Abstract
Pigs with X-linked severe combined immunodeficiency (X-SCID) caused by a mutation of the interleukin-2 receptor gamma chain gene (IL2RG) are of value for a wide range of studies. However, they do not survive longer than 8 weeks because of their susceptibility to infections. To allow longer survival of X-SCID pigs, the animals must be born and reared under germ-free conditions. Here, we established an efficient system for piglet derivation by hysterectomy and used it to obtain and maintain a germ-free X-SCID pig. In four trials using pregnant wild-type pigs, 66% of piglets after hysterectomy started spontaneous breathing (range of 20–100% per litter). The resuscitation rate was found to negatively correlate with elapsed time from the uterus excision to piglet derivation (r=−0.97, P<0.05). Therefore, it is critical to deliver piglets within 5 min to achieve a high resuscitation rate (82% estimated from regression analysis). In a fifth trial with an IL2RG+/− pig, four piglets were delivered within 4.2 min of uterus excision and three were alive (75%). One of the live born piglets was genotypically and phenotypically determined to be X-SCID and was reared for 12 weeks. The X-SCID piglet was free from both bacteria and fungi at all time points tested by microbial culture and grew without any abnormal signs or symptoms. This study showed successful production and rearing of germ-free pigs, enabling experiments involving long-term follow-up of X-SCID pigs.
Keywords: germ-free pig, gnotobiote, interleukin-2 receptor gamma chain gene (IL2RG) knock-out, X-linked severe combined immunodeficiency (X-SCID)
Introduction
Immunodeficient mice are widely used for generating xenograft disease models [5, 9] and assessing cell pluripotency [4, 12, 22]. The possibility of using immunodeficient pigs for similar purposes is attractive as it offers the advantages that pigs more closely resemble humans in terms of size, longevity and other physiological parameters [10, 20]. Recently, we generated X-linked severe combined immunodeficiency (X-SCID) pigs by disrupting the interleukin-2 receptor gamma chain gene (IL2RG) using genome editing and somatic cell nuclear transfer technologies [23]. T and NK cells are absent in X-SCID pigs although B cells are still present. This phenotypic profile is more similar to that of human X-SCID patients than X-SCID mice not carrying B cells [1, 2, 16]. Due to the immunodeficiency, X-SCID (IL2RG−/− or IL2RG−/Y) pigs all died by 54 days of age as reported previously [21]. This early death hinders availability of X-SCID pigs and spoils the chance of leaving offspring. Therefore, a robust system for derivation and rearing of germ-free pigs is needed to generate germ-free X-SCID pigs efficiently and to extend their survival.
The production of germ-free pigs was first reported in 1964 [13] and in subsequent studies [8, 14, 19]. Piglets were obtained from pregnant pigs by hysterotomy and/or hysterectomy. Hysterectomy appears to be better than hysterotomy because the former has achieved the shorter surgical time (30 min versus 4 h) and the higher germfree rate (91% versus 77%) [14]. The resuscitation rate of piglets delivered by hysterectomy, however, shows a wide range among studies [8, 14].
In this study, we established an efficient system for piglet derivation using hysterectomy of wild-type pigs to optimize the conditions for delivery of piglets. Then, we generated a germ-free X-SCID pig from a heterozygous (IL2RG+/−) pig and maintained one germ-free piglet for 12 weeks under strict microbial control. Based on the size of the isolator in which the pigs were reared, 12 weeks was the longest available period of rearing in this study.
Materials and Methods
Surgical derivation of piglets
The experimental protocols were approved by the animal experiment committee of Jichi Medical University and by the Institutional Animal Care and Use Committee of Meiji University. To generate wild-type piglets, female domestic pigs (Landrace x Large White) were mated with male domestic pigs (Duroc), and a female miniature pig (White miniature F1) was mated with a male miniature pig (Chinese miniature). To generate X-SCID pigs, a female IL2RG+/− pig, which was generated using sperm derived from a chimera between X-SCID and wild-type pigs (unpublished data) [23], was mated with a male domestic pig (Duroc). At 112 days of gestation (full term, 114 days), pregnant pigs were anesthetized either with 1–5% isoflurane (Pig ID, P1-3 and P5) or with sevoflurane (P4), depending on the anesthesia apparatuses equipped in the facilities where the piglets were delivered. Because our preliminary experiments have shown that additional treatment with atropine sulfate, medetomidine chloride, and midazolam prior to the anesthesia decreased the resuscitation rate of delivered piglets, the preanesthetic drugs were not administered in this study. The uterus was excised from the anesthetized pig and was transferred into a plastic isolator (Ishizawa Corporation of Medical Implement, Ibaraki, Japan) (Figs. 1A and B) through a tank containing 100 L of 0.1% peracetic acid (Animal Biosecurity Consulting Inc., Chiba, Japan) for decontamination of the uterus. This isolator was sterilized by fumigating with 1% peracetic acid in advance. The piglets were then taken from the uterus in the isolator. Resuscitation rates were calculated by dividing the number of piglets that started spontaneous breathing by those that were delivered. Fetuses in which hearts had already stopped beating at delivery were defined as stillborn and they were excluded from the calculation of the resuscitation rate. Elapsed time from the excision of uterus cervix (uterus excision) to the completion of piglet derivation was measured. Some of the live piglets were transferred to rearing isolators (Ishizawa Corporation of Medical Implement) (Fig. 1C) that had been sterilized by spraying with 1% peracetic acid in advance. They were fed with milk (Nosan Corp., Kanagawa, Japan) and water, which had been sterilized by gamma irradiation (50 kGy/box) and autoclaving, respectively. When the milk or water in the rearing isolators was consumed, a replacement container was provided through the transfer port, in which containers for milk or water were sterilized by spraying with 1% peracetic acid (Fig. 1C).
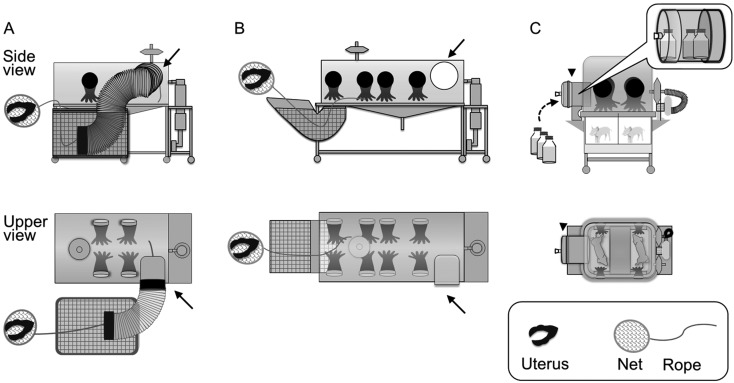
Fig. 1.
Side and upper views of isolators. (A) The original isolator for piglet derivation and a peracetic acid tank (indicated by mesh pattern) for sterilizing the uterus. The excised uterus was put inside a net, which was bound with a rope linking to the inside of the isolator. The net containing the excised uterus was brought into the isolator through a peracetic acid tank by pulling the rope. Arrows indicate connectors to rearing isolators. Two pairs of gloves are equipped. (B) An improved isolator for piglet derivation. A peracetic acid tank is directly attached to the isolator, which has four pairs of gloves. (C) A rearing isolator. One isolator can maintain a maximum of two piglets using a partition. Arrowheads indicate the transfer port through which containers for milk and water are sterilized before entry into the rearing isolators. The transfer port also serves as a connector to isolators for piglet derivation.
Microbial monitoring
The germ-free status of piglets was evaluated by microbial culture of samples at least every other week as described previously [11]. Specifically, samples were obtained from skin, oral mucosa, and stool swabs and inoculated into four different media: heart infusion broth (Becton Dickinson, BD, NJ, USA) for aerobic bacteria; cooked meat medium (BD) for anaerobic bacteria; thioglycollate medium (BD) for anaerobic bacteria; and potato dextrose broth (BD) for fungi. After 2 weeks of culture, microbial growth was assessed by visual checks of the media.
Identification of X-SCID pigs
Piglets delivered from the IL2RG+/− pig were subjected to immediate analyses to determine whether they were wildtype, heterozygotes, or X-SCID by PCR and flow cytometry of the umbilical cord blood cells as described previously [23]. Briefly, genomic DNA was extracted from the cord blood with a commercially available kit (Qiagen, Venlo, Netherlands). Then, the target region of IL2RG was amplified by PCR using Ex Taq (Takara Bio, Shiga, Japan) and the primer set 5′-ATAGTGGTGTCAGTGTGATTGAGC-3′ and 5′-TACGAACTGACTTATGACTTACC-3′. The amplified products were separated by agarose gel electrophoresis. For flow cytometric analysis, the cord blood was treated with erythrocyte-lysis buffer (155 mM NH4Cl, 100 mM KHCO3, and 1 mM EDTA) for 5 min at 37°C. Then, 1–10 × 105 cells were incubated with mouse anti-pig CD3e (Abcam, Cambridge, UK), CD16 (Bio-Rad, CA, USA), CD45RA (Bio-Rad), and monocyte and granulocyte (M/G) (Abcam) antibodies for 30 min at room temperature. The stained cells were analyzed using an SH800 flow cytometer (Sony, Tokyo, Japan).
Statistical analysis
The correlation of the resuscitation rate of piglets and the elapsed time from uterus excision to piglet derivation was analyzed by Pearson’s test using R software [18]. A value of P<0.05 was considered as statistically significant.
Results
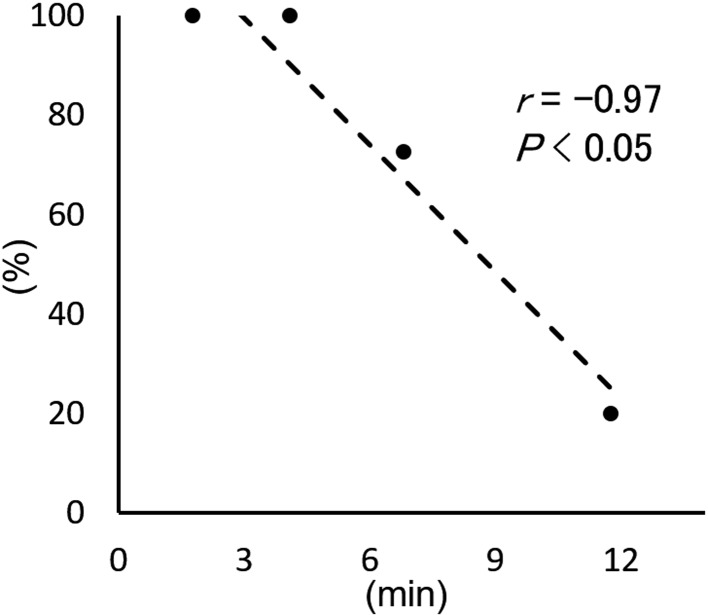
To determine the resuscitation rate of piglets by hysterectomy, pregnant wild-type pigs (domestic and miniature) were used. In the first trial (Pig ID, P1) with an original isolator (Fig. 1A), it took 11.8 min to deliver 10 piglets and the resuscitation rate of piglets was only 20% (Table 1). In the subsequent trials with the improved isolator (Fig. 1B), the derivation time became shortened (1.8–6.8 min) and the resuscitation rates became significantly increased (73–100%). We found that the resuscitation rates were negatively correlated with the elapsed time from uterus excision to completion of piglet derivation (r=−0.97, P<0.05) (Table 1 and Fig. 2). Therefore, shortening the derivation time is critically important for increasing the resuscitation rate. From the regression line in Fig. 2, an estimated resuscitation rate of 82% was obtained if the piglets were delivered within 5 min. On the other hand, the elapsed time from the beginning of anesthesia to the uterus excision (16–28 min) did not affect the resuscitation rates.
Table 1. Derivation of piglets by hysterectomy.
| Pig | No. (%) of piglets | Elapsed time to piglet derivation (min) | ||
|---|---|---|---|---|
| Genotype | ID | Delivered | Resuscitated | |
| Wild-type | P1 | 10 | 2 (20) | 11.8 |
| P2 | 11 | 8 (73) | 6.8 | |
| P3 | 8 | 8 (100) | 4.1 | |
| P4 | 3 | 3 (100) | 1.8 | |
| Overall | 32 | 21 (66) | 6.1 ± 3.7a | |
| IL2RG+/– | P5 | 4b | 3 (75) | 4.2 |
aMean ± SD. bFour stillborn piglets were also found in the uterus.
Fig. 2.
Scatter plots of the resuscitation rate versus elapsed time from the uterus excision to completion of derivation using wild-type pigs. The dashed line represents regression line. The rate was negatively correlated with the derivation time. Thus, we recommend the completion of piglet derivation within 5 min after the uterus excision.
Randomly selected live piglets were transferred into the rearing isolators and were maintained germfree. Microbial monitoring immediately after transfer into the isolators revealed that all five piglets were free from both bacteria and fungi. In the first trial of germ-free rearing, coagulase-negative staphylococci were detected from all swab samples at 2 weeks of rearing (Table 2). The bacteria were identified as Staphylococcus epidermidis using a commercially available kit for gram-positive bacteria (BBL Crystal GP ID kit, BD). To prevent microbial contamination in following trials, containers for milk and water, which were placed into the rearing isolator, were preliminarily decontaminated with 0.1% peracetic acid before sterilization with 1% peracetic acid in the transfer port (Fig. 1C). After the introduction of this decontamination procedure, in the next trial, four piglets were maintained germ-free for the scheduled 4 weeks (Table 2).
Table 2. Microbial monitoring of germ-free piglets.
| Litter ID | Genotype | Sex | Weeks old | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 4 | 6 to 12a | |||
| P1-1 | Wild-type | Female | – | – | +b | +b | Euthanized |
| P2-1 | Wild-type | Male | – | NT | – | – | Euthanized |
| P2-2 | Wild-type | Male | – | NT | – | – | Euthanized |
| P3-1 | Wild-type | Male | – | NT | – | – | Euthanized |
| P3-2 | Wild-type | Male | – | NT | – | – | Euthanized |
| P5-3 | IL2RG−/Y | Male | – | NT | – | – | – |
| Rate germ-free | 6/6 (100%) | 1/1 (100%) | 5/6 (83%) | 5/6 (83%) | 1/1 (100%) | ||
aMicrobial monitoring was performed every other week. bStaphylococcus epidermidis was detected. NT: Not tested.
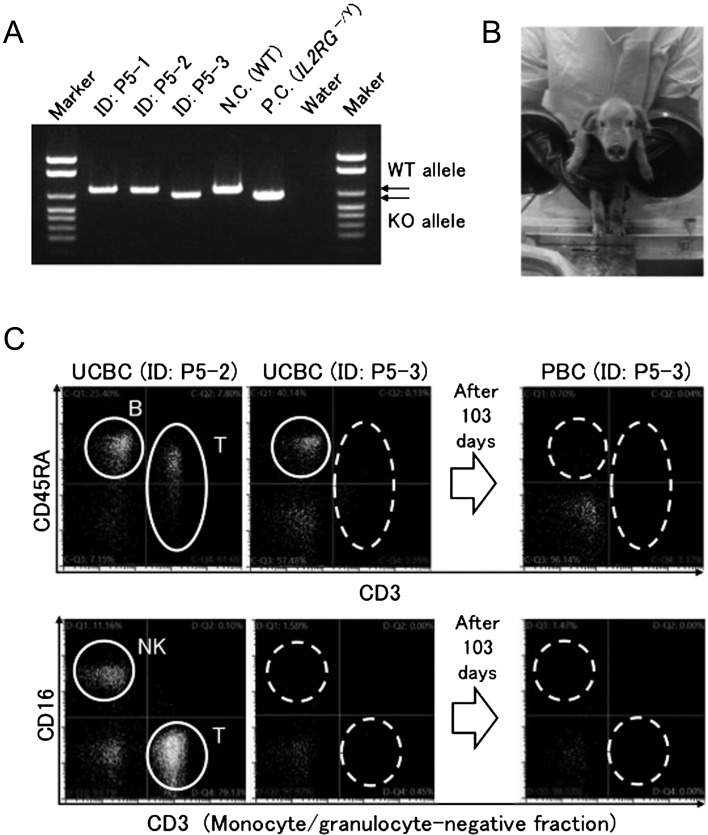
As wild type piglets were successfully delivered and maintained germ-free for 4 weeks, we then tried to generate germ-free X-SCID pigs. We carried out hysterectomy on a pregnant IL2RG+/− pig and successfully delivered three live male piglets (75%) within 4.2 min of uterus excision, although one female was dead (Table 1). Additionally, four stillborn piglets were also found in the uterus. One of the three live male piglets (ID, P5-3) was found to carry the IL2RG knock-out allele (IL2RG−/Y) (Figs. 3A and B). Flow cytometric analysis of the cord blood of this piglet showed that the animal lacked T and NK cells, although B cells were present (Fig. 3C). The profiles were phenotypically similar to those of human X-SCID. The proportion of X-SCID pigs (1/4) was as expected from Mendelian segregation. This X-SCID pig was transferred to the rearing isolator and was maintained germ-free for a scheduled 12 weeks; the germ-free status of the piglet was confirmed negative by microbial cultures every other week and the absence of abnormal signs or symptoms in the piglet (Table 2). As a result of the growth of the piglet, 12 weeks was the longest possible period for rearing in the isolator. At the time of euthanasia, B cells were no longer detectable in the peripheral blood of the animal (Fig. 3C).
Fig. 3.
Genotypic and phenotypic identification of an X-SCID piglet. (A) Genotyping of three live male piglets from a pregnant IL2RG+/− pig assessed by PCR targeting IL2RG. N.C., negative control. P.C., positive control. (B) A germ-free X-SCID piglet held by the hands of an operator, wearing gloves equipped to the rearing isolator. (C) Flow cytometric analysis of T, B and NK cells in the umbilical cord blood cells (UCBC) and the peripheral blood cells (PBC) from the piglets (P5-2 and P5-3). P5-3 was identified as X-SCID.
Discussion
X-SCID pigs are attractive for research use because they do not induce T cell- and NK cell-mediated immune responses against allogeneic or xenogeneic stimuli, for instance human cells or derivatives. However, as reported by Suzuki et al. [21], X-SCID pigs do not survive beyond 8 weeks of birth if they do not receive bone marrow transplantation from wild-type pigs to reconstruct their immune system. In this study, we maintained a germ-free X-SCID pig for a scheduled 12 weeks without any symptoms or signs of illness despite the fact that the animal lacked T and NK cells. These results indicate that germ-free X-SCID pigs produced and reared in this manner will enable us to monitor pigs for the requisite time for most chronic experiments. Although the rearing period of 12 weeks was determined by the limitation of piglet body size within the isolator, it can be extended either by increasing the isolator size or by miniaturization of X-SCID pigs.
When the X-SCID piglet was euthanized after the completion of the scheduled rearing period, B cells were absent in addition to T and NK cells, although B cells had been detected in the cord blood at birth (Fig. 3). The loss of B cells in the blood cell populations might be a natural progression in IL2RG knock-out pigs. In human subjects, B cells remain at least several years after birth [7]. On the other hand, B cells are absent from birth in X-SCID mice. The reason for the difference in B cell behavior in X-SCID species is unclear. In any case, the B cells present in X-SCID animals are not functional [6].
To establish a robust system for the efficient derivation of germ-free piglets, we performed hysterectomy on four pregnant wild-type pigs. As a result, we found that the resuscitation rate of piglets was negatively correlated with the elapsed time from uterus excision to derivation of the piglet (Fig. 2). This clearly indicates that rapid derivation is a key factor in achieving a high resuscitation rate. In our system, the plastic isolator used for piglet derivation was improved to facilitate the process of decontaminating the uterus and transferring it into the isolator (Figs. 1A and B). In addition, the equipment gloves, which limit the number of staff who could participate in the derivation and recovery of piglets, were doubled in number and thus four members of staff could participate in the procedure at the same time. The improved isolator allowed us to deliver piglets from a uterus within 5 min. The resuscitation rate of piglets delivered within 5 min was 93% (14/15) in this study, which was higher than that reported in a previous report (72%, 36/50) [8] and comparable to that of another report (90%, 1,267/1,410) [14]. Therefore, a maximum time of 5 min for piglet derivation can be set as a criterion for successful germ-free delivery.
The resuscitation rates were not affected by the methods of anesthesia either with isoflurane (Pig ID, P1-3 and P5) or with sevoflurane (P4) in this study (Table 1 and Fig. 2). In fact, a previous study showed that the methods of anesthesia did not affect physiological parameters including the uterine blood flow, fetal heart rates, blood pressure, and blood gases in sheep [17]. Germ-free piglets generated in this study could be used as a source of gnotobiotic pigs for the study of gut microbiota [3, 15]. The system for producing germ-free pigs can also be applied for generating clinical-grade xenografts or human tissues from pluripotent stem cells in pigs in vivo, such as pancreatic islets [24].
In conclusion, we successfully and efficiently generated germ-free wild-type and X-SCID pigs, and maintained an X-SCID pig for 12 weeks under germ-free conditions. This work will pave the way for the use of germ-free and X-SCID pigs.
Conflict of Interest
H.N. is a co-founder of PorMedTec Inc.
Acknowledgments
We wish to thank Shota Kono, Suvd Byambaa, and Nawin Chanthra (Jichi Medical University) for their help. This work was supported by grants from the Japan Agency for Medical Research and Development (AMED) for the Basic Science and Platform Technology Program for Innovative Biological Medicine, from AMED for Practical Application of Regenerative Medicine (formerly KAKENHI from the Ministry of Health, Labour and Welfare of Japan), and from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) for Supported Program for the Strategic Research Foundation at Private Universities.
References
- 1.Buckley R.H.2004. Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution. Annu. Rev. Immunol. 22: 625–655. doi: 10.1146/annurev.immunol.22.012703.104614 [DOI] [PubMed] [Google Scholar]
- 2.Cao X., Shores E.W., Hu-Li J., Anver M.R., Kelsall B.L., Russell S.M., Drago J., Noguchi M., Grinberg A., Bloom E.T., Paul W.E., Katz S.I., Love P.E., Leonard W.J.1995. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity 2: 223–238. doi: 10.1016/1074-7613(95)90047-0 [DOI] [PubMed] [Google Scholar]
- 3.Charbonneau M.R., O’Donnell D., Blanton L.V., Totten S.M., Davis J.C., Barratt M.J., Cheng J., Guruge J., Talcott M., Bain J.R., Muehlbauer M.J., Ilkayeva O., Wu C., Struckmeyer T., Barile D., Mangani C., Jorgensen J., Fan Y.M., Maleta K., Dewey K.G., Ashorn P., Newgard C.B., Lebrilla C., Mills D.A., Gordon J.I.2016. Sialylated milk oligosaccharides promote microbiota-dependent growth in models of infant undernutrition. Cell 164: 859–871. doi: 10.1016/j.cell.2016.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujishiro S.H., Nakano K., Mizukami Y., Azami T., Arai Y., Matsunari H., Ishino R., Nishimura T., Watanabe M., Abe T., Furukawa Y., Umeyama K., Yamanaka S., Ema M., Nagashima H., Hanazono Y.2013. Generation of naive-like porcine-induced pluripotent stem cells capable of contributing to embryonic and fetal development. Stem Cells Dev. 22: 473–482. doi: 10.1089/scd.2012.0173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giovanella B.C., Stehlin J.S., Wall M.E., Wani M.C., Nicholas A.W., Liu L.F., Silber R., Potmesil M.1989. DNA topoisomerase I--targeted chemotherapy of human colon cancer in xenografts. Science 246: 1046–1048. doi: 10.1126/science.2555920 [DOI] [PubMed] [Google Scholar]
- 6.Hacein-Bey-Abina S., Le Deist F., Carlier F., Bouneaud C., Hue C., De Villartay J.P., Thrasher A.J., Wulffraat N., Sorensen R., Dupuis-Girod S., Fischer A., Davies E.G., Kuis W., Leiva L., Cavazzana-Calvo M.2002. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N. Engl. J. Med. 346: 1185–1193. doi: 10.1056/NEJMoa012616 [DOI] [PubMed] [Google Scholar]
- 7.Haddad E., Le Deist F., Aucouturier P., Cavazzana-Calvo M., Blanche S., De Saint Basile G., Fischer A.1999. Long-term chimerism and B-cell function after bone marrow transplantation in patients with severe combined immunodeficiency with B cells: A single-center study of 22 patients. Blood 94: 2923–2930. [PubMed] [Google Scholar]
- 8.Hwang J.H., Gupta M.K., Park C.K., Kim Y.B., Lee H.T.2015. Establishment of major histocompatibility complex homozygous gnotobiotic miniature swine colony for xenotransplantation. Anim. Sci. J. 86: 468–475. doi: 10.1111/asj.12312 [DOI] [PubMed] [Google Scholar]
- 9.Karpinski J., Hauber I., Chemnitz J., Schäfer C., Paszkowski-Rogacz M., Chakraborty D., Beschorner N., Hofmann-Sieber H., Lange U.C., Grundhoff A., Hackmann K., Schrock E., Abi-Ghanem J., Pisabarro M.T., Surendranath V., Schambach A., Lindner C., van Lunzen J., Hauber J., Buchholz F.2016. Directed evolution of a recombinase that excises the provirus of most HIV-1 primary isolates with high specificity. Nat. Biotechnol. 34: 401–409. doi: 10.1038/nbt.3467 [DOI] [PubMed] [Google Scholar]
- 10.Lei S., Ryu J., Wen K., Twitchell E., Bui T., Ramesh A., Weiss M., Li G., Samuel H., Clark-Deener S., Jiang X., Lee K., Yuan L.2016. Increased and prolonged human norovirus infection in RAG2/IL2RG deficient gnotobiotic pigs with severe combined immunodeficiency. Sci. Rep. 6: 25222. doi: 10.1038/srep25222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maejima K., Nomura T.1975. [An experience of application of sterility test of germfree mice and rats recommended by JEARA. (AUTHOR’S TRANSL)]. Jikken Dobutsu 24: 177–181.(in Japanese) [PubMed] [Google Scholar]
- 12.Mandai M., Watanabe A., Kurimoto Y., Hirami Y., Morinaga C., Daimon T., Fujihara M., Akimaru H., Sakai N., Shibata Y., Terada M., Nomiya Y., Tanishima S., Nakamura M., Kamao H., Sugita S., Onishi A., Ito T., Fujita K., Kawamata S., Go M.J., Shinohara C., Hata K.I., Sawada M., Yamamoto M., Ohta S., Ohara Y., Yoshida K., Kuwahara J., Kitano Y., Amano N., Umekage M., Kitaoka F., Tanaka A., Okada C., Takasu N., Ogawa S., Yamanaka S., Takahashi M.2017. Autologous induced stem-cell-derived retinal cells for macular degeneration. N. Engl. J. Med. 376: 1038–1046. doi: 10.1056/NEJMoa1608368 [DOI] [PubMed] [Google Scholar]
- 13.Meyer R.C., Bohl E.H., Kohler E.M.1964. Procurement and maintenance of germ-free swine for microbiological investigations. Appl. Microbiol. 12: 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miniats O.P., Jol D.1978. Gnotobiotic pigs-derivation and rearing. Can. J. Comp. Med. 42: 428–437. [PMC free article] [PubMed] [Google Scholar]
- 15.Narushima S., Itoh K., Mitsuoka T., Nakayama H., Itoh T., Hioki K., Nomura T.1998. Effect of mouse intestinal bacteria on incidence of colorectal tumors induced by 1,2-dimethylhydrazine injection in gnotobiotic transgenic mice harboring human prototype c-Ha-ras genes. Exp. Anim. 47: 111–117. doi: 10.1538/expanim.47.111 [DOI] [PubMed] [Google Scholar]
- 16.Noguchi M., Yi H., Rosenblatt H.M., Filipovich A.H., Adelstein S., Modi W.S., McBride O.W., Leonard W.J.1993. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell 73: 147–157. doi: 10.1016/0092-8674(93)90167-O [DOI] [PubMed] [Google Scholar]
- 17.Okutomi T., Whittington R.A., Stein D.J., Morishima H.O.2009. Comparison of the effects of sevoflurane and isoflurane anesthesia on the maternal-fetal unit in sheep. J. Anesth. 23: 392–398. doi: 10.1007/s00540-009-0763-2 [DOI] [PubMed] [Google Scholar]
- 18.R Development Core Team. 2011. R: a language and environment for statistical computing. http://www.R-project.org/.
- 19.Ratcliffe B., Fordham J.P.1987. A technique for rearing germfree piglets obtained without surgery. Lab. Anim. 21: 53–59. doi: 10.1258/002367787780740680 [DOI] [PubMed] [Google Scholar]
- 20.Suzuki S., Iwamoto M., Hashimoto M., Suzuki M., Nakai M., Fuchimoto D., Sembon S., Eguchi-Ogawa T., Uenishi H., Onishi A.2016. Generation and characterization of RAG2 knockout pigs as animal model for severe combined immunodeficiency. Vet. Immunol. Immunopathol. 178: 37–49. doi: 10.1016/j.vetimm.2016.06.011 [DOI] [PubMed] [Google Scholar]
- 21.Suzuki S., Iwamoto M., Saito Y., Fuchimoto D., Sembon S., Suzuki M., Mikawa S., Hashimoto M., Aoki Y., Najima Y., Takagi S., Suzuki N., Suzuki E., Kubo M., Mimuro J., Kashiwakura Y., Madoiwa S., Sakata Y., Perry A.C., Ishikawa F., Onishi A.2012. Il2rg gene-targeted severe combined immunodeficiency pigs. Cell Stem Cell 10: 753–758. doi: 10.1016/j.stem.2012.04.021 [DOI] [PubMed] [Google Scholar]
- 22.Takahashi K., Yamanaka S.2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676. doi: 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- 23.Watanabe M., Nakano K., Matsunari H., Matsuda T., Maehara M., Kanai T., Kobayashi M., Matsumura Y., Sakai R., Kuramoto M., Hayashida G., Asano Y., Takayanagi S., Arai Y., Umeyama K., Nagaya M., Hanazono Y., Nagashima H.2013. Generation of interleukin-2 receptor gamma gene knockout pigs from somatic cells genetically modified by zinc finger nuclease-encoding mRNA. PLoS One 8: e76478. doi: 10.1371/journal.pone.0076478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamaguchi T., Sato H., Kato-Itoh M., Goto T., Hara H., Sanbo M., Mizuno N., Kobayashi T., Yanagida A., Umino A., Ota Y., Hamanaka S., Masaki H., Rashid S.T., Hirabayashi M., Nakauchi H.2017. Interspecies organogenesis generates autologous functional islets. Nature 542: 191–196. doi: 10.1038/nature21070 [DOI] [PubMed] [Google Scholar]