Abstract
For patients at risk of premature ovarian failure with cancer treatment, it is an important option to re-implant the ovarian tissue (OT) after cryopreservation to preserve endocrine function and fertility. With this technique, about 30% of pregnancy success rate and about 90 live births have been reported to date. However, there has been no case report of successful in vitro fertilization (IVF) and embryo transfer (ET) with oocytes collected from transplanted cryopreserved OT in Korea. We report a 30-year old woman with rectal cancer who underwent IVF and ET after cryopreserved OT thawing and re-implantation. She has been diagnosed with stage IIIC rectal cancer after surgery, and right ovary was removed and cryopreserved between cycles of chemotherapy. After completion of chemotherapy and radiotherapy, the patient underwent orthotopic transplantation of cryopreserved OTs. Three months after transplantation, the serum follicle-stimulating hormone level decreased from 91.11 mIU/mL to 43.69 mIU/mL. Thereafter, the patient underwent 11 ovarian stimulation cycles, and in 7 cycles, follicle growth was observed at the OT graft site. In one of these cycles, the oocyte was successfully retrieved and one embryo was transplanted after IVF. The patient was not pregnant, but the cryopreservation of OT can save the fertility after anticancer chemotherapy.
Keywords: Ovarian Tissue, Cryopreservation, Transplantation, Fertility Preservation
Graphical Abstract
INTRODUCTION
Cancer survivors at reproductive ages are increasing with the improvement of treatment.1 However, gonadotoxic cancer treatments induce ovarian failure.2,3 After recovery, childbirth is the factors that have a great influence on the quality of life. It is therefore important to give the opportunity to preserve their fertility. Ovarian tissue (OT) cryopreservation and transplantation are one of the fertility preservation options for female cancer patients.4 In 2004, the first successful live birth after OT transplantation was reported.5 Subsequently, about 90 live births were reported worldwide after frozen OT transplantation.6,7 Freezing and ischemic damage occur during OT cryopreservation and transplantation, and studies using various additives have been carried out to improve this limitation.8,9 As a result of these developments, there is an increasing number of reports on live births. Recently, we performed in vitro fertilization (IVF) and embryo transfer (ET) successfully after transplantation of cryopreserved OT for the first time in Korea, and we are to report this result.
CASE DESCRIPTION
Patient
A 30 years old married nulliparity woman was referred for fertility preservation. She had been healthy until diagnosed with rectal cancer, and her menstrual cycle was regular. She underwent a laparoscopic lower anterior resection on March 11, 2011. The postoperative pathologic staging was T4aN2bM0 (stage IIIC).
OT cryopreservation
The patient started to receive gonadotropin-releasing hormone (GnRH) agonist (Zoladex® depot, Goserelin, 3.6 mg; ICI, Melbourne, Australia) from one week after surgery and started chemotherapy with folic acid, fluorouracil, irinotecan hydrochloride (FOLFIRI) and bevacizumab regimen from 4 weeks after surgery. Prior to OT cryopreservation, 4 cycles of GnRH agonist and chemotherapy were administered. The serum anti-Mullerian hormone (AMH) level was 1.30 ng/mL on the day before ovarian cryopreservation. On June 29, 2011, laparoscopic right oophorectomy and left ovarian transposition was performed.
The removed ovary was collected in cold (4°C) Leibovitz's (L-15; WelGene, Daegu, Korea) medium and immediately delivered to the laboratory. The ovary was cut into small pieces and the medulla was removed from the cortex. A total of 33 ovarian cortex fragments of approximately 5 mm in length, 5 mm in width, and 1 mm in thickness were prepared. One fragment of OT was biopsied to confirm the absence of cancer metastasis. For cryopreservation, both vitrification and slow-freezing methods were used. During the vitrification process, 16 OT fragments were exposed into 7.5% ethylene glycol (EG; Sigma-Aldrich, St. Louis, MO, USA) and 7.5% dimethyl sulfoxide (DMSO; Sigma-Aldrich) in Dulbecco's phosphate-buffered saline (DPBS; Gibco, Paisley, UK) with 20% synthetic serum substitute (SSS; Irvine Scientific, Santa Ana, CA, USA) for 15 minutes at room temperature (RT) and transferred into a solution containing 20% EG, 20% DMSO, and 0.5 M of sucrose (Sigma-Aldrich) in DPBS medium with 20% SSS for 10 minutes. After putting the OTs into 1.8 mL cryo-vials (Nunc, Roskilde, Denmark), the vials were directly plunged into liquid nitrogen (LN2). For slow-freezing, 16 OT fragments were exposed to the freezing solution composed of DPBS, 20% SSS, 1.5 M DMSO, and 0.1 M sucrose for 20 minutes at RT. Then the OTs were put into the 1.8 mL cryo-vial pre-filled with 500 μL of the freezing solution. Then the cryo-vials were placed into an automated, computer-controlled freezing system (Kryo-360; Planer, Sunbury-on-Thames, UK). The initial cooling rate was −2°C/min to −7°C. At this stage, manual seeding was performed. After keeping the tissue for 10 minutes, cooling was continued at the rate of −0.3°C/min until −40°C and subsequently with −10°C/min until −150°C. Then the cryo-vials were plunged into LN2.
Adjuvant cancer therapy and follow up
Total 12 cycles of FOLFIRI and bevacizumab were administered. GnRH agonist was given 10 times at one-month intervals during the chemotherapy. During the treatment, there was a suspicion of metastasis of cancer in the left internal iliac lymph node and 56 Gy of radiation therapy was added.
After chemotherapy, menstruation was ceased. Serum levels of follicle-stimulating hormone (FSH) and estradiol at three months after completion of chemotherapy were 18.31 mIU/mL and < 10 pg/mL. The FSH level at 22 months after chemotherapy was 29.97 mIU/mL. A natural menstrual cycle returned at 30 months after chemotherapy, and subsequent menstruation occurred at the one-month interval for 6 months. Since then, however, no further menstruation has occurred. At this time, the patient wanted to have a child and decided to have OT transplantation. Serum hormone levels of FSH, estradiol, and AMH were 73.38 mIU/mL, 48 pg/mL, and 0.01 ng/mL, respectively.
OT transplantation
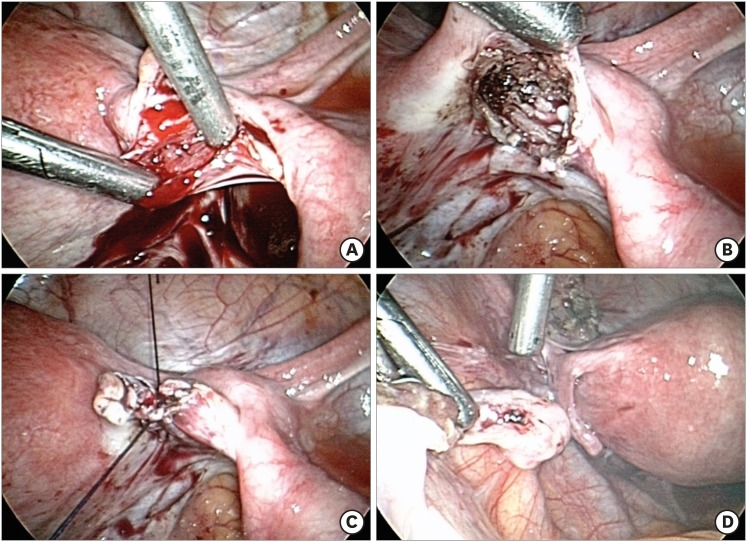
Laparoscopic OT transplantation was performed on July 8, 2015 (4 years after cryopreservation). We selected orthotopic transplantation because previous studies have shown that the IVF results were much better than heterotopic transplantation.10 The incision was made between the right ovarian ligament and the fallopian tube to create a space. Thawed and warmed OT fragments were inserted and then sutured (Fig. 1). One of the warmed ovarian pieces was pathologically examined and confirmed that there was no malignancy. For warming after vitrification, OTs were quickly immersed into 20% SSS supplemented DPBS containing 0.5M sucrose for 1 minute at 37°C and serially rinsed in 0.25 M and 0 M of sucrose for 5 minutes each at RT. For thawing after the slow-freezing procedure, the cryo-vials were taken from LN2 and exposed to RT for 30 seconds and then placed in 37°C water for 2 minutes. Then the OTs were immersed in 20% SSS supplemented DPBS containing 0.5 M, 0.25 M, and 0 M of sucrose for 5 minutes each at RT. The left ovary was repositioned to its original position in the operation however, no follicular growth was observed thereafter.
Fig. 1. Laparoscopic OT transplantation. (A) Incision between the right ovarian ligament and the fallopian tube to make space. (B) Insertion of the thawed and warmed OT fragments into the space. (C) Suture was done to envelop the space. (D) Left ovary reposition to its original site.
OT = ovarian tissue.
Ovarian function and infertility treatment
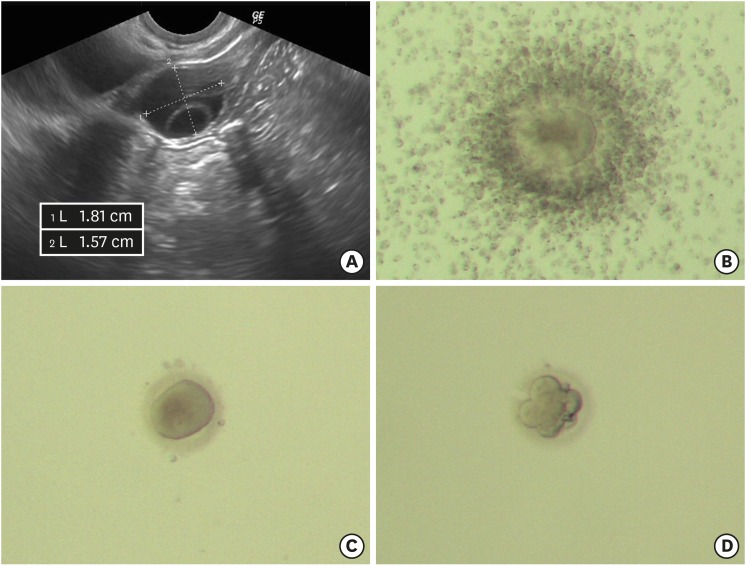
Hormone tests at one week after transplantation were FSH 91.11 mIU/mL, and estradiol 29 pg/mL. At three months after transplantation the hormonal level were FSH 43.69 mIU/mL, LH 16.16 mIU/mL, estradiol 57 pg/mL, and AMH 0.08 ng/mL. The patient underwent infertility treatment at a private fertility clinic. The results of ovarian stimulation are summarized in Table 1. Six cycles were canceled due to inappropriate follicular growth. Two cycles of intrauterine insemination were done due to follicle rupture on oocyte retrieval day. Transvaginal oocyte retrieval was performed in three cycles. No oocyte was collected in two cycles and successful oocyte retrieval was done in the cycle at 10 months after transplantation. One immature oocyte was retrieved from transplanted OT and fertilized by intracytoplasmic sperm injection. One 6-cell cleavage state embryo (grade III/IV) was transferred on the third day after fertilization (Fig. 2). The patient was not pregnant. The patient has not undergone menstruation since the last infertility treatment and, as shown in the Table 1, the last FSH and AMH results were considered little ovarian hormonal function. Currently, infertility treatment is temporarily stopped.
Table 1. Summary of the infertility treatment after ovarian tissue transplantation.
| Months after transplantation | MCD #3 E2, pg/mL; FSH, mIU/L; AMH, ng/mL | COS regimen, duration | Diameter of the developed follicles, mm | Peak E2, pg/mL | Results |
|---|---|---|---|---|---|
| 3 | E2 57; FSH 43.69; AMH 0.08 | Natural cycle | RO 15 | - | Cycle cancellation due to no further growth |
| 5 | CC 50 mg, 5 days | No growth | - | Cycle cancellation | |
| 7 | CC 100 mg, 5 days | RO 15 | 41 | Cycle cancellation due to no further growth | |
| → LT 2.5 mg, 5 days | |||||
| → hMG 225 IU, 3 days | |||||
| → rFSH 225 IU, 10 days | |||||
| 10 | CC 50 mg, 5 days | RO 18.5 | 276 | Oocyte retrieved (GV × 1) | |
| Embryo transferred (6 cell grade III/IV × 1, on day 3) | |||||
| → No pregnancy | |||||
| 11 | LT 2.5 mg, 5 days | RO 16.5, 14, 13 | 168 | Follicle ruptured before TVOA and changed to IUI | |
| → CC 50 mg, 5 days | → No pregnancy | ||||
| → hMG 225 IU, 9 days | |||||
| 13 | CC 50 mg, 5 days | RO 19 | 135 | Follicle ruptured before TVOA and changed to IUI | |
| → LT 2.5 mg, 5 days | → No pregnancy | ||||
| → hMG 75 IU, 1 days | |||||
| 14 | CC 50 mg, 5 days | RO 17 | 88 | No oocyte retrieved after TVOA | |
| → hMG 225 IU, 3 days | |||||
| 15 | CC 50 mg, 5 days | No growth | - | Cycle cancellation | |
| → CC 100 mg, 5 days | |||||
| → hMG 225 IU, 9 days | |||||
| 16 | CC 50 mg, 5 days | No growth | - | Cycle cancellation | |
| 18 | E2 89; FSH 42.06; AMH 0.03 | CC 100 mg, 5 days | RO 17.5 | 314 | No oocyte retrieved after TVOA |
| → CC 150 mg, 5 days | |||||
| → hMG 150 IU, 2 days | |||||
| 19 | CC 100 mg, 5 days | No growth | - | Cycle cancellation | |
| → LT 2.5 mg, 5 days |
MCD = menstrual cycle day, FSH = follicle-stimulating hormone, AMH = anti-microbial handwash, CC = clomiphene citrate, COS = controlled ovarian stimulation, GV = germinal vesicle, hMG = human menopausal gonadotropin (Menopur®; Ferring SA, Madrid, Spain), IUI = intrauterine insemination, LT = Letrozole, rFSH = recombinant follicle-stimulating hormone (Gonal-F®; Merck Serono, Darmstadt, Germany), RO = right ovary, TVOA = transvaginal oocyte aspiration.
Fig. 2. Grown follicle, collected oocyte, and the developing embryo. (A) Ultrasound findings of grown follicles. (B) Collected cumulus-oocyte complex. (C) Germinal vesicle oocyte after removing cumulus cells. (D) Six-cell embryo developed after three days of in vitro culture.
DISCUSSION
This is the first cryopreserved OT transplantation case in Korea. In this case, the endocrine function was not fully recovered after transplantation, though oocyte retrieval could be performed and IVF-ET was possible. Therefore, it is proven that ovulation is possible even if inadequate endocrine function recovery is observed after OT transplantation.11
Although the embryo could be successfully transferred after the oocyte retrieval, the oocyte retrieval was successful in only one of the 11 stimulation cycles. OT cryopreservation is a useful fertility preservation option which can preserve primordial follicles. However, the function of the cryopreserved and transplanted ovary is diminished comparing to the fresh state. The reason for this is that OT cryopreservation causes cryodamage to the cells and ischemic damage arises until revascularization occurs after transplantation.12,13 In addition, cryopreservation and transplantation cause accelerated follicular activation and depletion.14
In this case, OT cryopreservation was performed after 4 cycles of chemotherapy. It is suggested that OT cryopreservation after chemotherapy is not optimal due to the detrimental effect of chemotherapy and this could be one of the reasons for poor ovarian function in this case.15,16 It is important that fertility conservation procedures should be performed before chemotherapy or radiotherapy to obtain the favorable results. Therefore, it is crucial to ensure that young female patients receive appropriate fertility preservation modality at the right time, through the active participation of clinicians in various fields and patient education.17
We cryopreserved OTs using both slow freezing and vitrification methods. The live births reported so far are mainly from transplantation of OT cryopreserved by slow freezing.18 Vitrification has been introduced recently compared to slow freezing. Vitrification is a method to minimize cell damage due to ice crystal formation by extremely rapid cooling using a high concentration of cryoprotectant.19 Until recently, the number of live births reported from vitrification is relatively small.18 However, in many studies vitrification was comparable to slow freezing in cryopreservation of OT.20 Therefore, we used both methods to cryopreserve OT in order to take advantage of both. However, since the tissues were transplanted together, the comparison between the groups of different cryopreservation methods could not be performed.
We report the first case of IVF-ET in Korea after successful transplantation of cryopreserved OT in a patient with cancer therapy. If further clinical experience and the results of live birth are accumulated, the OT cryopreservation is expected to be beyond the status of innovative technique in near future.
ACKNOWLEDGMENTS
The authors thank Dr. Hye Won Youm, Director, Fertility Center, Seoul National University Bundang Hospital, for her technical assistance for this study.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
Author Contributions: Conceptualization: Lee JR. Data curation: Lee JR, Lee D, Park S, Paik EC, Kim SK. Investigation: Lee JR, Park S, Paik EC. Writing - original draft: Lee JR, Lee D. Writing - review & editing: Lee JR, Jee BC, Suh CS, Kim SH.
References
- 1.Tiong V, Rozita AM, Taib NA, Yip CH, Ng CH. Incidence of chemotherapy-induced ovarian failure in premenopausal women undergoing chemotherapy for breast cancer. World J Surg. 2014;38(9):2288–2296. doi: 10.1007/s00268-014-2542-y. [DOI] [PubMed] [Google Scholar]
- 2.Howell S, Shalet S. Gonadal damage from chemotherapy and radiotherapy. Endocrinol Metab Clin North Am. 1998;27(4):927–943. doi: 10.1016/s0889-8529(05)70048-7. [DOI] [PubMed] [Google Scholar]
- 3.Critchley HO, Wallace WH. Impact of cancer treatment on uterine function. J Natl Cancer Inst Monogr. 2005;(34):64–68. doi: 10.1093/jncimonographs/lgi022. [DOI] [PubMed] [Google Scholar]
- 4.Kim SY, Kim SK, Lee JR, Woodruff TK. Toward precision medicine for preserving fertility in cancer patients: existing and emerging fertility preservation options for women. J Gynecol Oncol. 2016;27(2):e22. doi: 10.3802/jgo.2016.27.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Live birth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364(9443):1405–1410. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 6.Kristensen SG, Veronica G, Peter H, Birgit A, Anne-Mette B, Erik E, et al. Fertility preservation and refreezing of transplanted ovarian tissue - a potential new way of managing patients with low risk of malignant cell recurrence. Fertil Steril. 2017;107(5):1206–1213. doi: 10.1016/j.fertnstert.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Jensen AK, Macklon KT, Fedder J, Ernst E, Humaidan P, Andersen CY. 86 successful births and 9 ongoing pregnancies worldwide in women transplanted with frozen-thawed ovarian tissue: focus on birth and perinatal outcome in 40 of these children. J Assist Reprod Genet. 2017;34(3):325–336. doi: 10.1007/s10815-016-0843-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee J, Lee JR, Youm HW, Suh CS, Kim SH. Effect of preoperative simvastatin treatment on transplantation of cryopreserved-warmed mouse ovarian tissue quality. Theriogenology. 2015;83(2):285–293. doi: 10.1016/j.theriogenology.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 9.Lee J, Kim SK, Youm HW, Kim HJ, Lee JR, Suh CS, et al. Effects of three different types of antifreeze proteins on mouse ovarian tissue cryopreservation and transplantation. PLoS One. 2015;10(5):e0126252. doi: 10.1371/journal.pone.0126252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gellert SE, Pors SE, Kristensen SG, Bay-Bjørn AM, Ernst E, Andersen CY. Transplantation of frozen-thawed ovarian tissue: an update on worldwide activity published in peer-reviewed papers and on the Danish cohort. J Assist Reprod Genet. 2018 doi: 10.1007/s10815-018-1144-2. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donnez J, Squifflet J, Van Eyck AS, Demylle D, Jadoul P, Van Langendonckt A, et al. Restoration of ovarian function in orthotopically transplanted cryopreserved ovarian tissue: a pilot experience. Reprod Biomed Online. 2008;16(5):694–704. doi: 10.1016/s1472-6483(10)60484-1. [DOI] [PubMed] [Google Scholar]
- 12.Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Orthotopic and heterotopic ovarian tissue transplantation. Hum Reprod Update. 2009;15(6):649–665. doi: 10.1093/humupd/dmp021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J, Kong HS, Kim EJ, Youm HW, Lee JR, Suh CS, et al. Ovarian injury during cryopreservation and transplantation in mice: a comparative study between cryoinjury and ischemic injury. Hum Reprod. 2016;31(8):1827–1837. doi: 10.1093/humrep/dew144. [DOI] [PubMed] [Google Scholar]
- 14.David A, Van Langendonckt A, Gilliaux S, Dolmans MM, Donnez J, Amorim CA. Effect of cryopreservation and transplantation on the expression of Kit ligand and anti-müllerian hormone in human ovarian tissue. Hum Reprod. 2012;27(4):1088–1095. doi: 10.1093/humrep/des013. [DOI] [PubMed] [Google Scholar]
- 15.Meirow D. Reproduction post-chemotherapy in young cancer patients. Mol Cell Endocrinol. 2000;169(1-2):123–131. doi: 10.1016/s0303-7207(00)00365-8. [DOI] [PubMed] [Google Scholar]
- 16.Donnez J, Silber S, Andersen CY, Demeestere I, Piver P, Meirow D, et al. Children born after autotransplantation of cryopreserved ovarian tissue. A review of 13 live births. Ann Med. 2011;43(6):437–450. doi: 10.3109/07853890.2010.546807. [DOI] [PubMed] [Google Scholar]
- 17.Lee S, Ozkavukcu S, Heytens E, Moy F, Oktay K. Value of early referral to fertility preservation in young women with breast cancer. J Clin Oncol. 2010;28(31):4683–4686. doi: 10.1200/JCO.2010.30.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Serrano MS, Schmidt KT, et al. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril. 2013;99(6):1503–1513. doi: 10.1016/j.fertnstert.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 19.Vajta G, Nagy ZP. Are programmable freezers still needed in the embryo laboratory? Review on vitrification. Reprod Biomed Online. 2006;12(6):779–796. doi: 10.1016/s1472-6483(10)61091-7. [DOI] [PubMed] [Google Scholar]
- 20.Klocke S, Bündgen N, Köster F, Eichenlaub-Ritter U, Griesinger G. Slow-freezing versus vitrification for human ovarian tissue cryopreservation. Arch Gynecol Obstet. 2015;291(2):419–426. doi: 10.1007/s00404-014-3390-6. [DOI] [PubMed] [Google Scholar]