Abstract
Triclosan (TCS) is used as an antimicrobial agent and has been widely dispersed and detected in the aquatic environment. However, it remains uncertain whether TCS is genotoxic or not. In this study, the acute toxicity of TCS in goldfish (Carassius auratus) was studied. Then, based on the results for acute toxicity, other goldfish were exposed to various concentrations of TCS (control, DMSO control, and 1/4, 1/2, and 1/8 LC50) for 14 days, and the effects on genetic toxicity were evaluated using micronucleus (MN) and nuclear abnormalities (NA) frequencies in peripheral blood and the comet assay in the liver of the goldfish. In addition, malondialdehyde (MDA), reduced glutathione (GSH), catalase (CAT), and total antioxidant capacity (T-AOC) in the liver were assayed to evaluate oxidative stress and the possible mechanism of genotoxicity. The 96 h median lethal concentration of TCS was 1111.9 µg/l. After 14 days of exposure, the MN and NA frequencies were significantly increased in peripheral blood of the TCS-treated groups compared with the solvent control, and the comet tail moment and MDA in the liver in the highest dose of TCS groups were also significantly high. Meanwhile, an evident change in GSH, CAT, and T-AOC of the liver was found as the TCS exposure concentration increased. The results showed that TCS caused oxidative stress and a genotoxic response in goldfish, suggesting that it presents a potential ecotoxicological risk to aquatic ecosystems.
Keywords: comet assay, micronucleus, nuclear abnormalities, oxidative stress, triclosan
Introduction
Pharmaceuticals and personal care products (PPCPs), an emerging class of environmental pollutants, are some of the most extensively used antibacterial agents, which are used not only for preventive and curative in humans and animals but also for the promotion of growth in poultry and aquatic animals species [11]. Triclosan (TCS; 2, 4, 4’-trichloro-2’-hydroxydiphenyl ether) is one of the main known PPCPs, and it is added to a wide variety of consumer products (e.g., toothpastes, hand sanitizers, soaps, deodorants, air fresheners, textiles, shoes, and cosmetics) [32]. Widespread use of this compound could lead to large-scale disposal of it in gray water via local sewer systems and ultimately result in it being found in river water and sediment [30, 34].
TCS can accumulate and subsequently cause adverse effects on nontarget organisms in the aquatic environment [5]. Toxicology research has confirmed that TCS is highly toxic to fish [7, 17, 19, 20]. The reproductive and developmental toxicity of TCS has been evaluated in some aquatic species under controlled laboratory conditions. The results concerning the early life-stage toxicity of TCS in rainbow trout (Oncorhynchus mykiss) showed that no statistical differences were observed in mean time to egg hatch among groups exposed to different concentrations of TCS in a water environment [28]. TCS exposure impairs lipid metabolism in zebrafish (Danio rerio) embryos [17], and has significant teratogenic effects on early-life stages of zebrafish [27]. It has been reported that TCS has potentially estrogenic and weak androgenic effects on Japanese medaka (Oryzias latipes) fry [14, 19] and can alter the swimming speed of fish [25]. Genotoxicity and mutagenicity studies using classical prokaryotic and eukaryotic systems have been reported [31], and researchers have concluded that TCS is neither genotoxic nor mutagenic according to the available evidence. However, there is some evidence to suggest that TCS may be genotoxic in some types of organisms and/or cell types. Acute toxicity experiments using hemocytes from zebra mussels (Dreissena polymorpha) that were exposed to environmentally relevant concentrations of TCS provided evidence concerning the genotoxicity of TCS after only 24 h of exposure [3]. The genotoxicity of TCS has also been evaluated in the algal species Closterium ehrenbergii [8]. At concentrations of 0.25 mg/land greater, the genetic toxicity of TCS was apparent. Researchers have also tested the genotoxicity and cytotoxicity of TCS in animal cell lines. The results showed that genetic damage accrued as a result of exposure to TCS at higher concentrations [20]. In summary, the genetic toxicity of TCS may be related to the species, dose of TCS, and exposure time.
The micronucleus (MN) and nuclear abnormalities (NA) assays, two of the most popular environmental genotoxicity tests, have served as indicators of cytogenetic damage [6, 18, 22]. The comet assay, a sensitive and fast method for DNA strand break testing in individual cells, has been widely used as a genotoxicity test in fish [23, 40]. It is reported that the MN frequency of zebrafish exposed to a high dose of TCS was slightly higher than that of zebrafish exposed to a low dose of TCS, though the difference was not significant [6]. The MN and comet assays have demonstrated that TCS induces genetic toxicity in zebra mussels [3].
Therefore, this study was conducted to evaluate the effects of TCS on genetic toxicity in goldfish using a chronic (14 days) assay. In this assay, at the end of the exposure period, MN, NA, and comet assays were performed to evaluate genetic toxicity. The oxidative stress levels in the liver were also examined to investigate the possible mechanism of genotoxicity.
Materials and Methods
Test chemicals
TCS (CAS NO. 3380–34-5; molecular weight,289.541; 289.541;>98.0% purity) was obtained from Wako Pure Chemical Industries Ltd., Tokyo, Japan, and dissolved in dimethyl sulfoxide (DMSO, Wako Pure Chemical Industries). TCS stock solution (2,000 mg/l was stored in the dark at 4°C and used to prepare the experiment solutions by serially diluting it to the required concentrations. DMSO in the experiment solution was kept at 0.1%.
Test organisms
Management of the fish used in the experiments conformed to the Rules of Experimental Animal Management, which are used for protection of experimental animal welfare and managed by the Chinese government. The fish were caught with the permission of the Luoyang Department of Environmental Protection and approved by the Luoyang Ethical Committee. They were handled in such a way as to relieve stress and discomfort.
Six hundred goldfish (Carassius auratus) with body lengths of 6.8 to 7.5 cm and body weights of 10.1 to 13.2 g were bought from the New village aquarium market in the Luolong District of Luoyang. Tap water was aerated continuously for 72 h to remove redundant chlorine, and the temperature and pH were determined to ensure that the water was suitable for the experiments. The fish were first acclimatized in 58 × 28 × 36 cm aquariums with light for 10 to 12 h each day at 21 ± 1°C for 10 days. They were fed daily with freshly hatched brine shrimp, and residual feeds and faces were removed. The water was replaced in a timely manner. Five hundred fish to be used in preliminary, acute, and chronic experiments were then randomly selected.
Acute toxicity test
Acute toxicity tests in our experiment were performed with reference to the procedure in a previous study [21]. Six groups of different TCS exposure concentrations based on the preliminary experiments (0.6000, 0.6900, 0.7935, 0.9125, 1.0494, and 1.2068 mg /l) were set. Each group (n=10) was assayed in triplicate. Meanwhile, controls and solvent controls using DMSO were also prepared for the tests. The 96 h acute toxicity tests were performed with no feeding in each aquarium, which contained 10 fish and 20 l water. Half of the solution in each aquarium was replaced daily with fresh solution. The toxic effects on the fish were observed carefully, and dead fish were removed in a timely manner. The goldfish were exposed to TCS for 4 consecutive days and solvent control groups were kept in 0.1% (v/v) DMSO solutions. The LC50 at 96 h was calculated by the modified Kaber method (95%confidence interval).
The concentrations of TCS in the present experimental solutions were determined during the exposure by highperformance liquid chromatography with ultraviolet detection. Analysis of the results revealed that the degradation of TCS in the tested tanks for 96 h was lower than 2%.
Genetic toxicity and oxidative stress after chronic exposure
On the basis of the results of the 96 h acute toxicity tests, the concentrations of TCS were set up at three levels: 0.1399 mg/l (1/8 LC50), 0.2798 mg/l (1/4 LC50), and 0.5596 mg/l (1/2 LC50). Blank and solvent controls were also prepared. Half of the solution in each aquarium was replaced with fresh solution each day, and then the fish were fed. The residual food and excrement were removed by siphon. After 14 days of exposure, peripheral blood samples were obtained from the caudal vein of 5 random fish from each group and smeared onto pre-cleaned slides for determination of MN and NA. Meanwhile, a small portion of the liver in fish of each group (n=5) was used to perform the comet assay, and the other liver of the same fish as mentioned above were immediately snap-frozen in liquid nitrogen and then stored at −80°C in a refrigerator for measurement of oxidative stress markers.
Analysis of micronuclei (MN) and other nuclear abnormalities (NA)
MN and NA were assayed according to a previous study protocol [4]. In brief, smears were fixed in absolute methanol for 10 min, air-dried, and stained with 10% Giemsa solution for 8 min, washed with PBS, and dried at room temperature. The frequencies of MN and NA were evaluated (per 1,000 cells) by scoring at a 1000× magnification usingOlympus CX 31 (Japan) bright-field microscopes. A total of 5,000 erythrocytes from peripheral blood with intact cellular and nuclear membranes were examined for each fish specimen.
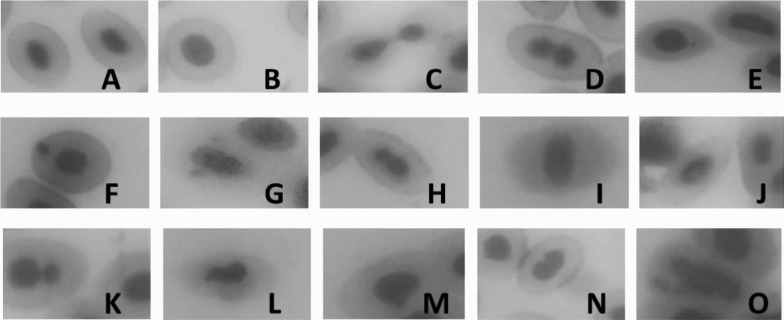
Based on the finding of previous research and our experimental results, we defined four types of the nucleus of normal erythrocytes in the peripheral blood of fish: mature nuclei, immature nuclei, cell cleavage-stage nuclei, and equally constricted nuclei (Figs. 1A–D). MN includes small micronuclei, large micronuclei, and double micronuclei (Figs. 1E–G). NA includes unequally constricted nuclei, abnormal located nuclei, vacuolated nuclei, double nuclei, irregular nuclei, bulging nuclei, notched nuclei, and fragmented nuclei (Figs. 1H–O).
Fig. 1.
Erythrocytes with normal and abnormal nuclei. (A) Mature nuclei, (B) immature nuclei, (C) cell-cleavage stage nuclei, (D) equally constricted nuclei, (E) small micronuclei, (F) large micronuclei, (G) double micronuclei, (H) unequally constricted nuclei, (I) abnormal location of nuclei, (J) vacuolated nuclei, (K) double nuclei, (L) irregular nuclei, (M) bulging nuclei, (N) notched nuclei, and (O) fragmented nuclei
Liver comet assay
The comet assay was performed as per established protocols [40]. Briefly, livers from each fish were put into 1.5 ml centrifuge tubes, rinsed with phosphate buffered solution (PBS) three times, and then digested to single cells with 400 µl trypsine. After digestion, the cells were dispersed into 800 µl Dulbecco’s Modified Eagle Medium (DMEM) and filtered through a 74 µm nylon cloth into 1.5 ml centrifuge tubes. Then, cells were collected by centrifugation for 5 min at 1,000 rpm and resuspended in DMEM medium. After adjusting the density of cells, the other cell suspensions were processed in the comet assay.
Thirty microliters of cell suspension was mixed with 90 µl 0.65% low melting agar (LMA) at 37°C and covered with a fully frosted slide precoated with a layer of 100 µl 1% normal melting agar (NMA). After solidification, slides were immersed into lysate (2.5 mol/l NaCl, 100 mmol/l EDTA, 10 mmol/l Tris, NaOH [pH 10.0] 1% Na-sarcosinate, 10% DMSO, and 1% Triton X-100) for 1.5 h. Slides were then incubated in an electrophoresis tank containing 300 mmol/l NaOH and 1 mmol/l EDTA for 20 min and subjected to electrophoresis for 20 min at 25 V (300 mA). Then, slides were neutralized (0.4 mol/l Tris, pH 7.5), stained with 40 µl Gelred (0.2 µl/ml, and viewed using a fluorescence microscope (Te2000-U, Eclipse, Nikon) equipped with a CCD camera. Nonoverlapping cells were captured at ×400 magnification. At least 100 cells per slide were analyzed using the Comet Assay Software Project (CASP).
Assay of oxidative stress marker in liver
Preparation of liver homogenate was performed as per established protocols [41]. In brief, the whole preparation process for goldfish liver homogenate was performed on ice. First, the livers were thawed, washed with 0.86% normal saline at 4°C, dried, weighed, and put into a tissue grinder immediately with 0.01 mol/l Tris–HCl buffer solution at pH 8.0 using a mass ratio of 1:9. The homogenate was collected in centrifuge tubes and centrifuged at 1,006 × g for 20 min. The supernatant was collected for determining the level of total antioxidant capacity (T-AOC), reduced glutathione (GSH), catalase (CAT), malondialdehyde (MDA), and protein.
T-AOC, GSH, MDA, CAT, and protein were measured by using T-AOC, GSH, MDA, CAT, and protein determination kits according to the manufacturer’s protocol (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). T-AOC was measured by the method of ferric reducing-antioxidant power assay method and detected at 520 nm with a spectrophotometer. T-AOC level was expressed as U/mg prot, with mg prot defined as mg protein in homogenate. GSH was determined spectrophotometrically by monitoring the chromophoric product resulting from reaction of the 5, 50-dithiobis-(2-nitrobenzoic acid) with GSH in the presence of reduced nicotinamide adenine dinucleotide phosphate (NADPH) and glutathione reductase at 412 nm. GSH content was expressed as mg/g prot. Degree of lipid peroxidation in liver tissue homogenates was determined in terms of thiobarbituric acid reactive substances (TBARS) formation with maximal absorbance at 532 nm by following the protocol. The concentration of MDA in the liver in fish was calculated by comparing the absorbance to that produced by the control standard 1,1,3,3-tetraethoxypropane and expressed as nmol/mg prot. CAT activity was measured using the ammonium molybdate spectrophotometric method, which is based on the fact that ammonium molybdate can rapidly terminate the hydrogen peroxide (H2O2) degradation reaction catalyzed by CAT and react with the residual H2O2 to generate a yellow complex, which can be monitored by its absorbance at 405 nm using a spectrophotometer. Values of CAT activity are expressed as U/mg prot.
Statistics analysis
Data analyses were performed using the SPSS Statistics software (Ver17.0, SPSS Inc., Chicago, IL, USA). Data were expressed as means ± SD. One-way ANOVA and LSD multiple comparisons were applied to evaluate the significance of differences. Differences were considered statistically significant when 0.01<P<0.05 and highly significant when P<0.01.
Results
Acute toxicity of TCS in goldfish
Goldfish in each group showed different intoxication symptoms during the first 6 h of the exposure experiment. In higher TCS exposure groups, goldfish were fully mobilized shortly after contact with the solution. Their opercula moved rapidly, attacking along aquariums. After 2 h, some goldfish seemed to show signs of toxicity and lost their balance, and their whole bodies occasionally shook. After 4 h, the goldfish swam slowly and appeared to flock together at the bottom of the aquariums. After 8 h, some of the fish were dead. By contrast, the symptoms appeared later in the low TCS exposure groups but the intoxication characteristics were the same as those in the high concentration groups. Dead fish were found after exposure for 32 h.
The mortality of goldfish was increased with the increase in TCS concentration within 96 h and the linearity of this relationship was remarkable. The 96 h mortality rates were 60%, 50%, and 10% at the concentrations of 1.2068 mg/l, 1.0496 mg/l, and 0.7935 mg/l, respectively. However, no dead fish was found at the lower concentration (0.6900 and 0.6000 mg/L). The 96 h LC50 value of TCS was calculated as 1.1119 mg/L for goldfish, with a 95% confidence interval from 1.0121 to 1.3558 mg/L.
Induction of MN and NA in peripheral blood
After 14 days of exposure, the activities in control fish were similar to those in the solvent control. Compared with the solvent control, total loss of equilibrium in fish was observed in the 0.1399 mg/l TCS-treated group, while goldfish in the 0.2798 and 0.5596 mg/l TCS-treated groups swam slowly. Furthermore, 30% of the fish were dead in the 0.5596 mg/l TCS-treated group, but there were no dead fish in the other groups.
MN and NA rates in peripheral blood of goldfish exposed to TCS are shown in Table 1. There were no significant differences in MN and NA rates between the control and solvent control. The rates gradually increased with the increase in TCS dosage. Dose-response relationships were found between MN and NA rates of erythrocytes in peripheral blood and TCS concentration. There were significant differences in MN and NA rates between the TCS-treated groups and solvent control (P<0.01).
Table 1. MN and NA frequencies in peripheral blood erythrocytes of goldfish exposed to TCS.
| Group | MN rate (%) | NA rate (%) |
|---|---|---|
| Control | 0.48 ± 0.11 | 0.83 ± 0.09 |
| Solvent control | 0.59 ± 0.14 | 0.91 ± 0.13 |
| 0.1399 mg/l | 1.22 ± 0.18** | 1.81 ± 0.08** |
| 0.2798 mg/l | 1.63 ± 0.22** | 2.34 ± 0.16** |
| 0.5596 mg/l | 1.81 ± 0.11** | 2.88 ± 0.26** |
Asterisks indicate statistically significant differences from the solvent control group (**P<0.01). Data are presented as the mean ± SD (n=5).
Assay of liver DNA damage (comet assay)
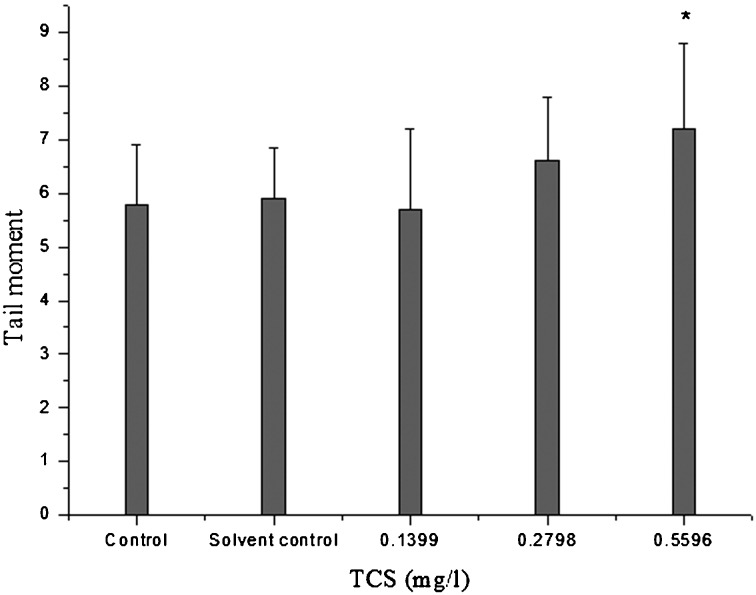
The parameter used to quantify the extent of DNA damage using the comet assay was the tail moment. The results for liver tail moment of goldfish exposed to TCS are shown in Fig. 2. There were no significant differences in tail moment of the hepatocytes between the control and the solvent control. However, the tail moment of hepatocytes in the 0.5596 mg/l TCS-treated group was significantly increased compared with that of the solvent control (P<0.05).
Fig. 2.
DNA damage determined by tail moment of the hepatocytes in goldfish exposed to TCS for 14 days. Asterisks indicate statistically significant differences from the control group (*P<0.05). Data are presented as the mean ±SD (n=5).
Influence of TCS exposure on lipid oxidative damage in the liver
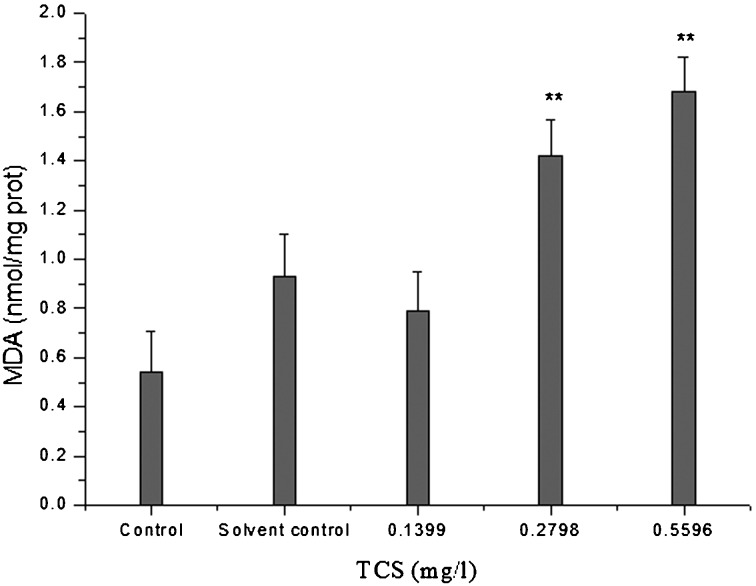
Lipid peroxidation is a process that is detected by the extent of the peroxide-forming free radicals mechanism and the peroxide-removing antioxidant system. MDA in liver homogenates was used as an indicator of lipid oxidation. The MAD levels in liver homogenates of goldfish exposed to TCS are shown in Fig. 3. There were no significant differences in MDA level between the control and solvent control. However, the MDA levels in the 0.2798 and 0.5596 mg/l TCS-treated groups were significantly increased compared with that of the solvent control (P<0.01).
Fig. 3.
MDA levels in the liver of goldfish exposed to TCS for 14 days. Asterisks indicate statistically significant differences from the control group (**P<0.01). Data are presented as the mean ± SD (n=5).
Influence of TCS exposure on the antioxidant system in the liver
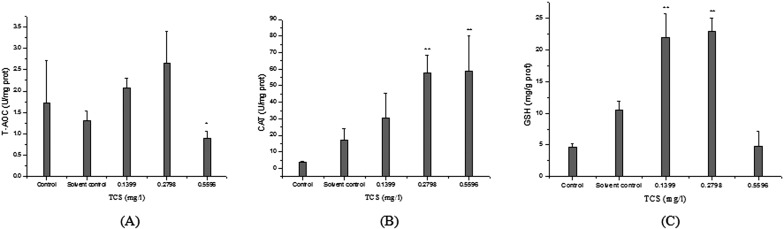
As shown in Fig. 4A, the T-AOC levels in the liver homogenates of the control and solvent control fish were 1.73 ± 0.98 and 1.32 ± 0.22 U/mg prot, and there were no significant differences between the control and solvent control. TCS at 0.1399 and 0.2798 mg/l led to a slight induction, but the changes were not significant (P>0.05). However, the T-AOC levels in the 0.5596 mg/l TCS-treated groups were significantly decreased compared with that of the solvent control (P<0.05). The CAT activities in the 0.2798 and 0.5596 mg/l TCS-treated groups were significantly increased compared with that of the solvent control (P<0.01), while there was no significant difference between the control and the solvent control (P>0.05) (Fig. 4B).The GSH contents in the 0.1399 and 0.2798 mg/l TCS-treated groups were significantly increased compared with that of the solvent control (P<0.01), while there was no significant difference between the control, solvent control, and 0.5596 mg/l TCS-treated group (P>0.05) (Fig. 4C).
Fig. 4.
Antioxidant system markers in the liver of goldfish exposed to TCS for 14 days. (A) T-AOC, (B) CAT, and (C) GSH. Asterisks indicate statistically significant differences from the control group (*P<0.05; **P<0.01). Data are presented as the mean ± SD (n=5).
Discussion
The MN test is one of the most widespread and promising tests of environmental genotoxicity [13, 29, 40]. In recent years, other NA have also served as an indicator of cytogenetic damage [4,5,6, 35]. In the present study, based on the results of 96 h acute toxicity testing, a chronic (14 days) TCS exposure experiment was performed to evaluate the genetic toxicity and oxidative stress of TCS in goldfish. Our results showed that 0.1399, 0.2798, and 0.5596mg/l TCS induce oxidative stress and increases in MN and NA and that only 0.5596 mg/l TCS causes an increase in tail moment after chronic (14 days) TCS exposure.
MN is produced from chromosome fragments or whole chromosomes that lag at cell division due to lack of a centromere, damage in the centromere, or a defect in cytokinesis. The MN test in fish has the potential to monitor clastogenic and aneugenic effects of environmental agents in the aquatic environment. Moreover, MN has been scored in fish erythrocytes as a measure of clastogenic activity [1]. NA may complement the scoring of MN in conventional genotoxicity surveys [4,5,6, 10]. In our study, MN and NA frequencies in TCS-treated groups were significantly increased. These results showed that TCS may be genotoxic in goldfish. In a previous study, the results for MN in zebrafish exposed to TCS showed that the MN frequency was slightly higher at a high TCS dose than that at a low dose [27]. Acute toxicity experiments using hemocytes from zebra mussels that were exposed to environmentally relevant concentrations of TCS (0.29, 0.58, and 0.87 µg/l) provided evidence of genotoxicity after only 24 h of exposure [2]. The genotoxicity of TCS in hemocytes was evaluated with the comet assay, the micronucleus assay, and the halo test, a measure of the apoptotic frequencies, while cytotoxicity was assessed with the neutral red retention assay. The genetic damage accrued in the hemocytes was significant at all three concentrations of TCS and followed a concentration-dependent and time-dependent pattern. The genotoxicity of TCS has also been evaluated using the comet assay in Artemia salina [39], Tetrahymena thermophila thermophile [15], and the algal species Closterium ehrenbergii [8]. Exposure of Artemia salina to 171.1 µg/l TCS for 96 h resulted in a significant increase in genotoxic biomarkers. Furthermore, 1.0 µg/l TCS can lead to statistically significant DNA damage in Tetrahymena thermophila after 2 h of exposure. Algal cells were exposed to TCS for 96 h at concentrations in the range of 0.125–1 mg/l. At concentrations of 0.25 mg/l and greater, the genetic toxicity of TCS was apparent, with the antimicrobial exerting its toxicity in a dose-dependent manner. Complete dissolution of the nucleus was observed at concentrations of 0.5 and 1 mg/l. Meanwhile, the genotoxicity of TCS has been tested in animal cell lines [20]. KB and Vero cell lines were treated with two concentrations of TCS, the 50% inhibition concentration (9.84 and 10.42 mg/l respectively) and the maximum concentration of TCS in personal care products (6.66 mg/l). In both cell lines, the number of comet cells increased as the concentration and duration of exposure to TCS increased. These results indicated TCS has genotoxic effects. However, a battery of 24 in vitro and in vivo mutagenicity and genotoxicity tests designed to evaluate the full range of potential to produce mutagenic or genotoxic effects in prokaryotic and eukaryotic systems indicated that neither TCS nor its metabolites are mutagenic or genotoxic [31]. Of these 24 experiments, only 3 yielded weakly positive responses for the endpoints evaluated. Two of these 3 assays used in vitro systems, whereas the third was an in vivo test. The few weakly positive results were not consistent with respect to the type of genetic alterations observed, and the observations have not been duplicated in the same or equivalent assays. Moreover, toxicogenomics analysis unequivocally shows that TCS is identified as a compound acting through non-DNA reactive mechanisms [12]. Therefore, in order to further confirm the genetic toxicity of TCS, a comet assay was performed in the liver of goldfish in our study. A measurement of tail moment was used to quantify the extent of DNA damage, as tail moment is generally used in this ecotoxicity field [38, 40]. We found that the tail moment at the highest dose of TCS was significantly so this result also showed that TCS is genotoxic in goldfish.
A previous study indicated that the genotoxicity of TCS in zebra mussels was probably due to a combination of oxidative stress and/or a direct effect on DNA [2]. In a follow-up study, we assayed the oxidative stress level in the liver of goldfish. An imbalance between reactive oxygen species (ROS) and antioxidant defenses in favor of the former has been defined as oxidative stress. Antioxidant defense in a living aerobiont is connected with a series of intracellular antioxidant enzymes and nonenzymatic antioxidant systems, the roles of which are to intercept and inactivate ROS [36]. CAT is one of the important enzymes in the antioxidant system, and it can eliminate the H2O2 produced with ROS catalyzed by SOD, alleviating the damages to organisms [26]. Our results showed that CAT activities in the liver were increased significantly in the 0.2798 (1/4 LC50) and 0.5596 mg/l (1/2 LC50) TCS groups. When CAT activity was induced, it was beneficial in that it eliminated excess H2O2, protecting cells from oxidative damage. GSH is an important nonenzymatic antioxidant in animals and is capable of preventing damage to important cellular components caused by ROS such as free radicals and lipid peroxides [33]. The GSH contents in the 0.1399 mg/l (1/8 LC50) and 0.2798 mg/l (1/4 LC50) TCS exposure groups were significantly increased in the present study. This showed that GSH was induced in the 0.2798 mg/l (1/4 LC50) and 0.1399 mg/l (1/8 LC50) TCS exposure groups, and this was beneficial for eliminate of excess ROS. However, the response at 0.5596 mg/L TCS decreased dramatically, which showed that excessive ROS may cause lipid peroxidation in the liver. T-AOC refers to the total antioxidative capacity. T-AOC was significantly decreased in the 0.5596 mg/l (1/2 LC50) TCS exposure group, suggesting an imbalance between ROS and antioxidant defenses. MDA is a product of lipid peroxidation, which can be used to evaluate the oxidative damage in lipids [24, 37]. In the present study, the MDA levels in the livers of goldfish exposed to 0.2798 (1/4 LC50) and 0.5596 mg/l (1/2 LC50) TCS were significantly increased compared with that of the solvent control. Our study showed that TCS led to an imbalance between oxidants and antioxidants in the livers of goldfish, resulting in lipid and DNA damage. A previous study also demonstrated that TCS exposure caused increases in of ROS content (%) and glutathione transferase (GST) enzymatic activity in the monogonont rotifer [16].
Based on the results of the MN, NA, and comet assays and evaluation of oxidative stress in our study, we conclude that TCS caused oxidative stress and genotoxicity in the goldfish and that oxidative stress is associated with genotoxicity in goldfish exposed to TCS. Due to the wide use of TCS, the levels of TCS in aquatic environments have been found range from nanograms/liter to micrograms/liter [9], which are below the concentrations in this experiment. However, its risk to the aquatic organisms cannot be ignored.
Conflict of Interest
All authors declare that there is no conflict of interest.
Acknowledgments
This study was supported by the Joint Fund for Fostering Talents of the National Natural Science Foundation of China and Henan Province (U1504303) and by the Foundation of Henan Provincial Youth Backbone Teachers (2016GGJS-119).
References
- 1.al-Sabti K., Metcalfe C.D.1995. Fish micronuclei for assessing genotoxicity in water. Mutat. Res. 343: 121–135. doi: 10.1016/0165-1218(95)90078-0 [DOI] [PubMed] [Google Scholar]
- 2.Binelli A., Cogni D., Parolini M., Riva C., Provini A.2009. Cytotoxic and genotoxic effects of in vitro exposure to triclosan and trimethoprim on zebra mussel (Dreissena polymorpha) hemocytes. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 150: 50–56. doi: 10.1016/j.cbpc.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 3.Binelli A., Cogni D., Parolini M., Riva C., Provini A.2009. In vivo experiments for the evaluation of genotoxic and cytotoxic effects of Triclosan in Zebra mussel hemocytes. Aquat. Toxicol. 91: 238–244. doi: 10.1016/j.aquatox.2008.11.008 [DOI] [PubMed] [Google Scholar]
- 4.Cavaş T., Ergene-Gözükara S.2005. Induction of micronuclei and nuclear abnormalities in Oreochromis niloticus following exposure to petroleum refinery and chromium processing plant effluents. Aquat. Toxicol. 74: 264–271. doi: 10.1016/j.aquatox.2005.06.001 [DOI] [PubMed] [Google Scholar]
- 5.Cavaş T., Ergene-Gözükara S.2005. Micronucleus test in fish cells: a bioassay for in situ monitoring of genotoxic pollution in the marine environment. Environ. Mol. Mutagen. 46: 64–70. doi: 10.1002/em.20130 [DOI] [PubMed] [Google Scholar]
- 6.Cavas T., Garanko N.N., Arkhipchuk V.V.2005. Induction of micronuclei and binuclei in blood, gill and liver cells of fishes subchronically exposed to cadmium chloride and copper sulphate. Food Chem. Toxicol. 43: 569–574. doi: 10.1016/j.fct.2004.12.014 [DOI] [PubMed] [Google Scholar]
- 7.Chen X., Xu B., Han X., Mao Z., Chen M., Du G., Talbot P., Wang X., Xia Y.2015. The effects of triclosan on pluripotency factors and development of mouse embryonic stem cells and zebrafish. Arch. Toxicol. 89: 635–646. doi: 10.1007/s00204-014-1270-2 [DOI] [PubMed] [Google Scholar]
- 8.Ciniglia C., Cascone C., Giudice R.L., Pinto G., Pollio A.2005. Application of methods for assessing the geno- and cytotoxicity of Triclosan to C. ehrenbergii. J. Hazard. Mater. 122: 227–232. doi: 10.1016/j.jhazmat.2005.03.002 [DOI] [PubMed] [Google Scholar]
- 9.Dann A.B., Hontela A.2011. Triclosan: environmental exposure, toxicity and mechanisms of action. J. Appl. Toxicol. 31: 285–311. doi: 10.1002/jat.1660 [DOI] [PubMed] [Google Scholar]
- 10.da Silva Souza T., Fontanetti C.S.2006. Micronucleus test and observation of nuclear alterations in erythrocytes of Nile tilapia exposed to waters affected by refinery effluent. Mutat. Res. 605: 87–93. doi: 10.1016/j.mrgentox.2006.02.010 [DOI] [PubMed] [Google Scholar]
- 11.Daughton C.G., Ternes T.A.1999. Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ. Health Perspect. 107:(Suppl 6): 907–938. doi: 10.1289/ehp.99107s6907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doktorova T.Y., Ates G., Vinken M., Vanhaecke T., Rogiers V.2014. Way forward in case of a false positive in vitro genotoxicity result for a cosmetic substance? Toxicol. In Vitro 28: 54–59. doi: 10.1016/j.tiv.2013.09.022 [DOI] [PubMed] [Google Scholar]
- 13.Fenech M., Chang W.P., Kirsch-Volders M., Holland N., Bonassi S., Zeiger E., HUman MicronNucleus project2003. HUMN project: detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte cultures. Mutat. Res. 534: 65–75. doi: 10.1016/S1383-5718(02)00249-8 [DOI] [PubMed] [Google Scholar]
- 14.Foran C.M., Bennett E.R., Benson W.H.2000. Developmental evaluation of a potential non-steroidal estrogen: triclosan. Mar. Environ. Res. 50: 153–156. doi: 10.1016/S0141-1136(00)00080-5 [DOI] [PubMed] [Google Scholar]
- 15.Gao L., Yuan T., Cheng P., Bai Q., Zhou C., Ao J., Wang W., Zhang H.2015. Effects of triclosan and triclocarban on the growth inhibition, cell viability, genotoxicity and multixenobiotic resistance responses of Tetrahymena thermophila. Chemosphere 139: 434–440. doi: 10.1016/j.chemosphere.2015.07.059 [DOI] [PubMed] [Google Scholar]
- 16.Han J., Won E.J., Hwang U.K., Kim I.C., Yim J.H., Lee J.S.2016. Triclosan (TCS) and Triclocarban (TCC) cause lifespan reduction and reproductive impairment through oxidative stress-mediated expression of the defensome in the monogonont rotifer (Brachionus koreanus). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 185-186: 131–137. doi: 10.1016/j.cbpc.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 17.Ho J.C.H., Hsiao C.D., Kawakami K., Tse W.K.F.2016. Triclosan (TCS) exposure impairs lipid metabolism in zebrafish embryos. Aquat. Toxicol. 173: 29–35. doi: 10.1016/j.aquatox.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 18.Iarmarcovai G., Bonassi S., Botta A., Baan R.A., Orsière T.2008. Genetic polymorphisms and micronucleus formation: a review of the literature. Mutat. Res. 658: 215–233. doi: 10.1016/j.mrrev.2007.10.001 [DOI] [PubMed] [Google Scholar]
- 19.Ishibashi H., Matsumura N., Hirano M., Matsuoka M., Shiratsuchi H., Ishibashi Y., Takao Y., Arizono K.2004. Effects of triclosan on the early life stages and reproduction of medaka Oryzias latipes and induction of hepatic vitellogenin. Aquat. Toxicol. 67: 167–179. doi: 10.1016/j.aquatox.2003.12.005 [DOI] [PubMed] [Google Scholar]
- 20.Jirasripongpun K., Wongarethornkul T., Mulliganavin S.2008. Risk assessment of triclosan using animal cell lines. Witthayasan Kasetsat Witthayasat 42: 353–359. [Google Scholar]
- 21.Kenaga E.E.1981. Aquatic test organisms and methods useful for assessment of chronic toxicity of chemicals. Regul. Toxicol. Pharmacol. 1: 277–292. doi: 10.1016/0273-2300(81)90077-5 [DOI] [Google Scholar]
- 22.Liu S., Zhang X., Zang X., Arunakumara K.K.2008. Genotoxic evaluation of the GH transgenic Synechocystis using mice and turbot (Scophthalmus maximus L.). Mutat. Res. 653: 113–116. doi: 10.1016/j.mrgentox.2008.04.003 [DOI] [PubMed] [Google Scholar]
- 23.Lorenzo Y., Costa S., Collins A.R., Azqueta A.2013. The comet assay, DNA damage, DNA repair and cytotoxicity: hedgehogs are not always dead. Mutagenesis 28: 427–432. doi: 10.1093/mutage/get018 [DOI] [PubMed] [Google Scholar]
- 24.Lu Z., Li C.M., Qiao Y., Yan Y., Yang X.2008. Effect of inhaled formaldehyde on learning and memory of mice. Indoor Air 18: 77–83. doi: 10.1111/j.1600-0668.2008.00524.x [DOI] [PubMed] [Google Scholar]
- 25.Nassef M., Matsumoto S., Seki M., Khalil F., Kang I.J., Shimasaki Y., Oshima Y., Honjo T.2010. Acute effects of triclosan, diclofenac and carbamazepine on feeding performance of Japanese medaka fish (Oryzias latipes). Chemosphere 80: 1095–1100. doi: 10.1016/j.chemosphere.2010.04.073 [DOI] [PubMed] [Google Scholar]
- 26.Nordberg J., Arnér E.S.2001. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 31: 1287–1312. doi: 10.1016/S0891-5849(01)00724-9 [DOI] [PubMed] [Google Scholar]
- 27.Oliveira R., Domingues I., Koppe Grisolia C., Soares A.M.V.M.2009. Effects of triclosan on zebrafish early-life stages and adults. Environ. Sci. Pollut. Res. Int. 16: 679–688. doi: 10.1007/s11356-009-0119-3 [DOI] [PubMed] [Google Scholar]
- 28.Orvos D.R., Versteeg D.J., Inauen J., Capdevielle M., Rothenstein A., Cunningham V.2002. Aquatic toxicity of triclosan. Environ. Toxicol. Chem. 21: 1338–1349. doi: 10.1002/etc.5620210703 [DOI] [PubMed] [Google Scholar]
- 29.Pacheco M., Santos M.A.2002. Biotransformation, genotoxic, and histopathological effects of environmental contaminants in European eel (Anguilla anguilla L.). Ecotoxicol. Environ. Saf. 53: 331–347. doi: 10.1016/S0147-6513(02)00017-9 [DOI] [PubMed] [Google Scholar]
- 30.Ramaswamy B.R., Shanmugam G., Velu G., Rengarajan B., Larsson D.G.J.2011. GC-MS analysis and ecotoxicological risk assessment of triclosan, carbamazepine and parabens in Indian rivers. J. Hazard. Mater. 186: 1586–1593. doi: 10.1016/j.jhazmat.2010.12.037 [DOI] [PubMed] [Google Scholar]
- 31.Rodricks J.V., Swenberg J.A., Borzelleca J.F., Maronpot R.R., Shipp A.M.2010. Triclosan: a critical review of the experimental data and development of margins of safety for consumer products. Crit. Rev. Toxicol. 40: 422–484. doi: 10.3109/10408441003667514 [DOI] [PubMed] [Google Scholar]
- 32.Sethuraman S., Kumar T.R., Vivekananthan T.2014. Effect of triclosan on histopathological changes in the hepatopancreas of zebra fish Brachydanio rerio hamilton-buchman. Asian J. Biochem. Pharmacol. Res. 4: 2231–2560. [Google Scholar]
- 33.Szalai G., Kellős T., Galiba G., Kocsy G.2009. Glutathione as an Antioxidant and Regulatory Molecule in Plants Under Abiotic Stress Conditions. J. Plant Growth Regul. 28: 66–80. doi: 10.1007/s00344-008-9075-2 [DOI] [Google Scholar]
- 34.Tong L., Wang Y., Li P., Wang J., Shao Y.2010. Headspace solidphase microextraction (hs-spme) and solid-phase extraction (SPE) concentration for quantification of malodorous substances in piggery wastewater. Environ. Forensics 11: 355–362. doi: 10.1080/15275922.2010.526533 [DOI] [Google Scholar]
- 35.Torres-Bugarín O., Zavala-Cerna M.G., Nava A., Flores-García A., Ramos-Ibarra M.L.2014. Potential uses, limitations, and basic procedures of micronuclei and nuclear abnormalities in buccal cells. Dis. Markers 2014: 956835. doi: 10.1155/2014/956835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ventura-Aguilar R.I., Rivera-Cabrera F., Méndez-Iturbide D., Pelayo-Zaldívar C., Bosquez-Molina E.2013. Enzymatic and non-enzymatic antioxidant systems of minimally processed cactus stems (Opuntia ficus-indica Mill.) packaged under modified atmospheres. Int. J. Food Sci. Technol. 48: 2603–2612. doi: 10.1111/ijfs.12256 [DOI] [Google Scholar]
- 37.Wang F., Li C., Liu W., Jin Y.2014. Potential mechanisms of neurobehavioral disturbances in mice caused by sub-chronic exposure to low-dose VOCs. Inhal. Toxicol. 26: 250–258. doi: 10.3109/08958378.2014.882447 [DOI] [PubMed] [Google Scholar]
- 38.Xu D., Li C., Wen Y., Liu W.2013. Antioxidant defense system responses and DNA damage of earthworms exposed to perfluorooctane sulfonate (PFOS). Environ. Pollut. 174: 121–127. doi: 10.1016/j.envpol.2012.10.030 [DOI] [PubMed] [Google Scholar]
- 39.Xu X., Lu Y., Zhang D., Wang Y., Zhou X., Xu H., Mei Y.2015. Toxic assessment of triclosan and triclocarban on Artemia salina. Bull. Environ. Contam. Toxicol. 95: 728–733. doi: 10.1007/s00128-015-1641-2 [DOI] [PubMed] [Google Scholar]
- 40.Zhang W., Liu W., Zhang J., Zhao H., Zhang Y., Quan X., Jin Y.2012. Characterisation of acute toxicity, genotoxicity and oxidative stress posed by textile effluent on zebrafish. J. Environ. Sci. (China) 24: 2019–2027. doi: 10.1016/S1001-0742(11)61030-9 [DOI] [PubMed] [Google Scholar]
- 41.Zhao X., Gao Y., Qi M.2014. Toxicity of phthalate esters exposure to carp (Cyprinus carpio) and antioxidant response by biomarker. Ecotoxicology 23: 626–632. doi: 10.1007/s10646-014-1194-x [DOI] [PMC free article] [PubMed] [Google Scholar]