Structured Abstract
Objectives
This study characterized cardiac rehabilitation (CR) utilization amongst ventricular assist device (VAD) recipients in the United States and the association of CR with one-year hospitalizations and mortality using the 2013–2015 Medicare files.
Background
Exercise-based CR is indicated in patients with heart failure with reduced ejection fraction, but there are no data regarding CR participation after VAD implantation.
Methods
The study included Medicare beneficiaries enrolled due to disability or age ≥65. We identified VAD patients by diagnosis codes and cumulated CR sessions occurring within one year after VAD implantation. We used multivariable-adjusted Andersen-Gill models to evaluate the association of CR with one-year hospitalization risk and Cox regression to evaluate the association of CR with one-year mortality.
Results
There were 1164 VADs implanted in Medicare beneficiaries in the United States in 2014. CR utilization was low, with 348 patients (30%) participating in CR programs. The Midwest had the highest proportion of VAD patients initiating CR (38%) while the Northeast had the lowest proportion of CR participants (25%). Each 5-year increase in age was associated with attending an additional 1.6 CR sessions (95% confidence interval (CI) 0.7–2.5, p<0.001). CR participation was associated with a 23% lower one-year hospitalization risk (95% CI 11%–33%, p<0.001) and a 47% lower one-year mortality risk (95% CI 18%–66%, p<0.01) after multivariable adjustment.
Conclusions
Approximately one third of VAD recipients attend CR. Though it is not possible to fully account for unmeasured confounding, VAD patients who participate in CR appear to have lower risk for hospitalization and mortality.
Keywords: Cardiac rehabilitation, ventricular assist device, hospitalizations, readmissions, mortality
Introduction
Cardiac rehabilitation (CR), a systematic, multidisciplinary program of prescribed exercise, nutritional counseling, psychosocial support, and cardiovascular risk factor control, is indicated in patients with stable heart failure with reduced ejection fraction (HFrEF) as well as after heart transplantation(1). CR decreases mortality and improves quality of life in patients with ischemic heart disease(2). Despite its known benefits, less than 20% of eligible patients participate in CR programs(3–7). To date, there are no data on CR participation after ventricular assist device (VAD) implantation.
Exercise training in VAD patients is feasible and safe, and improves self-reported health status, peak oxygen uptake and skeletal muscle function(8–11). Moreover, exercise capacity is significantly diminished after VAD implantation, increasing the relative benefit of improvements in exercise tolerance(12). VAD implantation is not currently one of the indications for CR covered by Medicare. However, many VAD patients are eligible for CR under the HFrEF indication, which covers patients with stable, chronic heart failure and a left ventricular ejection fraction (LVEF) of ≤35%(5). The Centers for Medicare & Medicaid Services (CMS) define stable chronic heart failure patients as those that have not had cardiovascular hospitalizations within the prior 6 weeks(13). Almost all patients who are candidates for VAD implantation meet medical criteria for disability benefits (LVEF ≤30% with symptoms impacting activities of daily living) and are thus eligible for Medicare(14).
Using CMS data, we evaluated CR utilization after VAD implantation in the United States. We also characterized the association of CR with one-year hospitalizations and mortality amongst VAD recipients. We hypothesized that CR is associated with a decreased risk of hospitalization and mortality in these patients.
Methods
Data source
We obtained data regarding CR utilization in VAD recipients in the United States from the 2014–2015 Medicare 100% Limited Data Set (LDS) files from CMS. These files contain all inpatient and institutional outpatient claims for fee-for-service Medicare beneficiaries. The institutional review board of Vanderbilt University Medical Center approved the study, which was carried out under the auspices of a data use agreement with CMS.
Patient population
The study population included Medicare beneficiaries enrolled in 2014 due to disability or age ≥65 who resided in the United States, had uninterrupted fee-for-service coverage until their death or for one year following discharge and did not attend any CR sessions in the year prior to VAD implantation. Inclusion in the study was based on a discharge diagnosis code (International Classification of Diseases 9th Revision (ICD-9) codes 37.60, 37.63, 37.65, 37.66) or procedure code (Current Procedure Terminology (CPT) codes 33975, 33976, 33979, 33981, 33982, 33983 0051T, 0052T, 0053T) for VAD implantation, replacement or repair.
Outcomes
Participation in CR programs, defined as a binary variable (yes/no), was the primary outcome. We searched the Medicare outpatient LDS files for CR claims (CPT codes 93797, 93798, G0422, G0423, or S9472) occurring within one year after the VAD hospitalization discharge date. Secondary outcomes included (1) CR as a continuous variable (number of sessions attended); (2) the number of hospitalizations that occurred in the one-year period after patients underwent VAD implantation, determined from the Inpatient file; and (3) all-cause mortality, determined from death dates in the Medicare denominator file.
Other variables
Patients receiving heart transplants within one year of discharge from the VAD hospitalization were identified by CPT code 33945 or ICD-9 code 37.51. We obtained demographic characteristics, including age, sex, race (Black, White, or Other) and geographic census division (Midwest, Northeast, West and South) from the denominator file. We characterized the burden of comorbidities with Elixhauser comorbidity groups present during the hospitalization for VAD implantation and the preceding 12 months using ICD-9 codes as described previously(15). We determined whether the VAD hospital had a CR program from the American Hospital Association Annual Survey of Hospitals(16). We characterized socioeconomic status with median income from the patient’s county of residence, obtained from the United States Census Bureau Small Area Income and Poverty Estimates for 2014(17).
Statistical analysis
Baseline demographic and geographic characteristics of VAD recipients participating in CR were compared to those who did not participate in CR programs using chi-square tests for categorical variables and Wilcoxon rank-sum tests for continuous variables. We used multivariable-adjusted logistic regression to evaluate the effect of individual covariates on CR initiation rates. We used linear regression to analyze predictors of the number of CR sessions attended. The Andersen-Gill model with a robust sandwich covariance estimator (also known as a proportional means model), a technique for the analysis of repeated events, was used to model the effect of participating in CR on one-year hospitalization risk after adjusting for covariates(18,19). Patients receiving heart transplants were censored at the time of the transplant admission. CR participation was used as a time-updated covariate such that all individuals in the sample were considered non-CR participants at baseline and remained so until beginning CR. For example, if an individual had no hospitalizations in the year following VAD implantation and did not initiate CR until 3 months after discharge, he or she would contribute 3 months of non-hospitalization time in the non-CR participant group and the remaining 9 months in the follow-up period would contribute to the CR participant group. This approach, known as the Mantel-Byar method, was chosen to minimize immortal person-time bias(20,21). The Mantel-Byar method has been shown to yield unbiased estimates even when event hazards change over time(20).
To evaluate the association of CR with one-year mortality risk, we constructed a Cox regression model adjusting for clinical characteristics and comorbidities and again used CR participation as a time-varying covariate in order to minimize immortal person-time bias. As a sensitivity analysis to address potential healthy cohort bias, we created a marginal structural model with inverse probability of treatment weighting(22). For the marginal structural model, the follow-up period was broken into one-week blocks starting at 3-weeks post discharge in order to accumulate enough CR participants, and the sample was reweighted at the beginning of each one-week interval. This weighting scheme allowed us to estimate the average treatment effect in the CR participants. Thus, the CR participants at each time point served as our reference population to which the sample was standardized.
We conducted an additional analysis to measure the sensitivity of the effect of CR on mortality to residual confounding from unmeasured variables, specifically frailty, after adjusting for observed confounders. This method makes statistical inferences about the true exposure effect of CR by specifying distributions of unmeasured confounders in CR participants and nonparticipants along with the effects of these confounders on the outcome, i.e. mortality(23). Frailty, defined as a score of >0.25 on the Rockwood Frailty Index(24), was found in a study of 99 VAD patients to have a prevalence of 61.6% prior to VAD implantation and a hazard ratio (HR) of 2.31 (95% confidence interval (CI) 1.18 to 4.98, p<0.05) for one-year mortality(25). These point estimates were used as the basis for the sensitivity analysis.
All analyses used SAS version 9.4(26) and R version 3.1.2(27).
Results
Cohort derivation
There was a total of 1647 Medicare beneficiaries receiving VADs in 2014. We excluded 95 patients who attended CR in the year prior to hospital admission for VAD implantation, 179 patients who did not have uninterrupted fee-for-service Medicare coverage, and 209 patients who died in the hospital or on the day of discharge for a final sample size of 1164 VAD recipients.
Cohort characteristics
A total of 348 (30%) of Medicare beneficiaries receiving VADs initiated CR (Table 1). The average age of the cohort was 61 and 20% of VAD recipients were female. Most patients undergoing VAD implantation were White (72%), while 23% were Black and 5% were in another racial category, including Asian and non-White Hispanics. Almost all patients in the study (96%) received VADs at hospitals that reported having CR programs. A total of 69 patients (6%) underwent heart transplantation within one year of VAD implantation, with a greater proportion receiving transplants amongst CR participants as compared to nonparticipants (8% vs 5%, p<0.05). One-year mortality was 22% amongst CR nonparticipants (179 deaths) compared to 7% amongst CR participants (25 deaths, p<0.0001). After VAD implantation, 31% of patients (363) were discharged to inpatient rehabilitation facilities (IRFs) or skilled nursing facilities (SNFs).
Table 1.
Baseline characteristics of Medicare beneficiaries receiving ventricular assist devices in 2014 (N=1164).
| Characteristic | VADs receiving Medicare | Medicare CR nonparticipants | Medicare CR participants | p-value* |
|---|---|---|---|---|
| All, n | 1164 | 816 (70%) | 348 (30%) | n/a |
| Demographic | ||||
| Age | 61.2 ± 11.8 | 60.7 ± 12.0 | 62.6 ± 11.2 | <0.01 |
| Sex | 0.35 | |||
| Female | 237 (20%) | 172 (21%) | 65 (19%) | -- |
| Male | 927 (80%) | 644 (79%) | 283 (81%) | -- |
| Race | 0.10 | |||
| White | 834 (72%) | 575 (70%) | 259 (74%) | -- |
| Black | 265 (23%) | 188 (23%) | 77 (22%) | -- |
| Other | 65 (5%) | 53 (7%) | 12 (4%) | -- |
| Median county income | $56,477 ±$14,986 | $56,077 ± $15,040 | $57,416 ± $14,836 | 0.11 |
| Census Region | <0.01 | |||
| Midwest | 302 (26%) | 187 (23%) | 115 (33%) | -- |
| Northeast | 209 (18%) | 157 (19%) | 52 (15%) | -- |
| South | 514 (44%) | 377 (46%) | 137 (39%) | -- |
| West | 139 (12%) | 95 (12%) | 44 (13%) | -- |
| Clinical | ||||
| CR program at VAD hospital | 1117 (96%) | 784 (96%) | 333 (96%) | 0.76 |
| Length of stay (days) | 33.8 ± 27.5 | 35.1 ± 29.7 | 30.9 ± 21.3 | 0.10 |
| Discharged to IRF or SNF | 363 (31%) | 244 (30%) | 119 (34%) | 0.15 |
| Cormorbidities | ||||
| Chronic pulmonary disease | 854 (73%) | 592 (73%) | 262 (75%) | 0.33 |
| Depression | 349 (30%) | 253 (31%) | 96 (28%) | 0.24 |
| Diabetes | 592 (51%) | 419 (51%) | 173 (50%) | 0.61 |
| Hypertension | 1019 (88%) | 713 (87%) | 306 (88%) | 0.79 |
| Hypothyroidism | 263 (23%) | 185 (23%) | 78 (22%) | 0.92 |
| Liver disease | 234 (20%) | 170 (21%) | 64 (18%) | 0.34 |
| Obesity | 391 (34%) | 267 (33%) | 124 (36%) | 0.34 |
| Other neurological disorders | 154 (13%) | 117 (14%) | 37 (11%) | 0.09 |
| Peripheral vascular disease | 212 (18%) | 154 (19%) | 58 (17%) | 0.37 |
| Pulmonary circulation disorders | 703 (60%) | 479 (59%) | 224 (64%) | 0.07 |
| Renal failure | 790 (68%) | 535 (66%) | 255 (73%) | <0.01 |
| Weight loss | 367 (32%) | 255 (31%) | 112 (32%) | 0.75 |
| VADs receiving transplants within one year | 69 (6%) | 41 (5%) | 28 (8%) | <0.05 |
| One-year hospitalizations | 2.3 ± 2.2 | 2.4 ± 2.2 | 1.9 ± 2.1 | <0.05 |
| One-year mortality | 204 (18%) | 179 (22%) | 25 (7%) | <0.0001 |
VAD, ventricular assist device; CR, cardiac rehabilitation; IRF, inpatient rehabilitation facility; SNF, skilled nursing facility.
Values are displayed as mean ± standard deviation or n (percentage) unless otherwise noted.
All p-values obtained by Pearson Chi-Square test or Wilcoxon test.
Cardiac rehabilitation utilization
The only significant predictor of CR initiation amongst VAD recipients was census region (Table 2). VAD patients in the Midwest had a higher odds of initiating CR than those in the South (OR 1.59, 95% CI 1.16–2.18, p<0.01). None of the comorbidities nor age was associated with CR initiation.
Table 2.
Predictors of cardiac rehabilitation initiation and number of sessions attended amongst Medicare beneficiaries receiving ventricular assist devices in 2014 (N=1164).
| Characteristic | Proportion of patients initiating CR, % | Participation in a CR program | CR sessions amongst participants, Mean ± SD | Change in sessions attended* | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|---|
| Odds Ratio* | 95% CI | p-value | ||||||
| All | 30% | 24.5 ± 15.0 | ||||||
| Demographic | ||||||||
| Age (5 year increase) | n/a | 1.06 | 0.99, 1.13 | 0.10 | n/a | 1.6 | 0.7, 2.5 | <0.001 |
| Sex | -- | -- | 0.45 | -- | -- | 0.34 | ||
| Female | 27% | -- | Referent | -- | 24.3 ± 15.5 | -2.2 | −.5, 2.0 | -- |
| Male | 31% | 1.14 | 0.81, 1.61 | -- | 25.1 ± 12.7 | -- | Referent | -- |
| Race | -- | -- | 0.13 | -- | -- | 0.96 | ||
| Black | 29% | 1.05 | 0.73, 1.50 | -- | 22.2 ± 13.9 | 1.2 | −.3, 5.8 | -- |
| Other | 18% | 0.52 | 0.27, 1.003 | -- | 22.4 ± 12.2 | 1.7 | −.1, 10.6 | -- |
| White | 31% | -- | Referent | -- | 25.3 ± 15.4 | -- | Referent | -- |
| Median county income (per $10,000 increase) | n/a | 1.07 | 0.97, 1.17 | 0.17 | n/a | 0.29 | −.8, 1.4 | 0.57 |
| Census Region | -- | -- | <0.05 | -- | -- | 0.31 | ||
| Midwest | 38% | 1.59 | 1.16, 2.18 | -- | 25.0 ± 13.5 | 0.7 | −.9, 4.4 | -- |
| Northeast | 25% | 0.88 | 0.59, 1.31 | -- | 27.1 ± 14.4 | 2.1 | −.1, 7.3 | -- |
| West | 32% | 1.18 | 0.76, 1.82 | -- | 21.8 ± 12.5 | −3.4 | −8.6, 1.8 | -- |
| South | 27% | -- | Referent | -- | 24.0 ± 17.1 | -- | Referent | -- |
| Clinical | ||||||||
| Length of stay (5 day increase) | n/a | 0.97 | 0.94, 1.01 | 0.10 | n/a | −0.5 | −0.9, −0.1 | <0.05 |
| Discharged to IRF or SNF | 33% | 1.25 | 0.93, 1.68 | 0.15 | 25.1 ± 13.9 | −1.2 | −4.7, 2.3 | 0.41 |
| Comorbidities | ||||||||
| Chronic pulmonary disease | 31% | 0.92 | 0.60, 1.40 | 0.69 | 24.6 ± 14.7 | 0.7 | −4.7, 6.1 | 0.86 |
| Depression | 28% | 0.87 | 0.65, 1.17 | 0.35 | 24.0 ± 14.7 | −0.6 | −4.1, 3.0 | 0.75 |
| Diabetes | 29% | 0.89 | 0.68, 1.17 | 0.41 | 22.6 ± 14.2 | −3.2 | −6.4, 0.06 | <0.05 |
| Hypertension | 30% | 0.95 | 0.63, 1.43 | 0.80 | 24.6 ± 15.3 | 3.7 | −1.4, 8.7 | 0.86 |
| Hypothyroidism | 30% | 0.95 | 0.70, 1.30 | 0.76 | 24.3 ± 11.7 | −1.3 | −5.2, 2.6 | 0.52 |
| Liver disease | 27% | 0.96 | 0.69, 1.34 | 0.81 | 24.2 ± 15.6 | 1.8 | −2.4, 5.9 | 0.38 |
| Obesity | 32% | 1.28 | 0.95, 1.71 | 0.10 | 21.6 ± 14.3 | −1.2 | −4.9, 2.6 | 0.48 |
| Other neurological disorders | 24% | 0.71 | 0.47, 1.08 | 0.11 | 23.6 ± 14.7 | −0.2 | −5.6, 5.1 | 0.92 |
| Peripheral vascular disease | 27% | 0.80 | 0.56, 1.13 | 0.20 | 27.2 ± 15.2 | 3.5 | −0.7, 7.8 | 0.24 |
| Pulmonary circulation disorders | 32% | 1.23 | 0.84, 1.80 | 0.30 | 24.6 ± 14.6 | 0.6 | −4.3, 5.5 | 0.82 |
| Renal failure | 32% | 1.32 | 0.98, 1.79 | 0.07 | 23.2 ± 14.0 | −7.3 | −11.0, −3.5 | <0.001 |
| Weight loss | 31% | 1.05 | 0.78, 1.40 | 0.75 | 24.6 ± 13.4 | 1.2 | −2.3, 4.8 | 0.50 |
CR, cardiac rehabilitation; CI, confidence interval; IRF, inpatient rehabilitation facility; SNF, skilled nursing facility.
Adjusted for all listed variables.
Those patients who did initiate CR attended a mean of 24.5 ± standard deviation (SD) 15.0 sessions, fewer than the generally recommended program of 36 sessions (Table 2). Less than one third of CR attendees participated in the full course of 36 sessions. Older patients attended more CR sessions, with a 1.6 session increase (95% CI 0.7–2.5, p<0.001) per 5-year increase in age. VAD recipients with renal failure attended an average of 7.3 fewer sessions (95% CI 3.5–11.0, p<0.001). There was a small but statistically significant inverse association between length of stay during the VAD hospitalization and the number of CR sessions attended (−.5 sessions per 5-day increase in length of stay, 95% CI −0.9 to −0.1, p<0.05). Amongst CR participants, the average time between discharge and the first CR session was 109 ± SD 84 days, with a median of 83 (interquartile range 44–155) days.
Cardiac rehabilitation and hospitalizations
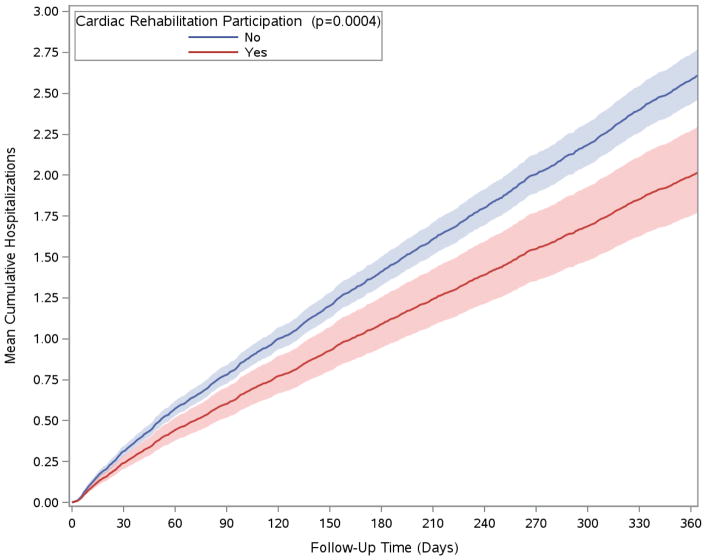
The median number of total hospitalizations within one year of VAD implantation in the cohort was 2 (interquartile range 1–3), with 914 patients (79%) hospitalized at least once during this time. After multivariable adjustment, participation in a CR program was associated with a 23% (95% CI 11%–33%, p<0.001) decrease in one-year hospitalizations (Table 3). Multivariable-adjusted cumulative hospitalizations over one year, stratified by CR participation, are displayed in the Figure. Patients with chronic pulmonary disease prior to VAD implantation were more likely to be readmitted (HR 1.42, 95% CI 1.20–1.68, p<0.0001), as were those with renal failure (HR 1.28, 95% CI 1.13–1.45, p<0.001). Patients discharged to a IRF or SNF were also more likely to be readmitted (HR 1.15, 95% CI 1.02–1.29, p<0.05). In contrast, patients with a prior history of pulmonary circulation disorders (including pulmonary hypertension) had a lower odds of being readmitted.
Table 3.
Predictors of cumulative one-year hospitalization risk amongst Medicare beneficiaries receiving ventricular assist devices in 2014 (N=1164).
| Cumulative one-year hospitalization risk | |||
|---|---|---|---|
| Characteristic | Hazard Ratio* | 95% CI | p-value |
| CR participation | 0.77 | 0.67, 0.89 | <0.001 |
| Demographics | |||
| Age (5 year increase) | 0.99 | 0.96, 1.01 | 0.31 |
| Sex | -- | -- | 0.76 |
| Male | 0.98 | 0.85, 1.12 | -- |
| Female | -- | Referent | -- |
| Race | -- | -- | 0.21 |
| Black | 1.06 | 0.92, 1.22 | -- |
| Other | 0.85 | 0.68, 1.07 | -- |
| White | -- | Referent | -- |
| Median county income (per $10,000 increase) | 1.01 | 0.98, 1.05 | 0.54 |
| Census Region | -- | -- | 0.37 |
| Midwest | 1.03 | 0.90, 1.18 | -- |
| Northeast | 0.94 | 0.80, 1.09 | -- |
| West | 0.88 | 0.74, 1.05 | -- |
| South | -- | Referent | -- |
| Clinical | |||
| Length of stay (5 day increase) | 1.003 | 0.995, 1.01 | 0.50 |
| Discharged to IRF or SNF | 1.15 | 1.02, 1.29 | <0.05 |
| Comorbidities | |||
| Chronic pulmonary disease | 1.42 | 1.20, 1.68 | <0.0001 |
| Depression | 1.14 | 1.01, 1.28 | <0.05 |
| Diabetes | 1.11 | 1.00, 1.24 | 0.06 |
| Hypertension | 1.00 | 0.84, 1.19 | 0.99 |
| Hypothyroidism | 0.96 | 0.84, 1.09 | 0.51 |
| Liver disease | 0.95 | 0.83, 1.09 | 0.46 |
| Obesity | 1.07 | 0.94, 1.20 | 0.31 |
| Other neurological disorders | 1.05 | 0.90, 1.23 | 0.54 |
| Peripheral vascular disease | 1.12 | 0.97, 1.29 | 0.12 |
| Pulmonary circulation disorders | 0.80 | 0.69, 0.94 | <0.01 |
| Renal failure | 1.28 | 1.13, 1.45 | <0.001 |
| Weight loss | 1.02 | 0.90, 1.15 | 0.77 |
CR, cardiac rehabilitation; CI, confidence interval; IRF, inpatient rehabilitation facility; SNF, skilled nursing facility.
Hazard ratios derived from the multivariable-adjusted Andersen-Gill model with robust sandwich covariance estimator (or proportional means model) adjusted for all listed covariates. Ventricular assist device recipients receiving transplants were censored at the time of the transplant admission.
Figure. Cumulative hospitalizations over time for Medicare beneficiaries receiving ventricular assist devices in 2014, stratified by participation in cardiac rehabilitation.
Cumulative hospitalizations were calculated using the Andersen-Gill model adjusted for age, sex, race, census region, comorbidities, discharge to an inpatient rehabilitation facility or skilled nursing facility, and length of stay. Shaded areas represent 95% confidence intervals.
Cardiac rehabilitation and mortality
After adjusting for demographics, clinical factors and comorbidities, CR was associated with a decreased risk of mortality in the year after VAD implantation (Table 4, HR 0.53, 95% CI 0.34–0.82, p<0.01). Factors associated with increased one-year mortality included age (HR 1.09 per 5-year increase, 95% CI 1.01–1.19, p<0.05), discharge to an IRF or SNF (HR 1.57, 95% CI 1.17–2.10, p<0.01), peripheral vascular disease (HR 1.45, 95% CI 1.04–2.02, p<0.05), and weight loss (HR 1.42, 95% CI 1.05–1.90, p<0.05). A sensitivity analysis using a marginal structural model demonstrated a similar association between CR and one-year mortality (HR 0.47, 95% CI 0.30–0.74, p=0.001). Plots of standardized mean differences for individual covariates after using inverse probability of treatment weighting are displayed in Supplementary Figures 1–2, demonstrating that the sample was well-balanced on covariates at 1 and 12 months post-discharge.
Table 4.
Predictors of one-year mortality risk amongst Medicare beneficiaries receiving ventricular assist devices in 2014 (N=1164).
| One-year mortality risk | |||
|---|---|---|---|
| Characteristic | Hazard Ratio* | 95% CI | p-value |
| CR participation | 0.53 | 0.34, 0.82 | <0.01 |
| Demographics | |||
| Age (5 year increase) | 1.09 | 1.01, 1.19 | <0.05 |
| Sex | -- | -- | 0.69 |
| Male | 1.04 | 0.71, 1.53 | -- |
| Female | -- | Referent | -- |
| Race | -- | -- | 0.14 |
| Black | 0.76 | 0.50, 1.14 | -- |
| Other | 1.44 | 0.82, 2.54 | -- |
| White | -- | Referent | -- |
| Median county income (per $10,000 increase) | 0.93 | 0.84, 1.04 | 0.20 |
| Census Region | -- | -- | 0.84 |
| Midwest | 0.85 | 0.59, 1.24 | -- |
| Northeast | 0.87 | 0.56, 1.36 | -- |
| West | 0.97 | 0.61, 1.64 | -- |
| South | -- | Referent | -- |
| Clinical | |||
| Length of stay (5 day increase) | 1.02 | 1.00, 1.04 | 0.07 |
| Discharged to IRF or SNF | 1.57 | 1.17, 2.10 | <0.01 |
| Comorbidities | |||
| Chronic pulmonary disease | 1.44 | 0.93, 2.24 | 0.10 |
| Depression | 0.96 | 0.69, 1.35 | 0.83 |
| Diabetes | 1.23 | 0.91, 1.67 | 0.18 |
| Hypertension | 1.00 | 0.63, 1.58 | 0.98 |
| Hypothyroidism | 0.96 | 0.69, 1.35 | 0.82 |
| Liver disease | 0.94 | 0.65, 1.34 | 0.72 |
| Obesity | 0.99 | 0.71, 1.38 | 0.94 |
| Other neurological disorders | 1.30 | 0.90, 1.89 | 0.17 |
| Peripheral vascular disease | 1.45 | 1.04, 2.02 | <0.05 |
| Pulmonary circulation disorders | 0.91 | 0.63, 1.32 | 0.62 |
| Renal failure | 1.06 | 0.76, 1.49 | 0.72 |
| Weight loss | 1.42 | 1.05, 1.90 | <0.05 |
CR, cardiac rehabilitation; CI, confidence interval; IRF, inpatient rehabilitation facility; SNF, skilled nursing facility; VAD, ventricular assist device.
Hazard ratios derived from a Cox regression model adjusted for all listed covariates.
Ventricular assist device recipients receiving transplants were censored at the time of the transplant admission.
An additional analysis was conducted to measure the sensitivity of the effect of CR on mortality to residual confounding from unmeasured variables(23), specifically frailty. Assuming frailty has an HR of 2.31 for one-year mortality and a prevalence of 61.6% in the VAD population based on prior work(25), CR participants would need to have a frailty prevalence of 36.8% or less to make the observed effect of CR on one-year mortality nonsignificant (i.e. a frailty prevalence of 36.8% would make the effect of CR nonsignificant exactly at p=0.05).
Discussion
This is the first study to report CR utilization rates in patients undergoing VAD implantation in the United States. Approximately one third of VAD patients participated in CR programs. There was geographic variation in CR after VAD implantation, with the Midwest having the highest CR initiation rates. VAD patients participating in CR programs began an average of three months after discharge and attended two thirds of the recommended course of 36 sessions. Younger CR participants attended significantly fewer CR sessions than older patients. Although it is not possible to fully account for all confounding variables, VAD patients who participate in CR appear to have lower risk for hospitalization and all-cause death.
Cardiac rehabilitation by indication
CR utilization varies by indication, with reported initiation rates ranging from less than 10% in patients with systolic heart failure(5), to 10–20% in patients with acute myocardial infarction (AMI) and percutaneous coronary intervention(6,28), and up to 50% in patients receiving heart transplants(29). Approximately one third of patients undergoing coronary artery bypass grafting (3,4,7,30) participate in CR programs, a proportion similar to that seen in VAD patients in the current study. Unlike the aforementioned indications, Medicare does not specifically cover CR after VAD implantation and these patients are often referred to CR programs under the auspices of other conditions. VAD patients could potentially be eligible for CR Medicare coverage under the HFrEF indication(13), stable angina pectoris(1) (which covers most patients with ischemic heart disease), and/or AMI(1) (which covers patients experiencing an AMI within the prior year). Cardiac rehabilitation initiation The only significant predictor of CR initiation amongst VAD recipients was census region. The Midwest census region had a significantly higher proportion of VAD patients initiating CR than the other regions. This geographic variation in CR utilization is consistent with prior studies of CR use after acute myocardial infarction and coronary artery bypass grafting(7). The fact that geographic location is more strongly associated with the odds of initiating CR than any of the clinical characteristics or comorbidities in this population underscores the importance of further research to characterize variation in CR referral patterns and access. For those initiating CR, the time between discharge and the first CR appointment was much longer in VAD patients (median 83 days) as compared to a recent study in patients with ischemic heart disease (median 42 days)(31). This delay is likely attributable to the significant postoperative recovery period after VAD implantation as well as the Medicare requirement that patients referred to CR programs for systolic heart failure be stable for 6 weeks (e.g. no cardiovascular hospitalizations) prior to attending the first session(13).
Cardiac rehabilitation dose
A dose-dependent relationship has been identified between the number of sessions attended and mortality in patients with ischemic heart disease(3,4). Interestingly, older CR participants were more likely to attend more sessions than younger participants. One might expect that older VAD recipients would be inclined to participate in fewer CR sessions due to a higher burden of comorbidities and frailty(24,25). It is possible that younger VAD patients may be more likely to return to work, and work responsibilities are a significant barrier to attending CR programs(32,33). Renal failure was associated with a significant decrease in the number of sessions attended amongst CR participants, but not with the odds of initiating CR. This is likely representative of the fact that the time demands of hemodialysis are a major barrier to attending CR sessions three times weekly.
Cardiac rehabilitation and one-year outcomes after VAD implantation
CR was associated with fewer hospitalizations in the year following VAD implantation. The magnitude of this association in our analyses (a 23% decrease, 95% CI 0.67–0.89) is similar to that in other studies(19). A recent meta-analysis of the effect of exercise-based CR versus usual care on hospitalizations demonstrated an 18% decrease in hospitalization risk (95% CI 4%–30%)(2). The etiology of this association is likely multifactorial. Beyond CR’s known beneficial effects on skeletal muscle function, peak oxygen uptake and health status in VAD recipients (8–10), CR offers an opportunity for healthcare professionals to serially monitor these patients, potentially averting unplanned hospitalizations.
In our adjusted analyses COPD and renal failure were the only comorbidities that were associated with an increased hospitalization risk in VAD patients. Curiously, pulmonary circulation disorders (including pulmonary hypertension) prior to VAD implantation were associated with a decreased risk of hospitalization. It is possible that such patients experience disproportionate benefit from a VAD as these devices significantly improve pulmonary arterial pressures(34), though this conclusion would be speculative with the available data and warrants further study.
The magnitude of the association between CR and one-year mortality risk (HR=0.53, 95% CI 0.32–0.76) is similar to that in prior studies as well. Suaya et al. identified a 56% reduction in one-year mortality risk in an analysis of over 600,000 Medicare beneficiaries hospitalized for acute myocardial infarction or CABG(4). Another study demonstrated a 46% reduction in all-cause mortality in a cohort of 846 CABG patients(30).
Sensitivity analyses
It is important to interpret all of these results in the context of potential confounding due to healthy cohort bias, which could overestimate of the effect of CR on mortality as well as hospitalizations. However, our analysis controlled extensively for sociodemographic and clinical factors, and Elixhauser comorbidity groups provide effective comorbidity adjustment in surgical populations(15), including heart transplant patients(35) and those receiving VADs(36). We also used multiple statistical techniques, including marginal structural models, to control for observed confounders.
Frailty or functional impairment represents one of the most significant unobserved confounders, as frailty cannot be well-characterized with administrative claims data(24). Using prior work by Dunlay et al.(25), who found that 62% of VAD recipients were frail (as defined by the Rockwood Frailty Index(24)) prior to device implantation, we demonstrated that the prevalence of frailty in CR participants would have to be very low (<37%) for the effect of CR on mortality to become nonsignificant. It is unlikely that frailty would be this infrequent in VAD recipients who participated in CR, as frailty prevalence is 37% after ten years of follow-up in a similarly-aged community cohort of myocardial infarction survivors(37) and is well over 35% in younger intensive care unit populations(38). Given this context the association of CR with one-year mortality appears to be quite robust, even the setting of unobserved confounding.
Clinical and policy implications
VAD patients necessitate multidisciplinary care and require an enormous amount of resources. Our results suggest that CR is associated with improved outcomes in this population. Further study is needed on the mechanisms by which VAD patients are being referred to CR (i.e. HFrEF, stable angina pectoris or AMI) and whether the six-week interval after discharge required for a patient to be deemed stable under the HFrEF indication is leading to delays in CR initiation.
Study limitations
Our study has limitations in addition to those previously addressed. First, we were only able to capture utilization data on VAD patients age ≥65 or with Medicare disability benefits. The fact that a significant number of patients in our cohort received disability coverage does not indicate that they were less likely to participate in CR than those eligible by age, as almost all patients receiving VADs would meet the chronic heart failure medical criteria for disability benefits. Second, our data are obtained from CMS administrative claims, which are not adjudicated. However, CMS data have been used to effectively study many cardiovascular therapies, including CR, in prior work(3,4,7). Third, our analyses were limited to VAD patients enrolled in fee-for-service Medicare and may not be generalizable to patients enrolled in Medicare private health plans. However, fee-for-service Medicare still accounted for 71% of Medicare beneficiaries in 2014(39). Lastly, the CMS decision memo approving HFrEF as an indication for CR was issued in February 2014, so it is possible that CR uptake in VAD recipients under the HFrEF indication has increased in the following years.
Conclusions
In summary, less than one third of Medicare beneficiaries receiving VADs participate in CR programs in the United States. Although it is not possible to fully account for all confounding variables, VAD patients who participate in CR appear to have lower risk for hospitalization and mortality. These exploratory results suggest opportunities for further, more definitive studies of the effectiveness of CR in this population, as well as to understand factors that drive patient and caregiver decisions regarding CR participation.
Supplementary Material
Supplementary Figure 1 - Title: Standardized mean differences for individual covariates at 1 month post-discharge. Caption: This plot illustrates individual covariates before and after inverse probability of treatment weighting. PVD, peripheral vascular disease; SNF, skilled nursing facility.
Supplementary Figure 2 - Title: Standardized mean differences for individual covariates at 12 months post-discharge. Caption: This plot illustrates individual covariates before and after inverse probability of treatment weighting. PVD, peripheral vascular disease; SNF, skilled nursing facility.
Clinical Perspectives.
Competency in Medical Knowledge
Cardiac rehabilitation is indicated in patients with stable heart failure with reduced ejection fraction, including those receiving ventricular assist devices. Participation in cardiac rehabilitation programs is associated with decreased one-year hospitalizations and mortality in ventricular assist device patients.
Translational Outlook
Further studies are needed to characterize the barriers to cardiac rehabilitation participation in patients receiving ventricular assist devices, along with quality improvement interventions to increase cardiac rehabilitation uptake in this population.
Acknowledgments
Funding Sources: This project was supported by the Vanderbilt Clinical and Translational Science grant UL1 TR000445 from the National Center for Advancing Translational Sciences at the National Institutes of Health and grant number K12HS022990 from the Agency for Healthcare Research and Quality. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the above agencies.
The authors would like to acknowledge the Million Hearts Initiative co-led by the Centers for Medicaid & Medicare Services and Centers for Disease Control and Prevention. Million Hearts hosts the Cardiac Rehabilitation Collaborative, within which the authors had many discussions with other cardiac rehabilitation professionals that helped shape this study. The authors would also like to acknowledge Benjamin D. Levine, MD, FACC, FAHA, FACSM, Chair of the American Heart Association’s Clinical Cardiology Council Exercise and Cardiac Rehabilitation Committee, who reviewed the manuscript and provided helpful guidance.
Abbreviations List
- CMS
Centers for Medicare & Medicaid Services
- CPT
Current Procedure Terminology
- CR
Cardiac rehabilitation
- HFrEF
Heart failure with reduced ejection fraction
- ICD-9
International Classification of Diseases 9th Revision
- IRF
Inpatient rehabilitation facility
- LDS
Limited Data Set
- LVEF
Left ventricular ejection fraction
- SNF
Skilled nursing facility
- VAD
Ventricular assist device
Footnotes
Disclosures:The authors report no disclosures or relationships with industry.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thomas RJ, King M, Lui K, et al. AACVPR/ACCF/AHA 2010 Update: Performance measures on cardiac rehabilitation for referral to cardiac rehabilitation/secondary prevention services: A report of the American Association of Cardiovascular and Pulmonary Rehabilitation and the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Clinical Performance Measures for Cardiac Rehabilitation) Journal of cardiopulmonary rehabilitation and prevention. 2010;30:279–88. doi: 10.1097/HCR.0b013e3181f5e36f. [DOI] [PubMed] [Google Scholar]
- 2.Anderson L, Oldridge N, Thompson DR, et al. Exercise-Based Cardiac Rehabilitation for Coronary Heart Disease: Cochrane Systematic Review and Meta-Analysis. Journal of the American College of Cardiology. 2016;67:1–12. doi: 10.1016/j.jacc.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 3.Hammill BG, Curtis LH, Schulman KA, Whellan DJ. Relationship between cardiac rehabilitation and long-term risks of death and myocardial infarction among elderly Medicare beneficiaries. Circulation. 2010;121:63–70. doi: 10.1161/CIRCULATIONAHA.109.876383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suaya JA, Stason WB, Ades PA, Normand SL, Shepard DS. Cardiac rehabilitation and survival in older coronary patients. Journal of the American College of Cardiology. 2009;54:25–33. doi: 10.1016/j.jacc.2009.01.078. [DOI] [PubMed] [Google Scholar]
- 5.Golwala H, Pandey A, Ju C, et al. Temporal Trends and Factors Associated With Cardiac Rehabilitation Referral Among Patients Hospitalized With Heart Failure: Findings From Get With The Guidelines-Heart Failure Registry. Journal of the American College of Cardiology. 2015;66:917–26. doi: 10.1016/j.jacc.2015.06.1089. [DOI] [PubMed] [Google Scholar]
- 6.Doll JA, Hellkamp A, Ho PM, et al. Participation in Cardiac Rehabilitation Programs Among Older Patients After Acute Myocardial Infarction. JAMA internal medicine. 2015:E1–3. doi: 10.1001/jamainternmed.2015.3819. [DOI] [PubMed] [Google Scholar]
- 7.Suaya JA, Shepard DS, Normand SL, Ades PA, Prottas J, Stason WB. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation. 2007;116:1653–62. doi: 10.1161/CIRCULATIONAHA.107.701466. [DOI] [PubMed] [Google Scholar]
- 8.Kerrigan DJ, Williams CT, Ehrman JK, et al. Cardiac rehabilitation improves functional capacity and patient-reported health status in patients with continuous-flow left ventricular assist devices: the Rehab-VAD randomized controlled trial. JACC Heart Fail. 2014;2:653–9. doi: 10.1016/j.jchf.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Hayes K, Leet AS, Bradley SJ, Holland AE. Effects of exercise training on exercise capacity and quality of life in patients with a left ventricular assist device: a preliminary randomized controlled trial. J Heart Lung Transplant. 2012;31:729–34. doi: 10.1016/j.healun.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 10.Laoutaris ID, Dritsas A, Adamopoulos S, et al. Benefits of physical training on exercise capacity, inspiratory muscle function, and quality of life in patients with ventricular assist devices long-term postimplantation. Eur J Cardiovasc Prev Rehabil. 2011;18:33–40. doi: 10.1097/HJR.0b013e32833c0320. [DOI] [PubMed] [Google Scholar]
- 11.Alsara O, Reeves RK, Pyfferoen MD, et al. Inpatient rehabilitation outcomes for patients receiving left ventricular assist device. Am J Phys Med Rehabil. 2014;93:860–8. doi: 10.1097/PHM.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 12.Jung MH, Gustafsson F. Exercise in heart failure patients supported with a left ventricular assist device. J Heart Lung Transplant. 2015;34:489–96. doi: 10.1016/j.healun.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Medicare & Medicaid Services. [Accessed March 31, 2017];Decision Memo for Cardiac Rehabilitation (CR) Programs - Chronic Heart Failure (CAG-00437N) 2014 https://www.cms.gov/medicare-coverage-database/details/nca-decision405memo.aspx?NCAId=270.
- 14.Social Security Administration. [Accessed May 27, 2017];Disability Evaluation Under Social Security (Blue Book - October 2008) 2008 https://www.ssa.gov/disability/professionals/bluebook/4.00-408Cardiovascular-Adult.htm-4_02.
- 15.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 16.American Hospital Association. [Accessed November 2, 2016];American Hospital Association Annual Survey of Hospitals. 2016 https://www.ahadataviewer.com/book-cd-products/AHA-Survey/
- 17.United States Census Bureau. [Accessed June 1, 2017];Small Area Income Estimates. 2014 https://www.census.gov/did/www/saipe/data/statecounty/data/2015.html.
- 18.Lin DY, Wei LJ, Yang I, Ying Z. Semiparametric regression for the mean and rate functions of recurrent events. J R Statist Soc B. 2000;62:711–30. [Google Scholar]
- 19.Dunlay SM, Pack QR, Thomas RJ, Killian JM, Roger VL. Participation in cardiac rehabilitation, readmissions, and death after acute myocardial infarction. The American journal of medicine. 2014;127:538–46. doi: 10.1016/j.amjmed.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mi X, Hammill BG, Curtis LH, Lai EC, Setoguchi S. Use of the landmark method to address immortal person-time bias in comparative effectiveness research: a simulation study. Stat Med. 2016;35:4824–4836. doi: 10.1002/sim.7019. [DOI] [PubMed] [Google Scholar]
- 21.Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008;167:492–9. doi: 10.1093/aje/kwm324. [DOI] [PubMed] [Google Scholar]
- 22.Cook NR, Cole SR, Hennekens CH. Use of a marginal structural model to determine the effect of aspirin on cardiovascular mortality in the Physicians' Health Study. Am J Epidemiol. 2002;155:1045–53. doi: 10.1093/aje/155.11.1045. [DOI] [PubMed] [Google Scholar]
- 23.Lin DY, Psaty BM, Kronmal RA. Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics. 1998;54:948–63. [PubMed] [Google Scholar]
- 24.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62:738–43. doi: 10.1093/gerona/62.7.738. [DOI] [PubMed] [Google Scholar]
- 25.Dunlay SM, Park SJ, Joyce LD, et al. Frailty and outcomes after implantation of left ventricular assist device as destination therapy. J Heart Lung Transplant. 2014;33:359–65. doi: 10.1016/j.healun.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.SAS Institute. Statistical Analysis Software, version 9.4. 2016. [Google Scholar]
- 27.R Core Team. [Accessed October 22, 2016];R: A language and environment for statistical computing. 2015 https://www.r-project.org/
- 28.Doll JA, Hellkamp A, Thomas L, et al. Effectiveness of cardiac rehabilitation among older patients after acute myocardial infarction. American heart journal. 2015;170:855–64. doi: 10.1016/j.ahj.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Bachmann JM, Shah AS, Duncan MS, et al. Cardiac rehabilitation and readmissions after heart transplantation. J Heart Lung Transplant. 2017 doi: 10.1016/j.healun.2017.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pack QR, Goel K, Lahr BD, et al. Participation in cardiac rehabilitation and survival after coronary artery bypass graft surgery: a community-based study. Circulation. 2013;128:590–7. doi: 10.1161/CIRCULATIONAHA.112.001365. [DOI] [PubMed] [Google Scholar]
- 31.Pack QR, Mansour M, Barboza JS, et al. An early appointment to outpatient cardiac rehabilitation at hospital discharge improves attendance at orientation: a randomized, single-blind, controlled trial. Circulation. 2013;127:349–55. doi: 10.1161/CIRCULATIONAHA.112.121996. [DOI] [PubMed] [Google Scholar]
- 32.Dunlay SM, Witt BJ, Allison TG, et al. Barriers to participation in cardiac rehabilitation. American heart journal. 2009;158:852–9. doi: 10.1016/j.ahj.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evenson KR, Fleury J. Barriers to outpatient cardiac rehabilitation participation and adherence. Journal of cardiopulmonary rehabilitation. 2000;20:241–6. doi: 10.1097/00008483-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Atluri P, Fairman AS, MacArthur JW, et al. Continuous flow left ventricular assist device implant significantly improves pulmonary hypertension, right ventricular contractility, and tricuspid valve competence. J Card Surg. 2013;28:770–5. doi: 10.1111/jocs.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mujib M, Khanna N, Mazumder NK, et al. Pretransplant coagulopathy and in-hospital outcomes among heart transplant recipients: a propensity-matched nationwide inpatient sample study. Clin Cardiol. 2015;38:300–8. doi: 10.1002/clc.22391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCarthy FH, Kobrin D, Rame JE, et al. Increasing Frequency of Left Ventricular Assist Device Exchanges in the United States. Ann Thorac Surg. 2015;100:1660–4. doi: 10.1016/j.athoracsur.2015.04.072. discussion 1665. [DOI] [PubMed] [Google Scholar]
- 37.Myers V, Drory Y, Gerber Y Israel Study Group on First Acute Myocardial I. Clinical relevance of frailty trajectory post myocardial infarction. Eur J Prev Cardiol. 2014;21:758–66. doi: 10.1177/2047487312462828. [DOI] [PubMed] [Google Scholar]
- 38.Muscedere J, Waters B, Varambally A, et al. The impact of frailty on intensive care unit outcomes: a systematic review and meta-analysis. Intensive Care Med. 2017;43:1105–1122. doi: 10.1007/s00134-017-4867-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaiser Family Foundation. [Accessed September 27, 2016];Fact Sheet: Medicare Advantage. 2016 http://files.kff.org/attachment/Fact-Sheet-Medicare-Advantage.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 - Title: Standardized mean differences for individual covariates at 1 month post-discharge. Caption: This plot illustrates individual covariates before and after inverse probability of treatment weighting. PVD, peripheral vascular disease; SNF, skilled nursing facility.
Supplementary Figure 2 - Title: Standardized mean differences for individual covariates at 12 months post-discharge. Caption: This plot illustrates individual covariates before and after inverse probability of treatment weighting. PVD, peripheral vascular disease; SNF, skilled nursing facility.