Abstract
Objectives
Sepsis-3 recommends using the quick Sequential Organ Failure Assessment (qSOFA) score followed by SOFA score for sepsis evaluation. The SOFA is complex and unfamiliar to most emergency physicians, while qSOFA is insensitive for sepsis screening and may result in missed cases of sepsis. The objective of this study was to devise an easy-to-use simple SOFA score for use in the emergency department (ED).
Methods
Retrospective study of ED patients with sepsis with in-hospital mortality as the primary outcome. A simple SOFA score was derived and validated and compared with SOFA and qSOFA.
Results
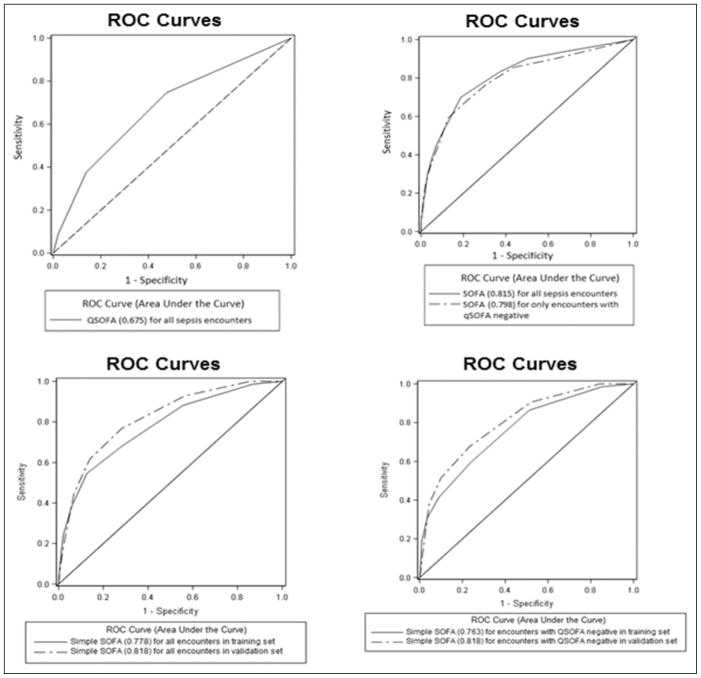
A total of 3297 patients with sepsis were included, and in-hospital mortality was 10.1%. Simple SOFA had a sensitivity and specificity of 88% and 44% in the derivation set and 93% and 44% in the validation set for in-hospital mortality, respectively. The sensitivity and specificity of qSOFA was 38% and 86% and for SOFA was 90% and 50%, respectively. There were 2760 (84%) of 3297 qSOFA-negative (<2) patients. In this group, simple SOFA had a sensitivity and specificity of 86% and 48% in the derivation set and 91% and 48% in the validation set, respectively. Sequential Organ Failure Assessment was 86% sensitive and 57% specific in qSOFA-negative patients. For all encounters, the areas under the receiver–operator characteristic curves (AUROC) were 0.82 for SOFA, 0.78 (derivation) and 0.82 (validation) for simple SOFA, and 0.68 for qSOFA. In qSOFA-negative patients, the AUROCs were 0.80 for SOFA and 0.76 (derivation) and 0.82 (validation) for simple SOFA.
Conclusions
Simple SOFA demonstrates similar predictive ability for in-hospital mortality from sepsis compared to SOFA. External validation of these findings is indicated.
Keywords: sepsis, septic shock, organ dysfunction, infection
Introduction
Despite years of research and emphasis on early recognition, sepsis remains a disease with a high morbidity and mortality that presents a diagnostic challenge. In the most recent revision of the sepsis definitions, Sepsis-3, an expert consensus panel redefined sepsis as a “dysregulated host response to infection,” rather than a “systemic response,”1 allowing for the concept of concomitant inflammation and immunosuppression.2–5 This shift represents the most significant change to the definition of sepsis since its inception.
Central to Sepsis-3 is a focus on organ dysfunction in the setting of infection, which is quantified using the Sequential (sepsis-related) Organ Failure Assessment (SOFA) score. Sequential Organ Failure Assessment was designed for patients with sepsis and has been validated as a potent predictor of clinical outcomes after sepsis.6–11 In their recommendations, the study group also derived a tool for bedside sepsis screening in non-intensive care unit (ICU) settings called quick SOFA, or qSOFA, which assigns points for respiratory rate ≥22 breaths/ minute, altered mental status, or systolic blood pressure ≤ 100 mm Hg.12 If qSOFA< 2, calculation of a full SOFA score using laboratory results is recommended,1 with SOFA ≥2 constituting sepsis.1
However, the new definitions have been criticized for being overly focused on existing organ dysfunction rather than detecting those at risk for subsequent dysfunction; for concerns that qSOFA is insensitive as a screening tool13; and for replacing previous definitions that led to improvements in clinical care and patient outcomes.14–16 Other barriers to translation to clinical practice include the need for arterial blood gas measurements for the ratio of arterial partial pressure of oxygen to the fraction of inspired oxygen (FiO2); the need for liver function tests for total bilirubin; the lack of familiarity of noncritical care practitioners with SOFA as well as it’s complexity; and the inability to apply the definitions in austere environments due to the lack of certain laboratory tests to make the diagnosis.
One major potential pitfall of using qSOFA as a screening test for sepsis is that whether qSOFA is negative (score < 2), patients will require a full SOFA score to diagnose sepsis. Clinical application of SOFA is therefore potentially problematic, as its complexity may lead to its abandonment. A simple SOFA score that uses similar but readily available quantifiable parameters would have enhanced clinical utility if test characteristics can be maintained. The primary objective of this study, therefore, was to derive and validate simple SOFA for prediction of in-hospital mortality in patients admitted from the emergency department (ED) with sepsis. Our goal was for simple SOFA to have similar predictive ability to full SOFA and be more sensitive than qSOFA, having particular utility in qSOFA-negative patients. We also sought to compare the prognostic value of SOFA, qSOFA, and the simple SOFA score. We hypothesize that the simple SOFA score will be an accurate substitute for SOFA, useful for predicting in-hospital mortality from sepsis.
Methods
Study Design and Selection of Participants
We performed a retrospective review of all adult patients (age ≥18 years) admitted through the UF Health Jacksonville ED with a diagnosis of sepsis, and with a primary discharge diagnosis of sepsis from October 1, 2013, to May 15, 2016. UF Health Jacksonville is a not-for-profit institution, a 696-bed level 1 trauma center with 142 intensive care beds, and a regional referral center.
Using methods similar to Angus et al, patients meeting clinical criteria for sepsis on admission, and with a discharge International Classification of Diseases, Ninth Revision or International Classification of Diseases, Tenth Revision code for sepsis were identified via the electronic health record.17 Patients who were incarcerated were excluded from the study. Review of this study and approval was obtained from the institutional review board of UF Health Jacksonville .
Data Collection and Variables
The retrospective electronic health record query yielded data on patient demographics (age, sex, and race), initial vital signs, and laboratory values for the calculation of scoring criteria within 24 hours of ED presentation. Additional data including culture results, inpatient disposition, ICU length of stay, mechanical ventilation use, vasopressor use, and in-hospital mortality were also collected. Comorbidities were quantified using the Charlson Comorbidity Score.18,19
Scoring
Sequential Organ Failure Assessment score is a quantitative score-based assessment of 6 organ systems with points assigned from 0 (no dysfunction) to 4 (severe dysfunction), with 24 being the highest possible total score. All data for SOFA score calculation were abstracted from the chart retrospectively. Data were taken from the earliest set of laboratory, vital signs, or clinical assessments from the first 24 hours of admission and preferentially from ED data. Glasgow coma scale was obtained from nursing documentation. Quick Sequential Organ Failure Assessment criteria were calculated as described earlier.3
Statistical Analysis
Simple SOFA criteria selection
The patient encounters were randomly assigned to 1 of 2 data sets, with 2209 encounters (two-thirds) in the derivation set and 1088 encounters in the validation set. Univariate testing of relevant clinical and laboratory criteria which are either easily attainable in the ED or already included in SOFA was performed on both derivation and validation sets. These included any vasopressor or inotrope use, FiO2 > .21 (room air), FiO2 > .27 (2 liters nasal cannula), FiO2 > .33 (4 liters nasal cannula), creatinine > 1.2 mg/dL, platelet count < 150 × 103/mm3, bands > 5%, heart rate > 100 beats/ min, heart rate > 90 beats/min, lactate > 2 mmol/L, and lactate > 4 mmol/L. We excluded total bilirubin measurement, as liver function tests are not frequently ordered for diagnosing sepsis in the ED and are least likely to add value in patients without scleral icterus or jaundice.20 Similarly, the neurologic component of the score based on GCS was eliminated due to confounding of intubation status secondary to sedative medications, previously reported inaccuracies in its calculation in routine clinical practice,21 and data suggesting that it adds little to the predictive value of the SOFA score.22 Bands >5% was included as relevant laboratory criteria, as there is data to suggest its predictive ability for mortality in ED patients with sepsis.23 As there were no differences in the discriminant abilities of higher FiO2 levels over FiO2 > 0.21 in the univariate analysis, FiO2 > .21 was chosen for inclusion in the regression analysis. Heart rate >100 beats/min was more strongly associated with in-hospital mortality from sepsis than a cutoff of 90 beats/min, and previous work from our group has shown that heart rate >100 beats/min on admission is associated with in-patient mortality in ED patients.24 Cut points for variables included from full SOFA were based on the minimal value required for a component SOFA score of 1 for creatinine and platelet count.
A multivariable logistic regression model was created using the derivation set to generate the scoring for simple SOFA with missing data imputed using multiple imputation. The Hosmer and Lemeshow goodness-of-fit test was used to test the model fit, and Pearson χ2 was applied to compare observed counts with expected counts. Scoring for each criterion was determined by rounding the unadjusted odds ratio to the nearest integer and dividing by 2, with a value ≥2 assigned a score of 2 and value <2 assigned a score of 1. Final criteria included in simple SOFA were any vasopressor or inotrope use (2 points), creatinine >1.2 mg/dL (1 point), platelet count <150 × 103/mm3 (1 point), FiO2 >21% (1 point), HR >100 beats/min (1 point), and bands >5% (1 point; Table 1).
Table 1.
Simple SOFA.a
| Simple SOFA Clinical Criteria for Diagnosing Sepsis | ||
|---|---|---|
|
| ||
| Criteria | Points | Odds Ratio (95% CI) |
| Any vasopressor or inotrope use | 2 | 3.5 (2.4–5.1) |
| FiO2 > 21% | 1 | 3.2 (2.2–4.7) |
| Creatinine > 1.2 mg/dL | 1 | 1.7 (1.2–2.5) |
| Platelet count < 150 × 103/mm3 | 1 | 1.5 (1.0–2.2) |
| Bands > 5% | 1 | 1.2 (0.8–1.8) |
| Heart Rate > 100 beats/min | 1 | 0.9 (0.7–1.3) |
Abbreviations: CI, confidence interval; FiO2, fraction of inspired oxygen; SOFA, sequential organ failure assessment.
Score ≥ 2 is considered positive.
Derivation and Validation Methods
To assess the performance of simple SOFA ≥ 2 in predicting the outcomes, receiver–operator characteristic (ROC) analysis using validation data was used. The performance of the same model fit (trained) using the derivation data set was then assessed by applying it to the validation data set. The fit of the model was assessed using the Hosmer and Lemeshow goodness-of-fit test.
Counts and percentages were used to summarize categorical data. Medians and lower and upper quartiles were used to summarize continuous data. The primary outcome was the rate of all-cause, in-hospital mortality. Sensitivities and specificities were calculated for patients with a discharge diagnosis of sepsis with a score of 2 or more for SOFA, qSOFA, or simple SOFA and were compared to patients with a score of <2. Areas under the ROC curves (AUROCs) were calculated for each outcome for all SOFA, qSOFA, and simple SOFA, respectively. All analyses were performed using SAS 9.4 (Cary, North Carolina).
Results
There were 3297 encounters for sepsis meeting study criteria. Mean age was 58 years (standard deviation [SD]: 17.3), with 51% female patients, 51% African American, and 44% caucasian patients. The overall in-hospital mortality was 332 (10.1%) of 3297 . There were 861 (26%) of 3297 patients who required vasopressors, while 189 (6%) of 3297 required mechanical ventilation and 748 (22.7%) of 3297 patients had an ICU length of stay ≥3 days. Mean lactate upon presentation was 2.8 mmoL/L (SD: 2.9). Demographic characteristics are presented in Table 2. Features of sepsis encounters are presented in Table 3.
Table 2.
Patient Characteristics for all Patients, Derivation Set, and Validation Set.
| Variable | Overall, n = 3297 | Derivation, n = 2209 | Validation, n = 1088 | P Value |
|---|---|---|---|---|
| Age | ||||
| Median (Q1, Q3) | 59 (48,70) | 59 (47, 59) | 59 (48, 70) | .594a |
| Gender | ||||
| Female | 1685 (51) | 1122 (51) | 563 (52) | .606b |
| Race | ||||
| African American | 1685 (51) | 1131 (51) | 554 (50) | .856b |
| White | 1455 (44) | 970 (44) | 485 (45) | |
| Other | 157 (5) | 108 (5) | 49 (5) | |
| Comorbidities (%) | ||||
| AIDS | 119 (4) | 30 (3) | 89 (4) | .065b |
| Cancer | 340 (10) | 108 (10) | 232 (11) | .600b |
| CHF | 689 (21) | 235 (22) | 454 (21) | .499b |
| COPD | 1095 (33) | 380 (35) | 715 (32) | .149b |
| CVD | 277 (8) | 100 (9) | 177 (8) | .256b |
| DM (no complications) | 1020 (31) | 336 (31) | 684 (31) | .943b |
| DM (complications) | 255 (8) | 82 (8) | 173 (8) | .758b |
| Dementia | 175 (5) | 55 (5) | 120 (5) | .643b |
| ESRD | 263 (8) | 95 (9) | 168 (8) | .262b |
| Liver (mild) | 342 (10) | 104 (10) | 238 (11) | .276b |
| Liver (moderate-severe) | 72 (2) | 21 (2) | 51 (2) | .481b |
| Myocardial infarction | 234 (7) | 80 (7) | 154 (7) | .696b |
| Metastatic cancer | 114 (3) | 36 (3) | 78 (4) | .738b |
| Charlson index | ||||
| Median (Q1, Q3) | 2 (1,4) | 2 (1,4) | 2 (1,4) | .752a |
Abbreviations: AIDS, acquired immunodeficiency syndrome; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; DM, diabetes mellitus; ESRD, end-stage renal disease; Q1, 1st quartile; Q3, 3rd quartile.
Wilcoxon rank-sum test.
Pearson χ2 test.
Table 3.
Features of Sepsis Encounters for all Patients, Derivation Set, and Validation Set.a
| Variable | Overall, n = 3297 | Derivation Set, n = 2209 | Validation Set, n = 1088 | P Value |
|---|---|---|---|---|
| Initial vital signs | ||||
| SBP (mm Hg) | 123.6 (30.5); n = 3013 | 123.8 (30.9); n = 2010 | 124.1 (30.2); n = 982 | .471b |
| HR (beats/min) | 103.4 (22.9); n = 3168 | 103.4 (23.0); n = 2124 | 103.5 (22.9); n = 1044 | .884b |
| RR (breaths/min) | 19.8 (5.1); n = 3292 | 19.8 (5.1); n = 2206 | 19.8 (5.2); n = 1086 | .954b |
| Temperature (°F) | 99.1 (2.3); n = 3294 | 99.0 (2.3); n = 2208 | 99.2 (2.3); n = 1086 | .092b |
| SpO2 (%) | 96.2 (5.4); n = 1135 | 96.2 (5.3); n = 2204 | 96.1 (5.5); n =1 083 | .186b |
| Laboratory findings | ||||
| Initial WBC (thousand/mm3) | 14.5 (7.8); n = 3296 | 14.4 (7.7); n = 2208 | 14.5 (8.1); n = 1088 | .973b |
| Lactate, (mmol/L) | 2.8 (2.9); n = 1647 | 2.9 (2.9); n = 1099 | 2.8 (2.9); n = 548 | .640b |
| Lactate, n (%) | ||||
| ≥ 4 mmol/L | 274 (17) | 184 (17) | 90 (16) | .982c |
| 2 to 3.9 mmol/L | 530 (32) | 354 (32) | 176 (32) | |
| < 2 mmol/L | 843 (5) | 561 (51) | 282 (52) | |
| Creatinine (mg/dL) | 1.8 (2.1); n = 3297 | 1.8 (2.0); n = 2209 | 1.9 (2.3); n = 1088 | .953b |
| Positive culture, n (%) | ||||
| Blood | 969 (36) | 642 (36) | 327 (35) | .668c |
| Respiratory | 666 (25) | 443 (25) | 223 (24) | |
| Urine | 911 (34) | 597 (33) | 314 (34) | |
| Wound | 164 (7) | 101 (6) | 63 (7) | |
| Outcomes | ||||
| Inpatient mortality | 332 (10.1) | 220 (10) | 112 (10) | .764c |
| ICU LOS ≥3 days | 748 (22.7) | 487 (22) | 261 (24) | .210c |
| Severity of Illness, n (%) | ||||
| qSOFA Positive (≥2) | 537 (16) | 371 (17) | 166 (15) | .505c |
| qSOFA negative (<2) and SOFA≥2 | 1287 (39) | 861 (39) | 426 (39) | |
| qSOFA negative (<2) and SOFA <2 | 1473 (44) | 977 (44) | 496 (46) | |
Abbreviations: HR, heart rate; ICU, intensive care unit; qSOFA, quick sequential organ failure assessment; RR, respiratory rate; SBP, systolic blood pressure; WBC, white blood cell count; LOS, length of stay.
Data are means (SDs), unless otherwise specified.
Wilcoxon rank sum test.
Pearson χ2 test.
Adjusting for all the other variables in the model, the odds ratios for simple SOFA components for in-patient mortality were 3.5 (95% confidence interval [CI]: 2.4–5.1) for any vasopressor or inotrope use, 3.2 (95% CI: 2.2–4.7) for FiO2 >21%, 1.7 (95% CI: 1.2–2.5) for creatinine >1.2 mg/dL, 1.5 (95% CI: 1.0–2.2) for platelet count <150 × 103/mm3, 1.2 (95% CI: 0.8–1.8) for bands >5%, and 0.9 (95% CI: 0.7–1.3) for heart rate >100 beats/min. Although the confidence intervals for bands >5% and heart rate >100 beats/min did not achieve significance, they were included in the final score because they improved score performance, enhancing both sensitivity and specificity.
For patients included in this study, 1783 (54.1%) of 3297 had SOFA ≥2 and 537 (16%) of 3297 had qSOFA ≥2. For all patients, the sensitivity and specificity of SOFA ≥2 for inhospital mortality were 90% and 50%, and for qSOFA ≥2 were 38% and 86%, respectively. Simple SOFA ≥2 had a sensitivity and specificity of 88% and 44% in the derivation set and 93% and 44% in the validation set for in-hospital mortality, respectively (Table 4).
Table 4.
Sensitivity and Specificity for Inpatient Mortality for all Sepsis Encounters and qSOFA-Negative Encounters.a,b,c
| Sensitivity and Specificity for Inpatient Mortality for All Sepsis Encounters (n = 3297) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Variable | Inpatient Mortality, N (%)
|
Sensitivity (95% CI)
|
Specificity (95% CI)
|
||||||
| Total | Derivation | Validation | Total | Derivation | Validation | Total | Derivation | Validation | |
| SOFA ≥ 2 | 299 (17) | 198 (16) | 101 (17) | 90%(87% to 93%) | 90%(86% to 94%) | 90%(85% to 96%) | 50%(48% to 52%) | 50%(47% to 52%) | 51%(48% to 54%) |
| qSOFA≥ 2 | 125 (23) | 87 (23) | 38 (23) | 38%(32% to 43%) | 40%(33% to 46%) | 34%(25% to 43%) | 86%(85% to 87%) | 86%(84% to 87%) | 87%(85% to 89%) |
| Simple SOFA ≥ 2 | 298 (15) | 194 (15) | 104 (16) | 90%(86% to 93%) | 88%(84% to 92%) | 93%(88% to 98%) | 44%(42% to 46%) | 44%(42% to 47%) | 44%(40% to 47%) |
|
| |||||||||
| Sensitivity and Specificity for Inpatient Mortality for qSOFA-Negative Encounter (n=2760) | |||||||||
|
| |||||||||
| SOFA score ≥ 2 | 177 (14) | 114 (13) | 63 (15) | 86%(81% to 90%) | 86%(80% to 92%) | 85%(77% to 93%) | 57%(55% to 58%) | 56%(54% to 59%) | 57%(54% to 61%) |
| Simple SOFA ≥ 2 | 182 (12) | 115 (12) | 67 (13) | 88%(83% to 92%) | 86%(81% to 92%) | 91%(84% to 97%) | 48%(47% to 50%) | 48%(46% to 51%) | 48%(45% to 51%) |
Abbreviations: CI, confidence interval; qSOFA, quick sequential organ failure assessment; SOFA, sequential organ failure assessment.
n = 3297.
Inpatient mortality by SOFA, qSOFA, and simple SOFA ≥ 2 for all septic encounters and encounters with qSOFA negative for derivation and validation sets.
Percentage is inpatient mortality divided by total number of patients with each score ≥2.
Of all sepsis admissions, 2760 (84%) of 3297 were qSOFA negative (< 2) and formed the basis of the subgroup of primary interest in this study. Of these, 1287 (47%) had a SOFA ≥2. In qSOFA-negative patients, simple SOFA had a sensitivity and specificity of 86% and 48% in the derivation set and 91% and 48% in the validation set for in-hospital mortatlity, respectively. This was very similar to the sensitivity and specificity of full SOFA at 86% and 57%, respectively.
Receiver–operator characteristic curves for all sepsis admissions and for qSOFA-negative encounters were generated and AUROCs calculated (Figure 1). For all encounters, AUROCs were 0.82 for SOFA, 0.78 (derivation) and 0.82 (validation) for simple SOFA, and 0.68 for qSOFA. In qSOFA-negative patients, the AUROCs were 0.80 for SOFA and 0.76 (derivation) and 0.82 (validation) for simple SOFA. Additionally, a graphical representation of mortality with increasing simple SOFA score is presented in Figure 2.
Figure 1.
Receiver–operator characteristic (ROC) curves with areas under the curve calculated. Encounters analyzed by all sepsis encounters and quick Sequential Organ Failure Assessment (qSOFA)-negative encounters for sequential organ failure assessment (SOFA). Simple SOFA analyzed by all sepsis encounters in derivation and validation sets, and qSOFA-negative encounters in derivation and validation sets. Receiver–operator characteristic curve for qSOFA is for all sepsis encounters. Areas under the ROC curves for each score are displayed parenthetically in the legend.
Figure 2.
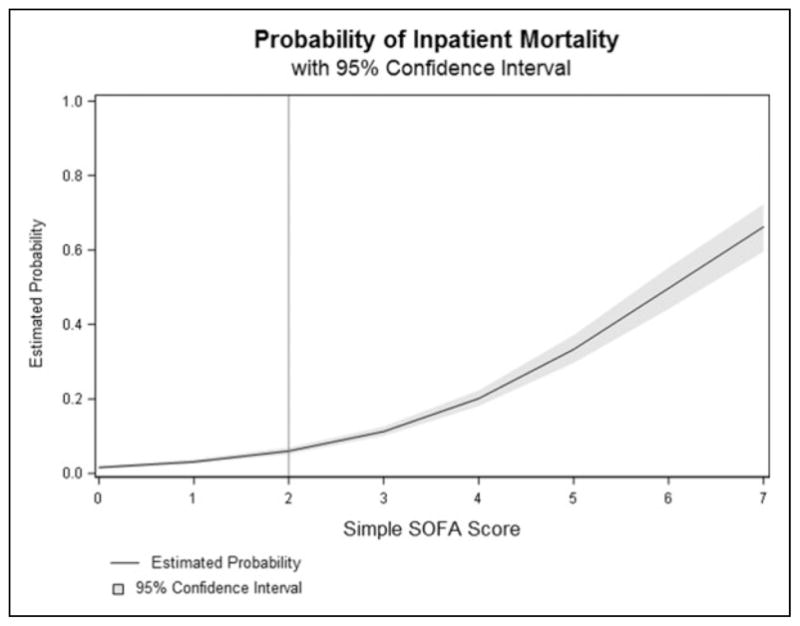
Estimated probability of death with increasing simple Sequential Organ Failure Assessment (SOFA) score with 95% confidence intervals.
Discussion
In this retrospective study of ED patients with sepsis, we have demonstrated that an easy-to-use simple SOFA score using widely available clinical criteria has similar sensitivity and specificity to SOFA for predicting in-hospital mortality. In addition, simple SOFA had very similar sensitivity and specificity to SOFA in qSOFA-negative patients, a population we believe is at risk of being missed for the diagnosis of sepsis.
The proposal to use SOFA score as part of the sepsis definition is based on the well-validated predictive ability of SOFA score for important outcomes after sepsis.9–14 However, SOFA was not intended for bedside or ED use and has several barriers that prevent its clinical adoption, which provided the rationale for qSOFA. As multiple groups have demonstrated, qSOFA is insensitive with a sensitivity of 38% for in-hospital mortality in this study.13,25–27 Therefore, qSOFA is clearly not a viable screening test but rather a rapid “rule-in” for identification of high-risk sepsis. This fact is concerning given that hospital EDs and general wards may tailor their sepsis screening protocols based on the poorly sensitive qSOFA criteria. Although the findings of this study must be validated, our concept offers a usable alternative to SOFA in qSOFA-negative patients with suspected sepsis.
Of note in this study was that of 332 in-hospital mortalities from sepsis, none of the evaluated scores was sufficiently sensitive to identify all patients. Sequential Organ Failure Assessment had a sensitivity of 90% and identified 299 patients, while simple SOFA had a sensitivity of 90% and identified 298 patients. Quick Sequential Organ Failure Assessment was least sensitive identifying only 125 patients from the general population. One must keep in mind that no scoring system is perfect, and while 2 of the 3 scores performed quite well, 33 patients who succumbed to sepsis would still have been missed by all scores.
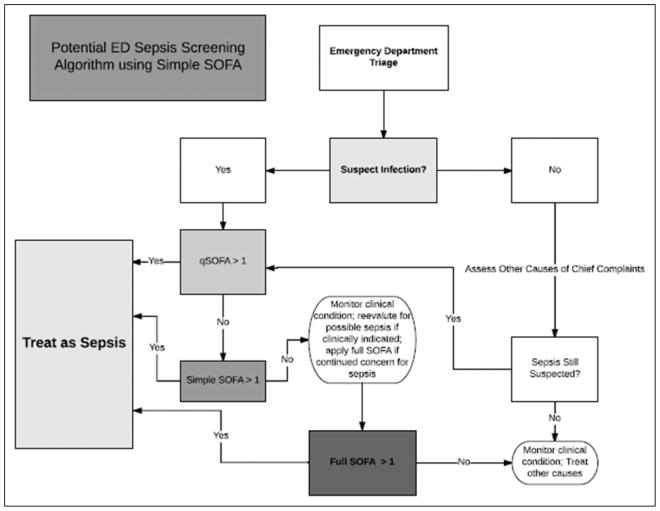
After further validation, we propose that the findings of this study could be practically applied during a patient’s initial evaluation for sepsis. First, qSOFA should be applied and if >1, this would prompt immediate treatment with early, aggressive care, given the high mortality of this subset of patients. If negative, a focused clinical and laboratory assessment would be initiated with application of the simple SOFA score which, if positive, would prompt early, aggressive care for sepsis (Figure 3). Patients who are both qSOFA negative and simple SOFA negative could be treated at the discretion of the providers based on clinical judgment, as full SOFA score would likely have marginal if any increased sensitivity for adverse clinical outcomes based on these data.
Figure 3.
Potential emergency department sepsis screening algorithm using simple Sequential Organ Failure Assessment (SOFA).
In comparison to the study by Seymour et al, which demonstrated an AUROC of 0.69 for qSOFA in the baseline model, the unadjusted AUROC of 0.68 from this study is nearly identical.12 Only after adjustment for decile of baseline risk did the AUROC improve to 0.81 in the Seymour study. The authors also note that “most encounters with infection had less than 2 qSOFA points, and mortality ranged from 1% to 24% over the score range” and that “24% of encounters with infection with 2 or 3 qSOFA points accounted for 70% of deaths or ICU stays.” In comparison with this study, the sensitivity and specificity in the general population of 38% and 86%, respectively, are comparable. However, if one considers that we are trying to identify patients at high risk of death, using qSOFA alone would have resulted in an unacceptably high number (207) of missed cases with sepsis. This reiterates the need for subsequent formalized risk assessment if qSOFA is negative.
This study had several limitations. This was a retrospective, single-center study of a new scoring system and external validation is recommended. However, procedures for identifying sepsis based on diagnosis codes were standardized and based on previously validated methods.17 This study was limited to determining predictive ability for meaningful outcomes after sepsis (mortality) rather than confirming the sepsis diagnosis and as such may have missed some sepsis cases that did not receive the sepsis diagnosis. However, we do believe that the mortalities, incidences of organ failure, and need for advanced care, such as vasopressors and ICU care are reflective of the bulk of the sepsis literature. Also, because patients were identified using billing diagnosis codes, the performance of this score is unknown among patients with sepsis who lack a billing code diagnosis of sepsis.
Conclusion
In this analysis, simple SOFA performed similar to SOFA for predicting in-hospital mortality in all patients admitted with sepsis as well as qSOFA-negative patients with sepsis. External validation of these findings is indicated.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr Guirgis (K23GM115690), Dr Puskarich (K23GM113041), Dr Moore (P50 GM111152), and Dr Jones (R01GM103799) have received support from the National Institutes of General Medical Sciences. Dr Puskar-ich also receives support from the NIH Loan Repayment Program. The authors have no conflicts of interest to disclose. Funding sources did not directly pay for the study but provided protected time for the authors in conducting the research.
We would like to thank the Director of the UF Health Jacksonville Research Division Dr Colleen J. Kalynych, as well as our Research Coordinators Jennifer Bowman MPH, Taylor Miller, and Morgan Henson and all of our staff for assistance with data collection and processing.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boomer JS, To K, Chang KC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306(23):2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walton AH, Muenzer JT, Rasche D, et al. Reactivation of multiple viruses in patients with sepsis. PLoS One. 2014;9(2):e98819. doi: 10.1371/journal.pone.0098819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immuno-suppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13(12):862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gentile LF, Cuenca AG, Efron PA, et al. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72(6):1491–1501. doi: 10.1097/TA.0b013e318256e000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones AE, Trzeciak S, Kline JA. The Sequential Organ Failure Assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation. Crit Care Med. 2009;37(5):1649–1654. doi: 10.1097/CCM.0b013e31819def97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vosylius S, Sipylaite J, Ivaskevicius J. Sequential organ failure assessment score as the determinant of outcome for patients with severe sepsis. Croat Med J. 2004;45(6):715–720. [PubMed] [Google Scholar]
- 8.Michalopoulos A, Falagas ME, Karatza DC, et al. Epidemiologic, clinical characteristics, and risk factors for adverse outcome in multiresistant gram-negative primary bacteremia of critically ill patients. Am J Infect Control. 2011;39(5):396–400. doi: 10.1016/j.ajic.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 9.Vincent JL, de Mendonca A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26(11):1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Sterling SA, Puskarich MA, Summers RL, Jones AE. The effect of early quantitative resuscitation on organ function in survivors of septic shock. J Crit Care. 2014;30(2):261–263. doi: 10.1016/j.jcrc.2014.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guirgis FW, Brakenridge S, Sutchu S, et al. The long-term burden of severe sepsis and septic shock: sepsis recidivism and organ dysfunction. J Trauma Acute Care Surg. 2016;81(3):525–532. doi: 10.1097/TA.0000000000001135. [DOI] [PubMed] [Google Scholar]
- 12.Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Churpek MM, Snyder A, Han X, et al. qSOFA, SIRS, and early warning scores for detecting clinical deterioration in infected patients outside the ICU. Am J Respir Crit Care Med. 2017;195(7):906–911. doi: 10.1164/rccm.201604-0854OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshall JC. Sepsis-3: what is the meaning of a definition? Crit Care Med. 2016;44(8):1459–1460. doi: 10.1097/CCM.0000000000001983. [DOI] [PubMed] [Google Scholar]
- 15.Simpson SQ. New sepsis criteria: a change we should not make. Chest. 2016;149(5):1117–1118. doi: 10.1016/j.chest.2016.02.653. [DOI] [PubMed] [Google Scholar]
- 16.Dellinger RP, Levy MM, Carlet JM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock. 2008. Intensive Care Med. 2008;36(1):17–60. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Sharabiani MT, Aylin P, Bottle A. Systematic review of comorbidity indices for administrative data. Med Care. 2012;50(12):1109–1118. doi: 10.1097/MLR.0b013e31825f64d0. [DOI] [PubMed] [Google Scholar]
- 19.Li P, Kim MM, Doshi JA. Comparison of the performance of the CMS Hierarchical Condition Category (CMS-HCC) risk adjuster with the Charlson and Elixhauser comorbidity measures in predicting mortality. BMC Health Serv Res. 2010;10:245. doi: 10.1186/1472-6963-10-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grissom CK, Brown SM, Kuttler KG, et al. A modified sequential organ failure assessment score for critical care triage. Disaster Med Public Health Prep. 2010;4(4):277–284. doi: 10.1001/dmp.2010.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reith FCM, Van den Brande R, Synnot A, Gruen R, Maas AIR. The reliability of the Glasgow Coma Scale: a systematic review. Intensive Care Med. 2016;42(1):3–15. doi: 10.1007/s00134-015-4124-3. [DOI] [PubMed] [Google Scholar]
- 22.Arnold RC, Sherwin R, Shapiro NI, et al. Multicenter observational study of the development of progressive organ dysfunction and therapeutic interventions in normotensive sepsis patients in the emergency department. Acad Emerg Med. 2013;20(5):433–440. doi: 10.1111/acem.12137. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro NI, Wolfe RE, Moore RB, Smith E, Burdick E, Bates DW. Mortality in Emergency Department Sepsis (MEDS) score: a prospectively derived and validated clinical prediction rule. Crit Care Med. 2003;31(3):670–675. doi: 10.1097/01.CCM.0000054867.01688.D1. [DOI] [PubMed] [Google Scholar]
- 24.Puskarich MA, Nandi U, Long BG, Jones AE. Association between persistent tachycardia and tachypnea and in-hospital mortality among non-hypotensive emergency department patients admitted to the hospital. Clin Exp Emerg Med. 2017;4(1):2–9. doi: 10.15441/ceem.16.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forward E, Konecny P, Burston J, Adhikari S, Doolan H, Jensen T. Predictive validity of the qSOFA criteria for sepsis in non-ICU inpatients. Intensive Care Med. 2017;46(6):945–946. doi: 10.1007/s00134-017-4776-2. [DOI] [PubMed] [Google Scholar]
- 26.Henning DJ, Puskarich MA, Self WH, et al. An emergency department validation of the SEP-3 sepsis and septic shock definitions and comparison with 1992 consensus definitions. Ann Emerg Med. 2017;70(4):544–552e5. doi: 10.1016/j.annemergmed.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donnelly JP, Safford MM, Shapiro NI, Baddley JW, Wang HE. Application of the third international consensus definitions for sepsis (Sepsis-3) classification: a retrospective population-based cohort study. Lancet Infect Dis. 2017;17(6):661–670. doi: 10.1016/S1473-3099(17)30117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]