Abstract
Mammalian fertilization is comprised of many steps including sperm survival in the uterus, sperm migration in the female reproductive tract, physiological and morphological changes to the spermatozoa, and sperm-egg interaction in the oviduct. In vitro studies have revealed essential factors for these fertilization steps for over half a century. However, the molecular mechanism of fertilization has recently been revised by the emergence of genetically modified animals. Here, we focus on essential factors for sperm fertilizing ability and describe recent advances in our knowledge of the mechanisms of mammalian fertilization, especially of sperm migration from the uterus into the oviduct.
Keywords: female reproductive tract, fertilization, male infertility, sperm motility, uterotubal junction
Introduction
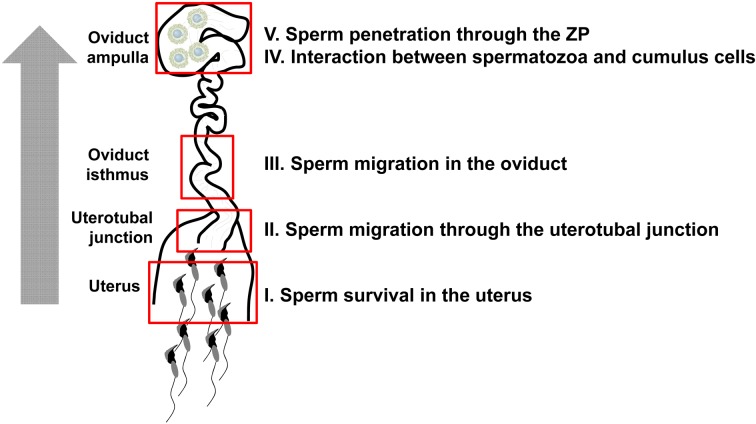
The human body is comprised of 60 trillion cells that originate from a fertilized egg produced by the fusion of a spermatozoon with an egg. Mammalian spermatozoa are morphologically differentiated in the testis, but freshly ejaculated spermatozoa are incapable of fertilization. Ejaculated spermatozoa gain their fertilizing ability in the female reproductive tract [2, 18]. The process that renders spermatozoa competent to fertilize an egg is called sperm capacitation [3]. These facts led to the possibility of performing in vitro fertilization (IVF) by mixing capacitated spermatozoa with eggs [20, 106]. In the past, factors important for fertilization were discovered via IVF experiments using biochemical approaches. However, recent studies using gene knockout (KO) methods have revealed that several sperm factors identified from the IVF system are not essential for in vivo fertilization [37, 76]. KO experiments have also unexpectedly revealed essential factors for fertilization in vivo. These essential factors play a role in spermatozoa gaining their fertilizing ability at stages such as spermatogenesis and epididymal transit. In this review, we focus on the molecular mechanism of the sperm fertilizing ability in the female reproductive tract revealed by in vivo analysis of genetically modified (GM) mice (Fig. 1).
Fig. 1.
Overview of the sperm journey into the female reproductive tract. Ejaculated spermatozoa overcome several hurdles in the uterus and the oviduct to fertilize eggs. This review summarizes in five sections the molecular dissection of sperm migration revealed by GM mouse models.
Sperm Survival in the Uterus
Mammalian spermatozoa must travel a long distance from the uterus to the oviduct, where fertilization takes place. Ejaculated spermatozoa gain their fertilizing ability after remaining in the female reproductive tract for a period of time. This process is called sperm capacitation and is specific to mammalian spermatozoa. Though over 60 years have passed since the discovery of capacitation [3], its molecular mechanism remains to be fully determined [8]. Sperm surface-bound glycoproteins, CD52, CD55, and CD59, transferred from epididymal luminal fluids were believed to protect spermatozoa from immunological attacks in the female reproductive tract [51]. Moreover, removal of these glycosylphosphatidylinositol-anchored proteins (GPI-APs) was also thought to induce sperm capacitation [13]. However, KO mouse experiments showed that CD52, CD55, CD59a, and CD59b are not essential for sperm fertilizing ability [34, 83, 91, 103]. Recently, Cd55b was identified in mice, but its physiological function has not been clarified.
Ejaculated spermatozoa mix together with a fluid, called seminal plasma, secreted from a male accessory sexual gland. Capacitated spermatozoa reversibly lose their fertilizing ability when treated with seminal plasma [19]. This finding suggests that seminal plasma contains a decapacitation factor that prevents sperm capacitation [11]. However, this phenomenon has been observed only in in vitro experiments that mixed capacitated spermatozoa with seminal plasma or candidate decapacitation factors [58, 61, 74]. Seminal vesicles, which secrete the main component of seminal plasma, contribute to reproduction because removal of seminal vesicles causes a significant reduction in male fertility [78]. Seminal vesicle protein secretion 2 (SVS2) is a major component of the seminal vesicle secretions, and it was found to be a decapacitation factor for mouse spermatozoa in vitro [1, 47]. Male Svs2 KO mice were severely subfertile because of a deficiency in copulatory plug formation and uterus-derived cytotoxicity that damaged the intrauterine spermatozoa [45]. Thus, the authors concluded that SVS2 protects ejaculated spermatozoa from immunological attack in the uterus and is required for spermatozoa to survive in the female reproductive tract. However, the decapacitation functions remain to be determined. Other functions of seminal fluid include influencing the growth and health of offspring [15]. Mammalian seminal plasma proteins may also have a key role in both fertilization and embryo development in vivo [64].
Sperm Migration through the Uterotubal Junction
The next obstacle for spermatozoa that survived in the uterus is passage through the uterotubal junction (UTJ) into the oviduct. The UTJ is the connection between the uterus and oviduct and is characterized as the distal portion of the oviduct. Although the structure of the UTJ varies among mammals, the passageway is usually narrow. In mice, the number of spermatozoa that pass through the UTJ is significantly reduced before reaching the oviduct ampulla. It has been proposed that most spermatozoa swim up through the UTJ into the oviduct by self-propulsion. However, KO mice experiments indicate that sperm motility alone is insufficient for sperm migration through the UTJ [37, 77]. There are more than 10 factors found to be essential for UTJ migration. In the following section, we discuss the interactions of these factors and the current understanding of the mechanism of UTJ migration (Fig. 2).
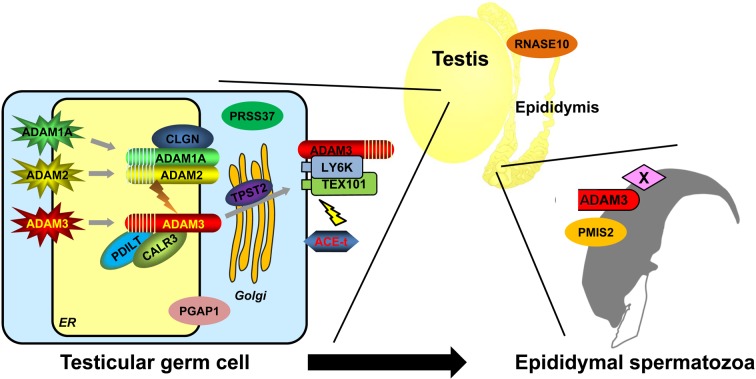
Fig. 2.
Essential factors for sperm migration through the uterotubal junction. Currently, fourteen genes are known to be required for sperm migration through the uterotubal junction (Table 1). Testis-specific ADAMs, ER chaperones, and GPI-anchored proteins allow testicular spermatozoa to obtain migrating ability. Epididymal protein RNASE10 works as a sperm maturation factor in the caput epididymis. Sperm protein ADAM3 disappears from spermatozoa in most cases (11/14 genes) and is the leading candidate in interactions with the female reproductive tract in mice. However, ADAM3 remains in Ly6k and Pgap1 KO spermatozoa, although Ly6k and Pgap1 KO mice show impaired sperm migration. Moreover, Adam3 and Pmis2 are not conserved in humans. It is possible that an unknown factor X localizes to the sperm membrane and is conserved in mice and humans. An unknown factor X may regulate sperm migrating ability through the uterotubal junction. All of the KO mice with impaired sperm migration have another phenotype, impaired ZP-binding ability in vitro. These phenotypes may be correlated with sperm attachment to the epithelial cells of the uterotubal junction.
Testis-specific ADAM proteins
A disintegrin and metalloproteinase (ADAM) family members are membrane-anchored metalloproteinases, and they regulate various events such as cell migration, cell adhesion, and cell interactions [86]. Testicular ADAMs, ADAM1B and ADAM2, heterodimerize to form fertilin. Fertilin is localized to the sperm plasma membrane and has been characterized as a sperm-egg fusion protein [12]. As expected, male Adam2 KO mice were found to be sterile [21], but the phenotype was not related to sperm-egg fusion [72]. When ADAM1B, a subunit of the fertilin heterodimer, was knocked out, both ADAM1B and ADAM2 disappeared from mature spermatozoa, but the mice were fully fertile [49]. Further investigations indicated that ADAM2 functions to form a dimer with ADAM1A in the endoplasmic reticulum (ER) of spermatogenic cells, leading to the localization of ADAM3 on the sperm surface [73]. Since male Adam3 KO mice were found to be sterile because of impaired sperm migration through the UTJ [87, 102], ADAM3 is thought to play a pivotal role in sperm migration through the UTJ. More than 10 proteins involved in sperm migration through the UTJ interact with ADAM3, affecting the protein amount and/or the localization of spermatozoa (Table 1).
Table 1. KO mouse lines with impaired sperm-ZP binding in vitro and impaired sperm migration from uterus into the oviduct.
| Gene | Expression pattern | Localization | Phenotype of KO mice | Human ortholog | References | ||
|---|---|---|---|---|---|---|---|
| In vitro sperm-ZP binding ability | Sperm migration ability to oviduct | Localization of ADAM3 on spermatozoa | |||||
| Ace-t | Sperm | Sperm surface | Impaired | Impaired | Aberrantly localized | + | Krege JH, et al. 1995; Yamaguchi R, et al. 2006 |
| Adam1a | Testis | Endoplasmic reticulum | Impaired | Impaired | Disappeared | – | Nishimura H, et al. 2004 |
| Adam2 | Sperm | Sperm surface | Impaired | Impaired | Disappeared | + | Cho C, et al. 1998 |
| Adam3 | Sperm | Sperm surface | Impaired | Impaired | Disappeared | – | Shamsadin R, et al. 1999; Yamaguchi R, et al. 2009 |
| Calr3 | Testis | Endoplasmic reticulum | Impaired | Impaired | Disappeared | + | Ikawa M, et al. 2011 |
| Clgn | Testis | Endoplasmic reticulum | Impaired | Impaired | Disappeared | + | Ikawa M, et al. 1997 |
| Ly6k | Testis | Testicular germ cell | Impaired | Impaired | Localized | + | Fujihara Y, et al. 2014 |
| Pdilt | Testis | Endoplasmic reticulum | Impaired | Impaired | Disappeared | + | Tokuhiro K, et al. 2012 |
| Pgap1 | Ubiquitous | Endoplasmic reticulum | Impaired | Impaired | Localized | + | Ueda Y, et al. 2007 |
| Pmis2 | Sperm | Sperm surface | Impaired | Impaired | Disappeared | – | Yamaguchi R, et al. 2012 |
| Prss37 | Testis | Testicular germ cell | Impaired | Impaired | Disappeared | + | Shen C, et al. 2013 |
| Rnase10 | Epididymis | Epididymis | Impaired | Impaired | Disappeared | + | Krutskikh A, et al. 2012 |
| Tex101 | Testis | Testicular germ cell | Impaired | Impaired | Disappeared | + | Fujihara Y, et al. 2013 |
| Tpst2 | Ubiquitous | Golgi apparatus | Impaired | Impaired | Disappeared | + | Marcello M, et al. 2011 |
Testis-specific ER chaperones
ADAM3 is a cysteine-rich, glycosylated membrane protein that is co-translationally translocated into the ER of spermatids, where numerous molecular chaperones and catalysts promote glycoprotein folding as well as the disposal of misfolded proteins. Membrane-bound calnexin (CANX) and soluble calreticulin (CALR) were originally found as homologous lectin chaperones that mainly mediate nascent glycoprotein folding in somatic cells. Testicular germ cell-specific homologues of CANX and CALR are calmegin (CLGN) and calsperin (CALR3), respectively. CLGN mediates the heterodimerization of ADAM1A/ADAM2 that is required for the maturation of ADAM3 [38]. CALR3 binds directly to ADAM3 and regulates its maturation. Both Clgn and Calr3 KO mice lack ADAM3 in sperm and are sterile [39, 40]. Other chaperones, such as those in the protein disulfide isomerase (PDI) family proteins, have also been implicated in the intra- and intermolecular disulfide bond formation in the ER [79]. Among this protein family, PDIA3 is associated with CANX/CALR and contributes to the quality control cycle of newly synthesized glycoproteins in the ER. Testis-specific PDI-like protein, PDILT, cooperates with CALR3 in testicular germ cells and plays an indispensable role in disulfide bond formation and folding of ADAM3 [95, 97]. Male Pdilt KO mice are infertile because of impaired transport of ADAM3 to the sperm surface [95]. Testicular germ cell-specific ER chaperones are essential for the folding and maturation of ADAM3.
Testis-specific GPI-anchored proteins
GPI-APs are anchored to the outer cell membrane by GPI and are critical at various points in mammalian fertilization [26, 52]. The GPI-AP complex, which consists of testis expressed gene 101 (TEX101) and lymphocyte antigen 6 complex locus k (LY6K), is present only in testicular germ cells, and it disappears from epididymal spermatozoa [54, 107]. TEX101 and LY6K are required for sperm migration into the oviduct [28, 29] (Fig. 3). Our study revealed that the transient interaction of the LY6K/TEX101 GPI-AP complex with ADAM3 is a critical step for ADAM3 maturation. Intriguingly, dissociation of the complex from ADAM3 is mediated by the GPI-AP releasing (GPIase) activity of angiotensin-converting enzyme (ACE). ACE is a well-characterized carboxy dipeptidase that regulates blood pressure. Ace-deficient mice showed low blood pressure, and the male mice were sterile [30, 53]. In vitro analysis demonstrated that TEX101 (but not LY6K) is the specific substrate for not only wild-type ACE but also zinc peptidase-defective ACE. These findings are consistent with the aberrantly remaining TEX101/LY6K protein complex on Ace KO mouse spermatozoa [28, 29]. As a result, ADAM3 dislocates from the Triton X-114 detergent-enriched phase to the detergent-depleted phase in Ace KO mouse spermatozoa, although ADAM3 localizes to both phases in wild-type mouse spermatozoa [104]. Therefore, ACE-mediated shedding of the GPI-AP complex, TEX101 and LY6K, is required for the correct localization of ADAM3 in epididymal spermatozoa and subsequent sperm fertilizing ability. The release of GPI-APs is one of the key events in activation of the sperm fertilizing ability [26].
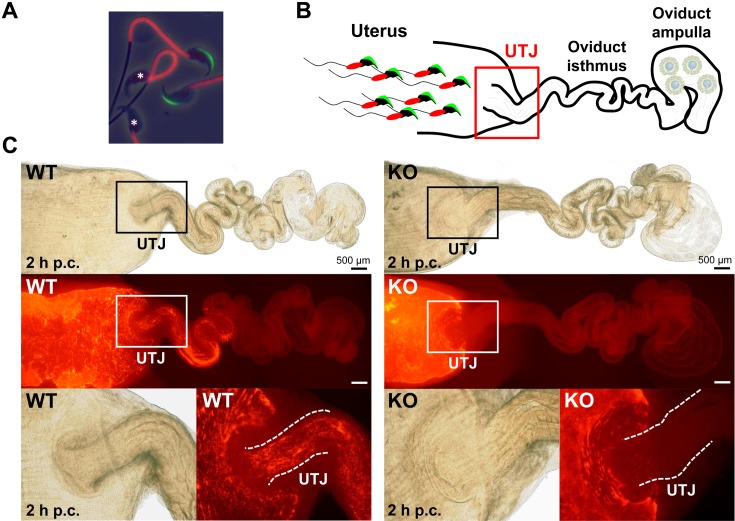
Fig. 3.
Observation of ejaculated spermatozoa into the female reproductive tract. (A) Visualization of the acrosome and midpiece of spermatozoa. A transgenic mouse line carrying Acr-Egfp and CAG-Su9/DsRed2 transgenes expressed both a green sperm acrosome and red mitochondria in the sperm midpiece [31]. It is easy to determine if the acrosome reaction occurred in these spermatozoa due to the green acrosome. *Acrosome-reacted spermatozoa. This transgenic mouse line [B6D2-Tg (CAG/su9-DsRed2, Acr3-Egfp) RBGS002Osb] is available from the RIKEN BioResource Center and the Center for Animal Resources and Development (CARD), Kumamoto University. (B) Scheme of observing sperm migration into the female reproductive tract using fluorescent spermatozoa. (C) Observation and visualization of ejaculated spermatozoa into the female reproductive tract two hours post coitus (p.c.). Observing the red signals, wild-type (WT) spermatozoa passed through the uterotubal junction (UTJ), but Tex101 KO spermatozoa were unable to migrate from the uterus to the oviduct [29]. Fluorescent spermatozoa could facilitate live imaging of localization and movement in vitro and in vivo.
Essential factors regulating sperm migration through the UTJ
To date, more than 10 factors have been reported to be essential for sperm migration through the UTJ (Table 1) and to be involved in ADAM3 maturation; however, there is no direct evidence that ADAM3 functions on the sperm surface during UTJ migration. Considering that ADAM3 is a pseudogene in humans, the contribution of undiscovered novel factors should be taken into account. This idea is also supported by the fact that ADAM3 localized normally in migration-defective Ly6k and Pgap1 KO spermatozoa [28, 96]. Because both LY6K and PGAP1 disappear during epididymal sperm maturation, these molecules do not directly function during UTJ migration. Recently, we identified sperm membrane proteins missing in Adam3 KO spermatozoa and found that protein missing in infertile sperm 2 (PMIS2) is a novel sperm protein required for UTJ migration [101]. Although many molecules have proven to be essential for sperm migration through the UTJ, the sperm migration mechanism per se is still unclear. Interestingly, migration-defective spermatozoa also show impaired binding to the zona pellucida (ZP) [77]. It is also reported that Adam3 KO spermatozoa are less adhesive than wild-type spermatozoa [87, 102]. To understand these defects, wild-type and Clgn KO chimeric mice were produced to test the migration ability of mixed spermatozoa. Although control wild-type spermatozoa could pass through the uterotubal junction, the mixed wild-type spermatozoa could not compensate for the inability of Clgn KO spermatozoa to migrate into the oviduct [71]. These data implicate that there is an initial interaction with the UTJ entrance that may be a critical step prior to sperm migration into the oviduct. Further study is needed to resolve the mystery of the factor (s) controlling sperm migration into the oviduct.
Contribution of sperm motility
Sperm motility is also important for the UTJ passage. For example, male mice that lack cation channel, sperm associated 1 (CATSPER1), a component of a Ca2+ channel localized in the principle piece, were infertile due to impaired sperm motility [84]. Migration of Catsper1 KO spermatozoa through the UTJ was inefficient as observed by transillumination [33] and fluorescence microscopy [22]. Some Catsper1 KO spermatozoa were observed in the oviduct isthmus a few hours after coitus but disappeared with time. This suggests that the Catsper1 KO spermatozoa can pass through the UTJ but that most of the ejaculated spermatozoa in the uterus lose their motility with time before entering the UTJ. Mice lacking protein phosphatase 3 catalytic subunit gamma (PPP3CC), a catalytic subunit of calcineurin localized in the sperm tail, are another example illustrating the importance of sperm motility for UTJ passage [68]. Ppp3cc KO spermatozoa showed a rigid midpiece (Fig. 4A), and when the oviduct isthmus was observed two hours after copulation, less KO spermatozoa were observed compared with the control (Fig. 4B). Although Ppp3cc KO spermatozoa showed impaired motility, their velocity parameters in a hybrid background were comparable with those in a wild-type C57BL/6 background. Further studies are required to understand the exact role of sperm motility in sperm migration, but flagellar movement patterns such as midpiece flexibility should also be taken into account for UTJ passage.
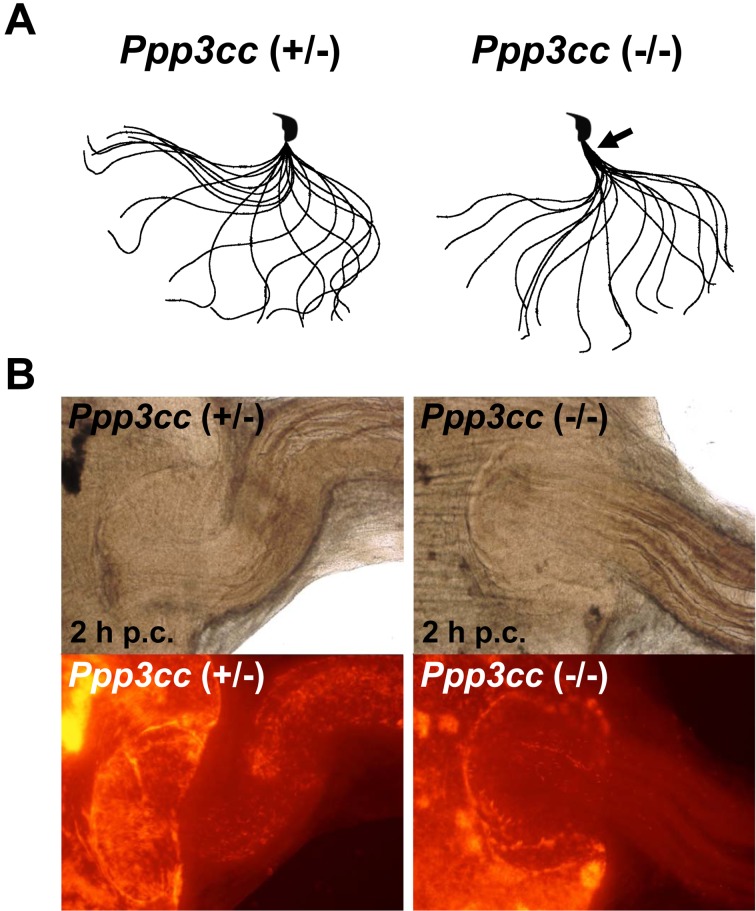
Fig. 4.
Waveform and migration of Ppp3cc KO spermatozoa. (A) Flagellar movement patterns. Sperm motility was videotaped at 200 frames per second. Single frames throughout one beating cycle are superimposed. The midpiece (black arrow) is rigid in the spermatozoa obtained from Ppp3cc KO mice [68]. (B) Visualization of ejaculated spermatozoa in the female reproductive tract two hours post coitus (p.c.). Fewer Ppp3cc KO spermatozoa were observed in the oviduct isthmus.
Recently, Muro et al. observed sperm migration in the female reproductive tract [70] using fluorescent spermatozoa that transgenically expressed green fluorescent protein (GFP) in the acrosome and red fluorescent protein from Discosoma sp. (DsRed2) in the midpiece [31]. They observed that the tail of spermatozoa migrating in the intramural UTJ seemed to be motionless. One explanation for this observation is that the midpiece that can be observed by DsRed2 fluorescence is motionless but that the principle piece and endpiece that cannot be observed with fluorescence are motile and play a role in sperm migration. However, analysis of Ppp3cc KO mice suggests that midpiece motility may in fact be important for UTJ passage [68]. Spermatozoa may pass through the UTJ with the midpiece “seemingly motionless” because of the viscous environment as suggested by Muro et al. [70].
Sperm Migration in the Oviduct
Sperm motility and hyperactivation for sperm migration
Once spermatozoa pass through the UTJ, they need to migrate through the oviduct to the ampulla. Sperm motility may be important for efficient sperm migration in the oviduct, as less Catsper1 and Ppp3cc KO spermatozoa were observed in the oviduct ampulla [22, 68]. A simple explanation for this importance is that spermatozoa swim by self-protrusion in the oviduct. However, there are several studies showing complex interactions between spermatozoa and the oviduct. Chang and Suarez observed that mouse spermatozoa attached to and detached from the epithelium of the oviduct isthmus [17], suggesting that spermatozoa may bind and unbind several times as they migrate through the oviduct. Detachment of spermatozoa may be caused by hyperactivated motility [17] characterized by a high amplitude and asymmetrical beating pattern of the sperm tail. It is interesting to mention that both Catsper1 and Ppp3cc KO spermatozoa cannot exhibit hyperactivated motility [16, 68]. Molecules that mediate the interaction between spermatozoa and the oviduct have not been identified yet using KO mouse models. In bulls, attachment of spermatozoa to the epithelium is mediated by binder of sperm protein 1 (BSP1), which is secreted by seminal vesicles [36]. There are two homologs of BSP in mice, Bsph1 and Bsph2 [57]; however, analyses of KO mice that lack these genes have not been performed.
Peristatic movement and sperm migration
Using fluorescent spermatozoa, Muro et al. observed that spermatozoa moved back and forth together with peristatic movement in the oviduct isthmus [70], suggesting that oviduct contractions may play a role in sperm migration. Ishikawa et al. observed a similar movement of sperm assemblage as well [42]. They showed that this movement was blocked and that fewer spermatozoa were found in the oviduct ampulla when peristatic movement was inhibited by the anticholinergic drug Padrin, suggesting that peristatic movement plays a role in sperm migration in the oviduct. However, a few spermatozoa can still reach the oviduct ampulla even with Padrin administration [42]. Another study that used Nicardipine to block oviduct contractions also showed that the spermatozoa could still reach the first loop of the oviduct isthmus or the oviduct ampulla [17]. These studies indicate that sperm motility may play a larger role in sperm migration in the oviduct isthmus rather than peristatic movement.
Oviductal fluid flow and sperm migration
How spermatozoa orient themselves in the oviduct remains an unanswered question. Miki and Clapham showed that mouse and human spermatozoa tend to swim against the flow (rheotaxis) and suggested that rheotaxis against oviductal flow is a major determinant of sperm guidance in the oviduct [65]. This is supported by an observation that Catsper1 KO spermatozoa cannot exhibit rheotaxic behavior and cannot migrate through the oviduct efficiently. In addition to rheotaxis, chemotaxis [60, 88] and thermotaxis [7] are also implicated in sperm migration. Because these hypotheses are based on in vitro studies, further in vivo experiments are necessary to understand how spermatozoa understand direction in the oviduct.
Interaction between Spermatozoa and Cumulus Cells
Sperm enzymes involved in sperm passage through cumulus cell layers
Spermatozoa move into the ampulla of the oviduct and encounter the cumulus-cell oocyte complex (COC). The COC consists of ovulated eggs covered by an extracellular matrix (ECM), the ZP, and a cumulus cell layer filled with hyaluronic acid (Figs 5A and B). Mouse spermatozoa have at least two hyaluronidases, sperm adhesion molecule 1 (SPAM1) and hyaluronoglucosaminidase 5 (HYAL5) [48]. SPAM1 was first identified as a sperm receptor for the ZP and was later reported to have hyaluronidase activity that enables spermatozoa to pass thorough the COC [59, 80, 81]. While Spam1 KO mice are fertile, Spam1 KO spermatozoa show a reduced ability to disperse cumulus cells in vitro [4]. Hyal5 KO mice are fertile both in vitro and in vivo [50], suggesting functional redundancies in these genes.
Fig. 5.
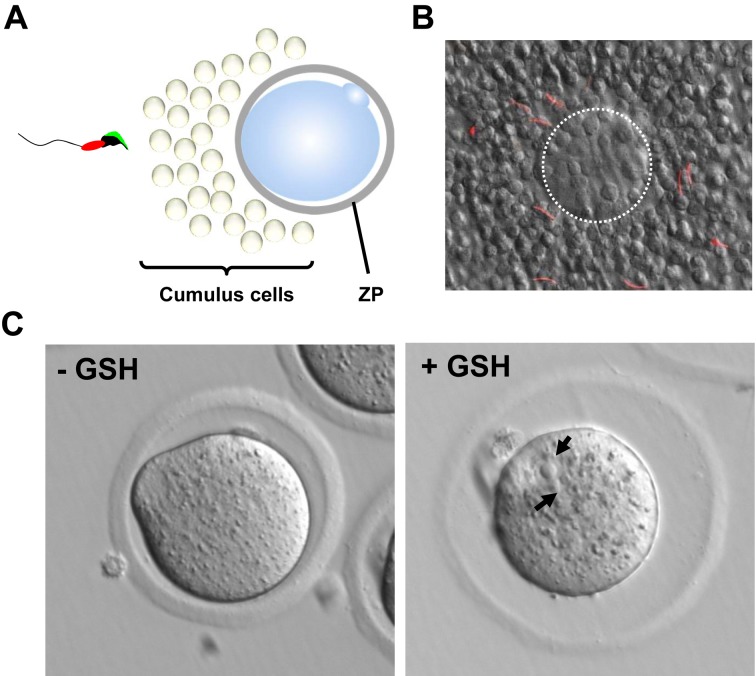
Sperm passage through the cumulus cell layer and the ZP. (A) The cumulus-cell oocyte complex (COC). Ovulated eggs are covered by a cumulus cell layer and the zona pellucida (ZP). (B) Sperm passage through the cumulus cell layer. The spermatozoa penetrating through the cumulus cell layer were observed using the red fluorescence localized in the midpiece. The egg is highlighted with a white dotted line. (C) Rescue of ZP penetration failure using glutathione (GSH). When GSH is used, the ZP is destabilized and expanded. Ppp3cc KO spermatozoa can penetrate the ZP in the presence of GSH and fertilize the egg. Black arrows indicate the pronuclei.
Proteinase activity is also implicated in COC penetration as shown below. Acrosin (ACR) and protease, serine 21 (PRSS21), are trypsin-like serine proteases and are localized on the sperm head [6, 35]. Although these sperm proteases were thought to play an essential role in ZP binding and penetration, Acr KO spermatozoa were fertile, albeit with a slight delay in ZP penetration in vitro [5]. Prss21 KO mouse lines were also fully fertile in vivo [105]. Moreover, Acr and Prss21 double KO mice were subfertile because of impaired sperm penetration through the cumulus matrix and ZP in vitro [46]. This indicates that the sperm trypsin-like activity is not essential for in vivo fertilization in mice. Therefore, the sperm factor required for penetration through the cumulus matrix in vivo remains to be determined.
COC factors that modulate sperm functions
Sperm chemotaxis is found in not only marine invertebrates but also mammals [44]. Chemoattractants are present in oviductal fluid and are also secreted from the COC [24]. In humans, the COC secretes sperm chemoattractants after ovulation [90]. Progesterone secreted from the COC influences several functions including hyperactivation and the acrosome reaction in human spermatozoa [9]. The extranuclear-mediated effects of progesterone stimulate an influx of calcium, tyrosine phosphorylation of proteins, and other signaling molecules [82]. Progesterone-induced calcium influx is mediated by a sperm-specific calcium channel CATSPER in human spermatozoa [60, 88]. It has recently been revealed that human CATSPER activation by progesterone is triggered by the steroid binding to a serine hydrolase, abhydrolase domain containing 2 (ABHD2) [66]. Furthermore, the CATSPER channel complex may serve as a polymodal sensor for multiple chemicals (odorants, 8-Br-cNMPs, or menthol) [14]. However, mouse Catsper does not react to progesterone induction in vitro, and mouse Abhd2 is not essential for male fertility [69]. Since the sequence similarity of Catsper orthologs is low (less than 50%) [85], the mechanism of sperm calcium entry may differ in each species.
Prostaglandin E2 (PGE2) is a key mediator of ovulation [23]. One of the four subtypes of PGE2 receptor, prostaglandin E receptor 2 (Ptger2), is expressed in the cumulus cells. Female Ptger2 KO mice are severely subfertile due to impaired cumulus expansion in the oviduct [32]. PGE2-PTGER2 signaling facilitates cumulus ECM assembly and sperm passage through cumulus cell layers. The gene expression profile indicates that cumulus cells upregulate a set of immune response- and chemokine-related genes during ovulation [93]. One of the chemokines, chemokine ligand 7 (Ccl7), is overexpressed abnormally in Ptger2 KO cumulus cells, and excessive cumulus ECM assembly interferes with sperm migration through the COC [94]. These results suggest that CCL7 promotes cumulus ECM assembly to protect the oocyte and functions as a chemoattractant for spermatozoa. While it is unclear why this occurs, proper interaction between prostaglandin and chemokine signaling is required for successful fertilization [89]. Further studies have shown that CCL7 facilitates sperm migration towards the COC in vitro. Recently, we found that Adam3 KO spermatozoa are able to fertilize cumulus-intact eggs but not cumulus-free eggs [95]. The supernatant of cumulus cells is able to partially restore Adam3 KO sperm fertilizing ability. These data also suggest that COC factors can modulate sperm fertilizing ability.
Sperm Penetration through the ZP
During in vitro fertilization, numerous spermatozoa bind to the ZP, and it has been long believed that ZP binding ability is critical for sperm fertilizing ability. However, when Adam3 KO spermatozoa, which cannot bind to the ZP [87], were deposited directly into the oviduct to circumvent sperm migration through the UTJ, the ovulated eggs were fertilized [95]. These data questioned the importance of ZP binding ability.
There are two possible factors that are necessary for sperm penetration through the ZP, namely, proteases and sperm motility. Proteases were thought to be important because the acrosome contains proteases that are released during the acrosome reaction. However, ACR and PRSS21, trypsin-like serine proteases in the sperm head, are not essential for male fertility in mice, as mentioned previously [5, 46, 105]. Further, recent live-imaging studies demonstrated that mouse spermatozoa underwent the acrosome reaction before contact with the ZP [43, 56, 70], and rabbit and mouse spermatozoa that penetrated the ZP once could penetrate the ZP again and fertilize ZP-intact eggs [41, 55]. These results suggest that the proteases that are released from the acrosome are not necessary for ZP penetration. However, careful interpretation is required because there is a possibility that proteases remain attached to the sperm head after the acrosome reaction, and this may contribute to ZP penetration.
There is positive evidence that sperm motility is crucial for ZP penetration. Field vole spermatozoa can penetrate through the ZP of mice and hamsters without the acrosome reaction [98], suggesting that the mechanical force generated by sperm motility is important for ZP penetration. This idea is supported by Catsper1 or Ppp3cc KO mice that exhibit impaired sperm motility and failure to penetrate the ZP [68, 84]. Both Catsper1 and Ppp3cc KO spermatozoa do not exhibit hyperactivated motility as mentioned previously. However, Ppp3cc KO spermatozoa could penetrate through the ZP when the eggs were treated with glutathione (GSH), which reduces disulfide bonds and destabilizes the ZP (Fig. 5C) [10, 68, 92]. The fertilized eggs developed to term when they were transplanted into the oviduct of pseudopregnant mice. This method using GSH in IVF could be useful to further identify factors that are involved in ZP penetration.
Conclusion
Use of GM animals is a powerful approach to clearly identify the in vivo function (s) of a given gene. In mammalian fertilization research especially, most findings based on biochemical in vitro approaches have been revised by the analyses of KO mouse models [37, 77]. However, the conventional KO method is expensive, laborious, and time-consuming to perform. Recently, the clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 system has emerged as a genome editing tool in mice, rats, and other animal models [99]. This system enables researchers to make GM mice easier and quicker than the conventional KO method [100]. We have also established a method to generate GM mice using a CRISPR/Cas9 expression plasmid [25, 62, 63] and have analyzed reproductive phenotypes of GM mice using this method [27, 67, 75, 108]. Reproductive biology is one of the most suitable research fields that can use GM animals. We therefore believe that mutant animals will soon unravel whole gene functions through gene-disruption experiments.
Acknowledgments
This work was supported by Ministry of Education, Culture, Sports, Science and Technology (MEXT)/Japan Society for the Promotion of Science (JSPS) KAKENHI grants (JP15H05573 to Y.F., JP17H04987 to H.M., JP25112007 and JP17H01394 to M.I.); Takeda Science Foundation grants to Y.F., H.M., and M.I.; the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD088412 and P01HD087157); and the Bill & Melinda Gates Foundation (Grand Challenges Explorations grant OPP1160866). We thank Julio M. Castaneda and Ferheen Abbasi for critical reading of this manuscript.
References
- 1.Araki N., Trencsényi G., Krasznai Z.T., Nizsalóczki E., Sakamoto A., Kawano N., Miyado K., Yoshida K., Yoshida M.2015. Seminal vesicle secretion 2 acts as a protectant of sperm sterols and prevents ectopic sperm capacitation in mice. Biol. Reprod. 92: 8. doi: 10.1095/biolreprod.114.120642 [DOI] [PubMed] [Google Scholar]
- 2.Austin C.R.1951. Observations on the penetration of the sperm in the mammalian egg. Aust. J. Sci. Res., B 4: 581–596. doi: 10.1071/BI9510581 [DOI] [PubMed] [Google Scholar]
- 3.Austin C.R.1952. The capacitation of the mammalian sperm. Nature 170: 326. doi: 10.1038/170326a0 [DOI] [PubMed] [Google Scholar]
- 4.Baba D., Kashiwabara S., Honda A., Yamagata K., Wu Q., Ikawa M., Okabe M., Baba T.2002. Mouse sperm lacking cell surface hyaluronidase PH-20 can pass through the layer of cumulus cells and fertilize the egg. J. Biol. Chem. 277: 30310–30314. doi: 10.1074/jbc.M204596200 [DOI] [PubMed] [Google Scholar]
- 5.Baba T., Azuma S., Kashiwabara S., Toyoda Y.1994. Sperm from mice carrying a targeted mutation of the acrosin gene can penetrate the oocyte zona pellucida and effect fertilization. J. Biol. Chem. 269: 31845–31849. [PubMed] [Google Scholar]
- 6.Baba T., Kashiwabara S., Watanabe K., Itoh H., Michikawa Y., Kimura K., Takada M., Fukamizu A., Arai Y.1989. Activation and maturation mechanisms of boar acrosin zymogen based on the deduced primary structure. J. Biol. Chem. 264: 11920–11927. [PubMed] [Google Scholar]
- 7.Bahat A., Tur-Kaspa I., Gakamsky A., Giojalas L.C., Breitbart H., Eisenbach M.2003. Thermotaxis of mammalian sperm cells: a potential navigation mechanism in the female genital tract. Nat. Med. 9: 149–150. doi: 10.1038/nm0203-149 [DOI] [PubMed] [Google Scholar]
- 8.Bailey J.L.2010. Factors regulating sperm capacitation. Syst Biol Reprod Med 56: 334–348. doi: 10.3109/19396368.2010.512377 [DOI] [PubMed] [Google Scholar]
- 9.Baldi E., Luconi M., Muratori M., Marchiani S., Tamburrino L., Forti G.2009. Nongenomic activation of spermatozoa by steroid hormones: facts and fictions. Mol. Cell. Endocrinol. 308: 39–46. doi: 10.1016/j.mce.2009.02.006 [DOI] [PubMed] [Google Scholar]
- 10.Bath M.L.2010. Inhibition of in vitro fertilizing capacity of cryopreserved mouse sperm by factors released by damaged sperm, and stimulation by glutathione. PLoS One 5: e9387. doi: 10.1371/journal.pone.0009387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bedford J.M., Chang M.C.1962. Removal of decapacitation factor from seminal plasma by high-speed centrifugation. Am. J. Physiol. 202: 179–181. [DOI] [PubMed] [Google Scholar]
- 12.Blobel C.P., Wolfsberg T.G., Turck C.W., Myles D.G., Primakoff P., White J.M.1992. A potential fusion peptide and an integrin ligand domain in a protein active in sperm-egg fusion. Nature 356: 248–252. doi: 10.1038/356248a0 [DOI] [PubMed] [Google Scholar]
- 13.Boerke A., van der Lit J., Lolicato F., Stout T.A., Helms J.B., Gadella B.M.2014. Removal of GPI-anchored membrane proteins causes clustering of lipid microdomains in the apical head area of porcine sperm. Theriogenology 81: 613–624. doi: 10.1016/j.theriogenology.2013.11.014 [DOI] [PubMed] [Google Scholar]
- 14.Brenker C., Goodwin N., Weyand I., Kashikar N.D., Naruse M., Krähling M., Müller A., Kaupp U.B., Strünker T.2012. The CatSper channel: a polymodal chemosensor in human sperm. EMBO J. 31: 1654–1665. doi: 10.1038/emboj.2012.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bromfield J.J., Schjenken J.E., Chin P.Y., Care A.S., Jasper M.J., Robertson S.A.2014. Maternal tract factors contribute to paternal seminal fluid impact on metabolic phenotype in offspring. Proc. Natl. Acad. Sci. USA 111: 2200–2205. doi: 10.1073/pnas.1305609111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlson A.E., Westenbroek R.E., Quill T., Ren D., Clapham D.E., Hille B., Garbers D.L., Babcock D.F.2003. CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proc. Natl. Acad. Sci. USA 100: 14864–14868. doi: 10.1073/pnas.2536658100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang H., Suarez S.S.2012. Unexpected flagellar movement patterns and epithelial binding behavior of mouse sperm in the oviduct. Biol. Reprod. 86: 140–, 1–8.. doi: 10.1095/biolreprod.111.096578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang M.C.1951. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature 168: 697–698. doi: 10.1038/168697b0 [DOI] [PubMed] [Google Scholar]
- 19.Chang M.C.1957. A detrimental effect of seminal plasma on the fertilizing capacity of sperm. Nature 179: 258–259. doi: 10.1038/179258a0 [DOI] [PubMed] [Google Scholar]
- 20.Chang M.C.1959. Fertilization of rabbit ova in vitro. Nature 184:(Suppl 7): 466–467. doi: 10.1038/184466a0 [DOI] [PubMed] [Google Scholar]
- 21.Cho C., Bunch D.O., Faure J.E., Goulding E.H., Eddy E.M., Primakoff P., Myles D.G.1998. Fertilization defects in sperm from mice lacking fertilin beta. Science 281: 1857–1859. doi: 10.1126/science.281.5384.1857 [DOI] [PubMed] [Google Scholar]
- 22.Chung J.J., Shim S.H., Everley R.A., Gygi S.P., Zhuang X., Clapham D.E.2014. Structurally distinct Ca(2+) signaling domains of sperm flagella orchestrate tyrosine phosphorylation and motility. Cell 157: 808–822. doi: 10.1016/j.cell.2014.02.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duffy D.M.2015. Novel contraceptive targets to inhibit ovulation: the prostaglandin E2 pathway. Hum. Reprod. Update 21: 652–670. doi: 10.1093/humupd/dmv026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisenbach M., Giojalas L.C.2006. Sperm guidance in mammals - an unpaved road to the egg. Nat. Rev. Mol. Cell Biol. 7: 276–285. doi: 10.1038/nrm1893 [DOI] [PubMed] [Google Scholar]
- 25.Fujihara Y., Ikawa M.2014. CRISPR/Cas9-based genome editing in mice by single plasmid injection. Methods Enzymol. 546: 319–336. doi: 10.1016/B978-0-12-801185-0.00015-5 [DOI] [PubMed] [Google Scholar]
- 26.Fujihara Y., Ikawa M.2016. GPI-AP release in cellular, developmental, and reproductive biology. J. Lipid Res. 57: 538–545. doi: 10.1194/jlr.R063032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujihara Y., Oji A., Larasati T., Kojima-Kita K., Ikawa M.2017. Human Globozoospermia-Related Gene Spata16 Is Required for Sperm Formation Revealed by CRISPR/Cas9-Mediated Mouse Models. Int. J. Mol. Sci. 18: 18. doi: 10.3390/ijms18102208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujihara Y., Okabe M., Ikawa M.2014. GPI-anchored protein complex, LY6K/TEX101, is required for sperm migration into the oviduct and male fertility in mice. Biol. Reprod. 90: 60. doi: 10.1095/biolreprod.113.112888 [DOI] [PubMed] [Google Scholar]
- 29.Fujihara Y., Tokuhiro K., Muro Y., Kondoh G., Araki Y., Ikawa M., Okabe M.2013. Expression of TEX101, regulated by ACE, is essential for the production of fertile mouse spermatozoa. Proc. Natl. Acad. Sci. USA 110: 8111–8116. doi: 10.1073/pnas.1222166110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hagaman J.R., Moyer J.S., Bachman E.S., Sibony M., Magyar P.L., Welch J.E., Smithies O., Krege J.H., O’Brien D.A.1998. Angiotensin-converting enzyme and male fertility. Proc. Natl. Acad. Sci. USA 95: 2552–2557. doi: 10.1073/pnas.95.5.2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hasuwa H., Muro Y., Ikawa M., Kato N., Tsujimoto Y., Okabe M.2010. Transgenic mouse sperm that have green acrosome and red mitochondria allow visualization of sperm and their acrosome reaction in vivo. Exp. Anim. 59: 105–107. doi: 10.1538/expanim.59.105 [DOI] [PubMed] [Google Scholar]
- 32.Hizaki H., Segi E., Sugimoto Y., Hirose M., Saji T., Ushikubi F., Matsuoka T., Noda Y., Tanaka T., Yoshida N., Narumiya S., Ichikawa A.1999. Abortive expansion of the cumulus and impaired fertility in mice lacking the prostaglandin E receptor subtype EP(2). Proc. Natl. Acad. Sci. USA 96: 10501–10506. doi: 10.1073/pnas.96.18.10501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho K., Wolff C.A., Suarez S.S.2009. CatSper-null mutant spermatozoa are unable to ascend beyond the oviductal reservoir. Reprod. Fertil. Dev. 21: 345–350. doi: 10.1071/RD08183 [DOI] [PubMed] [Google Scholar]
- 34.Holt D.S., Botto M., Bygrave A.E., Hanna S.M., Walport M.J., Morgan B.P.2001. Targeted deletion of the CD59 gene causes spontaneous intravascular hemolysis and hemoglobinuria. Blood 98: 442–449. doi: 10.1182/blood.V98.2.442 [DOI] [PubMed] [Google Scholar]
- 35.Honda A., Yamagata K., Sugiura S., Watanabe K., Baba T.2002. A mouse serine protease TESP5 is selectively included into lipid rafts of sperm membrane presumably as a glycosylphosphatidylinositol-anchored protein. J. Biol. Chem. 277: 16976–16984. doi: 10.1074/jbc.M112470200 [DOI] [PubMed] [Google Scholar]
- 36.Ignotz G.G., Lo M.C., Perez C.L., Gwathmey T.M., Suarez S.S.2001. Characterization of a fucose-binding protein from bull sperm and seminal plasma that may be responsible for formation of the oviductal sperm reservoir. Biol. Reprod. 64: 1806–1811. doi: 10.1095/biolreprod64.6.1806 [DOI] [PubMed] [Google Scholar]
- 37.Ikawa M., Inoue N., Benham A.M., Okabe M.2010. Fertilization: a sperm’s journey to and interaction with the oocyte. J. Clin. Invest. 120: 984–994. doi: 10.1172/JCI41585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ikawa M., Nakanishi T., Yamada S., Wada I., Kominami K., Tanaka H., Nozaki M., Nishimune Y., Okabe M.2001. Calmegin is required for fertilin alpha/beta heterodimerization and sperm fertility. Dev. Biol. 240: 254–261. doi: 10.1006/dbio.2001.0462 [DOI] [PubMed] [Google Scholar]
- 39.Ikawa M., Tokuhiro K., Yamaguchi R., Benham A.M., Tamura T., Wada I., Satouh Y., Inoue N., Okabe M.2011. Calsperin is a testis-specific chaperone required for sperm fertility. J. Biol. Chem. 286: 5639–5646. doi: 10.1074/jbc.M110.140152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ikawa M., Wada I., Kominami K., Watanabe D., Toshimori K., Nishimune Y., Okabe M.1997. The putative chaperone calmegin is required for sperm fertility. Nature 387: 607–611. doi: 10.1038/42484 [DOI] [PubMed] [Google Scholar]
- 41.Inoue N., Satouh Y., Ikawa M., Okabe M., Yanagimachi R.2011. Acrosome-reacted mouse spermatozoa recovered from the perivitelline space can fertilize other eggs. Proc. Natl. Acad. Sci. USA 108: 20008–20011. doi: 10.1073/pnas.1116965108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ishikawa Y., Usui T., Yamashita M., Kanemori Y., Baba T.2016. Surfing and Swimming of Ejaculated Sperm in the Mouse Oviduct. Biol. Reprod. 94: 89. doi: 10.1095/biolreprod.115.135418 [DOI] [PubMed] [Google Scholar]
- 43.Jin M., Fujiwara E., Kakiuchi Y., Okabe M., Satouh Y., Baba S.A., Chiba K., Hirohashi N.2011. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc. Natl. Acad. Sci. USA 108: 4892–4896. doi: 10.1073/pnas.1018202108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaupp U.B., Kashikar N.D., Weyand I.2008. Mechanisms of sperm chemotaxis. Annu. Rev. Physiol. 70: 93–117. doi: 10.1146/annurev.physiol.70.113006.100654 [DOI] [PubMed] [Google Scholar]
- 45.Kawano N., Araki N., Yoshida K., Hibino T., Ohnami N., Makino M., Kanai S., Hasuwa H., Yoshida M., Miyado K., Umezawa A.2014. Seminal vesicle protein SVS2 is required for sperm survival in the uterus. Proc. Natl. Acad. Sci. USA 111: 4145–4150. doi: 10.1073/pnas.1320715111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawano N., Kang W., Yamashita M., Koga Y., Yamazaki T., Hata T., Miyado K., Baba T.2010. Mice lacking two sperm serine proteases, ACR and PRSS21, are subfertile, but the mutant sperm are infertile in vitro. Biol. Reprod. 83: 359–369. doi: 10.1095/biolreprod.109.083089 [DOI] [PubMed] [Google Scholar]
- 47.Kawano N., Yoshida M.2007. Semen-coagulating protein, SVS2, in mouse seminal plasma controls sperm fertility. Biol. Reprod. 76: 353–361. doi: 10.1095/biolreprod.106.056887 [DOI] [PubMed] [Google Scholar]
- 48.Kim E., Baba D., Kimura M., Yamashita M., Kashiwabara S., Baba T.2005. Identification of a hyaluronidase, Hyal5, involved in penetration of mouse sperm through cumulus mass. Proc. Natl. Acad. Sci. USA 102: 18028–18033. doi: 10.1073/pnas.0506825102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim E., Yamashita M., Nakanishi T., Park K.E., Kimura M., Kashiwabara S., Baba T.2006. Mouse sperm lacking ADAM1b/ADAM2 fertilin can fuse with the egg plasma membrane and effect fertilization. J. Biol. Chem. 281: 5634–5639. doi: 10.1074/jbc.M510558200 [DOI] [PubMed] [Google Scholar]
- 50.Kimura M., Kim E., Kang W., Yamashita M., Saigo M., Yamazaki T., Nakanishi T., Kashiwabara S., Baba T.2009. Functional roles of mouse sperm hyaluronidases, HYAL5 and SPAM1, in fertilization. Biol. Reprod. 81: 939–947. doi: 10.1095/biolreprod.109.078816 [DOI] [PubMed] [Google Scholar]
- 51.Kirchhoff C., Hale G.1996. Cell-to-cell transfer of glycosylphosphatidylinositol-anchored membrane proteins during sperm maturation. Mol. Hum. Reprod. 2: 177–184. doi: 10.1093/molehr/2.3.177 [DOI] [PubMed] [Google Scholar]
- 52.Kondoh G., Tojo H., Nakatani Y., Komazawa N., Murata C., Yamagata K., Maeda Y., Kinoshita T., Okabe M., Taguchi R., Takeda J.2005. Angiotensin-converting enzyme is a GPI-anchored protein releasing factor crucial for fertilization. Nat. Med. 11: 160–166. doi: 10.1038/nm1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krege J.H., John S.W., Langenbach L.L., Hodgin J.B., Hagaman J.R., Bachman E.S., Jennette J.C., O’Brien D.A., Smithies O.1995. Male-female differences in fertility and blood pressure in ACE-deficient mice. Nature 375: 146–148. doi: 10.1038/375146a0 [DOI] [PubMed] [Google Scholar]
- 54.Kurita A., Takizawa T., Takayama T., Totsukawa K., Matsubara S., Shibahara H., Orgebin-Crist M.C., Sendo F., Shinkai Y., Araki Y.2001. Identification, cloning, and initial characterization of a novel mouse testicular germ cell-specific antigen. Biol. Reprod. 64: 935–945. doi: 10.1095/biolreprod64.3.935 [DOI] [PubMed] [Google Scholar]
- 55.Kuzan F.B., Fleming A.D., Seidel G.E., Jr1984. Successful fertilization in vitro of fresh intact oocytes by perivitelline (acrosome-reacted) spermatozoa of the rabbit. Fertil. Steril. 41: 766–770. doi: 10.1016/S0015-0282(16)47847-7 [DOI] [PubMed] [Google Scholar]
- 56.La Spina F.A., Puga Molina L.C., Romarowski A., Vitale A.M., Falzone T.L., Krapf D., Hirohashi N., Buffone M.G.2016. Mouse sperm begin to undergo acrosomal exocytosis in the upper isthmus of the oviduct. Dev. Biol. 411: 172–182. doi: 10.1016/j.ydbio.2016.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lefebvre J., Fan J., Chevalier S., Sullivan R., Carmona E., Manjunath P.2007. Genomic structure and tissue-specific expression of human and mouse genes encoding homologues of the major bovine seminal plasma proteins. Mol. Hum. Reprod. 13: 45–53. doi: 10.1093/molehr/gal098 [DOI] [PubMed] [Google Scholar]
- 58.Lin M.H., Lee R.K., Hwu Y.M., Lu C.H., Chu S.L., Chen Y.J., Chang W.C., Li S.H.2008. SPINKL, a Kazal-type serine protease inhibitor-like protein purified from mouse seminal vesicle fluid, is able to inhibit sperm capacitation. Reproduction 136: 559–571. doi: 10.1530/REP-07-0375 [DOI] [PubMed] [Google Scholar]
- 59.Lin Y., Mahan K., Lathrop W.F., Myles D.G., Primakoff P.1994. A hyaluronidase activity of the sperm plasma membrane protein PH-20 enables sperm to penetrate the cumulus cell layer surrounding the egg. J. Cell Biol. 125: 1157–1163. doi: 10.1083/jcb.125.5.1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lishko P.V., Botchkina I.L., Kirichok Y.2011. Progesterone activates the principal Ca2+ channel of human sperm. Nature 471: 387–391. doi: 10.1038/nature09767 [DOI] [PubMed] [Google Scholar]
- 61.Lu C.H., Lee R.K., Hwu Y.M., Chu S.L., Chen Y.J., Chang W.C., Lin S.P., Li S.H.2011. SERPINE2, a serine protease inhibitor extensively expressed in adult male mouse reproductive tissues, may serve as a murine sperm decapacitation factor. Biol. Reprod. 84: 514–525. doi: 10.1095/biolreprod.110.085100 [DOI] [PubMed] [Google Scholar]
- 62.Mashiko D., Fujihara Y., Satouh Y., Miyata H., Isotani A., Ikawa M.2013. Generation of mutant mice by pronuclear injection of circular plasmid expressing Cas9 and single guided RNA. Sci. Rep. 3: 3355. doi: 10.1038/srep03355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mashiko D., Young S.A., Muto M., Kato H., Nozawa K., Ogawa M., Noda T., Kim Y.J., Satouh Y., Fujihara Y., Ikawa M.2014. Feasibility for a large scale mouse mutagenesis by injecting CRISPR/Cas plasmid into zygotes. Dev. Growth Differ. 56: 122–129. doi: 10.1111/dgd.12113 [DOI] [PubMed] [Google Scholar]
- 64.McGraw L.A., Suarez S.S., Wolfner M.F.2015. On a matter of seminal importance. BioEssays 37: 142–147. doi: 10.1002/bies.201400117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miki K., Clapham D.E.2013. Rheotaxis guides mammalian sperm. Curr. Biol. 23: 443–452. doi: 10.1016/j.cub.2013.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miller M.R., Mannowetz N., Iavarone A.T., Safavi R., Gracheva E.O., Smith J.F., Hill R.Z., Bautista D.M., Kirichok Y., Lishko P.V.2016. Unconventional endocannabinoid signaling governs sperm activation via the sex hormone progesterone. Science 352: 555–559. doi: 10.1126/science.aad6887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miyata H., Castaneda J.M., Fujihara Y., Yu Z., Archambeault D.R., Isotani A., Kiyozumi D., Kriseman M.L., Mashiko D., Matsumura T., Matzuk R.M., Mori M., Noda T., Oji A., Okabe M., Prunskaite-Hyyrylainen R., Ramirez-Solis R., Satouh Y., Zhang Q., Ikawa M., Matzuk M.M.2016. Genome engineering uncovers 54 evolutionarily conserved and testis-enriched genes that are not required for male fertility in mice. Proc. Natl. Acad. Sci. USA 113: 7704–7710. doi: 10.1073/pnas.1608458113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miyata H., Satouh Y., Mashiko D., Muto M., Nozawa K., Shiba K., Fujihara Y., Isotani A., Inaba K., Ikawa M.2015. Sperm calcineurin inhibition prevents mouse fertility with implications for male contraceptive. Science 350: 442–445. doi: 10.1126/science.aad0836 [DOI] [PubMed] [Google Scholar]
- 69.Miyata K., Oike Y., Hoshii T., Maekawa H., Ogawa H., Suda T., Araki K., Yamamura K.2005. Increase of smooth muscle cell migration and of intimal hyperplasia in mice lacking the alpha/beta hydrolase domain containing 2 gene. Biochem. Biophys. Res. Commun. 329: 296–304. doi: 10.1016/j.bbrc.2005.01.127 [DOI] [PubMed] [Google Scholar]
- 70.Muro Y., Hasuwa H., Isotani A., Miyata H., Yamagata K., Ikawa M., Yanagimachi R., Okabe M.2016. Behavior of Mouse Spermatozoa in the Female Reproductive Tract from Soon after Mating to the Beginning of Fertilization. Biol. Reprod. 94: 80. doi: 10.1095/biolreprod.115.135368 [DOI] [PubMed] [Google Scholar]
- 71.Nakanishi T., Isotani A., Yamaguchi R., Ikawa M., Baba T., Suarez S.S., Okabe M.2004. Selective passage through the uterotubal junction of sperm from a mixed population produced by chimeras of calmegin-knockout and wild-type male mice. Biol. Reprod. 71: 959–965. doi: 10.1095/biolreprod.104.028647 [DOI] [PubMed] [Google Scholar]
- 72.Nishimura H., Cho C., Branciforte D.R., Myles D.G., Primakoff P.2001. Analysis of loss of adhesive function in sperm lacking cyritestin or fertilin beta. Dev. Biol. 233: 204–213. doi: 10.1006/dbio.2001.0166 [DOI] [PubMed] [Google Scholar]
- 73.Nishimura H., Kim E., Nakanishi T., Baba T.2004. Possible function of the ADAM1a/ADAM2 Fertilin complex in the appearance of ADAM3 on the sperm surface. J. Biol. Chem. 279: 34957–34962. doi: 10.1074/jbc.M314249200 [DOI] [PubMed] [Google Scholar]
- 74.Nixon B., MacIntyre D.A., Mitchell L.A., Gibbs G.M., O’Bryan M., Aitken R.J.2006. The identification of mouse sperm-surface-associated proteins and characterization of their ability to act as decapacitation factors. Biol. Reprod. 74: 275–287. doi: 10.1095/biolreprod.105.044644 [DOI] [PubMed] [Google Scholar]
- 75.Oji A., Noda T., Fujihara Y., Miyata H., Kim Y.J., Muto M., Nozawa K., Matsumura T., Isotani A., Ikawa M.2016. CRISPR/Cas9 mediated genome editing in ES cells and its application for chimeric analysis in mice. Sci. Rep. 6: 31666. doi: 10.1038/srep31666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Okabe M.2013. The cell biology of mammalian fertilization. Development 140: 4471–4479. doi: 10.1242/dev.090613 [DOI] [PubMed] [Google Scholar]
- 77.Okabe M.2015. Mechanisms of fertilization elucidated by gene-manipulated animals. Asian J. Androl. 17: 646–652. doi: 10.4103/1008-682X.153299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peitz B., Olds-Clarke P.1986. Effects of seminal vesicle removal on fertility and uterine sperm motility in the house mouse. Biol. Reprod. 35: 608–617. doi: 10.1095/biolreprod35.3.608 [DOI] [PubMed] [Google Scholar]
- 79.Persson S., Rosenquist M., Knoblach B., Khosravi-Far R., Sommarin M., Michalak M.2005. Diversity of the protein disulfide isomerase family: identification of breast tumor induced Hag2 and Hag3 as novel members of the protein family. Mol. Phylogenet. Evol. 36: 734–740. doi: 10.1016/j.ympev.2005.04.002 [DOI] [PubMed] [Google Scholar]
- 80.Phelps B.M., Primakoff P., Koppel D.E., Low M.G., Myles D.G.1988. Restricted lateral diffusion of PH-20, a PI-anchored sperm membrane protein. Science 240: 1780–1782. doi: 10.1126/science.3381102 [DOI] [PubMed] [Google Scholar]
- 81.Primakoff P., Lathrop W., Woolman L., Cowan A., Myles D.1988. Fully effective contraception in male and female guinea pigs immunized with the sperm protein PH-20. Nature 335: 543–546. doi: 10.1038/335543a0 [DOI] [PubMed] [Google Scholar]
- 82.Publicover S., Harper C.V., Barratt C.2007. [Ca2+]i signalling in sperm--making the most of what you’ve got. Nat. Cell Biol. 9: 235–242. doi: 10.1038/ncb0307-235 [DOI] [PubMed] [Google Scholar]
- 83.Qin X., Krumrei N., Grubissich L., Dobarro M., Aktas H., Perez G., Halperin J.A.2003. Deficiency of the mouse complement regulatory protein mCd59b results in spontaneous hemolytic anemia with platelet activation and progressive male infertility. Immunity 18: 217–227. doi: 10.1016/S1074-7613(03)00022-0 [DOI] [PubMed] [Google Scholar]
- 84.Ren D., Navarro B., Perez G., Jackson A.C., Hsu S., Shi Q., Tilly J.L., Clapham D.E.2001. A sperm ion channel required for sperm motility and male fertility. Nature 413: 603–609. doi: 10.1038/35098027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ren D., Xia J.2010. Calcium signaling through CatSper channels in mammalian fertilization. Physiology (Bethesda) 25: 165–175. [DOI] [PubMed] [Google Scholar]
- 86.Seals D.F., Courtneidge S.A.2003. The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev. 17: 7–30. doi: 10.1101/gad.1039703 [DOI] [PubMed] [Google Scholar]
- 87.Shamsadin R., Adham I.M., Nayernia K., Heinlein U.A., Oberwinkler H., Engel W.1999. Male mice deficient for germ-cell cyritestin are infertile. Biol. Reprod. 61: 1445–1451. doi: 10.1095/biolreprod61.6.1445 [DOI] [PubMed] [Google Scholar]
- 88.Strünker T., Goodwin N., Brenker C., Kashikar N.D., Weyand I., Seifert R., Kaupp U.B.2011. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature 471: 382–386. doi: 10.1038/nature09769 [DOI] [PubMed] [Google Scholar]
- 89.Sugimoto Y., Inazumi T., Tsuchiya S.2015. Roles of prostaglandin receptors in female reproduction. J. Biochem. 157: 73–80. doi: 10.1093/jb/mvu081 [DOI] [PubMed] [Google Scholar]
- 90.Sun F., Bahat A., Gakamsky A., Girsh E., Katz N., Giojalas L.C., Tur-Kaspa I., Eisenbach M.2005. Human sperm chemotaxis: both the oocyte and its surrounding cumulus cells secrete sperm chemoattractants. Hum. Reprod. 20: 761–767. doi: 10.1093/humrep/deh657 [DOI] [PubMed] [Google Scholar]
- 91.Sun X., Funk C.D., Deng C., Sahu A., Lambris J.D., Song W.C.1999. Role of decay-accelerating factor in regulating complement activation on the erythrocyte surface as revealed by gene targeting. Proc. Natl. Acad. Sci. USA 96: 628–633. doi: 10.1073/pnas.96.2.628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Takeo T., Nakagata N.2011. Reduced glutathione enhances fertility of frozen/thawed C57BL/6 mouse sperm after exposure to methyl-beta-cyclodextrin. Biol. Reprod. 85: 1066–1072. doi: 10.1095/biolreprod.111.092536 [DOI] [PubMed] [Google Scholar]
- 93.Tamba S., Yodoi R., Morimoto K., Inazumi T., Sukeno M., Segi-Nishida E., Okuno Y., Tsujimoto G., Narumiya S., Sugimoto Y.2010. Expression profiling of cumulus cells reveals functional changes during ovulation and central roles of prostaglandin EP2 receptor in cAMP signaling. Biochimie 92: 665–675. doi: 10.1016/j.biochi.2010.04.006 [DOI] [PubMed] [Google Scholar]
- 94.Tamba S., Yodoi R., Segi-Nishida E., Ichikawa A., Narumiya S., Sugimoto Y.2008. Timely interaction between prostaglandin and chemokine signaling is a prerequisite for successful fertilization. Proc. Natl. Acad. Sci. USA 105: 14539–14544. doi: 10.1073/pnas.0805699105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tokuhiro K., Ikawa M., Benham A.M., Okabe M.2012. Protein disulfide isomerase homolog PDILT is required for quality control of sperm membrane protein ADAM3 and male fertility [corrected]. Proc. Natl. Acad. Sci. USA 109: 3850–3855. doi: 10.1073/pnas.1117963109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ueda Y., Yamaguchi R., Ikawa M., Okabe M., Morii E., Maeda Y., Kinoshita T.2007. PGAP1 knock-out mice show otocephaly and male infertility. J. Biol. Chem. 282: 30373–30380. doi: 10.1074/jbc.M705601200 [DOI] [PubMed] [Google Scholar]
- 97.van Lith M., Hartigan N., Hatch J., Benham A.M.2005. PDILT, a divergent testis-specific protein disulfide isomerase with a non-classical SXXC motif that engages in disulfide-dependent interactions in the endoplasmic reticulum. J. Biol. Chem. 280: 1376–1383. doi: 10.1074/jbc.M408651200 [DOI] [PubMed] [Google Scholar]
- 98.Wakayama T., Ogura A., Suto J., Matsubara Y., Kurohmaru M., Hayashi Y., Yanagimachi R.1996. Penetration by field vole spermatozoa of mouse and hamster zonae pellucidae without acrosome reaction. J. Reprod. Fertil. 107: 97–102. doi: 10.1530/jrf.0.1070097 [DOI] [PubMed] [Google Scholar]
- 99.Wang H., La Russa M., Qi L.S.2016. CRISPR/Cas9 in Genome Editing and Beyond. Annu. Rev. Biochem. 85: 227–264. doi: 10.1146/annurev-biochem-060815-014607 [DOI] [PubMed] [Google Scholar]
- 100.Wang H., Yang H., Shivalila C.S., Dawlaty M.M., Cheng A.W., Zhang F., Jaenisch R.2013. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153: 910–918. doi: 10.1016/j.cell.2013.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yamaguchi R., Fujihara Y., Ikawa M., Okabe M.2012. Mice expressing aberrant sperm-specific protein PMIS2 produce normal-looking but fertilization-incompetent spermatozoa. Mol. Biol. Cell 23: 2671–2679. doi: 10.1091/mbc.E11-12-1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yamaguchi R., Muro Y., Isotani A., Tokuhiro K., Takumi K., Adham I., Ikawa M., Okabe M.2009. Disruption of ADAM3 impairs the migration of sperm into oviduct in mouse. Biol. Reprod. 81: 142–146. doi: 10.1095/biolreprod.108.074021 [DOI] [PubMed] [Google Scholar]
- 103.Yamaguchi R., Yamagata K., Hasuwa H., Inano E., Ikawa M., Okabe M.2008. Cd52, known as a major maturation-associated sperm membrane antigen secreted from the epididymis, is not required for fertilization in the mouse. Genes Cells 13: 851–861. doi: 10.1111/j.1365-2443.2008.01210.x [DOI] [PubMed] [Google Scholar]
- 104.Yamaguchi R., Yamagata K., Ikawa M., Moss S.B., Okabe M.2006. Aberrant distribution of ADAM3 in sperm from both angiotensin-converting enzyme (Ace)- and calmegin (Clgn)-deficient mice. Biol. Reprod. 75: 760–766. doi: 10.1095/biolreprod.106.052977 [DOI] [PubMed] [Google Scholar]
- 105.Yamashita M., Honda A., Ogura A., Kashiwabara S., Fukami K., Baba T.2008. Reduced fertility of mouse epididymal sperm lacking Prss21/Tesp5 is rescued by sperm exposure to uterine microenvironment. Genes Cells 13: 1001–1013. doi: 10.1111/j.1365-2443.2008.01222.x [DOI] [PubMed] [Google Scholar]
- 106.Yanagimachi R., Chang M.C.1963. Fertilization of Hamster Eggs in Vitro. Nature 200: 281–282. doi: 10.1038/200281b0 [DOI] [PubMed] [Google Scholar]
- 107.Yoshitake H., Tsukamoto H., Maruyama-Fukushima M., Takamori K., Ogawa H., Araki Y.2008. TEX101, a germ cell-marker glycoprotein, is associated with lymphocyte antigen 6 complex locus k within the mouse testis. Biochem. Biophys. Res. Commun. 372: 277–282. doi: 10.1016/j.bbrc.2008.05.088 [DOI] [PubMed] [Google Scholar]
- 108.Young S.A., Miyata H., Satouh Y., Kato H., Nozawa K., Isotani A., Aitken R.J., Baker M.A., Ikawa M.2015. CRISPR/Cas9-Mediated Rapid Generation of Multiple Mouse Lines Identified Ccdc63 as Essential for Spermiogenesis. Int. J. Mol. Sci. 16: 24732–24750. doi: 10.3390/ijms161024732 [DOI] [PMC free article] [PubMed] [Google Scholar]