro-inflammatory cytokines and regulation of blood pressure and cardiac function as discussed below, are all likely contributory pathways that underlie anti-calcific properties of vitamin D35.
Vitamin D deficiency has also been linked to clinically overt peripheral arterial disease (PAD). Results from the NHANES 2001 to 2004 study that examined 4839 participants found that low serum calcidiol levels were associated with a higher prevalence of PAD36. For each 10 ng/mL decline in calcidiol levels, the authors found a multivariable-adjusted prevalence ratio of PAD of 1.35 (95% CI 1.15–1.59). Moreover, there is evidence that racial differences in vitamin D concentrations could explain nearly one-third of excess risk of PAD in black adults beyond current traditional and novel risk factors for CVD37.
Given that PAD is largely caused by atherosclerosis, there is emerging data to suggest that VDR activation may have a role in preventing or ameliorating the pathogenesis of atherosclerosis. VDR knock-out mice develop enhanced platelet aggregation, reduced endothelial nitric-oxide (NO) synthase expression and exhibited multi-organ thrombus formation after exogenous lipopolysaccharide injection38. These mice had reduced expression of anti-thrombin in the liver and reduced expression of thrombomodulin in the aorta, liver and kidney. Molecular studies have shown that VDR activation results in inhibition of interferon-γ and upregulation of IL-1039,40. Inhibition of IL-1β and IL-6 occurs when VDRs are activated and this would prevent macrophage activation and plaque instability41. Additionally, activation of VDRs results in IL-4 synthesis and this can promote anti-atherogenic properties of Th2 cells42. These changes, though not exclusively likely underlie in part the potential atherogenic ameliorative effects of vitamin D (figure 3).
Highly prevalent in CKD patients is hypertension and many observational studies have shown an association between low 25-hydroxyvitamin D levels and hypertension. One systematic review that included 14 cross-sectional and 4 prospective studies representing 78,028 participants reported an inverse relationship between 25-hydroxyvitamin D levels and hypertension43. In a large mendelian randomisation study (n=49,363), a genetically determined increment of 10 nmol/l in circulating calcidiol was associated with a small but statistically significant reduction in systolic BP of 0.37mmHg44. A major mechanism by which vitamin D improves blood pressure likely involves inhibition of renin secretion which may be mediated by calcium intracellular increase45.
Vitamin D in cardiac disease and remodeling
A strong association exists between vitamin D deficiency and pathogenic processes underlying the development of cardiac failure, including slow coronary flow, endothelial dysfunction and subclinical atherosclerosis in patients with normal, or near-normal coronary arteries46. Observational studies have shown a relationship between low vitamin D levels and impaired left ventricular function in a cross-sectional study of patients referred for coronary angiography 47. Additionally, vitamin D deficiency is associated with overt coronary heart disease and myocardial infarction, and is prognostic for major post-infarction adverse events including, heart failure hospitalizations, recurrent acute myocardial infarction and death48,49. Examination of the Health Professionals Follow-up Study in a nested case-control study that included 18,225 men, showed that low levels of calcidiol (≤ 15 nmol/l) was associated with a higher risk of myocardial infarction compared to participants with sufficient levels (≥ 30 nmol/l) after adjustment for matched variables (RR 2.42, 95% CI 1.53–3.84).
Kong, et al. examined the therapeutic effects of paricalcitol and doxercalciferol, and in combination with losartan on the development of left ventricular hypertrophy in spontaneously hypertensive rats50. The authors found a 65 to 80% reduction in left ventricular wall thickness in rats treated with losartan, paricalcitol or doxercalciferol monotherapy. Impressively, in rats treated with combination losartan-paricalcitol or losartan-doxercalciferol, there was almost complete prevention of left ventricular hypertrophy. Renal and cardiac renin expression were markedly increased in losartan treated rats, but nearly normalized with combination therapy.
Another aspect is the association between vitamin D deficiency and arrhythmia. Large observational studies have shown that the incidence of atrial fibrillation (AF) is higher in the winter than in the summer 51,52 correlating with seasonal variations in calcidiol levels. Patients with nonvalvular AF have been found to have significantly lower calcidiol levels. In fact, patients with calcidiol levels < 20 ng/ml (50 nmol/l) have a twofold higher incidence of nonvalvular AF compared with patients with levels > 30 ng/ml (75 nmol/L) 53. One observational study showed that vitamin D deficiency is associated with new onset AF in hypertensive patients 54. However, these observational studies do not imply causality. On the contrary, no association between calcidiol levels and AF of any type were found in the Framingham Heart Study 55,56.
Vitamin D deficiency is also associated with sudden cardiac death (SCD) in hemodialysis patients 57. In one study, vitamin D treatment of hemodialysis patients was associated with a reduction of QTc dispersion, a significant risk factor for sudden cardiac death 58. In a large cross-sectional study that included 3299 Caucasian patients who were routinely referred to coronary angiography, investigators found that low calcidiol and calcitriol levels were associated with sudden cardiac death 47. Interventional trials are needed to determine the utility of vitamin D therapy for the management and prevention of arrhythmia.
Limitations of observational clinical studies
The human observational studies presented above fall short in their ability to establish a causal relationship between vitamin D levels and important cardiovascular outcomes, including the reduction of vascular calcification, blood pressure lowering and cardiac remodeling. Additionally, studies that suggest a favorable association between vitamin D levels and cardiovascular outcomes have been inconsistent. A number of reasons account for this such as the inability of these studies to fully adjust for confounding factors, factors related to study design, population differences and variations in definitions12. Vitamin D is a highly protein-bound hormone and less than <1% of circulating calcidiol exists in its free form59. This leads us to another explanation based on the free hormone hypothesis, that takes into consideration the levels of bioavailable vitamin D and their relationship with CVD12,60. Additionally, there is evidence that genetic polymorphisms influence vitamin D-binding protein levels and there is considerable ethnic variation in their allele frequencies12,61. Further studies are desperately needed to determine how these polymorphisms impact the definition of therapeutic levels considered of vitamin D sufficiency among race. While these observational studies taken together have provided a strong rationale for vitamin D therapy in CKD patients, clinical trials are critical to address their inherent limitations.
CLINICAL TRIALS OF VITAMIN D
Hypertension and other vascular outcomes
Several small interventional studies have specifically assessed the effects of vitamin D on blood pressure, while a number of larger studies have looked at other primary outcomes with blood pressure as a secondary outcome. In a small clinical trial that included 18 patients, ultraviolet B (UVB) light therapy in untreated hypertensive patients increased calcidiol levels by 162% and significantly decreased SBP (−6mmHg, 95% CI −14 to −1 mmHg) and DBP (−6mmHg, 95% CI −12 to −2) compared to ultraviolet A (UVA) therapy with SBP (0 mmHg, 95% CI −1 to −10 mmHg) and DBP (2mmHg, 95% CI −1 to −3) 62. The Vitamin D and Omega-3 (VITAL) trial was a double-blind multicenter study that enrolled 281 type 2-diabetic patients who were receiving a RAAS inhibitor 63. In this study, almost 100% of participants had hypertension at baseline. Patients were assigned to receive either 1-µg or 2-µg paricalcitol or placebo. The results showed a significant reduction in the urinary albumin/creatinine ratio and a dose-dependent decrease in SBP in the paricalcitol arm compared to placebo63.
The Paricalcitol and Endothelial Function in Chronic Kidney Disease (PENNY) study examined vascular endothelial function as measured in the brachial artery by nitric oxide (NO)-dependent flow-mediated dilation (FMD) response to increased shear stress by forearm ischemia, a recognized surrogate endpoint that predicts incident risk for cardiovascular events in patients with CKD64. This double-blinded randomized controlled trial including 88 patients with stage G3 and G4 CKD randomized to receive paricalcitol 2µg/day or placebo. The investigators reported an increased flow-mediated dilation in the paricalcitol group (mean proportional change of 61%) but not in the placebo group after 12 weeks. These effects were abolished 2 weeks after stopping the treatment.
Conversely, in the Women’s Health Initiative trial, 36,282 post-menopausal women were randomly assigned to receive 100mg of calcium and 400 IU of cholecalciferol daily or placebo with a median follow-up of 7 years65. The study showed no significant SBP change (0.22 mmHg, 95% CI −0.05 to 0.49) or DBP change (0.11 mmHg, 95% CI −0.04 to 0.27) after 7 years of therapy with a low dose of vitamin D. These results did not change after adjusting for nonadherence. The study however was confounded by several factors: firstly, the low dose of 400 IU provided of which 75% of patients had a calcidiol level below 25 ng/ml at baseline; more than half of the patients in the placebo arm took similar over-the-counter vitamin D doses; and only women were included in this study despite observational data of a stronger association between calcidiol level and blood pressure in men. In the Paricalcitol Capsule Benefits in Renal Failure-Induced Cardiac Morbidity (PRIMO) trial discussed in detail below, the study randomized 227 patients to 2µg of paricalcitol daily or matching placebo and did not show any difference in blood pressure between the two groups 66.
Cardiac outcomes
Despite the multiple experimental and observational data demonstrating an association of vitamin D with decreased cardiovascular-related morbidity and mortality, trial data to support this is severely lacking. The PRIMO study was a multinational double-blinded placebo-controlled trial that examined the effects of paricalcitol on left ventricular mass index (LVMI) 66. The trial included 227 patients followed over a period of 48 weeks in stage G3 and G4 CKD patients with mild-to-moderate left ventricular hypertrophy (LVH) and preserved LV ejection fraction at baseline. Cardiac magnetic resonance (CMR) imaging was used to determine changes in LVMI while echocardiography was used to determine left ventricular diastolic function. The study found that paricalcitol did not alter LVMI (0.34 g/m2.7, 95% CI −0.14–0.83 g/m2) compared to placebo (−0.07, g/m2.7, 95% CI −0.55–0.42 g/m2). There was also no change in pre-specified measures of diastolic function, or SBP. PTH levels were reduced to 30% of baseline in the paricalcitol arm, however this was associated with a significant increase in serum calcium and phosphate levels. However, an attenuated B-type natriuretic peptide (BNP) and a lower number of cardiovascular hospitalizations in the paricalcitol arm was found. In a post-hoc analysis of data from the PRIMO study, paricalcitol treatment in all subgroups was found to reduce left atrial volume index, a marker for diastolic dysfunction that is associated with significant cardiovascular risk67.
The Effect of Paricalcitol on Left Ventricular Mass and Function in CKD (the OPERA trial) showed similar results. The OPERA trial was a prospective, double-blinded, randomized, placebo-controlled trial to determine whether oral activated vitamin D reduced LV mass as examined by echocardiography 68. Patients were randomly assigned to receive either oral paricalcitol (n=30) or matching placebo (n=30) for 52 weeks. The study did not find a difference in LV mass between the two groups. However, there was a significant reduction in intact parathyroid hormone (p<0.001) and alkaline phosphatase levels (p=0.001) levels, as well as the number of cardiovascular-related hospitalizations (p=0.02) in the paricalcitol arm compared to placebo.
The burning of question of why both these trials were negative warrants a brief discussion here, particularly given that the results are in stark contrast to several animal models of LVH investigating vitamin D function including the spontaneously hypertensive rat model69, the Dahl salt sensitive rat70 and the pressure overload rat71. Several possibilities exist: Firstly, that the primary hypothesis that vitamin D is in fact null in humans, that LVH may have been too advanced, blood pressure too well controlled, or treatment duration too short to detect a difference. Another possibility is that the beneficial effects of paricalcitol are overridden by elevated FGF-23 concentrations in CKD, since experimental studies have shown that FGF-23 can directly induce LVH development72.
It should be noted that clinical trials to-date assessing the role of vitamin D in cardiac disease have mainly focused on morphological endpoints such as LVH, and such single-surrogate markers may be poorly reflective of cardiovascular performance. There is emerging evidence to suggest that impaired functional cardiovascular reserve occurs in CKD and this can be accurately and reproducibly assessed by cardiopulmonary exercise testing (CPET)73. CPET provides a significantly more robust marker for assessment of cardiovascular function compared to conventional static imaging by echocardiography or CMR, which focuses mainly on morphological alterations such as LVH as mentioned above. In a study that recruited 200 healthy adults subjected to CPET, serum vitamin D levels were found to predict maximal aerobic exercise capacity (VO2Max) which is a CPET index of cardiac functional reserve 74. There is evidence to suggest structural-functional uncoupling of the cardiovascular system in CKD73, which is suggestive of the need for trials assessing functional outcomes such as cardiovascular reserve in parallel with structural alterations. Furthermore, the precise relationship between alterations in functional cardiovascular reserve and left ventricular remodeling in CKD and following renal replacement therapy is unclear and further studies are desperately needed.
CALCIMIMETIC THERAPY
Calcimimetics are synthetic allosteric modulators of the calcium-sensing receptor (CaSRs). The CaSR is a 121 kDa that belongs to the G-protein coupled membrane-bound receptor protein superfamily. It plays a crucial role in the maintenance of normal calcium homeostasis, in particular in the regulation of extracellular ionized calcium concentrations75. At the cell surface, the CaSR is present constitutively in a dimeric configuration and contains a long extracellular N-terminal domain essential for interaction with its principal agonist, ionized calcium, seven hydrophobic membrane-spanning helices that anchor it to the plasma membrane and an intracellular C terminus that has protein kinase phosphorylation sites75. It is a low-affinity receptor requiring millimolar concentrations of agonists (3 mmol/l for calcium) for activation. Due to its limited selectivity, the CaSR can be activated by numerous divalent and trivalent cations, polycationic compounds such as neomycin and spermine, and numerous amino acids in addition to calcium76.
CaSRs are widely expressed in many tissue types that are involved in regulating systemic calcium homeostasis, including the kidney, intestine, bone, thyroid gland and parathyroid gland. and the cardiovascular system77. Activation of the CaSR regulates calcium homeostasis by inhibiting PTH secretion at the parathyroid gland, whereas in the thyroid C cells, it increases calcitonin secretion. Both of these effects results in a reduction of serum calcium concentration. At the kidney, activation of CaSRs increases urinary calcium excretion, in bone it decreases bone turnover and in inteinal cells it decreases intestinal absorption of calcium which taken together, further decreases serum calcium levels. CaSRs are also expressed in tissues not involved in calcium homeostasis such as the brain, skin, breast, testes and placenta where its function is not clearly elucidated. In the cardiovascular system, studies have shown that CaSRs are expressed in cardiac and arterial tissue, and emerging evidence suggest that CaSRs play a critical role in the regulation of cardiovascular health and disease as discussed in further detail below78,79.
Commercially available calcimimetics
There are currently two calcimimetics approved by the US Food and Drug Administration (FDA), Cinacalcet hydrochloride was the first calcimimetic to be approved by the FDA in 2004 and became the first allosteric G protein-coupled receptor modulator to enter the pharmaceutical market. It is FDA approved for the treatment of secondary hyperparathyroidism and for hypercalcemia in patients with parathyroid carcinoma. Cinacalcet is sold by Amgen under the name Senispar® in North America and Australia, Mimpara® in Europe, and Regpara® by Kyowa Hakko Kirin in Japan.
Etecalcetide is a novel second generation calcimimetic agent sold under the name Parsabiv® by Amgen80. The drug was developed by KAI Pharmaceuticals in the US and in July 2012, the company was acquired by Amgen. Recently Etelcalcetide became the second calcimimetic approved by the European Medicines Agency in November 2016, by Pharmaceuticals and Medical Devices Agency in Japan in December 2016 and by the FDA in February 2017 for the treatment of secondary hyperparathyroidism.
The role CaSRs and calcimimetics in the cardiovascular system
There is a growing body of experimental and observational evidence to suggest that activation of CaSRs by calcimimetic therapy can impart cardiovascular and survival benefit. In a study by Block et al., the investigators tested the hypothesis of whether prescription of the calcimimetic, cinacalcet to hemodialysis patients with secondary hyperparathyroidism improved survival81. This large prospective observational study collected data from a large hemodialysis provider that was merged with data in the United States Renal Data System. 19,186 patients were included in the study and 5976 received cinacalcet followed for 24 months. The study found a significant survival benefit with lower rates of adjusted cardiovascular mortality (HR 0.76, 95% CI 0.66–0.86) and all-cause mortality (HR 0.74, 95% CI 0.67–0.83) associated with cinacalcet prescription in patients receiving intravenous active vitamin D analogue.
Regulation of vascular calcification
Molostvov et al. showed that extracellular CaSRs are functionally expressed in human aortic smooth muscle cells (HA-SMCs), endothelial cells and in large and small arteries from healthy and ESRD patients78. The investigators found that expression of CaSRs were significantly reduced in calcified arteries from ESRD patients. High extracellular calcium reduces CaSR expression and promotes the development of calcification, ablation of CaSR function results in increased vascular smooth muscle calcification while calcimimetic therapy ameliorates calcification, in vitro82,83. Incubation of bovine VSMCs with the calcimimetic R-568 prevented the development of calcification, in vitro84. The same anti-calcific effects were found with incubation of calcimimetics using HA-SMCs, and this inhibitory effect was abolished following silencing of CaSRs by interference RNA85. Studies using various animal models have shown that the calcimetics AMG 64186, R-56885 and cinacalcet87 can inhibit the progression of vascular calcification.
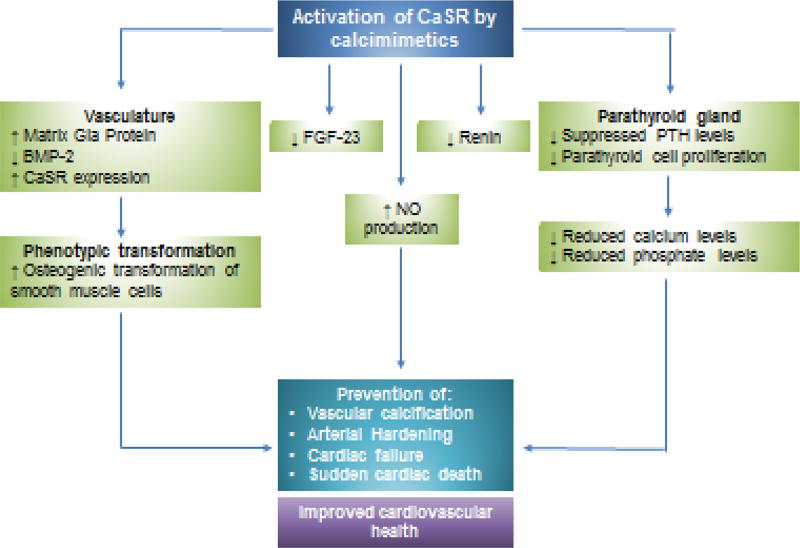
The anti-calcific effects of calcimimetics appear to involve modulation of important key pathogenic events and gene expression during the calcification process (figure 4). Vascular calcification is a regulated, cell-mediated process that resembles bone formation88. A major event in the calcification process is osteogenic transformation of VMSCs into a bone-like phenotype and apoptosis89. Molecular studies have shown that activation of CaSRs in HA-SMCs exerts anti-apoptotic effects while CaSR knock-down resulted in reduced proliferative potential of HA-SMCs under stress conditions suggesting that CaSR activity is crucial for cell survival, in vitro90. CaSR activation has been shown to stimulate matrix-Gla protein (MGP), an endogenous calcification inhibitor and attenuate upregulation of the osteoblast-specific gene bone morphogenetic protein (BMP)-2, which may provide another potential mechanism for their anti-calcific properties91,92. These cytoprotective effects may partly underlie the anti-calcific effects of CaSR activation and importantly, suggests that calcimimetics can exert vasculo-protective effects independent of PTH.
Figure 4. Proposed schematic diagram of potential cardiovascular benefits of CaSR activation by calcimimetics.
Activation of CaSRs by calcimimetics can potentially exert cardiovasculo-protective properties in CKD by regulating vascular smooth muscle phenotype involving prevention of ostegenic transformation and reducing FGF-23 levels. Calcimimetics have also been shown to increase nitric oxide (NO) production and reduce renin secretion, thereby regulating blood pressure and vascular tone. Importantly, activation of CaSRs reduce PTH leves and can help maintain normal mineral homeostasis.
It should be noted that studies have found that calcimimetics can also upregulate expression of CaSR in vascular cells in uremic rats93, thereby reversing a state of CaSR deficiency in CKD. Interestingly, there is evidence that cyclic stretch of HA-SMCs which usually results from physiological pulsatile blood pressure in health is important for maintaining vascular CaSR expression82. Loss of this pulsatile cyclic stretch of the arterial wall results in reduced CaSR expression and promotes a calcific process.
Regulation of volume and blood pressure
Calcium is associated with volume regulation and this is reflected by an increase in sodium excretion of approximately 150% following an infusion of calcium to raise serum levels by 25% in health94,95. Dietary calcium supplementation has also been associated with lower blood pressure95. Gain-of-function mutations of CaSRs can result in a Bartter-like phenotype that is characteristic for renal salt wasting associated with lower blood pressure96. Odenwalkd et al. showed that calcimemetic treatment of subtotally nephrectomized rats caused a marked and sustained antihypertensive effect97.
Several potential mechanisms underlie CaSR-mediated blood pressure effects (figure 4). Molecular studies have shown that CaSR activation is tightly associated with nitric oxide (NO) production in vascular endothelial cells. Knock-down of CaSRs in human aortic endothelial cells resulted in reduced or absent NO production, while stimulation of CaSR activity by polyamine spermine led to increased NO production98. CaSRs are also expressed in the juxtaglomerular cells of the renal afferent arteriole in the kidney that is responsible for secreting renin99. Animal studies have shown that activation of CaSRs administered acutely via intravenous bolus of cinacalcet decreased basal renin secretion and activity100.
Cardiac remodeling
FGF23 has found to be associated with cardiac remodeling and several studies have consistently shown that high FGF23 is associated with the development of left ventricular hypertrophy and/or increased LVMI 101–103. Elegant experiments utilizing animal models demonstrated that FGF23 can directly promote ventricular hypertrophy and contractility of the myocardium72. Interestingly, animal studies have shown that cinacalcet administration reduces serum FGF-23 levels104. The Cinacalcet study for Peritoneal Dialysis Patients In Double Arm on the Lowering Effect of iPTH Level (CUPID) study was a multicenter, open-labelled, randomized controlled study that enrolled 66 peritoneal dialysis patients105. Patients were randomly assigned to treatment with cinacalcet and oral vitamin D or vitamin D alone. The study included a 4-week screening for vitamin D washout, a 12-week dose-titration, and 4-week assessment phases. The CUPID study found that cinacalet treatment was independently associated with reduction of FGF-23 levels, however other cardiac-related clinical endpoints were not measured. Similar findings have been shown in several other trials involving cinacalcet administration106,107 however, further studies are needed to determine the role of calcimimetics in regulating cardiac remodeling.
CLINICAL TRIALS OF CALCIMIMETICS EXAMINING INTERMEDIATE AND HARD ENDPOINTS
The ample experimental and observational data available suggest that activation of CaSRs by calcimimetics imparts cardiovascular benefit and provides a strong rationale for conducting human trials. There is a great need for trials that examine intermediate endpoints such as cardiovascular structural and functional outcomes and hard clinical endpoints that includes cardiovascular and all-cause mortality. In the past few years, we have seen several important trials involving cinacalcet usage and cardiovascular outcomes as reviewed below.
The ADVANCE study (a randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in subjects with chronic kidney disease receiving dialysis) was a prospective, randomized, controlled trial that enrolled 360 hemodialysis patients with moderate-to-severe secondary hyperparathyroidism108. The study randomized patients to receive either cinacalcet combined with low-dose calcitriol or vitamin D analog or flexible vitamin D therapy, then followed for 52 weeks. All participants received calcium-based phosphate binders. Coronary artery calcification (CAC), and aorta and cardiac valve calcium scores were determined by multi-detector computed tomography and the primary end point was change in the Agatson CAC score from baseline to week 52. The trial demonstrated increased Agatson CAC scores of 24% (-22%, 119%) in the cinacalcet group and 31% (-9%-179%) in the flexible vitamin D group, that was not statistically different (P=0.073).
The Evaluation of Cinacalcet Hydrochloride Therapy to Lower Cardiovascular Events (EVOLVE) trial, a multicenter, prospective, randomized, placebo-controlled trial compared cinacalcet with placebo and followed for 64 months. The trial enrolled 3883 hemodialysis patients with moderate-to-severe hyperparathyroidism from 22 countries and is notably the largest trial ever conducted in hemodialysis patients109. The primary end points of the trial were all-cause mortality, myocardial infarction, hospitalization for unstable angina, heart failure or a peripheral vascular event. The median duration of cinacalcet exposure was 21.2 months in the experimental group versus 17.5 months in the placebo group. Unfortunately, no difference was observed in the primary composite end points in an unadjusted intention-to-treat analysis (HR 0.93, 95% CI 0.85–1.02, p=0.11). However, the pre-specified defined secondary end points of an adjusted intention-to-treat analysis (HR 0.88, 95% CI 0.79–0.97, P=0.008) was positive. This is a pivotal finding given an age imbalance between the two arms of the trial despite randomization and that age remains a strong predictor of death in dialysis patients. Two other important findings were that patients treated with cinacalcet were found to have a significantly reduced occurrence of unremitting hyperparathyroidism and reduced rate of parathyroidectomy by more than 50%110. Additionally, patients in the cinacalcet arm had nearly twice as many gastrointestinal side effects (nausea, 29.1 vs 15.5%; vomiting 25.6 vs 13.7%; diarrhea 20.5 vs 18.7%) compared to the placebo arm. In the cinalcalcet arm, 12.4% of patients developed hypocalcemia compared to 1.7% in the placebo arm.
In the EVOLVE trial, 66.7% of individuals discontinued the study drug compared to 70.5% in the placebo arm. In the placebo arm, 80% of patients achieved the maximum dose suggestive of appropriate titration and 19.8% of patients began receiving commercial cinacalcet before the occurrence of a primary event. Therefore, the drug exposure, high drop-out and high drop-in rates to the randomized arms ultimately impaired the study. With the observed study duration, drop-outs and drop-ins, the re-estimated statistical power of the EVOLVE study was only 54%111. Given that most patients who stopped cinacalcet stayed on vitamin D therapy, it is conceivable that some of the effects observed could be partially due to vitamin D. Other limitations that have hampered the interpretation of the findings include a lower-than-anticipated event rate and likely, the insufficient definition of secondary hyperparathyroidism given that many of the patients included may not have had histologic signs of secondary hyperparathyroidism (as suggested by normal bone-specific alkaline phosphatase levels and no differences in fracture rates)112,113.
The EVOLVE trial, is an addition to many important trials involving ESRD patients such as the Hemodialysis (HEMO) trial114, the Randomized Controlled Trial on the Efficacy and Safety of Atorvastatin in Patients with Type 2 Diabetes on Hemodialysis (4D Study)115 and the Study to Evaluate the Use of Rosuvastatin in Subjects on Regular Hemodialysis: An Assessment of Survival and Cardiovascular Events (AURORA) Study116. The European Renal Association (ERA) – European Dialysis and Transplant Association (EDTA) issued a position statement in 2015 following the EVOLVE trial recommending against the routine use of calcimimetic therapy to improve survival in patients with CKD stage G5 and biochemical evidence of secondary hyperparathyroidism117.
Due to the high gastrointestinal adverse side effects of cinacalcet that has limited its use in clinical practice, we look with an interest towards the second generation calcimimetics. One randomized, double-blind, double-dummy clinical trial compared IV etelcalcetide vs oral placebo and oral cinacalcet vs IV placebo in 683 patients receiving hemodialysis with serum PTH > 500 pg/mL118. The study found that the use of etelcalcetide was not inferior to cinacalcet in reducing serum PTH concentrations over 26 weeks. However, there have been no published clinical trials to-date examining its role in the management of cardiovascular disease in CKD.
CONCLUSIONS
Experimental and observational human studies have stimulated significant interest and provided fundamental rationale for the potential therapeutic role of vitamin D and calcimimetic therapy for the management of CVD in patients with reduced renal function. However, this has led to clinical trials that have yielded results requiring additional explanations. A number of possible explanations have led to discrepant cardiovascular outcomes data including potential confounding of human observational studies, and limitations of randomized controlled trials such as sample size, population characteristics and choice of intervention dosages.
At present, there are no conclusive trial data available that can support the hypothesis that native vitamin D treatment and correction of hypovitaminosis lowers the risk of cardiovascular events and mortality in CKD patients. Based on the conclusions by the ERA-EDTA, current trial data do not provide sufficient evidence of the superiority of calcimimetics over standard of care for the management of cardiovascular disease in CKD, although subgroup analyses are suggestive of a benefit.
There however remains strong experimental evidence suggesting that adaptations of cardiovascular structure and function can occur bi-directionally that is, that adverse regression of cardiac remodeling occurs during disease with the potential for recovery. Significant improvement involving regression of LV hypertrophy and LVMI, together with improvement in LV ejection fraction have been reported in kidney transplant patients 119–121. These data suggest that cardiovascular changes in CKD is a modifiable process that can be potentially controlled or halted as a decline in GFR ensures. The critical question is whether this process can be realistically controlled by medical therapy such as vitamin D, calcimimetics or a combination thereof. With the current available literature that we have, only suggestions can be made regarding vitamin D supplementation and calcimimetic use for the indication of modulating cardiovascular status and improving cardiovascular health in CKD. We therefore eagerly await the results of current ongoing trials in vitamin D (table 2), calcimimetic therapy (table 3) and future trials to be launched.
Table 2.
Ongoing Vitamin D Clinical Trials
| Trial name | Country | Population | Intervention | Main outcomes | Results expected |
|---|---|---|---|---|---|
| SIMPLIFIED (Survival Improvement with Cholecalciferol in Patients on Dialysis) | United Kingdom | 4,200 adults, aged > 18 years | Cholecalciferol 60,000 IU fortnightly | Mortality, health-relatedquality of life, hospital admission, cardiovascular events, cancer incidence | 2023 |
| VIDAL (Vitamin D and Longevity trial) | United Kingdom | 20,000 adults, 65–85 years old | 100,000 IU D3 monthly, placebo or open control | Mortality, morbidity (infections, doctor’s visits, cancer) and vitamin D levels | 2020 |
| FIND (Finnish Vitamin D Trial) | Finland | 18,000 adults, men > 60 years and women > 65 years | 3200 D3 daily, 1600 D3 daily or placebo | Cardiovascular disease, cancer and diabetes mellitus | 2019 |
| DOHealth (Vitamin D3 - Omega3 - Home Exercise - Healthy Ageing and Longevity Trial) | Europe | 2150 adults aged > 70 years | 2000 IU D2 or placebo | Bone fractures, infection, blood pressure, cognition and lower extremity function | 2017–2018 |
| TARGET-D (Trial Evaluating Vitamin D Normalization on Major Adverse Cardiovascular-Related Events Among Myocardial Infarction Patients) | United States | 890 adults, aged > 18 years | Target 25[OH]D levels > 40ng/mL with cholecalciferol or standard of care (no intervention) | Mortaliy, myocardial infarction, heart failure hospitalization and cerebral vascular accident | 2021 |
| Magnesium and Vitamin D Supplementation and Cardiometabolic Outcomes | United States | 123 adults, aged 30 to 70 years | 1000U vitamin D and 360 magnesium vs 1000U vitamin D vs placebo | PTH levels, inflammatory cytokines, lipid profile, blood pressure, serum osteocalcin | 2020 |
| Effect of Vitamin D on Ventricular Remodeling in Patients with Acute Myocardial Infarction (VITDAMI) | Spain | 144 adults, aged 40 to 85 years | 15,690 IU Calcifedioll vs placebo | Change in cardiac remodeling by MRI, echo parameters | 2017–2018 |
| Effect of Vitamin D Supplementation in Patients with Heart Failure and Vitamin D Deficiency | Mexico | 60 adults, aged 45 to 85 years | 5 000IU vitamin D D vs placebo | Myocardial structure and function by MRI, myocardial infarction | 2018 |
Table 3.
Ongoing calcimimetic clinical trials
| Trial name | Country | Population | Intervention | Main outcomes |
Results expected |
|---|---|---|---|---|---|
| Efficacy and safety of Cinacalcet in Ca, P and iPTH Levels in Patients With Mild, Moderate and Sever Secondary PTH | China | 750 adults, aged 18–75 years | Cinacalcet usage in severe vs moderate vs mild secondary hyperparathyroidism | PTH, calcium and phosphate levels | 2019 |
| Cinacalcet versus parathyroidectomy in peritoneal dialysis patients | Hong Kong | 70 adults, aged 18–75 years | Cinacalcet vs surgical total parathyroidectomy | Change in coronary artery calcium and heart valve calcium score, pulse wave velocity, cardiac structure | 2017 |
| Evaluating alternative medical therapies in primary hyperparathyroidism | United States | 60 adults, aged 18–80 years | Placebo + cinacalcet, amiloride + cinacalcet, eplerenone + cinacalcet | PTH, calcium levels | 2020 |
| Renal osteodystrophy: an individual management approach | United States | 120 adults, aged > 21 years | Teriparatide + cinacalcet vs no intervention: in low vs high turnover | Bone mineral density of hip, coronary artery calcification by MDCT, biochemical bone markers of bone activity | 2020 |
Whether vitamin D supplementation or calcimimetic therapy can alter the incidence of CVD has not been satisfactorily addressed by our current trial data. Overall, cardiovascular mortality in dialysis patients has improved in the US over the past decade122 and use of vitamin D and calcimimetics may have contributed to this observation. Despite this, survival of dialysis patients continues to be unsatisfactory in most countries. The adjusted annual mortality of patients on hemodialysis remains high in the US (22%), followed by Europe (16%) and Japan (7%)122. In order to better identify potential therapeutic strategies to change practice and improve the cardiovascular health of our CKD population, the field of nephrology is in desperate need for further well-designed randomized trials that examine cardiovascular and renal endpoints that will overcome current limitations as described above. Given the multi-systemic nature of ESRD, it would be too simplistic to think that a single agent is all that is needed and a combination therapy approach is likely required, however this type of trial presents further challenges.
Acknowledgments
Disclosures
K.L. is supported through an NIH National Research Service Award (NRSA) training award. R.T. consults for Fresenius Medical Care North America and is the principal investigator NIH NIDDK T32 DK007540-31A1 and R01 DK094486 grants. T.H is supported by grants from Chugai and Kyowa Hakko Kirin, which sells maxacalcitol and cinacalcet in Japan, respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nascimento BR, Brant LC, Moraes DN, Ribeiro AL. Global health and cardiovascular disease. Heart. 2014;100(22):1743–1749. doi: 10.1136/heartjnl-2014-306026. [DOI] [PubMed] [Google Scholar]
- 2.Kwan GF, Benjamin EJ. Global Health and Cardiovascular Disease. Circulation. 2015;132(13):1217. doi: 10.1161/CIRCULATIONAHA.115.019226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anavekar NS, McMurray JJ, Velazquez EJ, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351(13):1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 4.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104(22):2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 5.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108(17):2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 6.US Renal Data System (USRDS) Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage renal Disease in the United States. 2013 [Google Scholar]
- 7.Wright RS, Reeder GS, Herzog CA, et al. Acute myocardial infarction and renal dysfunction: a high-risk combination. Ann Intern Med. 2002;137(7):563–570. doi: 10.7326/0003-4819-137-7-200210010-00007. [DOI] [PubMed] [Google Scholar]
- 8.Szummer K, Lundman P, Jacobson SH, et al. Cockcroft-Gault is better than the Modification of Diet in Renal Disease study formula at predicting outcome after a myocardial infarction: data from the Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies (SWEDEHEART) Am Heart J. 2010;159(6):979–986. doi: 10.1016/j.ahj.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 9.Leonard O, Spaak J, Goldsmith D. Regression of vascular calcification in chronic kidney disease - feasible or fantasy? a review of the clinical evidence. Br J Clin Pharmacol. 2013;76(4):560–572. doi: 10.1111/bcp.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moe SM, Thadhani R. What have we learned about chronic kidney disease-mineral bone disorder from the EVOLVE and PRIMO trials? Curr Opin Nephrol Hypertens. 2013;22(6):651–655. doi: 10.1097/MNH.0b013e328365b3a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen NX, Moe SM. Vascular calcification: pathophysiology and risk factors. Curr Hypertens Rep. 2012;14(3):228–237. doi: 10.1007/s11906-012-0265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nigwekar SU, Thadhani R. Vitamin D receptor activation: cardiovascular and renal implications. Kidney Int Suppl (2011) 2013;3(5):427–430. doi: 10.1038/kisup.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodman WG. The consequences of uncontrolled secondary hyperparathyroidism and its treatment in chronic kidney disease. Semin Dial. 2004;17(3):209–216. doi: 10.1111/j.0894-0959.2004.17308.x. [DOI] [PubMed] [Google Scholar]
- 14.Kidney Disease: Improving Global Outcomes CKDMBDWG. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl. 2009;(113):S1–130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 15.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(4):1080S–1086S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 16.Oh YJ, Kim M, Lee H, et al. A threshold value of estimated glomerular filtration rate that predicts changes in serum 25-hydroxyvitamin D levels: 4th Korean National Health and Nutritional Examination Survey 2008. Nephrol Dial Transplant. 2012;27(6):2396–2403. doi: 10.1093/ndt/gfr763. [DOI] [PubMed] [Google Scholar]
- 17.Hiemstra TF. Vitamin D in renal disease. British Journal of Renal Medicine. 2012;17(4):23–27. [Google Scholar]
- 18.Bosworth C, de Boer IH. Impaired vitamin D metabolism in CKD. Semin Nephrol. 2013;33(2):158–168. doi: 10.1016/j.semnephrol.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289(1):F8–28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 20.Tripkovic L, Lambert H, Hart K, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95(6):1357–1364. doi: 10.3945/ajcn.111.031070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer SC, McGregor DO, Craig JC, Elder G, Macaskill P, Strippoli GF. Vitamin D compounds for people with chronic kidney disease requiring dialysis. Cochrane Database Syst Rev. 2009;(4):CD005633. doi: 10.1002/14651858.CD005633.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Palmer SC, McGregor DO, Macaskill P, Craig JC, Elder GJ, Strippoli GF. Meta-analysis: vitamin D compounds in chronic kidney disease. Ann Intern Med. 2007;147(12):840–853. doi: 10.7326/0003-4819-147-12-200712180-00004. [DOI] [PubMed] [Google Scholar]
- 23.Weishaar RE, Simpson RU. Vitamin D3 and cardiovascular function in rats. J Clin Invest. 1987;79(6):1706–1712. doi: 10.1172/JCI113010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weishaar RE, Kim SN, Saunders DE, Simpson RU. Involvement of vitamin D3 with cardiovascular function. III. Effects on physical and morphological properties. Am J Physiol. 1990;258(1 Pt 1):E134–142. doi: 10.1152/ajpendo.1990.258.1.E134. [DOI] [PubMed] [Google Scholar]
- 25.Chen S, Law CS, Grigsby CL, et al. Cardiomyocyte-specific deletion of the vitamin D receptor gene results in cardiac hypertrophy. Circulation. 2011;124(17):1838–1847. doi: 10.1161/CIRCULATIONAHA.111.032680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong MS, Delansorne R, Man RY, Svenningsen P, Vanhoutte PM. Chronic treatment with vitamin D lowers arterial blood pressure and reduces endothelium-dependent contractions in the aorta of the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol. 2010;299(4):H1226–1234. doi: 10.1152/ajpheart.00288.2010. [DOI] [PubMed] [Google Scholar]
- 27.Pilz S, Iodice S, Zittermann A, Grant WB, Gandini S. Vitamin D status and mortality risk in CKD: a meta-analysis of prospective studies. Am J Kidney Dis. 2011;58(3):374–382. doi: 10.1053/j.ajkd.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 28.Fiscella K, Franks P. Vitamin D, race, and cardiovascular mortality: findings from a national US sample. Ann Fam Med. 2010;8(1):11–18. doi: 10.1370/afm.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doherty TM, Tang W, Dascalos S, et al. Ethnic origin and serum levels of 1alpha,25-dihydroxyvitamin D3 are independent predictors of coronary calcium mass measured by electron-beam computed tomography. Circulation. 1997;96(5):1477–1481. doi: 10.1161/01.cir.96.5.1477. [DOI] [PubMed] [Google Scholar]
- 30.Watson KE, Abrolat ML, Malone LL, et al. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation. 1997;96(6):1755–1760. doi: 10.1161/01.cir.96.6.1755. [DOI] [PubMed] [Google Scholar]
- 31.Mizobuchi M, Finch JL, Martin DR, Slatopolsky E. Differential effects of vitamin D receptor activators on vascular calcification in uremic rats. Kidney Int. 2007;72(6):709–715. doi: 10.1038/sj.ki.5002406. [DOI] [PubMed] [Google Scholar]
- 32.Mathew S, Lund RJ, Chaudhary LR, Geurs T, Hruska KA. Vitamin D receptor activators can protect against vascular calcification. J Am Soc Nephrol. 2008;19(8):1509–1519. doi: 10.1681/ASN.2007080902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim K, Lu TS, Molostvov G, et al. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012;125(18):2243–2255. doi: 10.1161/CIRCULATIONAHA.111.053405. [DOI] [PubMed] [Google Scholar]
- 34.Lau WL, Leaf EM, Hu MC, et al. Vitamin D receptor agonists increase klotho and osteopontin while decreasing aortic calcification in mice with chronic kidney disease fed a high phosphate diet. Kidney Int. 2012;82(12):1261–1270. doi: 10.1038/ki.2012.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gunta SS, Thadhani RI, Mak RH. The effect of vitamin D status on risk factors for cardiovascular disease. Nat Rev Nephrol. 2013;9(6):337–347. doi: 10.1038/nrneph.2013.74. [DOI] [PubMed] [Google Scholar]
- 36.Melamed ML, Muntner P, Michos ED, et al. Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease: results from NHANES 2001 to 2004. Arterioscler Thromb Vasc Biol. 2008;28(6):1179–1185. doi: 10.1161/ATVBAHA.108.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reis JP, Michos ED, von Muhlen D, Miller ER., 3rd Differences in vitamin D status as a possible contributor to the racial disparity in peripheral arterial disease. Am J Clin Nutr. 2008;88(6):1469–1477. doi: 10.3945/ajcn.2008.26447. [DOI] [PubMed] [Google Scholar]
- 38.Aihara K, Azuma H, Akaike M, et al. Disruption of nuclear vitamin D receptor gene causes enhanced thrombogenicity in mice. J Biol Chem. 2004;279(34):35798–35802. doi: 10.1074/jbc.M404865200. [DOI] [PubMed] [Google Scholar]
- 39.Staeva-Vieira TP, Freedman LP. 1,25-dihydroxyvitamin D3 inhibits IFN-gamma and IL-4 levels during in vitro polarization of primary murine CD4+ T cells. J Immunol. 2002;168(3):1181–1189. doi: 10.4049/jimmunol.168.3.1181. [DOI] [PubMed] [Google Scholar]
- 40.Barrat FJ, Cua DJ, Boonstra A, et al. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195(5):603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panichi V, De Pietro S, Andreini B, et al. Calcitriol modulates in vivo and in vitro cytokine production: a role for intracellular calcium. Kidney Int. 1998;54(5):1463–1469. doi: 10.1046/j.1523-1755.1998.00152.x. [DOI] [PubMed] [Google Scholar]
- 42.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O’Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167(9):4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 43.Burgaz A, Orsini N, Larsson SC, Wolk A. Blood 25-hydroxyvitamin D concentration and hypertension: a meta-analysis. J Hypertens. 2011;29(4):636–645. doi: 10.1097/HJH.0b013e32834320f9. [DOI] [PubMed] [Google Scholar]
- 44.Vimaleswaran KS, Cavadino A, Berry DJ, et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2(9):719–729. doi: 10.1016/S2213-8587(14)70113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fryer RM, Rakestraw PA, Nakane M, et al. Differential inhibition of renin mRNA expression by paricalcitol and calcitriol in C57/BL6 mice. Nephron Physiol. 2007;106(4):76–81. doi: 10.1159/000104875. [DOI] [PubMed] [Google Scholar]
- 46.Oz F, Cizgici AY, Oflaz H, et al. Impact of vitamin D insufficiency on the epicardial coronary flow velocity and endothelial function. Coron Artery Dis. 2013;24(5):392–397. doi: 10.1097/MCA.0b013e328362b2c8. [DOI] [PubMed] [Google Scholar]
- 47.Pilz S, Marz W, Wellnitz B, et al. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab. 2008;93(10):3927–3935. doi: 10.1210/jc.2008-0784. [DOI] [PubMed] [Google Scholar]
- 48.Ng LL, Sandhu JK, Squire IB, Davies JE, Jones DJ. Vitamin D and prognosis in acute myocardial infarction. Int J Cardiol. 2013;168(3):2341–2346. doi: 10.1016/j.ijcard.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 49.Correia LC, Sodre F, Garcia G, et al. Relation of severe deficiency of vitamin D to cardiovascular mortality during acute coronary syndromes. The American journal of cardiology. 2013;111(3):324–327. doi: 10.1016/j.amjcard.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 50.Kong J, Kim GH, Wei M, et al. Therapeutic effects of vitamin D analogs on cardiac hypertrophy in spontaneously hypertensive rats. Am J Pathol. 2010;177(2):622–631. doi: 10.2353/ajpath.2010.091292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frost L, Johnsen SP, Pedersen L, et al. Seasonal variation in hospital discharge diagnosis of atrial fibrillation: a population-based study. Epidemiology. 2002;13(2):211–215. doi: 10.1097/00001648-200203000-00017. [DOI] [PubMed] [Google Scholar]
- 52.Murphy NF, Stewart S, MacIntyre K, Capewell S, McMurray JJ. Seasonal variation in morbidity and mortality related to atrial fibrillation. Int J Cardiol. 2004;97(2):283–288. doi: 10.1016/j.ijcard.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 53.Chen WR, Liu ZY, Shi Y, et al. Relation of low vitamin D to nonvalvular persistent atrial fibrillation in Chinese patients. Ann Noninvasive Electrocardiol. 2014;19(2):166–173. doi: 10.1111/anec.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ozcan OU, Gurlek A, Gursoy E, Gerede DM, Erol C. Relation of vitamin D deficiency and new-onset atrial fibrillation among hypertensive patients. J Am Soc Hypertens. 2015;9(4):307–312. doi: 10.1016/j.jash.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 55.Rienstra M, Cheng S, Larson MG, et al. Vitamin D status is not related to development of atrial fibrillation in the community. Am Heart J. 2011;162(3):538–541. doi: 10.1016/j.ahj.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qayyum F, Landex NL, Agner BR, Rasmussen M, Jons C, Dixen U. Vitamin D deficiency is unrelated to type of atrial fibrillation and its complications. Dan Med J. 2012;59(9):A4505. [PubMed] [Google Scholar]
- 57.Drechsler C, Pilz S, Obermayer-Pietsch B, et al. Vitamin D deficiency is associated with sudden cardiac death, combined cardiovascular events, and mortality in haemodialysis patients. Eur Heart J. 2010;31(18):2253–2261. doi: 10.1093/eurheartj/ehq246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim HW, Park CW, Shin YS, et al. Calcitriol regresses cardiac hypertrophy and QT dispersion in secondary hyperparathyroidism on hemodialysis. Nephron Clin Pract. 2006;102(1):c21–29. doi: 10.1159/000088295. [DOI] [PubMed] [Google Scholar]
- 59.Brown AJ, Coyne DW. Bioavailable vitamin D in chronic kidney disease. Kidney Int. 2012;82(1):5–7. doi: 10.1038/ki.2012.135. [DOI] [PubMed] [Google Scholar]
- 60.Mendel CM. The free hormone hypothesis: a physiologically based mathematical model. Endocr Rev. 1989;10(3):232–274. doi: 10.1210/edrv-10-3-232. [DOI] [PubMed] [Google Scholar]
- 61.Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376(9736):180–188. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krause R, Buhring M, Hopfenmuller W, Holick MF, Sharma AM. Ultraviolet B and blood pressure. Lancet. 1998;352(9129):709–710. doi: 10.1016/S0140-6736(05)60827-6. [DOI] [PubMed] [Google Scholar]
- 63.de Zeeuw D, Agarwal R, Amdahl M, et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet. 2010;376(9752):1543–1551. doi: 10.1016/S0140-6736(10)61032-X. [DOI] [PubMed] [Google Scholar]
- 64.Zoccali C, Curatola G, Panuccio V, et al. Paricalcitol and endothelial function in chronic kidney disease trial. Hypertension. 2014;64(5):1005–1011. doi: 10.1161/HYPERTENSIONAHA.114.03748. [DOI] [PubMed] [Google Scholar]
- 65.Margolis KL, Ray RM, Van Horn L, et al. Effect of calcium and vitamin D supplementation on blood pressure: the Women’s Health Initiative Randomized Trial. Hypertension. 2008;52(5):847–855. doi: 10.1161/HYPERTENSIONAHA.108.114991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thadhani R, Appelbaum E, Pritchett Y, et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. Jama. 2012;307(7):674–684. doi: 10.1001/jama.2012.120. [DOI] [PubMed] [Google Scholar]
- 67.Tamez H, Zoccali C, Packham D, et al. Vitamin D reduces left atrial volume in patients with left ventricular hypertrophy and chronic kidney disease. Am Heart J. 2012;164(6):902–909. e902. doi: 10.1016/j.ahj.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 68.Wang AY, Fang F, Chan J, et al. Effect of paricalcitol on left ventricular mass and function in CKD--the OPERA trial. J Am Soc Nephrol. 2014;25(1):175–186. doi: 10.1681/ASN.2013010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Przybylski R, McCune S, Hollis B, Simpson RU. Vitamin D deficiency in the spontaneously hypertensive heart failure [SHHF] prone rat. Nutr Metab Cardiovasc Dis. 2010;20(9):641–646. doi: 10.1016/j.numecd.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Choi JH, Ke Q, Bae S, et al. Doxercalciferol, a pro-hormone of vitamin D, prevents the development of cardiac hypertrophy in rats. J Card Fail. 2011;17(12):1051–1058. doi: 10.1016/j.cardfail.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meems LM, Cannon MV, Mahmud H, et al. The vitamin D receptor activator paricalcitol prevents fibrosis and diastolic dysfunction in a murine model of pressure overload. J Steroid Biochem Mol Biol. 2012;132(3–5):282–289. doi: 10.1016/j.jsbmb.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 72.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121(11):4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ting SM, Hamborg T, McGregor G, et al. Reduced Cardiovascular Reserve in Chronic Kidney Failure: A Matched Cohort Study. Am J Kidney Dis. 2015;66(2):274–284. doi: 10.1053/j.ajkd.2015.02.335. [DOI] [PubMed] [Google Scholar]
- 74.Ardestani A, Parker B, Mathur S, et al. Relation of vitamin D level to maximal oxygen uptake in adults. The American journal of cardiology. 2011;107(8):1246–1249. doi: 10.1016/j.amjcard.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brown EM, Gamba G, Riccardi D, et al. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993;366(6455):575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 76.Torres PA, De Broe M. Calcium-sensing receptor, calcimimetics, and cardiovascular calcifications in chronic kidney disease. Kidney Int. 2012;82(1):19–25. doi: 10.1038/ki.2012.69. [DOI] [PubMed] [Google Scholar]
- 77.Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81(1):239–297. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- 78.Molostvov G, James S, Fletcher S, et al. Extracellular calcium-sensing receptor is functionally expressed in human artery. Am J Physiol Renal Physiol. 2007;293(3):F946–955. doi: 10.1152/ajprenal.00474.2006. [DOI] [PubMed] [Google Scholar]
- 79.Massy ZA, Henaut L, Larsson TE, Vervloet MG. Calcium-sensing receptor activation in chronic kidney disease: effects beyond parathyroid hormone control. Semin Nephrol. 2014;34(6):648–659. doi: 10.1016/j.semnephrol.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 80.Blair HA. Etelcalcetide: First Global Approval. Drugs. 2016;76(18):1787–1792. doi: 10.1007/s40265-016-0671-3. [DOI] [PubMed] [Google Scholar]
- 81.Block GA, Zaun D, Smits G, et al. Cinacalcet hydrochloride treatment significantly improves all-cause and cardiovascular survival in a large cohort of hemodialysis patients. Kidney Int. 2010;78(6):578–589. doi: 10.1038/ki.2010.167. [DOI] [PubMed] [Google Scholar]
- 82.Molostvov G, Hiemstra TF, Fletcher S, Bland R, Zehnder D. Arterial Expression of the Calcium-Sensing Receptor Is Maintained by Physiological Pulsation and Protects against Calcification. PLoS One. 2015;10(10):e0138833. doi: 10.1371/journal.pone.0138833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Farzaneh-Far A, Proudfoot D, Weissberg PL, Shanahan CM. Matrix gla protein is regulated by a mechanism functionally related to the calcium-sensing receptor. Biochem Biophys Res Commun. 2000;277(3):736–740. doi: 10.1006/bbrc.2000.3747. [DOI] [PubMed] [Google Scholar]
- 84.Alam MU, Kirton JP, Wilkinson FL, et al. Calcification is associated with loss of functional calcium-sensing receptor in vascular smooth muscle cells. Cardiovasc Res. 2009;81(2):260–268. doi: 10.1093/cvr/cvn279. [DOI] [PubMed] [Google Scholar]
- 85.Ivanovski O, Nikolov IG, Joki N, et al. The calcimimetic R-568 retards uremia-enhanced vascular calcification and atherosclerosis in apolipoprotein E deficient (apoE−/−) mice. Atherosclerosis. 2009;205(1):55–62. doi: 10.1016/j.atherosclerosis.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 86.Lopez I, Mendoza FJ, Aguilera-Tejero E, et al. The effect of calcitriol, paricalcitol, and a calcimimetic on extraosseous calcifications in uremic rats. Kidney Int. 2008;73(3):300–307. doi: 10.1038/sj.ki.5002675. [DOI] [PubMed] [Google Scholar]
- 87.Jung S, Querfeld U, Muller D, Rudolph B, Peters H, Kramer S. Submaximal suppression of parathyroid hormone ameliorates calcitriol-induced aortic calcification and remodeling and myocardial fibrosis in uremic rats. J Hypertens. 2012;30(11):2182–2191. doi: 10.1097/HJH.0b013e328357c049. [DOI] [PubMed] [Google Scholar]
- 88.Shanahan CM, Crouthamel MH, Kapustin A, Giachelli CM. Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ Res. 2011;109(6):697–711. doi: 10.1161/CIRCRESAHA.110.234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Steitz SA, Speer MY, Curinga G, et al. Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res. 2001;89(12):1147–1154. doi: 10.1161/hh2401.101070. [DOI] [PubMed] [Google Scholar]
- 90.Molostvov G, Fletcher S, Bland R, Zehnder D. Extracellular calcium-sensing receptor mediated signalling is involved in human vascular smooth muscle cell proliferation and apoptosis. Cell Physiol Biochem. 2008;22(5–6):413–422. doi: 10.1159/000185484. [DOI] [PubMed] [Google Scholar]
- 91.Mendoza FJ, Martinez-Moreno J, Almaden Y, et al. Effect of calcium and the calcimimetic AMG 641 on matrix-Gla protein in vascular smooth muscle cells. Calcif Tissue Int. 2011;88(3):169–178. doi: 10.1007/s00223-010-9442-4. [DOI] [PubMed] [Google Scholar]
- 92.Ciceri P, Elli F, Brenna I, Volpi E, Brancaccio D, Cozzolino M. The calcimimetic calindol prevents high phosphate-induced vascular calcification by upregulating matrix GLA protein. Nephron Exp Nephrol. 2012;122(3–4):75–82. doi: 10.1159/000349935. [DOI] [PubMed] [Google Scholar]
- 93.Koleganova N, Piecha G, Ritz E, Schmitt CP, Gross ML. A calcimimetic (R-568), but not calcitriol, prevents vascular remodeling in uremia. Kidney Int. 2009;75(1):60–71. doi: 10.1038/ki.2008.490. [DOI] [PubMed] [Google Scholar]
- 94.el-Hajj Fuleihan G, Seifter J, Scott J, Brown EM. Calcium-regulated renal calcium handling in healthy men: relationship to sodium handling. J Clin Endocrinol Metab. 1998;83(7):2366–2372. doi: 10.1210/jcem.83.7.4915. [DOI] [PubMed] [Google Scholar]
- 95.Cha SK, Huang C, Ding Y, Qi X, Huang CL, Miller RT. Calcium-sensing receptor decreases cell surface expression of the inwardly rectifying K+ channel Kir4.1. J Biol Chem. 2011;286(3):1828–1835. doi: 10.1074/jbc.M110.160390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Toka HR, Koshy JM, Hariri A. The molecular basis of blood pressure variation. Pediatr Nephrol. 2013;28(3):387–399. doi: 10.1007/s00467-012-2206-9. [DOI] [PubMed] [Google Scholar]
- 97.Odenwald T, Nakagawa K, Hadtstein C, et al. Acute blood pressure effects and chronic hypotensive action of calcimimetics in uremic rats. J Am Soc Nephrol. 2006;17(3):655–662. doi: 10.1681/ASN.2005090914. [DOI] [PubMed] [Google Scholar]
- 98.Ziegelstein RC, Xiong Y, He C, Hu Q. Expression of a functional extracellular calcium-sensing receptor in human aortic endothelial cells. Biochem Biophys Res Commun. 2006;342(1):153–163. doi: 10.1016/j.bbrc.2006.01.135. [DOI] [PubMed] [Google Scholar]
- 99.Ortiz-Capisano MC, Ortiz PA, Garvin JL, Harding P, Beierwaltes WH. Expression and function of the calcium-sensing receptor in juxtaglomerular cells. Hypertension. 2007;50(4):737–743. doi: 10.1161/HYPERTENSIONAHA.107.095158. [DOI] [PubMed] [Google Scholar]
- 100.Atchison DK, Ortiz-Capisano MC, Beierwaltes WH. Acute activation of the calcium-sensing receptor inhibits plasma renin activity in vivo. Am J Physiol Regul Integr Comp Physiol. 2010;299(4):R1020–1026. doi: 10.1152/ajpregu.00238.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gutierrez OM, Januzzi JL, Isakova T, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119(19):2545–2552. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Knap B, Veceric-Haler Z, Benedik M, Buturovic-Ponikvar J, Ponikvar R, Bren AF. Fibroblast growth factor 23 and left ventricular mass index in maintenance hemodialysis patients: standard versus long nocturnal hemodialysis. Ther Apher Dial. 2013;17(4):407–411. doi: 10.1111/1744-9987.12087. [DOI] [PubMed] [Google Scholar]
- 103.Jovanovich A, Ix JH, Gottdiener J, et al. Fibroblast growth factor 23, left ventricular mass, and left ventricular hypertrophy in community-dwelling older adults. Atherosclerosis. 2013;231(1):114–119. doi: 10.1016/j.atherosclerosis.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Finch JL, Tokumoto M, Nakamura H, et al. Effect of paricalcitol and cinacalcet on serum phosphate, FGF-23, and bone in rats with chronic kidney disease. Am J Physiol Renal Physiol. 2010;298(6):F1315–1322. doi: 10.1152/ajprenal.00552.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim HJ, Kim H, Shin N, et al. Cinacalcet lowering of serum fibroblast growth factor-23 concentration may be independent from serum Ca, P, PTH and dose of active vitamin D in peritoneal dialysis patients: a randomized controlled study. BMC Nephrol. 2013;14:112. doi: 10.1186/1471-2369-14-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Koizumi M, Komaba H, Nakanishi S, Fujimori A, Fukagawa M. Cinacalcet treatment and serum FGF23 levels in haemodialysis patients with secondary hyperparathyroidism. Nephrol Dial Transplant. 2012;27(2):784–790. doi: 10.1093/ndt/gfr384. [DOI] [PubMed] [Google Scholar]
- 107.Wetmore JB, Liu S, Krebill R, Menard R, Quarles LD. Effects of cinacalcet and concurrent low-dose vitamin D on FGF23 levels in ESRD. Clin J Am Soc Nephrol. 2010;5(1):110–116. doi: 10.2215/CJN.03630509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Raggi P, Chertow GM, Torres PU, et al. The ADVANCE study: a randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol Dial Transplant. 2011;26(4):1327–1339. doi: 10.1093/ndt/gfq725. [DOI] [PubMed] [Google Scholar]
- 109.Investigators ET, Chertow GM, Block GA, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367(26):2482–2494. doi: 10.1056/NEJMoa1205624. [DOI] [PubMed] [Google Scholar]
- 110.Parfrey PS, Chertow GM, Block GA, et al. The clinical course of treated hyperparathyroidism among patients receiving hemodialysis and the effect of cinacalcet: the EVOLVE trial. J Clin Endocrinol Metab. 2013;98(12):4834–4844. doi: 10.1210/jc.2013-2975. [DOI] [PubMed] [Google Scholar]
- 111.Parfrey PS, Block GA, Correa-Rotter R, et al. Lessons Learned from EVOLVE for Planning of Future Randomized Trials in Patients on Dialysis. Clin J Am Soc Nephrol. 2016;11(3):539–546. doi: 10.2215/CJN.06370615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Urena-Torres P, Prie D, Souberbielle JC. Cinacalcet for cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2013;368(19):1842–1843. doi: 10.1056/NEJMc1301247. [DOI] [PubMed] [Google Scholar]
- 113.Goldsmith DJ, Lamb EJ. Cinacalcet for cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2013;368(19):1843. doi: 10.1056/NEJMc1301247. [DOI] [PubMed] [Google Scholar]
- 114.Eknoyan G, Beck GJ, Cheung AK, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347(25):2010–2019. doi: 10.1056/NEJMoa021583. [DOI] [PubMed] [Google Scholar]
- 115.Wanner C, Krane V, Marz W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353(3):238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 116.Fellstrom BC, Jardine AG, Schmieder RE, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360(14):1395–1407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 117.Goldsmith D, Covic A, Vervloet M, et al. Should patients with CKD stage 5D and biochemical evidence of secondary hyperparathyroidism be prescribed calcimimetic therapy? An ERA-EDTA position statement. Nephrol Dial Transplant. 2015;30(5):698–700. doi: 10.1093/ndt/gfv050. [DOI] [PubMed] [Google Scholar]
- 118.Block GA, Bushinsky DA, Cheng S, et al. Effect of Etelcalcetide vs Cinacalcet on Serum Parathyroid Hormone in Patients Receiving Hemodialysis With Secondary Hyperparathyroidism: A Randomized Clinical Trial. JAMA. 2017;317(2):156–164. doi: 10.1001/jama.2016.19468. [DOI] [PubMed] [Google Scholar]
- 119.Omran MT, Khakpour S, Oliaie F. Left ventricular function before and after kidney transplantation. Saudi Med J. 2009;30(6):821–823. [PubMed] [Google Scholar]
- 120.Dzemidzic J, Rasic S, Saracevic A, et al. Predictors of left ventricular remodelling in kidney transplant recipents in the first posttransplant year. Bosn J Basic Med Sci. 2010;10(Suppl 1):S51–55. doi: 10.17305/bjbms.2010.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Parfrey PS, Harnett JD, Foley RN, et al. Impact of renal transplantation on uremic cardiomyopathy. Transplantation. 1995;60(9):908–914. [PubMed] [Google Scholar]
- 122.Ritz E, Bommer J. Cardiovascular problems on hemodialysis: current deficits and potential improvement. Clin J Am Soc Nephrol. 2009;4(Suppl 1):S71–78. doi: 10.2215/CJN.01960309. [DOI] [PubMed] [Google Scholar]