Abstract
Hypoxia treatment enhances paracrine effect of mesenchymal stem cells (MSCs). The aim of this study was to investigate whether exosomes from hypoxia-treated MSCs (ExoH) are superior to those from normoxia-treated MSCs (ExoN) for myocardial repair. Mouse bone marrow-derived MSCs were cultured under hypoxia or normoxia for 24 h, and exosomes from conditioned media were intramyocardially injected into infarcted heart of C57BL/6 mouse. ExoH resulted in significantly higher survival, smaller scar size and better cardiac functions recovery. ExoH conferred increased vascular density, lower cardiomyocytes (CMs) apoptosis, reduced fibrosis and increased recruitment of cardiac progenitor cells in the infarcted heart relative to ExoN. MicroRNA analysis revealed significantly higher levels of microRNA-210 (miR-210) in ExoH compared with ExoN. Transfection of a miR-210 mimic into endothelial cells (ECs) and CMs conferred similar biological effects as ExoH. Hypoxia treatment of MSCs increased the expression of neutral sphingomyelinase 2 (nSMase2) which is crucial for exosome secretion. Blocking the activity of nSMase2 resulted in reduced miR-210 secretion and abrogated the beneficial effects of ExoH. In conclusion, hypoxic culture augments miR-210 and nSMase2 activities in MSCs and their secreted exosomes, and this is responsible at least in part for the enhanced cardioprotective actions of exosomes derived from hypoxia-treated cells.
Keywords: Exosomes, hypoxia, MSCs, microRNA210, nSMase2, myocardial infarction
Introduction
Stem cell transplantation is a promising strategy for myocardial repair that may help promote regeneration and reduce adverse remodelling after myocardial infarction (MI) [1]. Bone marrow-derived mesenchymal stem cells (MSCs) have been subject to vigorous preclinical and clinical tests for cardiovascular indications [2,3]. It is widely accepted that the modestly positive outcomes of cell therapy is attributed to paracrine effects of MSCs as opposed to direct cardiac regeneration [4]. Paracrine effects are typically mediated by bioactive components including cytokines, messenger RNAs and microRNAs that are present in secreted membrane vesicles, in particular exosomes [5]. Because cell-free therapies may be a choice of the procedures for treating heart disease, it is important to identify conditions for optimal production of cell free extracts for future clinical application.
Exosomes are biological nanovesicles of 30–120 nm in diameter that are secreted from different types of cells and contain messenger molecules including coding and non-coding RNAs that can regulate cell functions proximally and distally [6]. Various stem cell-derived exosomes have recently emerged as effective regulators of heart restorative process [7]. Studies have shown that exosomes derived from MSCs efficiently replicate the therapeutic activities of live cells in models of myocardial ischemia/reperfusion, infarction and remodelling [8–10]. The contents in exosomes are different, which reflects the parent cells in response to physiologic condition or stress stimuli [11]. Hypoxic precondition of MSCs can enhance their biological activities and, therefore, improve the efficacy of MSCs transplantation for treatment of MI [12–14]. However, it is not known whether such enhancement is mediated by exosomes or how hypoxia affects exosomal signalling.
MicroRNAs (miRNAs) are a class of small (18 ~ 22 nucleotides) single-stranded noncoding RNAs, which can downregulate the expression of target genes by either repressing mRNA translation or promoting mRNA degradation [15]. Exosomal miRNAs play important roles in mediating intracellular cell signalling, trafficking and cell/exosome therapy [16,17]. Furthermore, miRNA profiles in exosomes have been linked to disease pathologies [18]. Because hypoxia preconditioning enhances MSC-mediated therapy and regulates the expression of specific miRNAs, we sought to determine whether hypoxia augments the activity of exosomes by modulating component molecules including miRNAs.
We hypothesize that the salutary effects of hypoxia preconditioning on MSCs therapy for cardiovascular diseases are mediated in part or in whole by parallel effects on exosomes. By comparing the therapeutic efficacies of exosomes derived from MSCs under either normoxia (ExoN) or hypoxia (ExoH) culture condition, we found that ExoH had superior capability to promote angiogenesis, protect cardiomyocytes (CMs) from apoptosis and enhance cardiac regeneration in the infarcted heart. Exosomal enhancement by hypoxia is mediated at least in part by upregulation of miR-210 with a parallel augmentation of the secretory pathway through neutral sphingomyelinase-2 (nSMase2) activity.
Materials and methods
Detailed Methods are available in the Online Data Supplement.
Cell isolation and culture
All animal experiments were performed with approval of the Animal Ethics Committee of Zhejiang University, which complies with the Guide for the Care and Use of Laboratory Animals (2011). Mouse MSCs and Sca-1+ cardiac progenitor cells (CPCs) were isolated from bone marrow and heart, respectively, of adult C57BL/6 male mice, and used at passages 3–6. MSCs were characterized by a flow cytometry for the expression surface markers (Supplemental Figure S1). Neonatal cardiomyocytes (CMs) were isolated from the hearts of newborn mice by digestion with tryptase and collagenase II, and cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% foetal bovine serum (FBS). H9C2 myoblasts and CMs were maintained in DMEM with high glucose. Human umbilical vein endothelial cells (HUVECs) and MSCs were cultured in DMEM with low glucose.
Hypoxia preconditioning
Cultured MSCs were switched from normoxia (21% O2) to hypoxia condition (0.5% O2) and cultured for 24 h. Alternatively, cells were treated with the hypoxia-inducible factor-1 α (HIF-1α) hydroxylase inhibitor dimethyloxalylglycine (DMOG) at a final concentration of 1 mM. For the blockade of exosome generation, 10 μM GW4869, an inhibitor of neutral sphingomyelinase (nSMase2), was added to MSCs culture.
Exosome preparation
For isolation of exosomes from MSCs under normoxia, or hypoxia, or hypoxia with GW4869, or hypoxia with 50 nM miR-210 inhibitor (ExoN, ExoH, ExoH + GW, Exo210 KD, respectively), the conditioned supernatants were collected and centrifuged. Exosomes were isolated by repeated ultracentrifugation and resuspended in PBS. Exosomes were identified by transmission electron microscope (TEM), dynamic light scattering (DLS), Western blot and Nanoparticle Tracking Analysis (NTA).
In vitro cell assays
A Matrigel tube-formation assay was performed to assess in vitro angiogenesis of HUVECs after the cells were exposed to exosomes (ExoN, ExoH, ExoH+GW and Exo210 KD) or transfected with miR-210 mimic for 24 h prior to the Matrigel assay. The apoptosis of CMs was induced by hypoxia/reoxygenation (H/R) [19] and evaluated by TUNEL staining kit. In addition, after treatment with exosomes for 12 h (ExoN, ExoH, ExoH + GW and Exo210 KD), H9C2 apoptosis was induced by H2O2 and detected by flow cytometry assay after Annexin V/propidium iodide (PI) staining. Migration of CPCs after exosome treatment was assessed by using Transwell chambers.
Mouse model of myocardial infarction
Myocardial infarction surgery of male mice (C57BL/6, 6–8 week-old) was performed as described [12] by ligation of left anterior descending coronary artery. Exosomes (ExoN, ExoH derived from 2 × 107 MSCs, in 30 μL PBS) or PBS were injected at five sites around the border zone of infarcted heart.
Ultrasound analysis of cardiac function
After anesthetized, mice were subjected to transthoracic two-dimensional M-mode echocardiography to evaluate cardiac function. Left ventricular ejection fraction (LVEF) and fractional shortening (FS) were calculated as previously described [20] and shown in Supplemental Table S1.
Histochemical staining
Heart tissues were harvested at 3, 7, 14 and 28 d after MI for histological analysis. The infarct size was evaluated by Masson trichrome staining. Immunofluorescence staining was taken to detect capillaries, small arteries and cardiac progenitor cells. Proliferating CMs were detected by co-staining Ki67 with Troponin I (TnI). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI).
Exosomal miRNA array
The microRNA array was processed for three samples: ExoN, ExoH and ExoH + GW. Differentially expressed miRNAs were identified through fold change ≥1.5 with the threshold set for up- and down-regulated genes (Supplemental Figure S5(A) and Table S2). Microarray data have been deposited in NCBI’s Gene Expression Omnibus under accession code GSE102912 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE102912).
MiRNA/siRNA transfections
MiR-210 mimic and its non-specific miRNA negative control, small interfering RNA (siRNA) targeting mouse HIF-1α (siHIF-1α) and its scrambled control siRNA were transfected to target cells (HUVECs, H9C2s or MSCs) using lipofectamine 2000.
Statistical analysis
All data are presented as means ± standard deviation (SD). Continuous variables were compared by the Student t-test. Comparison of more than two groups was performed by 1-way ANOVA. Value of p < .05 was considered significant.
Results
Characterization of exosomes derived from MSCs under hypoxic and normoxic conditions
Exosomes from mouse MSCs under hypoxic or normoxic conditions were morphologically similar with a typical cup-shaped structure, and median 100 nm size as visualized by TEM (Figure 1(A,B)). Dynamic light scattering (DLS) analysis also showed no significant differences of exosome size distribution between ExoN and ExoH (Figure 1(C,D) and Table 1). Western blot analyses showed that exosomes were positive for the exosome-specific markers Alix, CD63 and CD9 (Figure 1(E)). Exposure of MSCs to hypoxia significantly increased the total exosome particles assessed by Nanoparticle Tracking Analysis (NTA) (Figure 1(F)). Both kinds of exosomes could be efficiently internalized into recipient cells as detected by uptaken of fluorescent dye labelled exosomes (Figure 1(G)).
Figure 1.
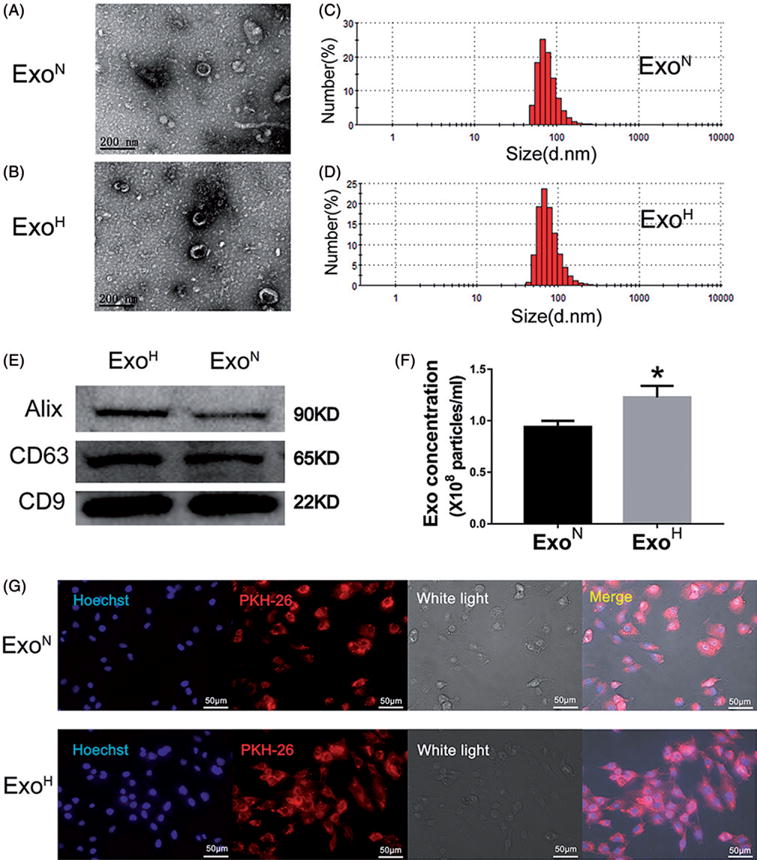
Characterization and functional validation of exosomes derived from mesenchymal stem cells (MSCs). (A,B) Cup-shaped morphology of purified exosomes assessed by TEM. Scale bar =200 nm. (C,D) DLS analysis of ExoN and ExoH shows that both exosomes had the typical size range (30–120 nm). (E) Exosomal markers assessed by Western blotting. (F) The number of exosomal particles assessed by NTA. *p < .05, vs. ExoN. (G) Uptake of red fluorescence dye PKH26 labelled exosomes into H9C2s. Scale bar =50 μm.
Table 1.
The size of exosomes from MSCs at different conditions.
| Size d.nm | ExoN | ExoH | ExoH+GW |
|---|---|---|---|
| 50.7≥ | 5.7 | 8.3 | 0 |
| 58.8 | 18.4 | 19.2 | 0 |
| 68.1 | 25.2 | 23.7 | 4.4 |
| 78.8 | 21.2 | 19.2 | 16.1 |
| 91.3 | 13.8 | 12.7 | 25.5 |
| 106 | 7.8 | 7.7 | 23.9 |
| 122 | 4 | 4.3 | 16.1 |
| 142 | 2.0 | 2.3 | 8.5 |
| ≥164 | 1.9 | 2.4 | 5.3 |
Numbers represent the percentage of fraction at specific size of exosome. Size is for diameter of exosomes.
ExoH are more efficient in pro-angiogenesis and anti-apoptosis in vitro
To investigate the biologic functions of MSC-derived exosomes, HUVECs and CMs were treated with exosomes and subjected to assays for angiogenesis or stress-induced cell apoptosis, respectively. Both types of exosomes promoted tube formation of HUVECs but treatment with ExoH promoted significantly more tubes than did ExoN (Figure 2(A,B)). MSC-derived exosomes also protected CMs from apoptosis caused by hypoxia/reoxygenation. A significant reduction in apoptosis was observed in ExoH-treated CMs (18.3 ± 4.8%) compared with ExoN-treated (25.2 ± 5.5%) and non-treated CMs (34.7 ± 3.9%) (Figure 2(C,D)).
Figure 2.
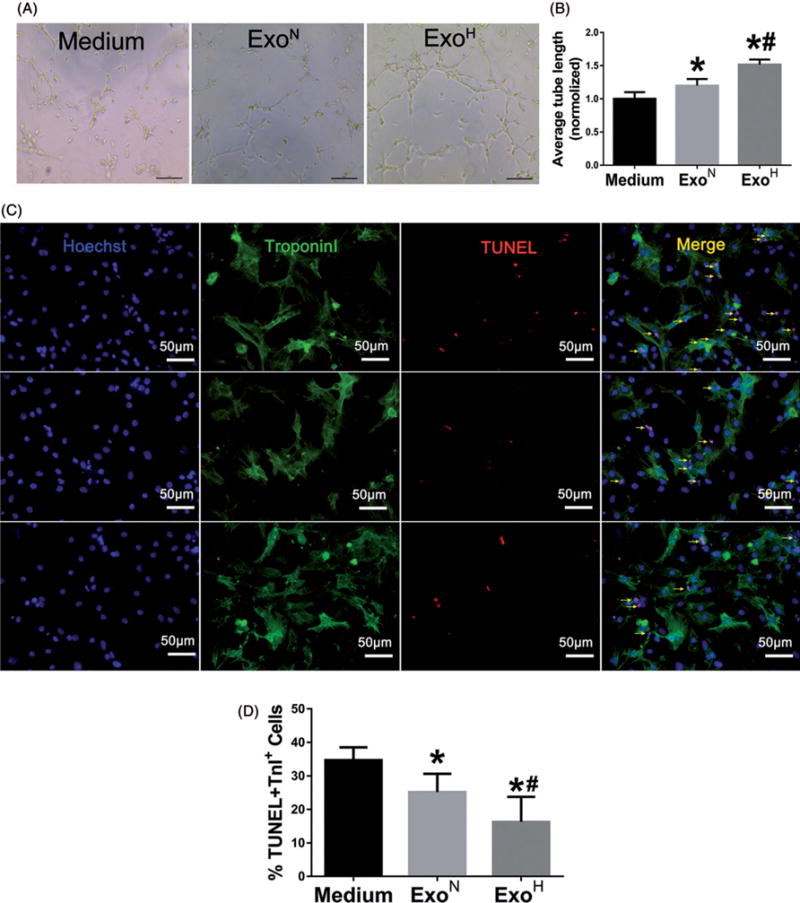
ExoH enhances angiogenic activity and survive of recipient cells. (A) Representative images showing tube formation in HUVECs treated with Medium, ExoN or ExoH. Bar =100 μm. (B) Quantification of tube length in each group (n = 12). (C) TUNEL staining for apoptosis of CMs after treated with Medium (top), ExoN (middle), and ExoH (bottom) and exposure to hypoxia/reoxygenation. Arrows point cells that are positive for both TUNEL (red) and Troponin I (green). Blue: Hoechst. Bar =50 μm. (D) Apoptotic cells were quantified as the percentage of CMs that were positive for TUNEL staining (n = 12), *p < .05, vs. Medium; #p < .05, vs. ExoN.
ExoH had superior ability in cardiac protection after MI
To further investigate the therapeutic efficacy of exosomes in vivo, ExoN, ExoH and PBS were intramyocardially injected into the border zone of mouse hearts in an infarct model (Supplemental Figure S2(A)). ExoH treated mice had the highest survival rate after MI until 4 weeks (Figure 3(A)). Echocardiography (Figure 3(B)) revealed that injection of ExoH limited left ventricular (LV) dilation and improved systolic function with higher EF and FS (Figure 3(C,D)). Histological analysis of the heart indicated significantly decreased scar size in ExoH-injected mice (33.27 ± 6. 77%) compared with ExoN (43.3 ± 4.9%) and PBS (52.6 ± 5. 69%) groups (Figure 3(E,F) and Supplemental Figure S2(B)). These results support a therapeutic superiority of ExoH for MI.
Figure 3.
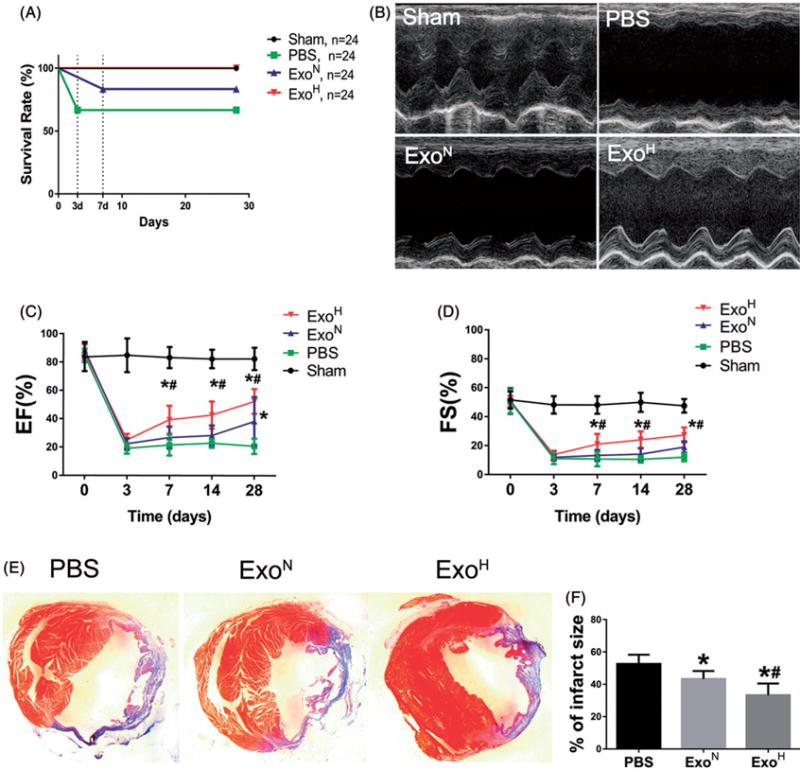
Augmenting cardiac function and ameliorating fibrosis after MI by ExoH. (A) The number of survival animals was recorded for each day of the study period (from 0 to 28 d). (B) Representative echocardiography images showing significantly increased wall motion in ExoH treated hearts. (C,D) Gradually increased ejection fraction (EF) and fractional shortening (FS) in mice transplanted with ExoH compared with other groups (n = 24 for Sham, 16 for PBS, 20 for ExoN and 24 for ExoH). (E) The cross-sections of infarcted hearts were analysed with Masson trichrome staining at 4 weeks after infarction. The fibrosis in the scar of infarcted hearts was shown in blue. (F) The fibrotic scar areas were quantified (n = 6). *p < .05, vs. PBS; #p < .05, vs. ExoN.
ExoH augment neovascularization and cardiomyocyte survive after MI
Immunofluorescence analysis of hearts was performed to determine the mechanism of exosomal therapy and augmented cardiac function after infarction. Capillary density was significantly increased in the ExoH group (Figure 4(A,B)) compared with ExoN or PBS groups 28 d after infarction. Similar trends were observed in arteriole density (Figure 4(C,D)). Treatment with either ExoH or ExoN effectively reduced the number of apoptotic cells in the myocardial peri-infarct areas after MI, and least TUNEL+ cells were observed in ExoH group (Figure 4(E), Supplemental Figure S3(A)).
Figure 4.

ExoH promotes angiogenesis and reduces cardiac apoptosis after infarction. (A) Capillary at the border zone on day 28 after MI was identified by staining with CD31 (red) and nuclei (blue). (B) Quantification of CD31+ cells in A (n = 8). (C) Arterioles at the border zone on day 28 after MI were identified by staining with α-SMA (green) and nuclei (blue). (D) Quantification of α-SMA+ cells in C (n = 8). (E) Quantification of TUNEL+ cells in Supplemental Figure S3(A). Apoptosis rate was quantified as the percentage of cells that were positive for TUNEL staining. (F) Quantification of Sca-1+ cells in Supplemental Figure S3(B) (n = 6). *p < .05, vs. PBS; #p < .05, vs. ExoN. Scale bar =100 μm.
Activation of resident CPCs within the heart may contribute to endogenous repair after MI injury. The number of Sca-1+ CPCs in the peri-infarct areas was greatest in heart injected with ExoH, although there was no statistically significance between the ExoH and ExoN groups (Figure 4(F), Supplemental Figure S3(B)). Cell proliferation was assessed by staining for Ki67+ cells in the recovered hearts 7 d after infarction. ExoH treated hearts had the highest number of Ki67+ cells. However, the number of both Ki67 and Troponin I positive cells representing the proliferating CMs were not significantly different between groups (Supplemental Figure S4(A–C)), suggesting low rates of CMs proliferation that were not affected by exosome treatments. These results indicate that ExoH conferred a significantly improved repair response after MI but activation of endogenous cardiac progenitors may not be the prime mechanisms of exosome therapy.
Roles of miR-210 in superior ExoH-mediated regenerative functions
To further investigate the underlying mechanism of the enhanced ability of ExoH in cardiac protection, microRNA expression profiles of exosomes were analysed by microarray-based miRNA profiling. There were 145 miRNAs upregulated (≥1.5 fold) in ExoH compared with ExoN (Supplemental Figure S5(A) and Supplemental Table S2). According to the miRNA array and published literatures, we focused on the most hypoxia-regulated miR-210. After cultured under hypoxic conditions for 24 h, MSCs had more than 10-fold increased miR-210 confirmed by RT-PCR. The levels of miR-210 in ExoH were also higher than those in ExoN (Supplemental Figure S5(B)).
To determine whether exosomal miR-210 contribute to the angiogenic and anti-apoptosis effects, HUVEC and H9C2 cells were transfected with a miR-210 mimic. Overexpression of miR-210 in HUVEC resulted in enhanced tube formation similar to that achieved by ExoH (Supplemental Figure 6(A)). The apoptosis marker protein cleaved caspase3 and the number of apoptotic cells were decreased in miR-210 mimic group as detected by Western blot and TUNEL staining, respectively (Supplemental Figure 6(B,C)). To further support a role for miR-210, we used inhibitor to selectively block the activity of miR-210 in MSCs. Exosomes derived from thereafter MSCs under hypoxic conditions were isolated and failed to provide the similar effects of pro-angiogenesis on HUVECs (Figure 5(A,B)) and anti-apoptosis on CMs as ExoH (Figure 5(C–F)). These data implicated miR-210 as an essential factor for ExoH executing the functions of pro-angiogenesis and anti-apoptosis.
Figure 5.
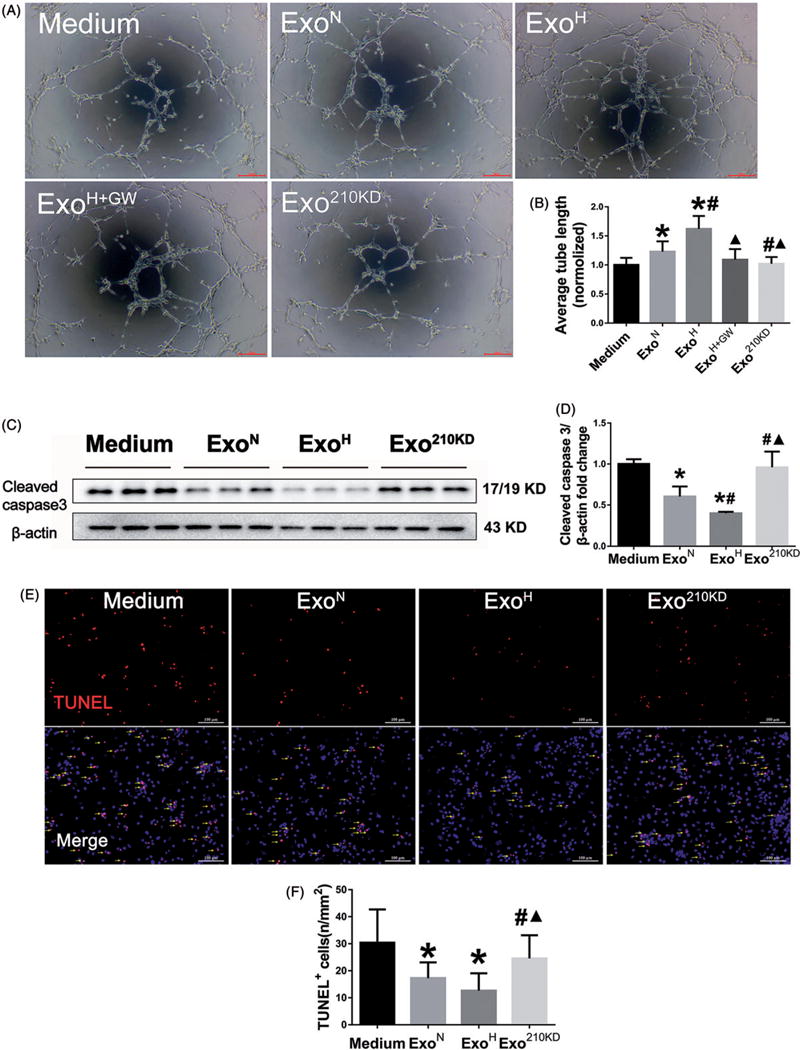
miR-210 mediates the improved biological function of recipient cells. (A) Tube formation assay using HUVECs treated with Medium, ExoN, ExoH, ExoH+GW or Exo210 KD. Scale bar =100 μm. (B) Quantification of tube length in A (n = 6). (C) Western blot for cleaved caspase3 protein in MSCs treated with different exosomes. (D) Quantification of cleaved caspase3 in C (n = 6). (E) TUNEL staining for apoptosis of CMs after treated with exosomes and exposure to hypoxia/reoxygenation. Arrows point cells that are positive for TUNEL. Scale bar = 100μm. (F) Quantification of apoptotic cells in E (n = 6). The number of apoptotic cells was increased in ExomiR210 KD group. *p <.05, vs. Medium; #p<.05, vs. ExoN; ▲p<.05, vs. ExoH.
Exosomal miR-210 transfer to recipient cells in an nSMase2-dependent way
It is known that nSMase2 and the Rab GTPase family Rab27a/b are crucial regulators of exosomal biogenesis and secretion [21]. Indeed, significantly increased expression of nSMase2 (gene:SMPD3) but not Rab27a was detected in hypoxia-treated MSCs in comparison with normoxia-treated MSCs (Figure 6(A,B)). To determine whether hypoxia-enhanced exosomal miR-210 expression is regulated in an nSMase2-dependent manner, nSMase2 activity in MSCs was blocked with a selective inhibitor GW4869 under the hypoxia condition. The levels of miR-210 in conditioned medium and purified exosome were decreased in parallel and in a GW4869 dose-dependent manner (Figure 6(C,D)). In contrast, miR210 levels in normoxic cells were not affected by GW4869 at all tested concentrations.
Figure 6.

nSMase2 modulates miR-210 salutary effects under hypoxia condition. (A) RT-PCR analysis of Rab27a and SMPD3 mRNA in MSCs after normoxia (Control) or hypoxia (HP) treatment (n = 6). (B) Western blot analysis of nSMase2 protein in MSCs (*p < .05, vs. Control). (C,D) Quantification of miR-210 by RT-PCR in conditioned medium (C) and exosomes (D) from MSCs treated with different dose of GW4869 under normoxia (Control) and hypoxia (HP) conditions (n = 6, p < .05, vs. 0 μM; #p < .05, vs. 5 μM). (E) Western blot analysis of apoptotic maker in H9C2s treated with Medium, ExoN, ExoH or ExoH+GW. (F) Quantification of cleaved caspase3
To gain further mechanistic support for roles of miR-210 and nSMase2 in therapy enhancement by hypoxic preconditioning, MSCs were treated with the effective does of GW4869 (10 mM) prior to hypoxic treatments and exosome purification. The effects of exosomes were examined again when nSMase2 activity was inhibited by GW4869. Apoptotic H9C2s cells treated with ExoH+GW were significantly increased as compared with those from the ExoH group by Annexin V-PI double-staining assay (Supplemental Figure S7), which was further confirmed by detection of increased apoptotic marker cleaved caspase3 in H9C2s treated with ExoH+GW (Figure 6(E, F)). The pro-tube formation effect observed in ExoH treated HUVECs was also abolished when HUVECs were treated ExoH+GW (Figure 5(A)). Migration of CPCs treated with different exosomes was analysed by transwell assays. Both ExoN and ExoH can significantly enhance the mobility of CPCs, which was also abrogated when ExoH+GW was used (Figure 6(G,H)).
Hif-1a is involved in modulating the SMPD3 expression
Because HIF-1α is a central regulator of responses to hypoxia and known to have key roles in hypoxia preconditioning, we asked whether normoxic activation of HIF-1α with DMOG, an inhibitor of HIF hydroxylase, affected nSMase2 and exosome release. The optimal concentration of DMOG for stabilized HIF-1α was 1 mM or greater (Supplemental Figure S8(A,B)). As shown in Figure 7(A,B), treatment of MSCs with 1 mM DMOG under normoxia conferred significantly increased expression of nSMase2. Furthermore, blocking HIF-1α with a selective siRNA significantly inhibited both HIF-1α and the nSMase2 activity in response to hypoxia (Figure 7(C,D)). Finally in support of a role for the HIF-1α, MSCs with knockdown of HIF-1α failed to promote enhanced exosomes release as quantified in hypoxia group (Supplemental Figure S9). The enhanced SMPD3 gene expression was also observed in MSCs that overexpressed with HIF-1α (Supplemental Figure S8(C)). Taken together, these results indicate that nSMase2 may be one of the hypoxia-sensitive genes and are directly regulated by HIF-1α.
Figure 7.

HIF-1α modulated nSMase2 expression in MSCs. (A) Western blot assay shows that stabilization of HIF-1α by DMOG increased nSMsase2 expression in MSCs under normoxia condition. (B) Quantification of HIF-1α and nSMase2 proteins in A (n = 3, repeated three times. *p < .05, vs. Control). (C) Western blot analysis of HIF-1α and nSMsase2 in MSCs that were incubated with two siRNA directed against HIF-1α (HIF-1α siRNA) or scrambled control as negative control (NC) and then subjected to hypoxia for 6 hours. (D) Quantification of HIF-1α and nSMase2 proteins in C. (n = 4, repeated two times. *p < .05, vs. NC).
Discussion
Although stem cell-based therapies have been well documented in a lot of preclinical researches, the weakness of low engraftment rate drives us to continue to find the optimal treatment for cardiovascular diseases. Emerging evidences have shown that the cardioprotective effect of MSCs is mainly through secreting paracrine factors including exosomes. Exosomes have been found to offer therapeutic benefit in various heart diseases [22,23]. Results described here suggest that the beneficial effects of hypoxia in augmenting MSC therapy for cardiovascular indications can largely be attributed to effects of hypoxia on exosomal miR-210 levels and the efficiency of secretion through nSMase2. These effects may be partially mediated by HIF-1α. This is supported by the following observations: (1) Hypoxia preconditioned MSCs significantly augmented the pro-angiogenic and cardioprotective properties of exosomes in vitro and rescue of infarction in vivo; (2) The enhanced properties of exosomes by hypoxia were mimicked by pro-miR-210 and eliminated by miR-210 knockdown; (3) Hypoxia activated nSMase2 and inhibition of nSMase2 blocked the enhanced properties of exosomes conferred by hypoxia preconditioning; (4) Pharmacological activation of HIF-1α mimicked the effects of hypoxia on nSMase2 suggesting a direct role of HIF-1α in nSMase2 gene regulation during hypoxia condition. Thus, this research represents a practical strategy to enhance MSCs secreted-exosome contribution to cardiac repair.
Several methods have been used to isolate exosomes, such as ultracentrifugation, precipitation with polymers, and immunoaffinity purification [24]. Exosomes for our studies were isolated by ultracentrifugation following ultrafiltration. They displayed the expected size range (30–120 nm), a traditional morphology and expression of selective exosomal specific markers including tetraspanins (CD9 and CD63) and Alix. Such characterization confirms the presence of exosomes as the dominant vesicle species used for all procedures and responsible for the biological/therapeutic actions described.
The unique properties of exosomes include low toxicity, high stability, hypo-immunogenic and high efficiency of transport to donor cells, which make them attractive agents for clinical therapy. Several approaches have been taken to optimize such therapy by treating donor cell prior to exosome extraction. For example, electroporation or genetic modifications of exosome-producing cells has been used to enhance exosome-mediated therapy through a selective enrichment of secreted factors [10,25–27]. Our results provide evidence for both enhanced therapy and mechanism of action of exosomes by hypoxia. Treating cultured cells or infarcted myocardium with the ExoH resulted in significantly greater angiogenic and cardioprotective results relative to the ExoN or PBS. The magnitude of fibrosis was also decreased and ventricular remodelling was attenuated to greater degrees in the ExoH group relative to ExoN after MI. In addition, hearts in ExoH treatment group showed improved cardiac function at earlier times relative to ExoN, and the therapeutic effects sustained at least to one month.
Microarrays of ExoH versus ExoN identified multiple miRNAs that were upregulated or downregulated by 1.5-fold or more (GEO code:GSE102912). Because the roles of miR-210 in cardio-protection under hypoxic condition have been reported [28,29], we focused on miR-210 and confirmed a robust >10-fold increase of the hypoxia-inducible miR-210 by hypoxic MSCs in the ExoH group. Meanwhile, we admit that other miRNAs in ExoH may also play critical roles for cardiac regeneration. The essential role of miR-210 in therapeutic enhancement by hypoxia was confirmed by gain and loss of function experiments. Exosomes secreted from MSCs that were transfected with miR-210 mimicked the enhanced protection and pro-angiogenic actions of hypoxia pretreatment. Conversely, survival rates of CMs and the capacities for tube formation of HUVEC were significantly reduced when exosomes were derived from MSCs with knockdown of miR-210.
The downstream target genes of miR-210 were not the concern in this study since previous studies have shown that hypoxia-induced miR-210 exhibits an anti-apoptotic effect by downregulating ephrin A3 and caspase-8 associated protein 2 [30,31], and pro-angiogenesis effect by upregulating VEGF [32]. Although the precise mechanism has not be identified, we presume that the increased miR-210 in donor cells by hypoxia is delivered to recipient cells by exosome transferring, and then suppress the downstream mRNA expression in target cells (Figure 8).
Figure 8.

Schematic representation of the effects and mechanisms of hypoxic-MSCs derived exosomes for cardiac repair after myocardial infarction. Hypoxia induced stabilization and nucleus translocation of HIF-1α causing the activation of downstream target nSMase2 (gene SMPD3), presumably through binding putative HREs in the promoter sequence of SMPD3 gene (The dotted line shows the hypothesis which is not confirmed). Multivesicular Bodies (MVBs) are late endosomal compartments that contain intraluminal vesicles as exosomes formed from the inward invagination of endosomal membranes, which requires ceramide generation by nSMase2. Increased miR-210 by hypoxia is encapsulated in the exosomes in an nSMase2 dependent way. Hypoxic-MSCs secreted exosomes deliver miR-210 to the infarct heart, resulting in modulation of angiogenesis, survival and migration of cardiac cells that ultimately leads significant augmentation of cardiac regeneration in the heart after MI.
Previous studies had shown that hypoxia induced enhancement of exosome release [33]. Exosomes are released by the fusion of multivesicular bodies (MVBs) to the plasma membrane. This procedure is modulated by the activities of sphingolipid-metabolizing enzymes, such as neutral sphingomyelinase 2 (nSMase2) and Rab GTPases such as Rab27a and Rab27b [34,35]. However, the mechanisms of hypoxia-regulated exosome release are not fully understood. Intriguing, our data demonstrate that hypoxia specifically increases expression of nSMase2, but not Rab27 in MSCs. Exosomal miR-210 is secreted from MSCs through nSMase2 regulated secretory machinery. Indeed, MSCs with inhibited nSMase2 by GW4869 fail to show the salutary phenotype of derived-exosomes. It is worthy to point out that hypoxia-augmented MV shedding from breast cancer cells was reported to be mediated by the HIF-dependent expression of the small GTPase Rab22A [36]. These results indicate that exosome releasing can be controled by multi-factors and could be cell type-specific.
As a transcriptional factor, the HIF-1 recognizes and binds to the consensus sequence 5′-(A/G) CGTG-3′ named hypoxia-responsive elements (HREs) to activate the transcriptional activity of target genes [37]. Analysis of the mouse SMPD3 promoter using JASPAR core database, we find the presence of putative HREs in the promoter sequence of SMPD3 gene, which indicates HIF-1α might modulate SMPD3 expression through this promoter sequence. In this study, we upregulate HIF-1α expression by stabilizer DMOG and observe the increased expression of nSMase2. In contrast, knockdown the HIF-1α in MSCs shows the decreased nSMase2 expression. These results indicate that under hypoxia condition, increased HIF-1α was translocated into nucleus to bind HRE in the SMPD3 promoter region. Then, the up-regulation of the SMPD3 expression enhances specifically encapsulated miR-210 in released exosomes (Figure 8). Further experiments, such as luciferase reporter assays, electrophoretic mobility shift assay (EMSA) or chromatin immunoprecipitation (ChIP) could be used to confirm the direct interaction of HIF-1α and SMPD3.
Taken together our results support a mechanism whereby hypoxia mediates enhanced production of miR-210 and activation nSMase2, possibly through the actions of HIF-1α. The enriched levels of miR-210 combined with enhanced secretory properties mediated by nSMase2 cause by hypoxia results in exosomes with markedly improved biological properties and therapeutic potential. Hypoxia preconditioning represents a safe, natural and effective approach to optimize the therapeutic actions of MSC-derived exosomes for cardiovascular indications. ExoH exerts their cardioprotection involving transferring miR-210 to the target cells in an nSMase2 dependent pathway.
Supplementary Material
Acknowledgments
We thank Yingchao Wang and Jing Zhao for doing the MI model and echocardiography and Zhongzhi Wei for technical assistance.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 31271585 and 81570251 to HY, 81528002 to YT, 81470382 and 81670257 to JC), Minister of Science and Technology of China (2012CBA1305 to HY), Zhejiang Provincial Natural Science Foundation (No. 2013C24009 to HY). The work in Miami, FL, USA was supported by the National Institutes of Health grants (HL072924 to KAW).
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data for this article can be accessed here.
References
- 1.Lee CY, Kim R, Ham O, et al. Therapeutic potential of stem cells strategy for cardiovascular diseases. Stem Cell Int. 2016;2016:4285938. doi: 10.1155/2016/4285938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahuja P, Perriard E, Perriard JC, et al. Sequential myofibrillar breakdown accompanies mitotic division of mammalian cardiomyocytes. J Cell Sci. 2004;117:3295–3306. doi: 10.1242/jcs.01159. [DOI] [PubMed] [Google Scholar]
- 3.Singla DK. Stem cells and exosomes in cardiac repair. Curr Opin Pharmacol. 2016;27:19–23. doi: 10.1016/j.coph.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Kochegarov A, Lemanski LF. New trends in heart regeneration: a review. J Stem Cells Regen Med. 2016;12:61–68. doi: 10.46582/jsrm.1202010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marote A, Teixeira FG, Mendes-Pinheiro B, et al. MSCs-derived exosomes: cell-secreted nanovesicles with regenerative potential. Front Pharmacol. 2016;7:231. doi: 10.3389/fphar.2016.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai RC, Yeo RW, Lim SK. Mesenchymal stem cell exosomes. Semin Cell Dev Biol. 2015;40:82–88. doi: 10.1016/j.semcdb.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Yellon DM, Davidson SM. Exosomes: nanoparticles involved in cardioprotection? Circ Res. 2014;114:325–332. doi: 10.1161/CIRCRESAHA.113.300636. [DOI] [PubMed] [Google Scholar]
- 8.Arslan F, Lai RC, Smeets MB, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10:301–312. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Yu B, Kim HW, Gong M, et al. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int J Cardiol. 2015;182:349–360. doi: 10.1016/j.ijcard.2014.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barile L, Moccetti T, Marban E, et al. Roles of exosomes in cardioprotection. Eur Heart J. 2016;38:1372–1379. doi: 10.1093/eurheartj/ehw304. [DOI] [PubMed] [Google Scholar]
- 12.Hu X, Xu Y, Zhong Z, et al. A large-scale investigation of hypoxia-preconditioned allogeneic mesenchymal stem cells for myocardial repair in nonhuman primates: paracrine activity without remuscularization. Circu Res. 2016;118:970–983. doi: 10.1161/CIRCRESAHA.115.307516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu X, Wu R, Shehadeh LA, et al. Severe hypoxia exerts parallel and cell-specific regulation of gene expression and alternative splicing in human mesenchymal stem cells. BMC Genomics. 2014;15:303. doi: 10.1186/1471-2164-15-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu X, Wu R, Jiang Z, et al. Leptin signaling is required for augmented therapeutic properties of mesenchymal stem cells conferred by hypoxia preconditioning. Stem Cells. 2014;32:2702–2713. doi: 10.1002/stem.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barwari T, Joshi A, Mayr M. MicroRNAs in cardiovascular disease. J Am Coll Cardiol. 2016;68:2577–2584. doi: 10.1016/j.jacc.2016.09.945. [DOI] [PubMed] [Google Scholar]
- 16.Gray WD, French KM, Ghosh-Choudhary S, et al. Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circ Res. 2015;116:255–263. doi: 10.1161/CIRCRESAHA.116.304360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ong SG, Lee WH, Huang M, et al. Cross talk of combined gene and cell therapy in ischemic heart disease: role of exosomal microRNA transfer. Circulation. 2014;130:S60–S69. doi: 10.1161/CIRCULATIONAHA.113.007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuwabara Y, Ono K, Horie T, et al. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ Cardiovasc Genet. 2011;4:446–454. doi: 10.1161/CIRCGENETICS.110.958975. [DOI] [PubMed] [Google Scholar]
- 19.Zhang N, Ye F, Zhu W, et al. Cardiac ankyrin repeat protein attenuates cardiomyocyte apoptosis by upregulation of Bcl-2 expression. Biochim Biophys Acta. 2016;1863:3040–3049. doi: 10.1016/j.bbamcr.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 20.Wang K, Jiang Z, Webster KA, et al. Enhanced cardioprotection by human endometrium mesenchymal stem cells driven by exosomal MicroRNA-21. Stem Cells Transl Med. 2016;6:209–222. doi: 10.5966/sctm.2015-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahoo S, Losordo DW. Exosomes and cardiac repair after myocardial infarction. Circ Res. 2014;114:333–344. doi: 10.1161/CIRCRESAHA.114.300639. [DOI] [PubMed] [Google Scholar]
- 22.Lee C, Mitsialis SA, Aslam M, et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126:2601–2611. doi: 10.1161/CIRCULATIONAHA.112.114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aliotta JM, Pereira M, Wen S, et al. Exosomes induce and reverse monocrotaline-induced pulmonary hypertension in mice. Cardiovasc Res. 2016;110:319–330. doi: 10.1093/cvr/cvw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Momen-Heravi F, Balaj L, Alian S, et al. Current methods for the isolation of extracellular vesicles. Biol Chem. 2013;394:1253–1262. doi: 10.1515/hsz-2013-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell CR, Berman AE, Weintraub NL, et al. Electrical stimulation to optimize cardioprotective exosomes from cardiac stem cells. Med Hypotheses. 2016;88:6–9. doi: 10.1016/j.mehy.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mackie AR, Klyachko E, Thorne T, et al. Sonic hedgehog-modified human CD34+ cells preserve cardiac function after acute myocardial infarction. Circ Res. 2012;111:312–321. doi: 10.1161/CIRCRESAHA.112.266015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang K, Ma R, Cai W, et al. Exosomes Secreted from CXCR4 overexpressing mesenchymal stem cells promote cardioprotection via Akt signaling pathway following myocardial infarction. Stem Cells Int. 2015;2015:659890. doi: 10.1155/2015/659890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goettsch C, Hutcheson JD, Aikawa E. MicroRNA in cardiovascular calcification: focus on targets and extracellular vesicle delivery mechanisms. Circ Res. 2013;112:1073–1084. doi: 10.1161/CIRCRESAHA.113.300937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barile L, Lionetti V, Cervio E, et al. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc Res. 2014;103:530–541. doi: 10.1093/cvr/cvu167. [DOI] [PubMed] [Google Scholar]
- 30.Kim HW, Haider HK, Jiang S, et al. Ischemic preconditioning augments survival of stem cells via miR-210 expression by targeting caspase-8-associated protein 2. J Biol Chem. 2009;284:33161–33168. doi: 10.1074/jbc.M109.020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fasanaro P, D’Alessandra Y, Di Stefano V, et al. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem. 2008;283:15878–15883. doi: 10.1074/jbc.M800731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng L, He X, Wang Y, et al. MicroRNA-210 overexpression induces angiogenesis and neurogenesis in the normal adult mouse brain. Gene Ther. 2014;21:37–43. doi: 10.1038/gt.2013.55. [DOI] [PubMed] [Google Scholar]
- 33.King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ostrowski M, Carmo NB, Krumeich S, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19–30. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- 35.Kowal J, Tkach M, Thery C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Wang T, Gilkes DM, Takano N, et al. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc Natl Acad Sci USA. 2014;111:E3234–E3242. doi: 10.1073/pnas.1410041111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaluz S, Kaluzova M, Stanbridge EJ. Regulation of gene expression by hypoxia: integration of the HIF-transduced hypoxic signal at the hypoxia-responsive element. Clin Chim Acta. 2008;395:6–13. doi: 10.1016/j.cca.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


