Abstract
Calcium signals arise by multiple mechanisms, including mechanisms of release of intracellular stored Ca2+, and the influx of Ca2+ through channels in the plasma membrane. One mechanism that links these two sources of Ca2+ is store-operated Ca2+ entry, the most commonly encountered version of which involves the extensively studied calcium-release-activated Ca2+ (CRAC) channel. The minimal and essential molecular components of the CRAC channel are the STIM proteins that function as Ca2+ sensors in the endoplasmic reticulum, and the Orai proteins that comprise the pore forming subunits of the CRAC channel. CRAC channels are known to play significant roles in a wide variety of physiological functions. This review discusses the multiple forms of STIM and Orai proteins encountered in mammalian cells, and discusses some specific examples of how these proteins modulate or mediate important physiological processes.
Introduction
Aberrant Ca2+ signaling underlies or contributes to numerous pathological conditions (Berridge, 1994; Feske, 2007; Missiaen et al., 2000; Mooren and Kinne, 1998). In particular, and pertinent to the theme of this volume, numerous studies implicate Ca2+ signaling in the development and progression of cancer cells (Baldi et al., 2003; Bodding et al., 2003; El Boustany et al., 2008; Flourakis et al., 2010; Jaffe, 2005; Kohn et al., 1995; Kohn et al., 1996; Lee et al., 2006; McAndrew et al., 2011; Tannheimer et al., 1997; Trump and Berezesky, 1996; Wissenbach et al., 2001; Yang et al., 2009; Zhuang et al., 2002). Virtually all mechanisms and pathways of Ca2+ signaling have been implicated in either the pathology or potential therapy of diseases, including Ca2+ pumps, intracellular Ca2+ mobilization, and Ca2+ channels. One widely encountered signaling mechanism involves the phosphoinositide-derived second messenger, inositol 1,4,5-trisphospate (IP3) (Mikoshiba, 2015). This mode of signaling involves IP3-induced activation of a receptor-channel on the endoplasmic reticulum resulting in release of stored Ca2+ and activation of downstream Ca2+-sensitive pathways. A large number of physiological processes, and disease states can be associated with IP3-induced signaling (Mikoshiba, 2015). Calcium signals can also result from the opening of Ca2+ channels in the plasma membrane. One specific and widely distributed Ca2+ channel, and the focus of this chapter, is the store-operated or calcium-release-activated-Ca2+ (CRAC) channel, implicated in a number of diseases (Feske, 2007; Feske, 2010), including cancer (Chen et al., 2011; Davis et al., 2014; McAndrew et al., 2011; Motiani et al., 2013; Yang et al., 2009).
Store-operated Ca2+ channels are activated when the Ca2+ concentration in the endoplasmic (or sarcoplasmic) reticulum is low (Parekh and Putney, 2005; Putney, 1986). Physiologically, this generally occurs through activation of a Ca2+ release signaling mechanism, usually inositol 1,4,5-trisphospate-induced release, or Ca2+-induced Ca2+ release through the ryanodine receptor. Experimentally, influx through store-operated channels results in a rise in steady-state Ca2+, a process sometimes referred to as store-operated Ca2+ entry (SOCE). In electrophysiological studies, SOCE presents as a small inwardly rectifying current termed calcium-release activating Ca2+ current (Icrac) (Hoth and Penner, 1992). The level of Ca2+ in the endoplasmic reticulum is sensed by a transmembrane endoplasmic reticulum resident protein, STIM1, and to a less well defined extent, its homolog STIM2 (Liou et al., 2005; Roos et al., 2005). STIM1 is a single pass transmembrane protein with an EF hand Ca2+ binding motif in the N-terminus directed to the lumen of the endoplasmic reticulum. When free Ca2+ in the endoplasmic reticulum falls, Ca2+ dissociates from STIM1 resulting in its aggregation into multimers and consequent confirmation change. This confirmation change exposes sequences that can interact with and activate store-operated Ca2+ channels, composed of Orai1 subunits, in the plasma membrane ((Feske et al., 2006; Vig et al., 2006; Zhang et al., 2006), reviewed in (Fahrner et al., 2009; Frischauf et al., 2008; Hogan et al., 2010; Prakriya, 2009)). In addition, Ca2+ entering through Orai1 channels can recruit some members of the TRPC cation channel family to the plasma membrane where they interact with STIM1 producing additional, albeit less specific, Ca2+ entry (Cheng et al., 2011).
STIM
STIM, or STromal Interacting Molecule, was first identified as a gene linked to certain tumors (Parker et al., 1996; Sabbioni et al., 1997) and almost simultaneously as a stromal cell derived plasma membrane protein capable of interacting with B-lymphocytes (Oritani and Kincade, 1996). Limited screens of Drosophila (Roos et al., 2005) and mammalian (Liou et al., 2005) siRNA libraries identified STIM proteins as requisite for SOCE. Drosophila has a single STIM, while mammalian cells express, to varying degrees, two proteins, STIM1 and STIM1. Both proteins contain Ca2+ binding EF hand motifs directed to the lumen of the endoplasmic reticulum. STIM1 is also expressed in the plasma membrane, but its function there remains poorly defined. In the endoplasmic reticulum, both proteins bind Ca2+ in the endoplasmic reticulum and lose the bound Ca2+ when luminal Ca2+ drops below a certain level. By far, much more work has been done with STIM1 (together with the Ca2+ channel subunit Orai1) than STIM2. Overexpression of STIM1 with Orai1 produces huge CRAC currents in response to Ca2+ store depletion, whether by phospholipase C-activating agonists or by the endoplasmic reticulum Ca2+ ATPase inhibitor, thapsigargin (Mercer et al., 2006; Peinelt et al., 2006; Soboloff et al., 2006). STIM2 on the other hand, when co-expressed with Orai1, produces constitutively active Ca2+ influx (Bird et al., 2009; Brandman et al., 2007; Parvez et al., 2008), consistent with one view that its function is largely maintenance of endoplasmic reticulum Ca2+ levels (Brandman et al., 2007). However, there also appears to be a role for STIM2 in producing Ca2+ signal-dependent activation of downstream pathways as well (Kar et al., 2012; Oh-Hora et al., 2008).
At least one additional splice variant each of STIM1 and STIM2 has been identified. A longer version of STIM1, STIM1L, arises from splicing into the C-terminus of an additional 106 amino acids (Darbellay et al., 2011). The additional sequence in STIM1L contains an actin-binding domain that anchors STIM1L to the actin cytoskeleton and concentrates STIM1L at the endoplasmic reticulum-plasma membrane contact sites for rapid Orai1 activation (Darbellay et al., 2011). STIM1L was found to be necessary for maintenance of intracellular stores when Ca2+ signaling involved brief spikes of Ca2+ release in myotubes, leading to the conclusion that anchoring of STIM1L resulted in faster and more efficient store refilling (Darbellay et al., 2011). However, a subsequent study found that STIM1L, while somewhat pre-localized to near plasma membrane junctions, was still further recruited upon Ca2+ store depletion with thapsigargin (Sauc et al., 2015). STIM1L and STIM1S supported SOCE equally well and at similar speeds in a paradigm utilizing cyclopiazonic acid to slowly deplete endoplasmic reticulum stores (Sauc et al., 2015). STIM1L differed from STIM1S in that the latter, but not the former, recruited and enlarged cortical endoplasmic reticulum cisternae when overexpressed (Sauc et al., 2015). In comparing the rates of these two STIM1 forms in moving to the plasma membrane junctions and activating SOCE, it is worth noting that both the aforementioned studies were carried out at room temperature. An earlier publication demonstrated that at physiological temperatures, STIM1 is pre-clustered at endoplasmic reticulum junction suggesting that the apparent diffuse distribution of STIM proteins when stores are full, seen in room temperature experiments, may be artifactual (Xiao et al., 2011).
By database mining, Miederer et al. (Miederer et al., 2015) found a splice variant of STIM2 (STIM2.1) that contains an eight amino acid sequence inserted in the STIM-Orai Activating Region (SOAR (Yuan et al., 2009)). STIM2.1 failed to activate Orai1 in an overexpression protocol, and appears to negatively regulate SOCE. STIM2.1 expression relative to the more common STIM2 (STIM2.2) varied among tissues and cell type, indicating that it can contribute to shaping SOCE signaling.
STIM1 activity can be modified by post-translational modifications. STIM1 contains two N-linked luminal glycosylation sites, and STIM2 contains one. Mutation of the sites in STIM1 can lead to either decreased or increased SOCE, likely due to alterations in STIM1 oligomerization (Kilch et al., 2013; Miederer et al., 2015; Mignen et al., 2007). A small amount of STIM1 gets to the plasma membrane where it is required for activation of non-store-operated Arachidonate-Regulated Ca2+ current (Icrac (Mignen et al., 2007; Shuttleworth et al., 2007). Mutants of STIM1 that cannot be glycosylated can still activate CRAC channels, but do not go the plasma membrane and thus fail to activate ARC channels. It is not clear, however, whether alterations in the glycosylation state of STIM proteins is regulated or varies in any way in vivo.
STIM1 is phosphorylated in vivo although the specific sites are unclear (Manji et al., 2000). However, it is clear that the phosphorylation state of STIM1 and STIM2 changes during the cell cycle and this change in phosphorylation alters the function of STIM1 in two significant ways. It is a relatively old observation, dating prior to the discoveries of STIM and Orai, that SOCE is diminished or absent in cells undergoing mitosis (Preston et al., 1991; Volpi and Berlin, 1988). Smyth et al. (Smyth et al., 2009) demonstrated that STIM1 is phosphorylated on multiple sites during mitosis, and mutation or deletion of candidate sites restores SOCE in mitotic cells (Smyth et al., 2012; Smyth et al., 2009).
There is an additional consequence of the phosphorylation of STIM1 during mitosis. In interphase cells, STIM1 associates with the growing tips of microtubules, through interaction with the microtubule growing tip protein, EB1 (Honnappa et al., 2009; Mimori-Kiyosue et al., 2000), and thus plays a role in remodeling of cortical endoplasmic reticulum (Honnappa et al., 2009). However, mutation of a specific EB1 binding site in STIM1 had no effect on its ability to support SOCE (Honnappa et al., 2009). During mitosis, the function of microtubules is dramatically altered, and microtubules rearrange and organize in the pro-nucleus to form the mitotic spindle that direct appropriate chromosomal segregation during cell division (Inoue, 1981). Phosphorylation of STIM1 prevents association with EB1, and thus with the mitotic spindle, allowing for appropriate segregation of STIM1 to the periphery during mitosis (Smyth et al., 2012). Mutation of candidate phosphorylation sites results in continued association of endoplasmic reticulum with microtubules during mitosis such that endoplasmic reticulum elements can be seen to decorate the mitotic spindle (Smyth et al., 2012). However, cells overexpressing this mutant form of STIM1 undergo normal cell division and the rate of cell division appears unaffected. Thus, it is not yet clear which, if either, of the underlying effects of STIM1 phosphorylation are functionally more important, the shutting down of SOCE, or the segregation of endoplasmic reticulum from the mitotic spindle. Likely more subtle experimental models, such as mutations in phosphorylation sites in model organisms may provide answers to this enigmatic question.
Orai
In mammals, there are three Orai gene products, Orai1, 2 and 3 (Feske et al., 2006). By far the most is known of the functions and regulation of Orai1, null mutations of which produce severe phenotypes in humans and mice (Feske et al., 2006; Vig et al., 2008). All three forms produce enhanced SOCE when co-expressed with STIM1, although quantitatively, Orai2 is much weaker than Orai1 in this regard, and Orai3 much weaker still. Mice lacking Orai1 die perinatally, likely due to poor skeletal muscle development, but can survive in less than Mendelian numbers in a mixed genetic background (Gwack et al., 2008; Vig et al., 2008). T-cells from Orai1 knockout mice have only partial loss of SOCE, and compensatory increased expression of Orai2 may account for this (REF**).
Orai1 can function in other signaling pathways other than SOCE. A subfamily of the large Transient Receptor Potential (TRP) family includes 7 Canonical TRPPs, or TRPC channels. These channels are known to be activated downstream of phospholipase C coupled receptors, and can clearly be activated independently of calcium store depletion (DeHaven et al., 2009; Vazquez et al., 2004). However, as mentioned above, Orai1 can interact with TRPC channel subunits in a poorly understood manner to produce a moderately selective Ca2+ current termed Isoc (Cheng et al., 2011; Cheng et al., 2008; Ong et al., 2007). Following Ca2+ store depletion, Ca2+ entering through CRAC channels recruits TRPC (in particular TRPC1) to the plasma membrane. There, TRPC1 interacts with STIM1 via a domain distinct from that which interacts with Orai1. However, it is unclear how prevalent this mode of signaling is in vivo. Mice lacking all 7 TRPC channel subunits show a significantly milder phenotype than mice lacking Orai1, and cells from these mice have apparent normal SOCE (http://grantome.com/grant/NIH/ZIA-ES101684-12).
Orai1 is also a necessary pore-forming subunit for a non-store-operated channel activated by arachidonic acid (Iarc) (Mignen et al., 2008)or a metabolite of arachidonic acid, leukotriene C4 (Gonzalez-Cobos et al., 2013). The Shuttleworth laboratory demonstrated that ARC channels are a pentamer of three Orai1 and two Orai3 subunits (Mignen et al., 2009). STIM1 is required for ARC channel activation; however, Ca2+ dissociation is unnecessary (Mignen et al., 2007). Rather, a pool of STIM1 targeted to the plasma membrane is required (Mignen et al., 2007). This scenario was called into question by Zhang et al. (Gonzalez-Cobos et al., 2013; Zhang et al., 2014). These authors identified a similar current in vascular smooth muscle cells activated by arachidonic acid, but more potently by its metabolite, leukotriene C4 (Ilarc). A comparison of the arachidonic acid and leukotriene currents in both HEK293 cells and vascular smooth muscle cells indicated that the two currents are essentially the same channels (Gonzalez-Cobos et al., 2013). Interestingly, STIM1 in the plasma membrane was required for channel activation when recording currents in whole-cell mode, but with perforated patch STIM1 in the ER was sufficient (Zhang et al., 2014).
In mammary glands, Orai1 appears to play a role in mediating basal flux of Ca2+ into milk (Feng et al., 2010; Ross et al., 2013). This Ca2+ flux appears to be independent of intracellular Ca2+ stores, does not depend on STIM1 or STIM2, but rather results from a constitutive interaction by a form of a Golgi Ca2+ pump, SPCA2 (Cross et al., 2013; Feng et al., 2010).
Much less is known of the functions of Orai2 and Orai3 in comparison to Orai1, owing in part to less experimental work with these Orai forms. Both Orai2 and Orai3 can be activated by store-depletion when overexpressed with STIM1, but the resulting currents are substantially less than those seen with Orai1 (Lis et al., 2007; Mercer et al., 2006). In the majority, knockdown or knockout seems to fully prevent SOCE (for example, (Davis et al., 2016; Davis et al., 2015; Hwang et al., 2012; Hwang and Putney, 2012; Steinckwich et al., 2015; Xing et al., 2013)). However, mast cells (Vig et al., 2008) show significant but incomplete loss of SOCE in Orai1 knockout mice. T-cells are even less affected (Gwack et al., 2008; Vig et al., 2008), and a recent report demonstrates roles for both Orai1 and Orai2 in T-cell function (Vaeth et al., 2017). Additionally, although there have been few studies of SOCE in the central nervous, Orai2 appears to be the major Orai form in the brain (Chauvet et al., 2016). A major role for Orai3 appears to be as a pore-forming subunit of the non-store-operated ARC channels. However, Saul et al. (Saul et al., 2016) provided evidence that Orai3 can function as a subunit of SOC channels, together with Orai1, and the presence of Orai3 reduces the redox sensitivity of the channels. In addition, Motiani et al. (Motiani et al., 2013) demonstrated SOCE in breast cancer cells mediated by Orai3, and also showed that estrogen receptor alpha activation led to increased expression of Orai3 and increased SOCE. A more detailed discussion of potential roles of Orai2 and Orai3 can be found in a review by Hoth and Niemeyer (Hoth and Niemeyer, 2013).
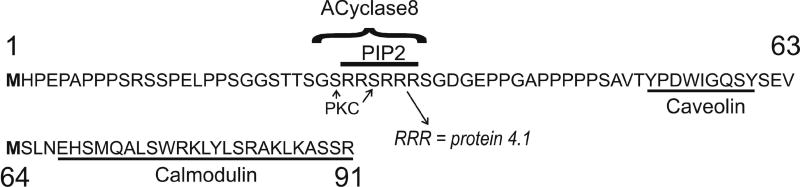
In humans and presumably other mammals, Orai1 protein is expressed in a long and short form termed Orai1α and Orai1β (Fukushima et al., 2012). The longer Orai1α when deglycosylated runs as a 33 kD protein, corresponding to the molecular size predicted from the coding sequence. The shorter Orai1β runs at 23 kD, and arises from alternative translation initiation at methionine 64. Deletion of the first 63 amino acids in Orai1 results in the loss of potentially important signaling domains, including a caveolin binding site (Yu et al., 2010), an adenylyl cyclase 8 binding site (Willoughby et al., 2012), a potential phosphatidylinositol 4,5-bisphosphate binding site, two potential protein kinase C phosphorylation sites (Kawasaki et al., 2010), and a recognition site for the membrane skeletal protein 4.1 (GASCARD et al., 1993) (Figure 1). Examination of the Kozak sequences at methionine 1 and 64 reveal that at methionine 1 the Kozak sequence is rather weak, while at methionine 64 it is strong. Strengthening of the Kozak sequence at methionine 1 results in formation of Orai1α exclusively indicating that the shorter Orai1β arises from skipping of the first initiation site. When overexpressed together with STIM1, the two forms appear to coalesce into puncta with STIM1 equally well, and produce similarly large CRAC currents and large SOCE (Fukushima et al., 2012). However, fluorescence recovery after photobleaching (FRAP) measurements indicated that plasma membrane mobility of Orai1α was considerably slower than Orai1β. Consistent with this finding, Orai1α was shown to distribute and traffic to intracellular structures to a greater extent than did Orai1β (Fukushima et al., 2012). This may result from the presence of a caveolin binding domain in the extended sequence of Orai1α (Yu et al., 2010). Interestingly, discreet segregation of Orai1α-like mobilities from Orai1β mobilities suggests that the two forms do not associate with one another to form heteromeric channels.
Figure 1. N-terminal sequence of Orai1.
Two methionines in the N-terminus can serve as initiation sites, at position 1 and position 64, resulting in two forms of Orai1. Potential sequences in the longer version include a caveolin binding site (Yu et al., 2010), an adenylyl cyclase 8 binding site (Willoughby et al., 2012), a potential phosphatidylinositol 4,5-bisphosphate binding site, two potential protein kinase C phosphorylation sites (Kawasaki et al., 2010), and a recognition site for the membrane skeletal protein 4.1 (GASCARD et al., 1993).
The cell biology and channel properties of Orai1α and Orai1β channels suggest they may subtend somewhat distinct physiological functions. (Desai et al., 2015). In experiments employing murine embryonic fibroblasts (MEFs) from Orai1-null mice (Vig et al., 2008), both Orai1α and Orai1β were capable of rescuing SOCE and Icrac. Extensive overexpression of Orai1 produces diminished SOCE (Mercer et al., 2006), presumably due to inappropriate stoichiometry, and so for these rescue experiments it was necessary to utilize constructs driven by the moderately active thymidine kinase promoter (Korzeniowski et al., 2010). The function of Orai1 forms in the TRPC-dependent current Isoc was assessed by transfection of HEK293 cells with TRPC1, STIM1 and wild-type Orai1, Orai1α or Orai1β. In each case, store depletion resulted in relatively non-selective inward currents, reminiscent of Isoc, and the currents in cells expressing the different Orai1 constructs were quantitatively and qualitatively similar (Desai et al., 2015). Omission of TRPC1 resulted in inwardly rectifying Icrac, similar in magnitude for all three constructs, consistent with the findings in MEFs (Desai et al., 2015).
Orai1-dependent CRAC currents undergo complex regulation by Ca2+, including a rapid negative feedback inhibition involving Ca2+ action in close proximity of the mouth of the channel termed fast inactivation (Fierro and Parekh, 1999; Hoth and Penner, 1993). In HEK293 cells co-transfected with STIM1 and either Orai1α or Orai1β, only cells transfected with Orai1α showed fast inactivation (Desai et al., 2015).
The relative ability of Orai1 forms to support the non-store-operated current, Iarc, was evaluated by using HEK293 cells, a line known to express endogenous Icrac and Iarc. Knockdown of Orai1 by siRNA essentially eliminated both endogenous currents. Transfection with constructs coding specifically Orai1α or Orai1β fully rescued store-operated Icrac. However, only Orai1α was capable of rescuing the non-store-operated Iarc (Desai et al., 2015).
Physiological Functions of Store-operated Channels
Despite the various roles for STIM and Orai dependent channels, it is primarily the store-operated channels for which there is information regarding roles in physiological processes. For example, to the author’s knowledge, no publications have appeared utilizing animal models to investigate the importance of ARC channels or SOC channels at the organ system or organism level. Animal models with TRPC channel deletions have revealed numerous physiological functions subtended by these channels, but without experimentally demonstrating that these involve the store-operated activation mode.
Mouse Models
Orai1 knockout mice die perinatally, but survive for prolonged periods in mixed genetic backgrounds (Gwack et al., 2008; Vig et al., 2008). As is the case for humans lacking functional Orai1, Orai1 knockout mice are deficient in multiple aspects of the immune system (Feske, 2009; Gwack et al., 2008; Vig et al., 2008). The mice are small in stature, which likely results from deficient muscle development (Lyfenko and Dirksen, 2008), and possibly also in deficient development of bone (Hwang et al., 2012; Robinson et al., 2012). The precursor cells for both osteoclasts (bone resorbing cells) and osteoblasts (bone forming cells) have reduced SOCE, and the differentiation and functions bone are impaired, such that the mice exhibit osteopenia (decreased bone density) (Hwang et al., 2012; Robinson et al., 2012).
SOCE is well known to play a prominent role in the immune system (Feske et al., 2003). Much of the research in this area has involved acquired immunity despite the fact that the original discovery of Icrac was in mast cells (Hoth et al., 1992). Mast cells from mice lacking either Orai1 (Vig et al., 2008) or STIM1 (Baba et al., 2008) exhibited a deficiency in SOCE and loss of mast cell function.
Neutrophils play a major role in innate immunity (McDonald and Kubes, 2011). Neutrophils migrate to sites of infection and inflammation by sensing gradients of small molecules, a process of chemotaxis (Maxfield, 1993). The well-studied chemoattractant, fMLF, has long been known to require Ca2+ to function. The tripeptide fMLF activates a plasma membrane G-protein-coupled receptor that is linked to phospholipase C and the production of the Ca2+ releasing signal molecule, IP3 (Dougherty et al., 1984; Jaconi et al., 1988). The Ca2+ requirement for chemotaxis apparently involves entry of Ca2+, and that entry mechanism seems to be SOCE (Alvarez et al., 1994; Andersson et al., 1986; Demaurex et al., 1992). Reduction of Orai1 or STIM1 expression in HL-60 neutrophils significantly impaired Ca2+ signaling and chemotaxis in response to fMLF (Steinckwich et al., 2015). Not surprisingly, when neutrophils become polarized in a chemoattractant gradient, it appears that SOCE signaling also distributes itself in an asymmetric manner. What is surprising is that STIM 1 seems to distribute to the rear of the cells (Putney et al., 2017).
Psoriasis is a skin disease resulting from a complex autoimmunity involving, among other components, neutrophils (Toichi et al., 2000). In mice exhibiting the symptoms of psoriasis, knockout of STIM1 increased the speed of reversal of psoriasis symptoms (Steinckwich et al., 2015). Thus, SOCE might be a useful pharmacological target for treatment of psoriasis and other autoimmunity-involved disorders.
The maintenance and development of the epidermis requires calcium, and Ca2+ signaling controlled primarily by a calcium sensing receptor (Tu et al., 2004). An extracellular calcium gradient in some manner signals differentiation of keratinocytes to their differentiated state. In HaCaT keratinocytes, increasing extracellular Ca2+ induced expression of keratinocyte-associated genes, , and slowed cell growth in the process of terminal differentiation (Tu et al., 2004). A calcium sensing receptor, signaling through phospholipase C, mediates this process (Hofer and Brown, 2003). Reduced expression of either Orai1 or STIM1 diminished SOCE, Icrac and [Ca2+]i signaling in response to elevation of extracellular Ca2+ (Numaga-Tomita and Putney, 2013).
1. Exocrine Glands
SOCE has been investigated with Orai1 knockout mice in two different exocrine glands, mammary glands and lacrimal glands. Calcium signaling is essential for mammalian oocyte fertilization in mammals (Miao and Williams, 2012; Miyazaki et al., 1993; Swann and Yu, 2008). Intracellular IP3 is generated through the action of sperm-delivered phospholipase C zeta, resulting periodic intracellular Ca2+ oscillations (Kashir et al., 2017). There is evidence implicating a role for Orai1 and SOCE as playing a role in mammalian oocyte fertilization (Gómez-Fernández et al., 2012). But surprisingly, female Orai1 knockout female mice are fertile, and with normal size litters. However, pups are small and survive only about four days, unless transferred to a wild type for fostering (Davis et al., 2015). The indication then is a failure of adequate lactation. Mammary glands secrete milk by a mechanism that differs from most other exocrine glands. At or around birth, hormones stimulate constitutive secretion of milk calcium and nutrients into alveoli. Through a mechanism involving oxytocin receptors, suckling stimulates contraction of alveolar basket myoepithelial cells (Crowley and Armstrong, 1992; Shennan and Peaker, 2000). Milk from Orai1 knockout females had a Ca2+ concentration of only about 50% of controls (Davis et al., 2015). Thus an additional function of Orai1 involves transporting Ca2+ into alveoli (Feng et al., 2010). However, this decrease in milk Ca2+ is unlikely to account for the observed phenotype. Engorged mammary glands of Orai1 knockouts suggested that constitutive milk formation was normal but discharge of milk was inhibited. In support of this interpretation, Ca2+ oscillations induced by oxytocin in myoepithelial cells were reduced substantially in Orai1 knockout mice. When alveolar contractions were examined microscopically, the alveolar contractions due to oxytocin were also substantially diminished (Davis et al., 2015). Thus, lactation in Orai1 knockout female mice likely fails due to a loss of Ca2+ entry in myoepithelial cells.
The original concept of SOCE emerged from Ca2+ signaling studies in exocrine glands, both parotid gland and lacrimal acinar cells (Putney and Bird, 2014; Putney et al., 1998; Takemura et al., 1989; Takemura and Putney, 1989). lacrimal gland acinar cells from Orai1 knockout mice do not show SOCE in response to muscarinic receptor activation or to the Ca2+ pump inhibitor, thapsigargin, and exhibit no detectable Icrac (Xing et al., 2013). Lacrimal secretion activated by pilocarpine in vivo was significantly reduced in knockout animals. Histological analysis of the lacrimal glands revealed that development and structure of the glands were normal. Secretion of peroxidase from wild-type lacrimal gland fragments was increased in response to the muscarinic agonist, methacholine, in the presence of Ca2+ and to a lesser degree in when Ca2+ was absent. When fragments from Orai1 knockout mice were utilized, peroxidase secretion in the presence of Ca2+ was decreased to that in the absence of Ca2+. However, secretion in the absence of extracellular Ca2+, resulting from IP3-induced Ca2+ release, as well as constitutive secretion, were unaffected in the cells from knockout mice (Xing et al., 2013). This illustrates how highly specific is the role of Orai1 in signaling calcium entry. Lacrimal glands of Orai1 mice lacking Orai1 also lack SOCE, Icrac, and the component of exocytotic secretion that depends on external Ca2+. However, the morphology and size, as well asCa2+-independent secretion remain unaffected. Thus, the basic mechanism of storage and synthesis and storage of secretory granules product, as well as the mechanism of Ca2+-dependent exocytosis, and signaling through the IP3 and release of intracellular Ca2+ are quantitatively unchanged.
2. Conclusions
This review focused on distinct forms of the major molecular players in store-operated Ca2+ entry, STIM1 and Orai1. A graphic summary of the mechanisms of action of these key molecules is shown in Figure 2. To better understand the physiological functions of these key molecules, mouse models lacking a specific molecule in the store-operated Ca2+ entry pathway have been developed. To illustrate the utility of such models, this review has discussed some key findings from such mouse models, providing new insights into the roles of SOCE in mammalian physiology. Hopefully such an approach will eventually allow modulation of this pathway to ameliorate any of the number of diseases known to result from aberrant Ca2+ signaling (Missiaen et al., 2000; Mooren et al., 1998; Targos et al., 2005).
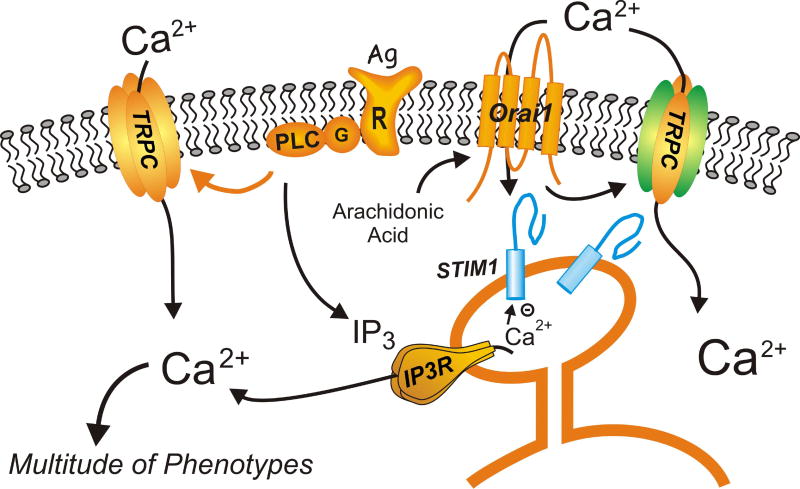
Figure 2. Actions of the key SOCE mediators, STIM1 and Orai1.
Signaling is generally initiated by agonist (Ag) acting through a receptor (R) and G-protein (G) to activate phospholipase C (PLC) and produce the Ca2+-mobilizing messenger, inositol 1,4,5-trisphosphate (IP3). IP3 in turn activates the IP3 receptor (IP3R) in the endoplasmic reticulum. The fall in Ca2+ in the endoplasmic reticulum activates STIM1 to aggregate and migrate to specific sites near the plasma membrane where Orai1 channels are activated resulting in SOCE. Channels composed of Orai1 and Orai3 (not shown) subunits can also be activated in a non-store-operated mode by arachidonic acid or a metabolite, leukotriene C4. Calcium entering through Orai1 channels can recruit and together with STIM1 activated certain members of the TRPC cation channel family. Some members of the TRPC cation family can also be activated more directly by products of phospholipase C. Calcium entering the cytoplasm through all of these routes regulates and activates a myriad of cell responses, some of which were discussed in the current review.
Supplementary Material
Acknowledgments
Work from the author’s laboratory discussed in this review was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The author declares no conflicts of interest with respect to this submission.
References
- 1.Alvarez J, Montero M, García-Sancho J. Agonist-induced Ca2+ influx in human neutrophils is not mediated by production of inositol polyphosphates but by emptying of the intracellular Ca2+ stores. Biochem. Soc. Trans. 1994;22:809–813. doi: 10.1042/bst0220809. [DOI] [PubMed] [Google Scholar]
- 2.Andersson T, Dahlgren C, Pozzan T, Stendahl O, Lew PD. Characterization of fMet-Leu-Phe receptor-mediated Ca2+ influx across the plasma membrane of human neutrophils. Mol. Pharmacol. 1986;30:437–443. [PubMed] [Google Scholar]
- 3.Baba Y, Nishida K, Fujii Y, Hirano T, Hikida M, Kurosaki T. Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses. Nat Immunol. 2008;9:81–88. doi: 10.1038/ni1546. [DOI] [PubMed] [Google Scholar]
- 4.Baldi C, Vazquez G, Boland R. Capacitative calcium influx in human epithelial breast cancer and non-tumorigenic cells occurs through Ca2+ entry pathways with different permeabilities to divalent cations. J. Cell Biochem. 2003;88:1265–1272. doi: 10.1002/jcb.10471. [DOI] [PubMed] [Google Scholar]
- 5.Berridge MJ. The biology and medicine of calcium signalling. Mol. Cell. Endocrinol. 1994;98:119–124. doi: 10.1016/0303-7207(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 6.Bird GS, Hwang SY, Smyth JT, Fukushima M, Boyles RR, Putney JW., Jr STIM1 is a calcium sensor specialized for digital signaling. Curr Biol. 2009;19:1724–1729. doi: 10.1016/j.cub.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bodding M, Fecher-Trost C, Flockerzi V. Store-operated Ca2+ current and TRPV6 channels in lymph node prostate cancer cells. J. Biol. Chem. 2003;278:50872–50879. doi: 10.1074/jbc.M308800200. [DOI] [PubMed] [Google Scholar]
- 8.Brandman O, Liou J, Park WS, Meyer T. STIM2 Is a Feedback Regulator that Stabilizes Basal Cytosolic and Endoplasmic Reticulum Ca2+ Levels. Cell. 2007;131:1327–1339. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chauvet S, Jarvis L, Chevallet M, Shrestha N, Groschner K, Bouron A. Pharmacological Characterization of the Native Store-Operated Calcium Channels of Cortical Neurons from Embryonic Mouse Brain. Front Pharmacol. 2016;7:486. doi: 10.3389/fphar.2016.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen YF, Chiu WT, Chen YT, Lin PY, Huang HJ, Chou CY, Chang HC, Tang MJ, Shen MR. Calcium store sensor stromal-interaction molecule 1-dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis. Proc. Natl. Acad. Sci. U. S. A. 2011;108:15225–15230. doi: 10.1073/pnas.1103315108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng KT, Liu X, Ong HL, Swaim W, Ambudkar IS. Local Ca(2)+ entry via Orai1 regulates plasma membrane recruitment of TRPC1 and controls cytosolic Ca(2)+ signals required for specific cell functions. PLoS. Biol. 2011;9:e1001025. doi: 10.1371/journal.pbio.1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng KT, Liu X, Ong HL, Ambudkar IS. Functional Requirement for Orai1 in Store-operated TRPC1-STIM1 Channels. J. Biol. Chem. 2008;283:12935–12940. doi: 10.1074/jbc.C800008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cross BM, Hack A, Reinhardt TA, Rao R. SPCA2 regulates Orai1 trafficking and store independent Ca2+ entry in a model of lactation. PLoS. One. 2013;8:e67348. doi: 10.1371/journal.pone.0067348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crowley WR, Armstrong WE. Neurochemical regulation of oxytocin secretion in lactation. Endocr. Rev. 1992;13:33–65. doi: 10.1210/edrv-13-1-33. [DOI] [PubMed] [Google Scholar]
- 15.Darbellay B, Arnaudeau S, Bader CR, Konig S, Bernheim L. STIM1L is a new actin-binding splice variant involved in fast repetitive Ca2+ release. The Journal of Cell Biology. 2011;194:335. doi: 10.1083/jcb.201012157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis FM, Azimi I, Faville RA, Peters AA, Jalink K, Putney JW, Jr, Goodhill GJ, Thompson EW, Roberts-Thomson SJ, Monteith GR. Induction of epithelial-mesenchymal transition (EMT) in breast cancer cells is calcium signal dependent. Oncogene. 2014;33:2307–2316. doi: 10.1038/onc.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis FM, Goulding EH, D’Agostin DM, Janardhan KS, Cummings CA, Bird GS, Eddy EM, Putney JW. Male infertility in mice lacking the store-operated Ca(2+) channel Orai1. Cell Calcium. 2016;59:189–197. doi: 10.1016/j.ceca.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis FM, Janoshazi A, Janardhan KS, Steinckwich N, D’Agostin DM, Petranka JG, Desai PN, Roberts-Thomson SJ, Bird GS, Tucker DK, Fenton SE, Feske S, Monteith GR, Putney JW., Jr Essential role of Orai1 store-operated calcium channels in lactation. Proc. Natl. Acad. Sci. U. S. A. 2015;112:5827–5832. doi: 10.1073/pnas.1502264112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeHaven WI, Jones BF, Petranka JG, Smyth JT, Tomita T, Bird GS, Putney JW. TRPC channels function independently of STIM1 and Orai1. The Journal of Physiology. 2009;587:2275–2298. doi: 10.1113/jphysiol.2009.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demaurex N, Lew DP, Krause K-H. Cyclopiazonic acid depletes intracellular Ca2+ stores and activates an influx pathway for divalent cations in HL-60 cells. J. Biol. Chem. 1992;267:2318–2324. [PubMed] [Google Scholar]
- 21.Desai PN, Zhang X, Wu S, Janoshazi A, Bolimuntha S, Putney JW, Trebak M. Multiple types of calcium channels arising from alternative translation initiation of the Orai1 message. Sci. Signal. 2015;8:ra74. doi: 10.1126/scisignal.aaa8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dougherty RW, Godfrey PP, Hoyle PC, Putney JW, Freer RJ. Secretagogue-induced phosphoinositide metabolism in human leucocytes. Biochem. J. 1984;222:307–314. doi: 10.1042/bj2220307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El Boustany C, Bidaux G, Enfissi A, Delcourt P, Prevarskaya N, Capiod T. Capacitative calcium entry and transient receptor potential canonical 6 expression control human hepatoma cell proliferation. Hepatol. 2008;47:2068–2077. doi: 10.1002/hep.22263. [DOI] [PubMed] [Google Scholar]
- 24.Fahrner M, Muik M, Derler I, Schindl R, Fritsch R, Frischauf I, Romanin C. Mechanistic view on domains mediating STIM1-Orai coupling. Immunol Rev. 2009;231:99–112. doi: 10.1111/j.1600-065X.2009.00815.x. [DOI] [PubMed] [Google Scholar]
- 25.Feng M, Grice DM, Faddy HM, Nguyen N, Leitch S, Wang Y, Muend S, Kenny PA, Sukumar S, Roberts-Thomson SJ, Monteith GR, Rao R. Store-independent activation of Orai1 by SPCA2 in mammary tumors. Cell. 2010;143:84–98. doi: 10.1016/j.cell.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev. Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 27.Feske S. ORAI1 and STIM1 deficiency in human and mice: roles of store-operated Ca2+ entry in the immune system and beyond. Immunol Rev. 2009;231:189–209. doi: 10.1111/j.1600-065X.2009.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feske S. CRAC channelopathies. Pflugers Arch. 2010 doi: 10.1007/s00424-009-0777-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feske S, Okamura H, Hogan PG, Rao A. Ca2+/calcineurin signalling in cells of the immune system. Biochem. Biophys. Res Commun. 2003;311:1117–1132. doi: 10.1016/j.bbrc.2003.09.174. [DOI] [PubMed] [Google Scholar]
- 30.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 31.Fierro L, Parekh AB. Fast calcium-dependent inactivation of calcium release-activated calcium current (CRAC) in RBL-1 cells. J. Membr. Biol. 1999;168:9–17. doi: 10.1007/s002329900493. [DOI] [PubMed] [Google Scholar]
- 32.Flourakis M, Lehen’kyi V, Beck B, Raphael M, Vandenberghe M, Abeele FV, Roudbaraki M, Lepage G, Mauroy B, Romanin C, Shuba Y, Skryma R, Prevarskaya N. Orai1 contributes to the establishment of an apoptosis-resistant phenotype in prostate cancer cells. Cell Death. Dis. 2010;1:e75. doi: 10.1038/cddis.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frischauf I, Schindl R, Derler I, Bergsmann J, Fahrner M, Romanin C. The STIM/Orai coupling machinery. Channels (Austin) 2008;2:261–268. doi: 10.4161/chan.2.4.6705. [DOI] [PubMed] [Google Scholar]
- 34.Fukushima M, Tomita T, Janoshazi A, Putney JW. Alternative translation initiation gives rise to two isoforms of orai1 with distinct plasma membrane mobilities. J. Cell Sci. 2012 doi: 10.1242/jcs.104919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.GASCARD P, PAWELCZYK T, LOWENSTEIN JM, COHEN CM. The role of inositol phospholipids in the association of band 4.1 with the human erythrocyte membrane. Eur. J. Biochem. 1993;211:671–681. doi: 10.1111/j.1432-1033.1993.tb17595.x. [DOI] [PubMed] [Google Scholar]
- 36.Gómez-Fernández C, López-Guerrero AM, Pozo-Guisado E, Álvarez IS, Martín-Romero FJ. Calcium signaling in mouse oocyte maturation: the roles of STIM1, ORAI1 and SOCE. Molecular Human Reproduction. 2012;18:194–203. doi: 10.1093/molehr/gar071. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez-Cobos JC, Zhang X, Zhang W, Ruhle B, Motiani RK, Schindl R, Muik M, Spinelli AM, Bisaillon JM, Shinde AV, Fahrner M, Singer HA, Matrougui K, Barroso M, Romanin C, Trebak M. Store-independent Orai1/3 channels activated by intracrine leukotriene C4: role in neointimal hyperplasia. Circ. Res. 2013;112:1013–1025. doi: 10.1161/CIRCRESAHA.111.300220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gwack Y, Srikanth S, Oh-Hora M, Hogan PG, Lamperti ED, Yamashita M, Gelinas C, Neems DS, Sasaki Y, Feske S, Prakriya M, Rajewsky K, Rao A. Hair loss and defective T- and B-cell function in mice lacking ORAI1. Mol. Cell Biol. 2008;28:5209–5222. doi: 10.1128/MCB.00360-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hofer AM, Brown EM. Extracellular calcium sensing and signalling. Nat Rev. Mol. Cell Biol. 2003;4:530–538. doi: 10.1038/nrm1154. [DOI] [PubMed] [Google Scholar]
- 40.Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu Rev Immunol. 2010;28:491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Honnappa S, Gouveia SM, Weisbrich A, Damberger FF, Bhavesh NS, Jawhari H, Grigoriev I, van Rijssel FJ, Buey RM, Lawera A, Jelesarov I, Winkler FK, Wuthrich K, Akhmanova A, Steinmetz MO. An EB1-binding motif acts as a microtubule tip localization signal. Cell. 2009;138:366–376. doi: 10.1016/j.cell.2009.04.065. [DOI] [PubMed] [Google Scholar]
- 42.Hoth M, Niemeyer BA. The neglected CRAC proteins: Orai2, Orai3, and STIM2. Curr. Top. Membr. 2013;71:237–271. doi: 10.1016/B978-0-12-407870-3.00010-X. [DOI] [PubMed] [Google Scholar]
- 43.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–355. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 44.Hoth M, Penner R. Calcium release-activated calcium current in rat mast cells. J. Physiol. (Lond. ) 1993;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hwang SY, Foley J, Numaga-Tomita T, Petranka JG, Bird GS, Putney JW., Jr Deletion of Orai1 alters expression of multiple genes during osteoclast and osteoblast maturation. Cell Calcium. 2012;52:488–500. doi: 10.1016/j.ceca.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hwang SY, Putney JW. Orai1-mediated calcium entry plays a critical role in osteoclast differentiation and function by regulating activation of the transcription factor NFATc1. FASEB J. 2012;26:1484–1492. doi: 10.1096/fj.11-194399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inoue S. Cell division and the mitotic spindle. The Journal of Cell Biology. 1981;91:131s–147s. doi: 10.1083/jcb.91.3.131s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jaconi MEE, Rives RW, Schlegel W, Wollheim CB, Pittet D, Lew PD. Spontaneous and chemoattractant-induced oscillations of cytosolic free calcium in single adherent human neutrophils. J. Biol. Chem. 1988;263:10557–10560. [PubMed] [Google Scholar]
- 49.Jaffe LF. A calcium-based theory of carcinogenesis. Adv. Cancer Res. 2005;94:231–263. doi: 10.1016/S0065-230X(05)94006-2. [DOI] [PubMed] [Google Scholar]
- 50.Kar P, Bakowski D, Di CJ, Nelson C, Parekh AB. Different agonists recruit different stromal interaction molecule proteins to support cytoplasmic Ca2+ oscillations and gene expression. Proc. Natl. Acad. Sci. U. S. A. 2012;109:6969–6974. doi: 10.1073/pnas.1201204109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kashir J, Nomikos M, Lai FA. Phospholipase C zeta and calcium oscillations at fertilisation: The evidence, applications, and further questions. Adv. Biol. Regul. 2017 doi: 10.1016/j.jbior.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 52.Kawasaki T, Ueyama T, Lange I, Feske S, Saito N. Protein kinase C-induced phosphorylation of Orai1 regulates the intracellular Ca2+ level via the store-operated Ca2+ channel. J. Biol Chem. 2010;285:25720–25730. doi: 10.1074/jbc.M109.022996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kilch T, Alansary D, Peglow M, Dorr K, Rychkov G, Rieger H, Peinelt C, Niemeyer BA. Mutations of the Ca2+-sensing stromal interaction molecule STIM1 regulate Ca2+ influx by altered oligomerization of STIM1 and by destabilization of the Ca2+ channel Orai1. J. Biol. Chem. 2013;288:1653–1664. doi: 10.1074/jbc.M112.417246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kohn EC, Alessandro R, Spoonster J, Wersto RP, Liotta LA. Angiogenesis: Role of calcium-mediated signal transduction. Proc. Nat. Acad. Sci. USA. 1995;92:1307–1311. doi: 10.1073/pnas.92.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kohn EC, Reed E, Sarosy G, Christian M, Link CJ, Cole K, Figg WD, Davis PA, Jacob J, Goldspiel B, Liotta LA. Clinical investigation of a cytostatic calcium influx inhibitor in patients with refractory cancers. Cancer Res. 1996;56:569–573. [PubMed] [Google Scholar]
- 56.Korzeniowski MK, Manjarres IM, Varnai P, Balla T. Activation of STIM1-Orai1 Involves an Intramolecular Switching Mechanism. Sci. Signal. 2010;3:ra82. doi: 10.1126/scisignal.2001122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee WJ, Monteith GR, Roberts-Thomson SJ. Calcium transport and signaling in the mammary gland: targets for breast cancer. Biochim. Biophys. Acta. 2006;1765:235–255. doi: 10.1016/j.bbcan.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 58.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lis A, Peinelt C, Beck A, Parvez S, Monteilh-Zoller M, Fleig A, Penner R. CRACM1, CRACM2, and CRACM3 are store-operated Ca2+ channels with distinct functional properties. Curr Biol. 2007;17:794–800. doi: 10.1016/j.cub.2007.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lyfenko AD, Dirksen RT. Differential dependence of store-operated and excitation-coupled Ca2+ entry in skeletal muscle on STIM1 and Orai1. J Physiol (Lond) 2008;586:4815–4824. doi: 10.1113/jphysiol.2008.160481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Manji SS, Parker NJ, Williams RT, Van SL, Pearson RB, Dziadek M, Smith PJ. STIM1: a novel phosphoprotein located at the cell surface. Biochim. Biophys. Acta. 2000;1481:147–155. doi: 10.1016/s0167-4838(00)00105-9. [DOI] [PubMed] [Google Scholar]
- 62.Maxfield FR. Regulation of leukocyte locomotion by Ca2+ Trends Cell Biol. 1993;3:386–391. doi: 10.1016/0962-8924(93)90088-i. [DOI] [PubMed] [Google Scholar]
- 63.McAndrew D, Grice DM, Peters AA, Davis FM, Stewart T, Rice M, Smart CE, Brown MA, Kenny PA, Roberts-Thomson SJ, Monteith GR. ORAI1-mediated calcium influx in lactation and in breast cancer. Mol. Cancer Ther. 2011;10:448–460. doi: 10.1158/1535-7163.MCT-10-0923. [DOI] [PubMed] [Google Scholar]
- 64.McDonald B, Kubes P. Cellular and molecular choreography of neutrophil recruitment to sites of sterile inflammation. J. Mol. Med. (Berl) 2011;89:1079–1088. doi: 10.1007/s00109-011-0784-9. [DOI] [PubMed] [Google Scholar]
- 65.Mercer JC, DeHaven WI, Smyth JT, Wedel B, Boyles RR, Bird GS, Putney JW. Large store-operated calcium-selected currents due to co-expression of orai1 or orai2 with the intracellular calcium sensor, stim1. J. Biol. Chem. 2006;281:24979–24990. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miao YL, Williams CJ. Calcium signaling in mammalian egg activation and embryo development: The influence of subcellular localization. Mol. Reprod. Dev. 2012;79:742–756. doi: 10.1002/mrd.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miederer AM, Alansary D, Schwar G, Lee PH, Jung M, Helms V, Niemeyer BA. A STIM2 splice variant negatively regulates store-operated calcium entry. Nat. Commun. 2015;6:6899. doi: 10.1038/ncomms7899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mignen O, Thompson JL, Shuttleworth TJ. STIM1 regulates Ca2+ entry via arachidonate-regulated Ca2+-selective (ARC) channels without store depletion or translocation to the plasma membrane. J. Physiol. (Lond. ) 2007;579:703–715. doi: 10.1113/jphysiol.2006.122432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mignen O, Thompson JL, Shuttleworth TJ. Both Orai1 and Orai3 are essential components of the arachidonate-regulated Ca2+-selective (ARC) channels. J Physiol (Lond) 2008;586:185–195. doi: 10.1113/jphysiol.2007.146258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mignen O, Thompson JL, Shuttleworth TJ. The molecular architecture of the arachidonate-regulated Ca2+-selective ARC channel is a pentameric assembly of Orai1 and Orai3 subunits. The Journal of Physiology. 2009;587:4181–4197. doi: 10.1113/jphysiol.2009.174193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mikoshiba K. Role of IP3 receptor signaling in cell functions and diseases. Adv. Biol. Regul. 2015;57:217–227. doi: 10.1016/j.jbior.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 72.Mimori-Kiyosue Y, Shiina N, Tsukita S. The dynamic behavior of the APC-binding protein EB1 on the distal ends of microtubules. Curr. Biol. 2000;10:865–868. doi: 10.1016/s0960-9822(00)00600-x. [DOI] [PubMed] [Google Scholar]
- 73.Missiaen L, Robberecht W, Van Den Bosch L, Callewaert G, Parys JB, Wuytack F, Raeymaekers L, Nilius B, Eggermont J, De Smedt H. Abnormal intracellular Ca2+ homeostasis and disease. Cell Calcium. 2000;28:1–21. doi: 10.1054/ceca.2000.0131. [DOI] [PubMed] [Google Scholar]
- 74.Miyazaki S, Shirakawa H, Nakada K, Honda Y. Essential role of the inositol 1,4,5-trisphosphate receptor/Ca2+ release channel in Ca2+ waves amd Ca2+ oscillations at fertilization in mammalian eggs. Dev. Biol. 1993;158:62–78. doi: 10.1006/dbio.1993.1168. [DOI] [PubMed] [Google Scholar]
- 75.Mooren FC, Kinne RKH. Cellular calcium in health and disease. Biochim. Biophys. Acta. 1998;1406:127–151. doi: 10.1016/s0925-4439(98)00006-4. [DOI] [PubMed] [Google Scholar]
- 76.Motiani RK, Zhang X, Harmon KE, Keller RS, Matrougui K, Bennett JA, Trebak M. Orai3 is an estrogen receptor alpha-regulated Ca(2)(+) channel that promotes tumorigenesis. FASEB J. 2013;27:63–75. doi: 10.1096/fj.12-213801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Numaga-Tomita T, Putney JW. Role of STIM1- and Orai1-mediated Ca2+ entry in Ca2+-induced epidermal keratinocyte differentiation. J Cell Sci. 2013;126:605–612. doi: 10.1242/jcs.115980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, Prakriya M, Feske S, Rao A. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol. 2008;9:432–443. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ong HL, Cheng KT, Liu X, Bandyopadhyay BC, Paria BC, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh BB, Gill D, Ambudkar IS. Dynamic assembly of TRPC1/STIM1/Orai1 ternary complex is involved in store operated calcium influx: Evidence for similarities in SOC and CRAC channel components. J. Biol Chem. 2007;282:9105–9116. doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oritani K, Kincade PW. Identification of stromal cell products that interact with pre-B cells. J. Cell Biol. 1996;134:771–782. doi: 10.1083/jcb.134.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Parekh AB, Putney JW. Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 82.Parker NJ, Begley CG, Smith PJ, Fox RM. Molecular cloning of a novel human gene (D11S4896E) at chromosomal region 11p15.5. Genomics. 1996;37:253–256. doi: 10.1006/geno.1996.0553. [DOI] [PubMed] [Google Scholar]
- 83.Parvez S, Beck A, Peinelt C, Soboloff J, Lis A, Monteilh-Zoller M, Gill DL, Fleig A, Penner R. STIM2 protein mediates distinct store-dependent and store-independent modes of CRAC channel activation. FASEB J. 2008;22:752–761. doi: 10.1096/fj.07-9449com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJS, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet JP. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Prakriya M. The molecular physiology of CRAC channels. Immunol Rev. 2009;231:88–98. doi: 10.1111/j.1600-065X.2009.00820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Preston SF, Sha’afi RI, Berlin RD. Regulation of Ca2+ influx during mitosis: Ca2+ influx and depletion of intracellular Ca2+ stores are coupled in interphase but not mitosis. Cell Regul. 1991;2:915–925. doi: 10.1091/mbc.2.11.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Putney JW. A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 88.Putney JW, Bird GS. Calcium signaling in lacrimal glands. Cell Calcium. 2014;55:290–296. doi: 10.1016/j.ceca.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Putney JW, Huang Y, Bird GStJ. Calcium signalling in lacrimal acinar cells. Adv. Exp. Med. Biol. 1998;438:123–128. doi: 10.1007/978-1-4615-5359-5_16. [DOI] [PubMed] [Google Scholar]
- 90.Putney JW, Steinckwich-Besancon N, Numaga-Tomita T, Davis FM, Desai PN, D’Agostin DM, Wu S, Bird GS. The functions of store-operated calcium channels. Biochim. Biophys. Acta. 2017;1864:900–906. doi: 10.1016/j.bbamcr.2016.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Robinson LJ, Mancarella S, Songsawad D, Tourkova IL, Barnett JB, Gill DL, Soboloff J, Blair HC. Gene disruption of the calcium channel Orai1 results in inhibition of osteoclast and osteoblast differentiation and impairs skeletal development. Lab Invest. 2012 doi: 10.1038/labinvest.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ross DG, Smart CE, Azimi I, Roberts-Thomson SJ, Monteith GR. Assessment of ORAI1-mediated basal calcium influx in mammary epithelial cells. BMC. Cell Biol. 2013;14:57. doi: 10.1186/1471-2121-14-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sabbioni S, Barbanti-Brodano G, Croce CM, Negrini M. GOK: a gene at 11p15 involved in rhabdomyosarcoma and rhabdoid tumor development. Cancer Res. 1997;57:4493–4497. [PubMed] [Google Scholar]
- 95.Sauc S, Bulla M, Nunes P, Orci L, Marchetti A, Antigny F, Bernheim L, Cosson P, Frieden M, Demaurex N. STIM1L traps and gates Orai1 channels without remodeling the cortical ER. J. Cell Sci. 2015;128:1568–1579. doi: 10.1242/jcs.164228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Saul S, Gibhardt CS, Schmidt B, Lis A, Pasieka B, Conrad D, Jung P, Gaupp R, Wonnenberg B, Diler E, Stanisz H, Vogt T, Schwarz EC, Bischoff M, Herrmann M, Tschernig T, Kappl R, Rieger H, Niemeyer BA, Bogeski I. A calcium-redox feedback loop controls human monocyte immune responses: The role of ORAI Ca<sup>2+</sup> channels. Sci. Signal. 2016;9:ra26. doi: 10.1126/scisignal.aaf1639. [DOI] [PubMed] [Google Scholar]
- 97.Shennan DB, Peaker M. Transport of milk constituents by the mammary gland. Physiol Rev. 2000;80:925–951. doi: 10.1152/physrev.2000.80.3.925. [DOI] [PubMed] [Google Scholar]
- 98.Shuttleworth TJ, Thompson JL, Mignen O. STIM1 and the noncapacitative ARC channels. Cell Calcium. 2007;42:183–191. doi: 10.1016/j.ceca.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Smyth JT, Beg AM, Wu S, Putney JW, Jr, Rusan NM. Phosphoregulation of STIM1 leads to exclusion of the endoplasmic reticulum from the mitotic spindle. Curr. Biol. 2012;22:1487–1493. doi: 10.1016/j.cub.2012.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smyth JT, Petranka JG, Boyles RR, DeHaven WI, Fukushima M, Johnson KL, Williams JG, Putney JW. Phosphorylation of STIM1 underlies suppression of store-operated calcium entry during mitosis. Nat Cell Biol. 2009;11:1465–1472. doi: 10.1038/ncb1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Orai1 and STIM Reconstitute Store-operated Calcium Channel Function. J. Biol. Chem. 2006;281:20661–20665. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- 102.Steinckwich N, Myers P, Janardhan KS, Flagler ND, King D, Petranka JG, Putney JW. Role of the store-operated calcium entry protein, STIM1, in neutrophil chemotaxis and infiltration into a murine model of psoriasis-inflamed skin. FASEB J. 2015;29:3003–3013. doi: 10.1096/fj.14-265215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Swann K, Yu Y. The dynamics of calcium oscillations that activate mammalian eggs. Int J Dev Biol. 2008;52:585–594. doi: 10.1387/ijdb.072530ks. [DOI] [PubMed] [Google Scholar]
- 104.Takemura H, Hughes AR, Thastrup O, Putney JW. Activation of calcium entry by the tumor promoter, thapsigargin, in parotid acinar cells. Evidence that an intracellular calcium pool, and not an inositol phosphate, regulates calcium fluxes at the plasma membrane. J. Biol. Chem. 1989;264:12266–12271. [PubMed] [Google Scholar]
- 105.Takemura H, Putney JW. Capacitative calcium entry in parotid acinar cells. Biochem. J. 1989;258:409–412. doi: 10.1042/bj2580409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tannheimer SL, Barton SL, Ethier SP, Burchiel SW. Carcinogenic polycyclic aromatic hydrocarbons increase intracellular Ca2+ and cell proliferation in primary human mammary epithelial cells. Carcinogen. 1997;18:1177–1182. doi: 10.1093/carcin/18.6.1177. [DOI] [PubMed] [Google Scholar]
- 107.Targos B, Baranska J, Pomorski P. Store-operated calcium entry in physiology and pathology of mammalian cells. Acta Biochim. Pol. 2005 doi: 10.18388/abp.2005_3452. [DOI] [PubMed] [Google Scholar]
- 108.Toichi E, Tachibana T, Furukawa F. Rapid improvement of psoriasis vulgaris during drug-induced agranulocytosis. J. Am. Acad. Dermatol. 2000;43:391–395. doi: 10.1067/mjd.2000.103264. [DOI] [PubMed] [Google Scholar]
- 109.Trump BF, Berezesky IK. The role of altered [Ca2+]i regulation in apoptosis, oncosis, and necrosis. Biochim. Biophys. Acta. 1996;1313:173–178. doi: 10.1016/0167-4889(96)00086-9. [DOI] [PubMed] [Google Scholar]
- 110.Tu CL, Oda Y, Komuves L, Bikle DD. The role of the calcium-sensing receptor in epidermal differentiation. Cell Calcium. 2004;35:265–273. doi: 10.1016/j.ceca.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 111.Vaeth M, Yang J, Yamashita M, Zee I, Eckstein M, Knosp C, Kaufmann U, Karoly JP, Lacruz RS, Flockerzi V, Kacskovics I, Prakriya M, Feske S. ORAI2 modulates store-operated calcium entry and T cell-mediated immunity. Nat. Commun. 2017;8:14714. doi: 10.1038/ncomms14714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vazquez G, Wedel BJ, Aziz O, Trebak M, Putney JW. The mammalian TRPC cation channels. Biochim. Biophys. Acta. 2004;1742:21–36. doi: 10.1016/j.bbamcr.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 113.Vig M, DeHaven WI, Bird GS, Billingsley JM, Wang H, Rao PE, Hutchings AB, Jouvin MH, Putney JW, Kinet JP. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat Immunol. 2008;9:89–96. doi: 10.1038/ni1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 Is a Plasma Membrane Protein Essential for Store-Operated Ca2+ Entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Volpi M, Berlin RD. Intracellular elevations of free calcium induced by activation of histamine H1 receptors in interphase and mitotic HeLa cells: hormone signal transduction is altered during mitosis. J Cell Biol. 1988;107:2533–2539. doi: 10.1083/jcb.107.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Willoughby D, Everett KL, Halls ML, Pacheco J, Skroblin P, Vaca L, Klussmann E, Cooper DM. Direct binding between Orai1 and AC8 mediates dynamic interplay between Ca2+ and cAMP signaling. Sci. Signal. 2012;5:ra29. doi: 10.1126/scisignal.2002299. [DOI] [PubMed] [Google Scholar]
- 117.Wissenbach U, Niemeyer B, Fixemer T, Schneidewind A, Trost C, Cavalié A, Reus K, Meese E, Bonkhoff H, Flockerzi V. Expression of CaTlike, a novel calcium-selective channel, correlates with the malignancy of prostate cancer. J. Biol. Chem. 2001;276:19461–19468. doi: 10.1074/jbc.M009895200. [DOI] [PubMed] [Google Scholar]
- 118.Xiao B, Coste B, Mathur J, Patapoutian A. Temperature-dependent STIM1 activation induces Ca2+ influx and modulates gene expression. Nat Chem Biol advance online publication. 2011 doi: 10.1038/nchembio.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xing J, Petranka JG, Davis FM, Desai PN, Putney JW, Bird GS. Role of orai1 and store-operated calcium entry in mouse lacrimal gland signaling and function. J. Physiol. (Lond. ) 2013;592:927–939. doi: 10.1113/jphysiol.2013.267740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yang S, Zhang JJ, Huang XY. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell. 2009;15:124–134. doi: 10.1016/j.ccr.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 121.Yu F, Sun L, Machaca K. Constitutive recycling of the store-operated Ca2+ channel Orai1 and its internalization during meiosis. The Journal of Cell Biology. 2010;191:523–535. doi: 10.1083/jcb.201006022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol. 2009 doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc. Natl. Acad. Sci U. S. A. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang X, Zhang W, Gonzalez-Cobos JC, Jardin I, Romanin C, Matrougui K, Trebak M. Complex role of STIM1 in the activation of store-independent Orai1/3 channels. J. Gen. Physiol. 2014;143:345–359. doi: 10.1085/jgp.201311084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhuang L, Peng J-B, Tou L, Takanaga H, Adam RM, Hediger MA, Freeman MR. Calcium-selective ion channel, CaT1, is apically localized in gastrointestinal tract epithelia and is aberrantly expressed in human malignancies. Lab. Invest. 2002;82:1755–1764. doi: 10.1097/01.lab.0000043910.41414.e7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.