Abstract
Background
This study was performed to assess serial cytokine changes and their clinical impact in children with cerebral palsy (CP) who received granulocyte-colony stimulating factor (G-CSF) followed by infusion of autologous mobilized peripheral blood mononuclear cells (mPBMCs).
Methods
Peripheral blood (PB) samples were collected from 16 CP children at enrollment, and 1 month and 7 months after G-CSF infusion as well as at the end of the study. Cytokine levels were measured by enzyme-linked immunosorbent assays with plasma samples.
Results
There were no significant differences in cytokine levels between the mPBMC and placebo groups over 6 months. However, when clinical responders and non-responders were compared, interleukin (IL)-6 (P = 0.050) as well as G-CSF (P = 0.010) were higher in the responders than the non-responders at 1 month, while brain-derived neurotrophic factor (BDNF) (P = 0.030) and insulin-like growth factor (IGF)-1 (P = 0.001) were lower. In addition, BDNF was higher at baseline in the responders than the non-responders (P = 0.030).
Conclusion
The changes of G-CSF itself, as well as G-CSF-induced cytokines such as IL-6, may be associated with the clinical improvement of neurologic functions. The G-CSF-induced changes of IL-6, BDNF and IGF-1, and BDNF levels before treatment, could be used as prognostic factors in G-CSF trials in CP children.
Keywords: Cytokines, Granulocyte-colony Stimulating Factor (G-CSF), Peripheral Blood Mononuclear Cells, Cerebral Palsy
Graphical Abstract

INTRODUCTION
Cell therapy has emerged as an advanced medical technology for restoring damaged tissues and organs. Of the various cell sources, mesenchymal stem cells (MSCs) have been used most frequently in experiments and in clinical trials in the field of regenerative medicine. In addition to the effects of the cell types into which the MSCs could differentiate, the MSCs themselves have been shown to exert paracrine actions that promote the repair of damaged tissues.1,2,3,4 There is increasing evidence that cell therapy produces positive effects largely via the secretion of various cytokines, such as growth factors, cytokines and chemokines with anti-scarring, anti-apoptotic, angiogenesis, immunomodulatory, and chemoattractant functions.1
There have been suggestions that mononuclear cells (MNCs) might also be used to regenerate tissues because they can be isolated and cultured from the MNC fractions of bone marrow (BM), cord blood (CB), and granulocyte-colony stimulating factor (G-CSF)-mobilized peripheral blood (mPB). The effects of MSCs and MNCs have been compared in various experimental settings.5,6,7 Furthermore, in clinical trials of intravenous CB MNCs in children with cerebral palsy (CP) some patients gave partial responses without any significant toxicity.8,9
However, there have been few reports of the paracrine effects of intravenously infused MNCs in improving neurological functions. This work was aimed to measure the serial cytokine changes in children with CP who participated in a clinical trial of cell therapy with G-CSF followed by autologous mobilized peripheral blood mononuclear cells (mPBMCs).
METHODS
Study subjects
Peripheral blood (PB) samples were collected from 16 CP children who participated in a randomized, double-blind, cross-over clinical study of intravenous infusion of G-CSF followed by mPBMCs. The study design is schematized as follows. Fifty-seven children with the non-severe type of CP were enrolled. After baseline studies (M0), intravenous G-CSF was administered for 5 days, at which time mPBMCs were collected by apheresis and cryopreserved. One month later (M1), the children were randomized for infusion of mPBMCs or placebo. After a further 6 months, cross-over infusion was performed and the children were observed for another 6 months. PB samples for cytokine measurements were collected at baseline (M0), before infusion of mPBMC/placebo (M1, M7) as well as at the end of the study (M13).
Sample preparation and measurement of cytokines
The PB samples were centrifuged at 1,800 rpm for 30 minutes and plasma was separated and stored at −80°C until the end of the clinical study. Thereafter, it was thawed and the following cytokines were measured using a Human Quantikine ELISA kit (R & D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions; G-CSF, brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF)-1, interleukin (IL)-6, IL-8, IL-10.
Statistical analysis
The cytokine levels were compared between T1 (mPBMC infusion 1 month after G-CSF infusion, n = 8) and T7 (mPBMC infusion 7 months after G-CSF infusion, n = 8) groups, mPBMC and placebo groups, clinical responders and non-responders. Clinical responses were assessed by a rehabilitation medicine specialist according to scores in various evaluation tools such as Gross Motor Function Measure (GMFM), Gross Motor Function Classification System (GMFCS), Manual Ability Classification System (MACS), Denver Developmental Screening Test (DDST), Pediatric Evaluation of Disability Inventory (PEDI). The patients were classified as responders and non-responders according to their clinical outcomes. Clinical responders were defined as having score changes of GMFM > 4 points and score changes in PEDI of > 7 points in 3 items.
Groups were compared using the Wilcoxon rank sum test and Wilcoxon signed rank test, which are nonparametric methods, because there were not many enrolled patients. All statistical analyses were conducted with SAS software (version 9.3; SAS Institute Inc., Cary, NC, USA). P values < 0.05 were accepted as statistically significant.
Ethics statement
This study was approved by the Institutional Review Board of Hanyang University Hospital (2011-03-002). All enrolled patients submitted their informed consent.
RESULTS
Changing patterns of cytokine levels in T1 and T7 groups
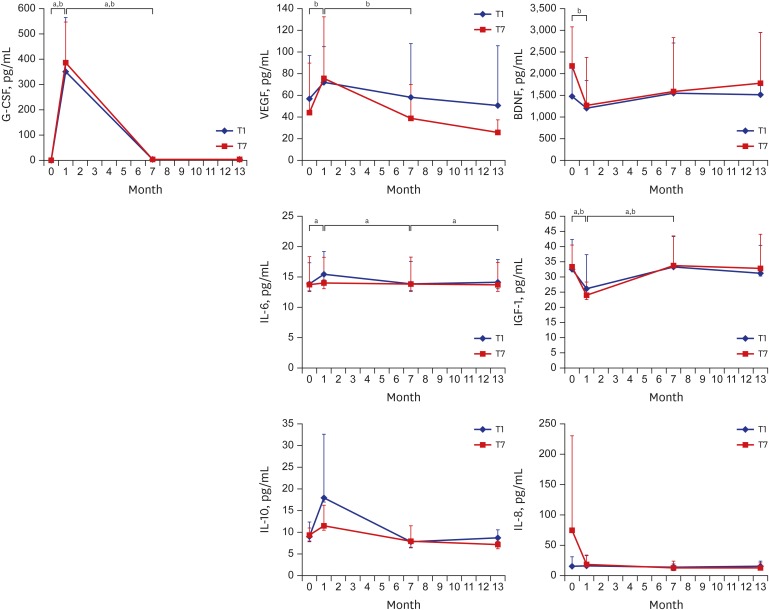
G-CSF levels increased significantly in T1 at 1 month after G-CSF infusion and decreased to baseline levels at 7, 13 months. VEGF, IL-6, and IL-10 levels underwent similar changes and even the VEGF level in the T7 group, like the G-CSF level, was reduced at 7 months, while that of the T1 group was not. On the other hand, BDNF and IGF-1 levels followed the opposite pattern to G-CSF; they decreased significantly at 1 month after G-CSF infusion and recovered at 7 months. Apart from IL-6, which increased after mPBMC infusion at 7 months in the T1 group, there were no changes of cytokine levels following mPBMC infusion at 7 months (Fig. 1).
Fig. 1.
Serum cytokine levels according to the time of infusion of mPBMC in CP children who received intravenous infusion of G-CSF-mPBMCs or placebo. G-CSF levels increased significantly in T1 at 1 month after G-CSF infusion and decreased to baseline levels at 7, 13 months. VEGF, IL-6, and IL-10 levels underwent similar changes and even the VEGF level in the T7 group, like the G-CSF level, was reduced at 7 months, while that of the T1 group was not. On the other hand, BDNF and IGF-1 levels followed the opposite pattern to G-CSF; they decreased significantly at 1 month after G-CSF infusion and recovered at 7 months. Apart from IL-6, which increased after mPBMC infusion at 7 months in the T1 group, there were no changes of cytokine levels following mPBMC infusion at 7 months.
G-CSF = granulocyte-colony stimulating factor, BDNF = brain-derived neurotrophic factor, VEGF = vascular endothelial growth factor, IGF = insulin-like growth factor, IL = interleukin, T1 = PBMC infusion at 1 month after G-CSF infusion, T7 = PBMC infusion at 7 months after G-CSF infusion, CP = cerebral palsy, mPBMC = mobilized peripheral blood mononuclear cell.
P values < 0.05 were accepted as statistically significant in aT1 and bT2 groups.
Cytokine levels in clinical responders versus non-responders
When the responders and non-responders were compared, IL-6 (P = 0.050) and G-CSF (P = 0.001) were higher in the responders than the non-responders at 1 month after G-CSF infusion, while BDNF (P = 0.030) and IGF-1 (P = 0.001) were lower. Interestingly, BDNF was higher at baseline in the responders than the non-responders (P = 0.030) (Fig. 2).
Fig. 2.
Serum cytokine levels in the clinical responder/non-responder groups in CP children who received intravenous infusion of G-CSF-mPBMCs or placebo. IL-6 and G-CSF were higher in the responders than the non-responders at 1 month after G-CSF infusion, while BDNF and IGF-1 were lower. Interestingly, BDNF was higher at baseline in the responders than the non-responders.
G-CSF = granulocyte-colony stimulating factor, BDNF = brain-derived neurotrophic factor, VEGF = vascular endothelial growth factor, IGF = insulin-like growth factor, IL = interleukin, CP = cerebral palsy, mPBMC = mobilized peripheral blood mononuclear cell.
P values < 0.05 were accepted as statistically significant in aresponder and bnon-responder groups.
Cytokine levels in the mPBMC and placebo groups
To reveal the effect of mPBMCs infusion on the cytokines, the analysis was performed during 6 months after infusion of mPBMCs or placebo. However, there were no significant differences of cytokine levels over 6 months between the mPBMC and placebo groups (Fig. 3).
Fig. 3.
Serum cytokine levels after randomization of CP children who received intravenous infusion of G-CSF-mPBMCs or placebo. There were no significant differences of cytokine levels over 6 months between the mPBMC and placebo groups.
G-CSF = granulocyte-colony stimulating factor, BDNF = brain-derived neurotrophic factor, VEGF = vascular endothelial growth factor, IGF = insulin-like growth factor, IL = interleukin, CP = cerebral palsy, mPBMC = mobilized peripheral blood mononuclear cell.
DISCUSSION
Both G-CSF and its receptor are widely expressed by neurons in the central nervous system, and their expression is induced by ischemia, which points to an autocrine protective signaling mechanism. Thus, G-CSF has been revealed to be an endogenous ligand in the CNS that has a dual activity countering acute neuronal degeneration and contributing to long-term plasticity after cerebral ischemia.10
G-CSF also has a neuroprotective role via Th2 switching and regulatory T cell production. There is compelling evidence that it exerts profound immunoregulatory effects by polarizing T cell differentiation via the production of IL-4 and IL-10, which is accompanied by reduced production of interferon-γ and IL-2.11,12,13,14 Deboy et al.15 showed that IL-4 is important for facial motor neuron survival after nerve injury, and Frenkel et al.16 also revealed a role of IL-10 produced by CD4+ T cells in neuroprotection. The cytokine IL-6 is also recognized as a trophic factor in the maintenance of normal neurons and in promoting neuronal survival and angiogenesis.17 Ohki et al.18 observed that G-CSF increased plasma VEGF release from neutrophils in vivo together with an increase in circulating neutrophils. The emerging evidence for an etiologic role of VEGF in (at least some types of) neurodegeneration provides the rationale for considering the therapeutic potential of VEGF in neurodegenerative disorders, which are mostly incurable. In this study, the production of VEGF, IL-6, and IL-10 increased in the first month after G-CSF infusion, along with a similar pattern for G-CSF, even after harvesting most of mPBMCs. Based on this study and the previous data regarding the immunoregulatory effects of G-CSF, it appears that G-CSF-derived cytokines as well as G-CSF itself may promote regenerative processes countering neurodevelopmental dysfunction.
BDNF plays a significant role in neurogenesis. It can promote the protective effects of neural stem cells that contribute to the brain's neurogenic response by enhancing their survival.19 IGF-1 also plays a significant role in neuronal development,20,21 recovery from neuronal injury,22,23 neuronal survival,24 and neurite outgrowth following crush injury.25 In vitro studies have suggested that IGF-1 is produced locally by non-neuronal cells following injury, and that it stimulates regeneration.26 Although BDNF is more concentrated in brain tissue, it is present in the bloodstream and derives from a variety of sources, including platelets and the brain.27,28,29 There have been reports that BDNF can cross the blood-brain barrier30 and a positive correlation between peripheral BDNF protein levels and brain levels have been reported in rodents,31 suggesting that peripheral BDNF levels may reflect BDNF levels in the brain. BDNF blood levels have also been shown to correlate with cortical integrity.32 Hence in clinical settings, PB levels (i.e., serum or plasma) are widely used as a proxy for central levels. However, we observed that G-CSF lowered plasma levels of BDNF and IGF-1, which is not consistent with previous studies that demonstrated that BDNF and IGF-1 were released from endothelial cells activated by G-CSF, and that they contributed to neural regeneration.33,34 Therefore, further studies are required to reveal the relation between plasma levels of G-CSF and BDNF/IGF-1.
Other studies have shown that CD34+ cells mobilized by G-CSF can home from the circulating blood to ischemic brain tissues and contribute to the improvement of neurological functions.35 In addition, we recently observed, in a previous study, that G-CSF modified the intracellular expression of inflammatory cytokines in G-CSF-mPBMCs of CP children, and that IL-6 levels were significantly elevated in mPBMCs rather than in PBMCs.36 However, in this study, against our expectations, there were no significant changes of cytokine secretion following booster mPBMC infusion compared to placebo. Nevertheless, VEGF levels did not decrease in the T1 group which received mPBMC at 1 month, but even decreased significantly at 7 months in the same pattern as G-CSF in the T7 group. Therefore, we tentatively suggest that mPBMC may have a booster effect, particularly when infused during elevated G-CSF levels.
We also observed, in the clinically responding group, that IL-6 as well as G-CSF were elevated at 1 month after G-CSF infusion, along with reduced BDNF and IGF-1 levels. In addition, BDNF levels were significantly higher at baseline in the responders than the non-responders. These findings suggest that clinical responses to G-CSF infusion in CP patients could be predicted by measuring BDNF before treatment, and serial changes of IL-6, BDNF, and IGF-1 after treatment.
In summary, the changes of G-CSF itself, as well as G-CSF-induced cytokines such as IL-6, may be associated with the clinical improvement of neurologic functions. The G-CSF-induced changes of IL-6, BDNF, and IGF-1, and BDNF levels before treatment, could be used as prognostic factors in G-CSF trials in CP children. Although a definite effect of mPBMC reinfusion was not detected in this study, further study is needed to clarify the effect of G-CSF or mPBMCs on their own in CP children.
Footnotes
Funding: This work was supported by the Research Fund of Hanyang University (HY-2014).
Disclosure: The authors have no potential conflicts of interest to disclose.
Author Contributions: Conceptualization: Lee YH. Data curation: Rah WJ, Kim YJ, Moon JH, Kim MJ. Formal analysis: Koh H, Rah WJ, Lee YH. Funding acquisition: Lee YH. Investigation: Koh H. Writing - original draft: Koh H. Writing - review & editing: Kim MJ, Lee YH.
References
- 1.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98(5):1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 2.Mezey E. Bone marrow-derived stem cells in neurological diseases: stones or masons? Regen Med. 2007;2(1):37–49. doi: 10.2217/17460751.2.1.37. [DOI] [PubMed] [Google Scholar]
- 3.Uccelli A, Laroni A, Freedman MS. Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. Lancet Neurol. 2011;10(7):649–656. doi: 10.1016/S1474-4422(11)70121-1. [DOI] [PubMed] [Google Scholar]
- 4.Oh YS, Kim SH, Cho GW. Functional restoration of amyotrophic lateral sclerosis patient-derived mesenchymal stromal cells through inhibition of DNA methyltransferase. Cell Mol Neurobiol. 2016;36(4):613–620. doi: 10.1007/s10571-015-0242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu D, Chen B, Liang Z, Deng W, Jiang Y, Li S, et al. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Res Clin Pract. 2011;92(1):26–36. doi: 10.1016/j.diabres.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Giraldi-Guimardes A, Rezende-Lima M, Bruno FP, Mendez-Otero R. Treatment with bone marrow mononuclear cells induces functional recovery and decreases neurodegeneration after sensorimotor cortical ischemia in rats. Brain Res. 2009;1266:108–120. doi: 10.1016/j.brainres.2009.01.062. [DOI] [PubMed] [Google Scholar]
- 7.Reich DM, Hau S, Stahl T, Scholz M, Naumann W, Emmrich F, et al. Neuronal hypoxia in vitro: investigation of therapeutic principles of HUCB-MNC and CD133+ stem cells. BMC Neurosci. 2008;9(1):91. doi: 10.1186/1471-2202-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee YH, Choi KV, Moon JH, Jun HJ, Kang HR, Oh SI, et al. Safety and feasibility of countering neurological impairment by intravenous administration of autologous cord blood in cerebral palsy. J Transl Med. 2012;10(1):58. doi: 10.1186/1479-5876-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Min K, Song J, Kang JY, Ko J, Ryu JS, Kang MS, et al. Umbilical cord blood therapy potentiated with erythropoietin for children with cerebral palsy: a double-blind, randomized, placebo-controlled trial. Stem Cells. 2013;31(3):581–591. doi: 10.1002/stem.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider A, Krüger C, Steigleder T, Weber D, Pitzer C, Laage R, et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest. 2005;115(8):2083–2098. doi: 10.1172/JCI23559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klangsinsirikul P, Russell NH. Peripheral blood stem cell harvests from G-CSF-stimulated donors contain a skewed Th2 CD4 phenotype and a predominance of type 2 dendritic cells. Exp Hematol. 2002;30(5):495–501. doi: 10.1016/s0301-472x(02)00785-3. [DOI] [PubMed] [Google Scholar]
- 12.Agnello D, Mascagni P, Bertini R, Villa P, Senaldi G, Ghezzi P. Granulocyte colony-stimulating factor decreases tumor necrosis factor production in whole blood: role of interleukin-10 and prostaglandin E(2) Eur Cytokine Netw. 2004;15(4):323–326. [PubMed] [Google Scholar]
- 13.Rutella S, Pierelli L, Bonanno G, Sica S, Ameglio F, Capoluongo E, et al. Role for granulocyte colony-stimulating factor in the generation of human T regulatory type 1 cells. Blood. 2002;100(7):2562–2571. doi: 10.1182/blood-2001-12-0291. [DOI] [PubMed] [Google Scholar]
- 14.Pan L, Delmonte J, Jr, Jalonen CK, Ferrara JL. Pretreatment of donor mice with granulocyte colony-stimulating factor polarizes donor T lymphocytes toward type-2 cytokine production and reduces severity of experimental graft-versus-host disease. Blood. 1995;86(12):4422–4429. [PubMed] [Google Scholar]
- 15.Deboy CA, Xin J, Byram SC, Serpe CJ, Sanders VM, Jones KJ. Immune-mediated neuroprotection of axotomized mouse facial motoneurons is dependent on the IL-4/STAT6 signaling pathway in CD4+ T cells. Exp Neurol. 2006;201(1):212–224. doi: 10.1016/j.expneurol.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 16.Frenkel D, Huang Z, Maron R, Koldzic DN, Moskowitz MA, Weiner HL. Neuroprotection by IL-10-producing MOG CD4+ T cells following ischemic stroke. J Neurol Sci. 2005;233(1-2):125–132. doi: 10.1016/j.jns.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Gertz K, Kronenberg G, Kälin RE, Baldinger T, Werner C, Balkaya M, et al. Essential role of interleukin-6 in post-stroke angiogenesis. Brain. 2012;135(Pt 6):1964–1980. doi: 10.1093/brain/aws075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohki Y, Heissig B, Sato Y, Akiyama H, Zhu Z, Hicklin DJ, et al. Granulocyte colony-stimulating factor promotes neovascularization by releasing vascular endothelial growth factor from neutrophils. FASEB J. 2005;19(14):2005–2007. doi: 10.1096/fj.04-3496fje. [DOI] [PubMed] [Google Scholar]
- 19.Bartkowska K, Paquin A, Gauthier AS, Kaplan DR, Miller FD. Trk signaling regulates neural precursor cell proliferation and differentiation during cortical development. Development. 2007;134(24):4369–4380. doi: 10.1242/dev.008227. [DOI] [PubMed] [Google Scholar]
- 20.D'Costa AP, Prevette DM, Houenou LJ, Wang S, Zackenfels K, Rohrer H, et al. Mechanisms of insulin-like growth factor regulation of programmed cell death of developing avian motoneurons. J Neurobiol. 1998;36(3):379–394. [PubMed] [Google Scholar]
- 21.Zackenfels K, Oppenheim RW, Rohrer H. Evidence for an important role of IGF-I and IGF-II for the early development of chick sympathetic neurons. Neuron. 1995;14(4):731–741. doi: 10.1016/0896-6273(95)90217-1. [DOI] [PubMed] [Google Scholar]
- 22.Hansson HA, Dahlin LB, Danielsen N, Fryklund L, Nachemson AK, Polleryd P, et al. Evidence indicating trophic importance of IGF-I in regenerating peripheral nerves. Acta Physiol Scand. 1986;126(4):609–614. doi: 10.1111/j.1748-1716.1986.tb07862.x. [DOI] [PubMed] [Google Scholar]
- 23.Yuan Q, Wu W, So KF, Cheung AL, Prevette DM, Oppenheim RW. Effects of neurotrophic factors on motoneuron survival following axonal injury in newborn rats. Neuroreport. 2000;11(10):2237–2241. doi: 10.1097/00001756-200007140-00035. [DOI] [PubMed] [Google Scholar]
- 24.Li L, Oppenheim RW, Lei M, Houenou LJ. Neurotrophic agents prevent motoneuron death following sciatic nerve section in the neonatal mouse. J Neurobiol. 1994;25(7):759–766. doi: 10.1002/neu.480250702. [DOI] [PubMed] [Google Scholar]
- 25.Rabinovsky ED, Gelir E, Gelir S, Lui H, Kattash M, DeMayo FJ, et al. Targeted expression of IGF-1 transgene to skeletal muscle accelerates muscle and motor neuron regeneration. FASEB J. 2003;17(1):53–55. doi: 10.1096/fj.02-0183fje. [DOI] [PubMed] [Google Scholar]
- 26.Cheng HL, Randolph A, Yee D, Delafontaine P, Tennekoon G, Feldman EL. Characterization of insulin-like growth factor-I and its receptor and binding proteins in transected nerves and cultured Schwann cells. J Neurochem. 1996;66(2):525–536. doi: 10.1046/j.1471-4159.1996.66020525.x. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto H, Gurney ME. Human platelets contain brain-derived neurotrophic factor. J Neurosci. 1990;10(11):3469–3478. doi: 10.1523/JNEUROSCI.10-11-03469.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radka SF, Holst PA, Fritsche M, Altar CA. Presence of brain-derived neurotrophic factor in brain and human and rat but not mouse serum detected by a sensitive and specific immunoassay. Brain Res. 1996;709(1):122–301. doi: 10.1016/0006-8993(95)01321-0. [DOI] [PubMed] [Google Scholar]
- 29.Lommatzsch M, Zingler D, Schuhbaeck K, Schloetcke K, Zingler C, Schuff-Werner P, et al. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging. 2005;26(1):115–123. doi: 10.1016/j.neurobiolaging.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37(12):1553–1561. doi: 10.1016/s0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 31.Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109(2):143–148. doi: 10.1016/s0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- 32.Lang UE, Hellweg R, Seifert F, Schubert F, Gallinat J. Correlation between serum brain-derived neurotrophic factor level and an in vivo marker of cortical integrity. Biol Psychiatry. 2007;62(5):530–535. doi: 10.1016/j.biopsych.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Dupraz S, Grassi D, Karnas D, Nieto Guil AF, Hicks D, Quiroga S. The insulin-like growth factor 1 receptor is essential for axonal regeneration in adult central nervous system neurons. PLoS One. 2013;8(1):e54462. doi: 10.1371/journal.pone.0054462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiba Y, Kuroda S, Osanai T, Shichinohe H, Houkin K, Iwasaki Y. Impact of ageing on biological features of bone marrow stromal cells (BMSC) in cell transplantation therapy for CNS disorders: functional enhancement by granulocyte-colony stimulating factor (G-CSF) Neuropathology. 2012;32(2):139–148. doi: 10.1111/j.1440-1789.2011.01255.x. [DOI] [PubMed] [Google Scholar]
- 35.Williams LR, Varon S, Peterson GM, Wictorin K, Fischer W, Bjorklund A, et al. Continuous infusion of nerve growth factor prevents basal forebrain neuronal death after fimbria fornix transection. Proc Natl Acad Sci U S A. 1986;83(23):9231–9235. doi: 10.1073/pnas.83.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koh H, Hwang K, Lim HY, Kim YJ, Lee YH. Mononuclear cells from the cord blood and granulocytecolony stimulating factor-mobilized peripheral blood: is there a potential for treatment of cerebral palsy? Neural Regen Res. 2015;10(12):2018–2024. doi: 10.4103/1673-5374.172321. [DOI] [PMC free article] [PubMed] [Google Scholar]