Abstract
Transorbital neuroendoscopic surgery (TONES) is a relatively new technique that not only allows access to the contents of the orbit but also the intracranial compartment, including the anterior cranial fossa, middle fossa and lateral cavernous sinus. In this study, we aimed to retrospectively review the largest experience to our knowledge with regards to surgical outcomes of skull base pathologies treated with a TONES procedure. Forty patients (aged 3–89 years) underwent 45 TONES procedures between the years of 2006–2013. Pathologies were cerebrospinal fluid leak repair (n = 16), traumatic fracture (n = 8), tumor (n = 11), meningoencephalocele (n = 5), hematoma (n = 1), and infection (n = 4). Three patients had a persistent complication at 3 months, including a case each of enophthalmos (unnoticed by patient), epiphora (delayed presentation at 2 months requiring dacryocystorhinostomy), and ptosis (improved at 1 year). Surgical success was achieved in all patients. Of special import, there were no cases of visual decline, diplopia, or stroke. There was no mortality. To our knowledge this is the first study and largest experience of TONES (level 4 evidence) to detail outcomes with respect to skull base pathologies. Our results indicate that TONES procedures can be performed with minimal morbidity. Further studies are needed to assess equivalency with craniotomy based approaches though this initial report is encouraging.
Keywords: CSF leak, Endoscope, Neuroendoscopy, Neurosurgery, Pituitary, Skull base, Transorbital
1. Introduction
Transorbital neuroendoscopic surgery (TONES) is a relatively new technique that not only allows access to the contents of the orbit but also the intracranial compartment, including the anterior cranial fossa, middle fossa, and lateral cavernous sinus [1–14]. In highly selected cases, the advantages of including TONES in a treatment armamentarium are multifold. First, TONES allows a multi-portal, multi-angled approach to lesions of the skull base that are extremely difficult through even expanded transnasal endoscopic procedures [13]. Second, surgical morbidity and cosmetic deformity are minimized compared to standard open, craniofacial approaches [15,16]. Third, two surgeons can work comfortably in a coplanar manner to address the lesion of interest rather than struggle to work through the relatively narrow transnasal route. Here we report the largest experience to our knowledge treating a variety of intracranial pathologies using the TONES approach as well as detailing the relevant patient outcomes associated with each procedure.
2. Overview of TONES
We previously have reported on the use of TONES in both cadaver studies and small clinical series [1,3–5]. Briefly, TONES represents a group of minimally disruptive approaches to the orbit and skull base that do not functionally compromise the eyelid. These are the precaruncular (PC), preseptal lower eyelid (PS), superior eyelid crease (SLC), and lateral retrocanthal approaches (LRC). The SLC approach allows access to the anterior cranial fossa and orbital roof (Fig. 1, quadrant A). The PC approach affords access to the anterior cranial fossa, lateral nasal cavity, cavernous sinus, and optic nerve (Fig. 1, quadrant B). The PS approach allows access to the orbital floor, infraorbital nerve, inferior orbital fissure and middle fossa floor including the foramen rotundum (Fig. 1, quadrant C). Finally, the LRC approach allows access to the deep orbit, cavernous sinus, middle fossa, and infratemporal fossa (Fig. 1, quadrant D). When combined with transnasal approaches or even in isolation, TONES provides access that is minimally disruptive and avoids crossing neurovascular structures.
Fig. 1.

Four quadrants of the orbit. Each quadrant affords access to a different area of the skull base. A = superior eyelid crease approach, B = precaruncular approach, C = preseptal lower eyelid approach, D = lateral retrocanthal approach.
With this framework in mind, we present our series of cases where TONES was utilized either in isolation or in conjunction with traditional transnasal endoscopic surgery.
3. Methods
Institutional Review Board approval for this study was obtained from the University of Washington Human Subjects Division (#44884). A prospectively maintained database was analyzed retrospectively for patients who had undergone a TONES procedure for intracranial pathology between 2006 and 2013. Inclusion criteria were the presence of a skull base defect requiring repair, optic nerve compression, cerebrospinal fluid (CSF) leak, meningoen-cephalocele, and skull base tumor. No patients were excluded and one was lost to follow-up (Supp. Table 1). All patients were operated on at an academic tertiary center, Harborview Medical Center, by the senior otolaryngologist (K.S.M.) and either senior neurosurgeon (L.J.K. or M.F.J.). Patients were followed for a minimum of 6 weeks. Outcome measures included the presence of orbital asymmetry, extraocular movement dysfunction, decline in visual acuity, continued CSF leak, diplopia, and eyelid malposition. Follow-up consisted of evaluation by a head and neck specialist, neurosurgeon, and ophthalmologist where necessary. For tumor cases, extent of resection and time to recurrence (where available) were noted. Procedures were completed using standard endoscopic equipment. Additional equipment utilized included ultrasonic bone aspirators. Skull base reconstructions were performed using irradiated cadaveric dermis, nasoseptal flaps, and fat for additional buttressing where necessary.
Table 1.
Pathology of 40 patients treated with transorbital neuroendoscopic surgery
| Pathology | N |
|---|---|
| Complex CSF leak | 16 |
| Traumatic fracture | 8 |
| Tumor/inflammatory condition | 11 |
| Meningoencephalocele | 5 |
| Hematoma | 1 |
| Infection | 4 |
| Total | 45* |
Total >40 as many cases of trauma had dual pathologies of CSF leak and displaced bony fractures requiring open reduction internal fixation.
CSF = cerebrospinal fluid.
In terms of patient selection, a number of factors were considered. First, if TONES in isolation was considered, the expected surgical result and morbidity had to be at least equivalent if not better than standard craniotomy or endonasal approaches. Second, if TONES was considered as an adjunct to endonasal surgery, it had to provide working angles and operative trajectories difficult to obtain through expanded endonasal corridors. Of note, some of these patients have been published elsewhere [1,3,5] by our group but this report focuses only on neurosurgically relevant pathologies and outcomes.
4. Illustrative patients
4.1. Patient 1
A 56-year-old woman presented with a history of spontaneous CSF rhinorrhea. Subsequent evaluation revealed a Sternberg canal encephalocele eroding through the anterior skull base (Fig. 2). Given the accessibility of the lesion from a combined transorbital/transnasal approach, the patient was advised to undergo surgical treatment.
Fig. 2.

Patient 1. (Left) Axial T1-weighted post-contrast and (center) coronal T2-weighted preoperative MRI showing encephalocele. (Right) Postoperative coronal T2-weighted MRI showing encephalocele repair.
The patient underwent both traditional endoscopic transnasal surgery as well as a TONES procedure with a PC approach. The encephalocele was endoscopically circumscribed using corridors supplied by the transnasal and transorbital approaches. The encephalocele was then cauterized and resected. A dural patch of irradiated cadaveric dermis was placed, along with a nasoseptal mucosal flap. A lumbar drain was placed intraoperatively and continued for 5 days.
Postoperatively, the patient had no complications and her leak repair proved durable. During follow-up, she had no diplopia, orbital asymmetry, decline in visual acuity, or difficulty with extraocular movements.
4.2. Patient 2
A 52-year-old man with a history of a right frontal ganglioma treated at the age of 19 with surgery and radiation presented with an enlarging right orbital mass. Workup revealed an enlarging anterior fossa mass with extension into the orbit, consistent with a radiation-induced meningioma (Fig. 3). The patient had a poorly vascularized scalp that was impressively atrophic, further complicating a craniotomy-based approach.
Fig. 3.

Patient 2. Preoperative coronal, sagittal and axial CT views, as well a reconstructed surface view with colorized tumor model of the meningioma.
The patient underwent a combination of endoscopic transnasal surgery as well as endoscopic transorbital surgery through a PC approach (Supp. Video 1). Removal of the mass was completed using standard endoscopic transnasal equipment. A small frontal craniotomy was performed through the posterior orbital wall to access the intracranial tumor components. The dura was repaired using irradiated cadaveric dermis and fibrin glue (Supp. Video 1).
During follow-up the patient had no visual complaints, objective abnormalities on examination or CSF leak. MRI revealed gross total resection (Fig. 4) of a World Health Organization grade I radiation-induced meningioma.
Fig. 4.

Patient 2. Postoperative coronal T1-weighted post-contrast MRI demonstrating complete resection of the meningioma.
4.3. Patient 3
A 67-year-old woman presented with sinus congestion and subsequent workup revealed an extensive sinonasal mass with extension through the anterior and middle cranial fossa (Fig. 5). Biopsy revealed sinonasal melanoma. The patient was offered both a traditional craniofacial approach for the resection of the lesion in addition to combined transnasal/TONES procedures, the latter of which she elected for.
Fig. 5.
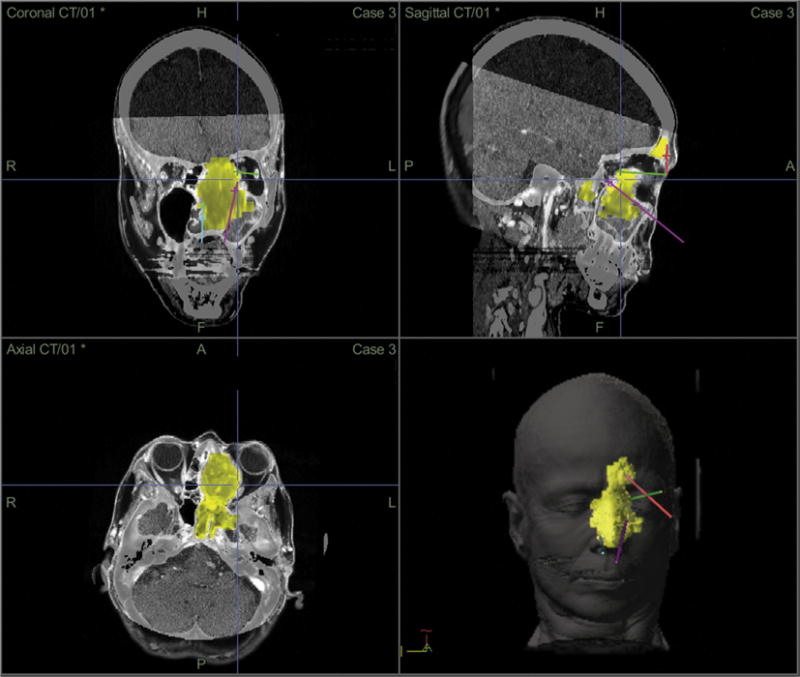
Patient 3. Preoperative coronal, sagittal and axial CT views, as well a reconstructed surface view with colorized tumor model of the sinonasal melanoma and trajectory planning through the transnasal and transorbital routes.
Preoperatively, the imaging were used to plan the most efficient pathways for tumor access using computer guided trajectory planning [17]. The patient underwent a combination of endoscopic transnasal surgery as well as endoscopic transorbital surgery through a LRC approach. Removal of the mass was completed using standard endoscopic transnasal equipment. The left olfactory nerve was infiltrated with tumor and thus resected. Margins of the resection, including periorbita, were negative for tumor on final pathology. The dura was repaired using irradiated cadaveric dermis and fibrin glue. The patient underwent neutron beam therapy to the tumor bed with Gamma Knife radiosurgery (Elekta AB, Stockholm, Sweden) to the margins.
During follow-up the patient had no visual complaints, objective abnormalities on examination or evidence of CSF leak. MRI revealed a gross total resection of tumor (Fig. 6). At 12 months, she has not developed local recurrence but did develop spinal metastases.
Fig. 6.
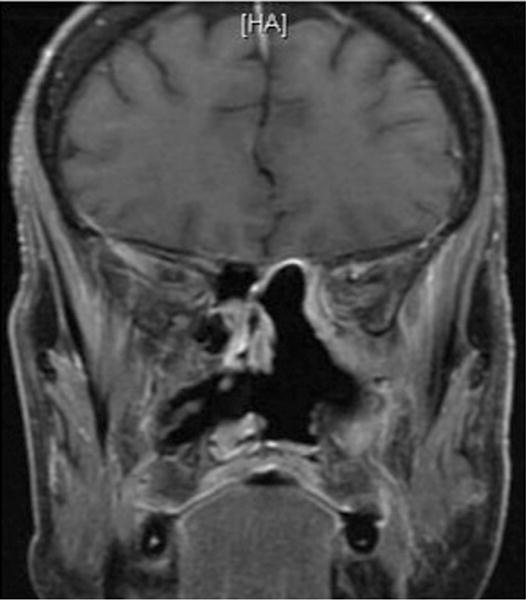
Patient 3. Postoperative coronal T1-weighted post-contrast MRI demonstrating complete resection of the sinonasal melanoma.
5. Results
Forty consecutive patients (average age 45 years, 15 females, 25 males) between 2006 and 2013 met our inclusion criteria. Follow-up ranged from 6 weeks to 12 months with an average follow-up of 3 months. One patient was lost to follow-up. The details of the approaches used have been described elsewhere [3]. The pathologies ranged from CSF leak (spontaneous or traumatically induced), traumatic fracture, tumor, inflammatory conditions, meningoencephalocele, hematoma, and spontaneous infection (Table 1). Table 2 lists unresolved complications post-procedure (6%). The PC approach was used in 37% of patients, the LRC approach in 9%, the SLC approach in 40%, and a combined SLC/PS approach in 13%. Transnasal endoscopy was combined with the transorbital procedure in 40% of patients. There were no CSF leaks. There was one case of enophthalmos, unnoticed by the patient and untreated. There was another case of epiphora requiring conjunctival dacry-ocystorhinostomy. There were four cases of temporary hypoesthesia involving the infraorbital nerve.
Table 2.
Persistent complications (>1 week post-procedure) following intervention with transorbital neuroendoscopic surgery
| Complications | N |
|---|---|
| CSF leak | 0 |
| Stroke | 0 |
| Hematoma | 0 |
| Infection | 0 |
| Visual deficits | 0 |
| Persistent eyelid malposition | 0 |
| Diplopia | 0 |
| Enophthalmos | 1 |
| Epiphora | 1 |
| Ptosis | 1 |
CSF = cerebrospinal fluid.
6. Discussion
This retrospective review presents our experience with 40 patients and 45 TONES procedures over a 6 year period involving neurosurgical pathologies. In all cases, surgical success was achieved with acceptable morbidity and no procedurally related mortality.
Periorbital and transorbital surgery have long been recognized as important modalities for the treatment of a variety of indications. These include cosmetic eyelid repair, orbital fracture, orbital tumor, maxillectomy, and even ventricular access [18–21]. Previous descriptions of endoscopic intraorbital surgery have concentrated on the medial orbit via transcaruncular and medial transconjunctival incisions for fracture repair and access to the orbital roof and optic nerve [1,6–8,10,22]. Some have found the transorbital approach to be limited, but these limitations have arisen from failing to recognize the orbit as a four quadrant system with angles and trajectories that must be deliberately chosen to suit the pathology [10]. Our current report, in conjunction with others from our group [1–5,17,23], expands the indications for TONES procedures to include not only traumatic CSF leaks, but also spontaneous CSF leaks from meningoencephaloceles, mucoceles, and extra-axial skull base tumors of the anterior and middle fossa. Additional indications not attempted in this series but deserving of further study include intradural lesions of the anterior and middle cranial fossa [12]. The advantages of expanding the indications for TONES procedures are many and include increased working angles, increased ease of four handed operating, an absence of a cranial incision, limited or absent brain retraction, and similar outcomes from more traditional open approaches [1,3,5]. Moreover, TONES avoids crossing critical neurovascular structures in contradistinction to expanded endonasal approaches. When selected appropriately, the addition of transorbital portal(s) allows for optimal visualization and instrumentation at the target location while minimizing collateral tissue damage.
There are a number of reasons transorbital surgery should be considered over craniotomy and standard endonasal procedures for suitable pathologies. First, lesions of the anterior skull base traditionally require bifrontal craniotomies, often necessitating exposure of the sagittal sinus and disruption of the frontal sinus. Midline pathologies of the anterior skull base accessed endonasally require disruption of the olfactory apparatus given the midline trajectory of the endonasal route. When orbitotomies and naso-orbital bar removal are added to these craniotomy based approaches, the risks, though small, of injury to the orbit itself and supraorbital nerve increase. Second, lesions of the middle fossa require mobilization of the temporalis muscle and risk to the frontalis branch of the facial nerve. Moreover cosmesis can be troublesome, as temporal craniectomy and sphenoid drilling can result in obvious temporal depression, despite a variety of reconstruction techniques. Although craniotomies are often necessary, transorbital approaches to appropriate lesions of the skull base avoids many of these craniotomy-based risks. Third, TONES most often represents an operative adjunct to endoscopic endonasal surgery. Patient 1 presented with a Sternberg canal encephalocele. These lesions can certainly be approached through an endoscopic, endonasal transpterygoid route or transcranial route [24–26]. However, the addition of the transorbital corridor to a standard endoscopic transsphenoidal approach is relatively quick and avoids the extensive sinonasal work performed through a transpterygoid corridor. In addition, the working angles afforded by the transorbital corridor allow ease in resecting the encephalocele and constructing the repair. In Patient 2, it is arguable that the lesion could have been resected through a transethmoidal approach involving the medial orbit and fovea ethmoidalis. However, the working angles required for such a resection are challenging and greatly enhanced from the transorbital corridor. Another approach to consider is the supraorbital keyhole craniotomy [27]. For selected lesions, this approach is versatile and can access a wide range of frontal fossa, parasellar, and some middle fossa tumors. However, lesions that traverse the orbit and sinonasal cavity are difficult with this approach and require unnecessary intracranial exposure. Thus, we do not advocate the use of TONES for standard pathologies, where the additional working angles do not add benefit. Rather, for lesions that require challenging working angles (such as combined orbital/nasal tumors, lesions of anterolateral anterior cranial fossa, lateral cavernous sinus, middle fossa dura, etc) and complex endoscopic visualization (30, 45, 60 degree endoscopes), the addition of a TONES port can greatly simplify the mechanics of the operation.
Orbital morbidity from TONES procedures is certainly a concern, both from a cosmetic and functional point of view. As expected, nearly all patients have some ocular dysfunction (though no visual dysfunction) in the immediate perioperative period. However, in our follow-up period there was only one case of prolonged partial ptosis (improved at 1 year) and one case of mild enophthalmos (unnoticed by the patient). The patient with epiphora presented 2 months post-procedure complaining of inadequate tear drainage, likely resulting from inflammation and subsequent scarring post-procedure. Additionally, the latter two complications occurred early in our experience and seemed to have been subsequently prevented by allowing more time for orbital relaxation and by improved posterior orbital wall reconstruction. Three technical maneuvers have helped minimize the complication rate, namely conservative approach to patient selection, orbital retraction of less than 10 millimeters, and frequent removal of intraorbital instruments to allow for orbital relaxation (every 15–20 minutes). Additionally, the use of ultrasonic bone aspirators (Sonopet [Stryker, Kalamazoo, MI, USA] or similar) avoids rotating drill bits within the orbit. The employment of these maneuvers has permitted the use of TONES with minimal morbidity, even in cases of high complexity and long operative times (greater than 6 hours).
This report represents our series of highly selected consecutive patients who underwent a TONES procedure for neurosurgical pathologies. Its limitations include its basis as a single institution experience. Moreover, this study does not robustly compare TONES associated procedures with standard craniotomy based approaches or endonasal only approaches in terms of the durability of surgical success, morbidity, and patient satisfaction in an adjudicated fashion. Future studies comparing TONES with supraorbital craniotomy and endonasal only approaches will help clarify the true role, if any, of TONES in the skull base toolkit.
The techniques described herein build on technical skills gained in already widespread transnasal endoscopic operations. A criticism of this approach pertains to its relevance in the setting of expanded endoscopic approaches (including transpterygoid, trans-fovea ethmoidalis [28–31]). Certainly, access to the areas of the skull base afforded by expanded endonasal approaches and even supraorbital keyhole craniotomies can be obtained. The point of this study is to illustrate that access, in and of itself, is not sufficient to facilitate the most effective surgery. As our trajectory map illustrates in Figure 5, the addition of the transorbital port allowed unencumbered access to the orbital apex thereby facilitating safety, two-surgeon operating, and a minimization of unfavorable working angles. While not intended as a replacement for expanded endonasal or craniotomy approaches, TONES provides a supplementary and complementary tool to enhance visualization and operative maneuverability, particularly when assessed preop-eratively with trajectory planning [17]. Indeed, 40% of cases in this series used the transorbital port as an adjunct to the endoscopic corridor. By combining transnasal and transorbital ports, surgeons can now optimize their views of the pathology, preoperatively choose the trajectory and associated working distance, and maximize the operative working space. As indications for complex conditions arise, the combination of TONES with transnasal surgery offers the closest alternative to traditional craniotomy based approaches to skull base lesions in terms of visualization and two handed maneuverability. Further studies will need to be completed to assess the efficacy and safety of TONES procedures when directly compared to traditional craniotomy based approaches and expanded endonasal procedures in a prospective fashion, however. However, these preliminary results indicate an important role for TONES as part of the neurosurgical treatment armamentarium.
7. Conclusion
TONES represents a new direction in skull base surgery. In this report, we have outlined the largest series to our knowledge of patients from a single institution who have undergone a TONES procedure for neurosurgical pathology with reasonable morbidity and no mortality. Future directions require direct comparison of TONES with traditional craniotomy based approaches in terms of durability, long-term morbidity and patient satisfaction.
Supplementary Material
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jocn.2015.07.021.
Footnotes
Conflicts of Interest/Disclosures
The authors declare that they have no financial or other conflicts of interest in relation to this research and its publication.
References
- 1.Balakrishnan K, Moe KS. Applications and outcomes of orbital and transorbital endoscopic surgery. Otolaryngol Head Neck Surg. 2011;144:815–20. doi: 10.1177/0194599810397285. [DOI] [PubMed] [Google Scholar]
- 2.Ciporen JN, Moe KS, Ramanathan D, et al. Multiportal endoscopic approaches to the central skull base: a cadaveric study. World Neurosurg. 2010;73:705–12. doi: 10.1016/j.wneu.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 3.Moe KS, Bergeron CM, Ellenbogen RG. Transorbital neuroendoscopic surgery. Neurosurgery. 2010;67:ons16–28. doi: 10.1227/01.NEU.0000373431.08464.43. [DOI] [PubMed] [Google Scholar]
- 4.Moe KS, Jothi S, Stern R, et al. Lateral retrocanthal orbitotomy: a minimally invasive, canthus-sparing approach. Arch Facial Plast Surg. 2007;9:419–26. doi: 10.1001/archfaci.9.6.419. [DOI] [PubMed] [Google Scholar]
- 5.Moe KS, Kim LJ, Bergeron CM. Transorbital endoscopic repair of cerebrospinal fluid leaks. Laryngoscope. 2011;121:13–30. doi: 10.1002/lary.21280. [DOI] [PubMed] [Google Scholar]
- 6.Chen CT, Chen YR. Endoscopic orbital surgery. Atlas Oral Maxillofac Surg Clin North Am. 2003;11:179–208. doi: 10.1016/s1061-3315(03)00016-7. [DOI] [PubMed] [Google Scholar]
- 7.Rhee JS, Chen CT. Endoscopic approach to medial orbital wall fractures. Facial Plast Surg Clin North Am. 2006;14:17–23. doi: 10.1016/j.fsc.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Prabhakaran VC, Selva D. Orbital endoscopic surgery. Indian J Ophthalmol. 2008;56:5–8. doi: 10.4103/0301-4738.37587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murchison AP, Rosen MR, Evans JJ, et al. Endoscopic approach to the orbital apex and periorbital skull base. Laryngoscope. 2011;121:463–7. doi: 10.1002/lary.21357. [DOI] [PubMed] [Google Scholar]
- 10.Raza SM, Quinones-Hinojosa A, Lim M, et al. The transconjunctival transorbital approach: a keyhole approach to the midline anterior skull base. World Neurosurg. 2013;80:864–71. doi: 10.1016/j.wneu.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kenyon B, Antisdel JL. Anatomic evaluation of endoscopic transnasal transorbital approach to the lateral orbital apex. Am J Rhinol Allergy. 2014;28:82–5. doi: 10.2500/ajra.2014.28.4000. [DOI] [PubMed] [Google Scholar]
- 12.Chen HI, Bohman LE, Loevner LA, et al. Transorbital endoscopic amygdalohippocampectomy: a feasibility investigation. J Neurosurg. 2014;120:1428–36. doi: 10.3171/2014.2.JNS131060. [DOI] [PubMed] [Google Scholar]
- 13.Doglietto F, Lauretti L, Frank G, et al. Microscopic and endoscopic extracranial approaches to the cavernous sinus: anatomic study. Neurosurgery. 2009;64:413–21. doi: 10.1227/01.NEU.0000338943.08985.73. [discussion 421–2] [DOI] [PubMed] [Google Scholar]
- 14.Bly RA, Ramakrishna R, Ferreira M, et al. Lateral transorbital neuroendoscopic approach to the lateral cavernous sinus. J Neurol Surg B Skull Base. 2014;75:11–7. doi: 10.1055/s-0033-1353363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solero CL, DiMeco F, Sampath P, et al. Combined anterior craniofacial resection for tumors involving the cribriform plate: early postoperative complications and technical considerations. Neurosurgery. 2000;47:1296–304. [discussion 1304–5] [PubMed] [Google Scholar]
- 16.Zabramski JM, Kiris T, Sankhla SK, et al. Orbitozygomatic craniotomy. Technical note. J Neurosurg. 1998;89:336–41. doi: 10.3171/jns.1998.89.2.0336. [DOI] [PubMed] [Google Scholar]
- 17.Bly RA, Chang SH, Cudejkova M, et al. Computer-guided orbital reconstruction to improve outcomes. JAMA Facial Plast Surg. 2013;15:113–20. doi: 10.1001/jamafacial.2013.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Owusu Boahene KD, Lim M, Chu E, et al. Transpalpebral orbitofrontal craniotomy: a minimally invasive approach to anterior cranial vault lesions. Skull Base. 2010;20:237–44. doi: 10.1055/s-0030-1249247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bourguet J. Les hernies graissueses de l’orbite. Noitre traitement chirurgicul. Bull Acad Med Paris. 1924:1270–2. [Google Scholar]
- 20.Goyal A, Tyagi I, Jain S, et al. Transconjunctival incision for total maxillectomy– an alternative for subciliary incision. Br J Oral Maxillofac Surg. 2011;49:442–6. doi: 10.1016/j.bjoms.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Tubbs RS, Loukas M, Shoja MM, et al. Emergency transorbital ventricular puncture: refinement of external landmarks. J Neurosurg. 2009;111:1191–2. doi: 10.3171/2009.3.JNS081651. [DOI] [PubMed] [Google Scholar]
- 22.Chu EA, Quinones-Hinojosa A, Boahene KD. Trans-blepharoplasty orbitofrontal craniotomy for repair of lateral and posterior frontal sinus cerebrospinal fluid leak. Otolaryngol Head Neck Surg. 2010;142:906–8. doi: 10.1016/j.otohns.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Bly RA, Su D, Hannaford B, et al. Computer modeled multiportal approaches to the skull base. J Neurol Surg Skull Base. 2012;73:415–23. doi: 10.1055/s-0032-1329623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bachmann-Harildstad G, Kloster R, Bajic R. Transpterygoid trans-sphenoid approach to the lateral extension of the sphenoid sinus to repair a spontaneous CSF leak. Skull Base. 2006;16:207–12. doi: 10.1055/s-2006-950389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bendersky DC, Landriel FA, Ajler PM, et al. Sternberg’s canal as a cause of encephalocele within the lateral recess of the sphenoid sinus: a report of two cases. Surg Neurol Int. 2011;2:171. doi: 10.4103/2152-7806.90034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt RF, Choudhry OJ, Raviv J, et al. Surgical nuances for the endoscopic endonasal transpterygoid approach to lateral sphenoid sinus encephaloceles. Neurosurg Focus. 2012;32:E5. doi: 10.3171/2012.3.FOCUS1267. [DOI] [PubMed] [Google Scholar]
- 27.Wilson DA, Duong H, Teo C, et al. The supraorbital endoscopic approach for tumors. World Neurosurg. 2014;82:S72–80. doi: 10.1016/j.wneu.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 28.Greenfield JP, Anand VK, Kacker A, et al. Endoscopic endonasal transethmoidal transcribriform transfovea ethmoidalis approach to the anterior cranial fossa and skull base. Neurosurgery. 2010;66:883–92. doi: 10.1227/01.neu.0000368395.82329.c4. [discussion 892] [DOI] [PubMed] [Google Scholar]
- 29.de Lara D, Ditzel Filho LF, Prevedello DM, et al. Endonasal endoscopic approaches to the paramedian skull base. World Neurosurg. 2014;82:S121–9. doi: 10.1016/j.wneu.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 30.Pinheiro-Neto CD, Fernandez-Miranda JC, Prevedello DM, et al. Transposition of the pterygopalatine fossa during endoscopic endonasal transpterygoid approaches. J Neurol Surg B Skull Base. 2013;74:266–70. doi: 10.1055/s-0033-1347367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Battaglia P, Turri-Zanoni M, Dallan I, et al. Endoscopic endonasal transpterygoid transmaxillary approach to the infratemporal and upper parapharyngeal tumors. Otolaryngol Head Neck Surg. 2014;150:696–702. doi: 10.1177/0194599813520290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


