Abstract
Unilateral cervical C2 hemisection (C2Hx) is a classic model of spinal cord injury (SCI) for studying respiratory dysfunction and plasticity. However, most previous studies were performed under anesthesia, which significantly alters respiratory network. Therefore, the goal of this work was to assess spontaneous diaphragm recovery post-C2Hx in awake, freely behaving animals. Adult rats were chronically implanted with diaphragm EMG electrodes and recorded during 8 weeks post-C2Hx. Our results reveal that ipsilateral diaphragm activity partially recovers within days post-injury and reaches pre-injury amplitude in a few weeks. However, the full extent of spontaneous ipsilateral recovery is significantly attenuated by anesthesia (ketamine/xylazine, isoflurane, and urethane). This suggests that the observed recovery may be attributed in part to activation of NMDA receptors which are suppressed by anesthesia. Despite spontaneous recovery in awake animals, ipsilateral hemidiaphragm dysfunction still persists: i) Inspiratory bursts during basal (slow) breathing exhibit an altered pattern, ii) the amplitude of sighs – or augmented breaths – is significantly decreased, and iii) the injured hemidiaphragm exhibits spontaneous events of hyperexcitation. The results from this study offer an under-appreciated insight into spontaneous diaphragm activity and recovery following high cervical spinal cord injury in awake animals.
Keywords: spinal cord injury, respiration, plasticity, phrenic, anesthesia
Introduction
High cervical spinal cord injury disrupts suprapspinal input to phrenic motor circuity, resulting in impaired diaphragm function and venitlation. Extensive preclinical research has shown that despite these devastating consequences, there is significant plasticity within the injured respiratory networks. The most commonly used animal model of respiratory dysfunction after spinal cord injury (SCI) is a lateral cervical C2 hemisection (C2Hx) (Bezdudnaya et al., 2017; Dougherty et al., 2012; Fuller et al., 2008; Goshgarian, 1979, 2003, 2009; Lane et al., 2008a; Mantilla et al., 2017; Mantilla et al., 2013; Porter, 1895; Sieck and Mantilla, 2009; Vinit et al., 2006). C2Hx interrupts unilateral projections from the brainstem to ipsilateral phrenic motoneurons resulting in hemidiaphragm paralysis. Immediately after injury, animals adopt a frequent and shallow breathing pattern (Fuller et al., 2008; Fuller et al., 2006; Golder et al., 2001b; Hoh et al., 2013). However, acute partial recovery of phrenic and hemidiaphragm activity after C2Hx can be elicited by contralateral phrenicotomy (Goshgarian, 2003; O’Hara and Goshgarian, 1991; Porter, 1895;) or complete termination of ventilator support (asphyxia) in paralyzed animals (Lewis and Brookhart, 1951; O’Hara and Goshgarian, 1991; Zhou et al, 2001). This recovery - referred to as the “crossed-phrenic phenomenon (CPP)” (Rosenbleuth and Ortiz, 1936) – has been attributed to activation of otherwise latent crossed bulbospinal pathways (at C3–C6 level), which become active during increased respiratory drive (Goshgarian, 2003; O’Hara and Goshgarian, 1991). In fact, reducing the respiratory drive abolishes recovery associated with the CPP (Goshgarian, 2009; Lewis and Brookhart, 1951). Spontaneous, long-lasting recovery of phrenic activity, albeit to a limited extent, has also been observed in chronic C2Hx animals and occurs within weeks to months (Fuller et al., 2006; Fuller et al., 2008; Mantilla et al., 2013; Nantwi et al., 1999; Sieck and Mantilla, 2009; Vinit et al., 2006). This type of plasticity is known as the “spontaneous crossed-phrenic phenomenon” (sCPP) (Fuller et al., 2008). It is unclear whether the mechanisms underlying the sCPP differ from the CPP, but recovery has been attributed to progressive actiavtion of crossed bulbospinal pathways, axonal sprouting, rerouting of bulbospinal projections (Darlot et al., 2012; Vinit et al., 2005; Vinit et al., 2011) and forming new polysynaptic connections with phrenic motoneurons via cervical spinal interneurons (Darlot et al., 2012; Fuller et al., 2009; Lane et al., 2009; Lane et al., 2008b; Sandhu et al., 2009; Zholudeva et al., 2017).
However, diaphragm recovery via sCPP has been shown to be dependent on plasticity of glutamatergic (Alilain and Goshgarian, 2007, 2008; Gransee et al., 2017; Mantilla et al., 2012; Mantilla et al., 2017), serotonergic (Basura et al., 2001; Fuller et al., 2005; Lee and Gonzalez-Rothi, 2017; Mantilla et al., 2012; Mantilla et al., 2017) and adenosinergic (Golder et al., 2008; Minic et al., 2017; Nantwi, 2009; Nantwi and Goshgarian, 2002) inputs to phrenic motoneurons. Glutamate is the primary excitatory neurotransmitter in the CNS and mediates respiratory synaptic inputs to phrenic motoneurons from the medulla (Alheid and McCrimmon, 2008; Chitravanshi and Sapru, 1996; Liu et al., 1990; McCrimmon et al., 1989). Different types of glutamate receptors were identified within phrenic motoneurons including ionotropic α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA), N-methyl-D-aspartate (NMDA) (Alilain and Goshgarian, 2008; Gransee et al., 2017; Mantilla et al., 2012; Robinson and Ellenberger, 1997), and metabotropic (mGluRs) (Dong and Feldman, 1999; Gransee et al., 2017) receptors. Previous studies have reported increased expression of NMDA receptors (Alilain and Goshgarian, 2008) in spinal cord tissue containing the phrenic nucleus and directly in the phrenic motoneurons (Gransee et al., 2017; Mantilla et al., 2012) following spontaneous recovery post-C2Hx. Pharmacological blocking of NMDA receptors decreased the amplitude of recovered diaphragm (Mantilla et al., 2017) with no effect on contralateral side and abolished the long-term facilitation of phrenic activity following intermittent hypoxia (McGuire et al., 2005).
A caveat with the majority of experimental studies using the C2Hx is that plasticity and recovery of phrenic and diaphragm function were evaluated under various types of anesthesia. Anesthesia suppresses respiratory drive and activity of spinal respiratory neurons by affecting multiple neurotransmitter systems, including blockade of NMDA receptors (Dickinson et al., 2007; Hara and Harris, 2002; Zorumski et al., 2016). Such adverse effects of anesthesia can significantly alter the obtained results. Therefore, the goal of the current study was to assess spontaneous phrenic motor system recovery using chronic diaphragm electromyography (EMG) recordings in awake, behaving animals following C2Hx up to 8 weeks. The results of the present work demonstrates for the first time that the extent of phrenic recovery following C2Hx may be much greater than previously appreciated.
Materials and Methods
Female, Sprague-Dawley rats (Envigo; 230–260g) were used for this study. 7 rats total were used for chronic recording of diaphragm EMG activity. All experiments were performed with approval from the Institutional Animal Care and Use Committee at Drexel University, and following the National Research Council’s Guidelines (USA).
Diaphragm EMG electrode implantation
Diaphragm EMG electrode implantation was modified from that previously described by Mantilla (Mantilla et al., 2011). The electrodes are comprised of insulated, stranded stainless steel wires (CoonerWire, part AS631), ~20 cm long, with a small 1–2 mm uninsulated area and anchor at one end (ball of dental cement – Grip Cement, Dentsply International Inc, #675571 and liquid #675572). The opposite end of the wire was also uninsulated (3 mm), inserted into the cut end of a 26 5/8 gauge needle (0.45mm × 16mm). The proximal (unbeveled) end was crimped to fix the wire inside, and the needle was manually bent into a curve (Supplemental Figure 1A).
For electrode implantation, all animals were anesthetized with xylazine (10mg/kg, s.q.) and ketamine (120mg/kg, i. p.), and received analgesia (buprenorphine; 0.03 mg/kg, s.q.). The skin overlying the rat’s abdomen and skull were shaved and disinfected with antiseptics (betadine and 70% ethanol wipes) for surgery. A midline longitudinal incision was made on the head, and the skin retracted to expose the skull. Three holes were made in the skull (Supplemental Figure 1B) using a drill (Saeyang Co). Surgical bone screws (Component Supply Co, part# SHCX-080-03) were inserted into each hole (Supplemental Figure 1B). Silver wire was attached to one screw, which served as a ground. Then dental cement was applied over the skull and screws to create a platform for electrode connectors.
A skin incision was made along the midline of the animal’s abdomen and chest to expose the abdominal and caudal-most external intercostal muscles. A median incision was made through the abdominal wall along to the linea alba to expose the abdominal surface of the diaphragm. Two electrodes (~1cm apart) were sutured (dorsal-ventral direction) through the medial costal part of hemidiaphragm on each side for bipolar recording. The cement anchor ball at the end of each wire held the electrode in place in the diaphragm (Supplemental Figure 1C). The needles and attached electrodes were pulled through the lateral space of xiphoideus to externalize the wires, and the abdominal muscles were sutured (4-0 Vicryl). The wires on each side of sternum were gently braided and needles removed. A loop was created from each pair of wires to avoid tension during chest movements. The free ends of the electrodes were then guided under the skin towards the head, and retrieved in front of each ear. This left the wires lateral to the neck musculature which would be later surgically exposed for C2Hx. The muscle and skin on the abdomen were sutured and closed with sterile wound clips, respectively. Wires from the EMG electrodes were connected to a 4-pin connector (Digi-key, #A104980-ND), and the silver wire that was attached to one of the three skull screws was connected to a separate 2 pin-connector. All connectors for recording electrodes and ground were then fixed to the head with dental cement (Supplemental Figure 1D). After surgery, animals received yohimbine (1.2 mg/kg, s.q.) to reverse effect of xylazine and lactated Ringers solution (5 ml, s.q.) to prevent dehydration. Additional analgesia (buprenorphine), lactated Ringers injections (5 ml/day, s.q.) and oral dietary supplement (Nutrical; 1–3 ml) were given daily as needed.
C2Hx Surgery
One week after diaphragm EMG electrode implantation, all animals received a C2Hx. The skin on the dorsal side of neck was shaved and cleaned as described above. An incision was made from the base of the skull to the shoulder blades through the skin and muscle overlying the cervical vertebrae. A partial laminectomy was done at the C2 vertebral level. An incision (~2mm) was made in the dura mater rostrocaudally along to the midline of the dorsal spinal cord. A C2Hx was made immediately caudal to the C2 rootlets, from the midline to lateral edge of the spinal cord using a No. 11 scalpel blade. To visually confirm the extent of the injury, a fine tipped glass pipette connected to a vacuum pump was used to gently aspirate spinal tissue at the lesion site. Dura and surrounding muscles were sutured (10-0 and 4-0 sterile suture, respectively) and skin was closed using wound clips. Yohimbine (1.2 mg/kg, s.q.), lactated Ringers solution (5ml, s.q.), and buprenorphine (0.03 mg/kg, s.q.) were given postoperatively, as described above.
Diaphragm EMG recordings
Awake diaphragm EMG recordings were made in standard rodent cages over a 10–20 minute period of exploration/rest, using a CED digitizer (Cambridge Electronic Design LTD (CED), model 1401), differential AC amplifier (A-M System, Model 1700), and digital video camera (Logitech) with Spike2 (CED) software. EMG signal was amplified by x1000 and filtered 100 – 5,000 Hz. Long, flexible, insulated wires were connected to the head stage (Supplemental Figure 1D), and the animal was allowed to move freely within the cage. These recordings allowed us to monitor diaphragm muscle activity pre-, acutely post- (<1hr) and weekly post-C2Hx for up to 8 weeks.
Terminal Phrenic Nerve Recordings
For terminal recordings of phrenic nerve activity, animals were anesthetized with isoflurane (4% in O2 100%) in an induction chamber and then via nose mask (Isoflurane vaporizer VIP3000, MDS Matrix, Scavenger Fluovac 50206, Stoelting). After tracheostomy, the animal was transferred to mechanical ventilation (MV, ~60 cycles/min, 2–2.5 ml tidal volume; 2% isoflurane, Columbus Apparatus) and two catheters (PE-50 tubing, BD Intramedic, NJ) were inserted into the femoral artery and vein for measurement of arterial blood pressure and infusion of drugs. Rectal temperature was maintained at 37.0±0.2 °C via a servo-controlled heating blanket (Harvard Apparatus) during surgery. Ventral neck muscles (sternomastoideus, sternohyoideus, and medial scaleneus were removed, and phrenic nerves were carefully dissected from surrounding tissue and cut below subclavian artery. Bilateral vagotomy was performed by transection below the vagal-laryngeal branch point, to prevent lung stretch receptor-dependent breathing during ventilation. Following surgery, the animal was placed in a stereotaxic frame under mechanical ventilation (2% Isoflurane with a gas mixture of 50% O2 + 50% N2). Arterial blood pressure (>100 mm Hg), lung inflation pressure (10 cm H2O), end-tidal CO2 (4.5–5.0 %), and body temperature (37.0±0.2 °C) were monitored via pressure transducers (CDXII; Argon Medical, TX), Capnostar CO2-meter (CWE, INC, PA) and Physitemp-200 servo controller (Fine Science Tools, CA), respectively. Then rat was slowly transferred from isoflurane to urethane (1.2 g/kg) anesthesia via i.v. injection. The muscle relaxant (vecuronium bromide, 3–4 mg/kg/h in Ringer-Locke solution) was continuously infused i.v. (1ml/h/100g of body weight) to remove all movement artifacts during phrenic recording. Bipolar silver electrodes (0.38 mm thickness, A-M Systems, Cat#787500) for the recording of phrenic activity were immersed in the mineral oil bath made by elevation of skin flaps on both sides of the neck. Phrenic neurograms were amplified (X10,000), filtered (50–5,000 Hz, Neurolog Digitizer, UK), digitized (10,000 samples/s) and stored on a PC hard drive using Chart5 software (AD Instruments, Australia).
Histology
At the end of each experiment, animals were transcardially perfused with saline, then paraformaldehyde (4% in 0.1M phosphate-buffered saline). The spinal cord was dissected and cryoprotected by sequentially soaking tissue in 15% and 30% sucrose (in 0.1M PBS). Transverse sections (20μm) were made directly on slide throughout the injury site. Then, the sections were counterstained with cresyl violet and pictures taken with Zeiss Imager M2 microscope to determine the extent of C2Hx (complete or incomplete).
Data Analysis
All obtained data were analyzed using Spike 2 (CED, UK) and OriginPro 9 (OriginLab Corporation, USA). Diaphragm EMG and phrenic neurograms were integrated (DC removed, rectified and smoothed with a 0.03 s of window width). Basal breathing bursts (Moore et al., 2013) with frequencies less than 3 Hz were identified through all EMG recordings and onset of each breath was tagged manually. Averaged integrated diaphragm and phrenic activities were taken over a minimum of 30–50 breaths. Ipsilateral phrenic amplitudes were normalized to the contralateral side of the same animal. Diaphragm EMG amplitudes were normalized to pre-injury level for the left and right sides separately. To eliminate tonic background activity, final amplitudes were determined as a difference between maximum and minimum points of integrated activity. Sighs were analyzed as amplitude ratio of a sigh to preceding breath. Significant differences between mean values were established using Wilcoxon signed ranks test for non-normally distributed data sets and one-way repeated measures ANOVA followed by post hoc Tukey for normally distributed data. P<0.05 was used as a level of significance. Lesion size analysis was performed with ImageJ software (1.51r, NIH, USA) using images of cross-sections at the epicenter of the lesion. Percentage of spared tissue was calculated as a ratio of spared area to the total area on the contralateral side and multiplied by 100. All data are presented as a mean ± standard deviation.
Results
Diaphragm EMG activity in naïve rats
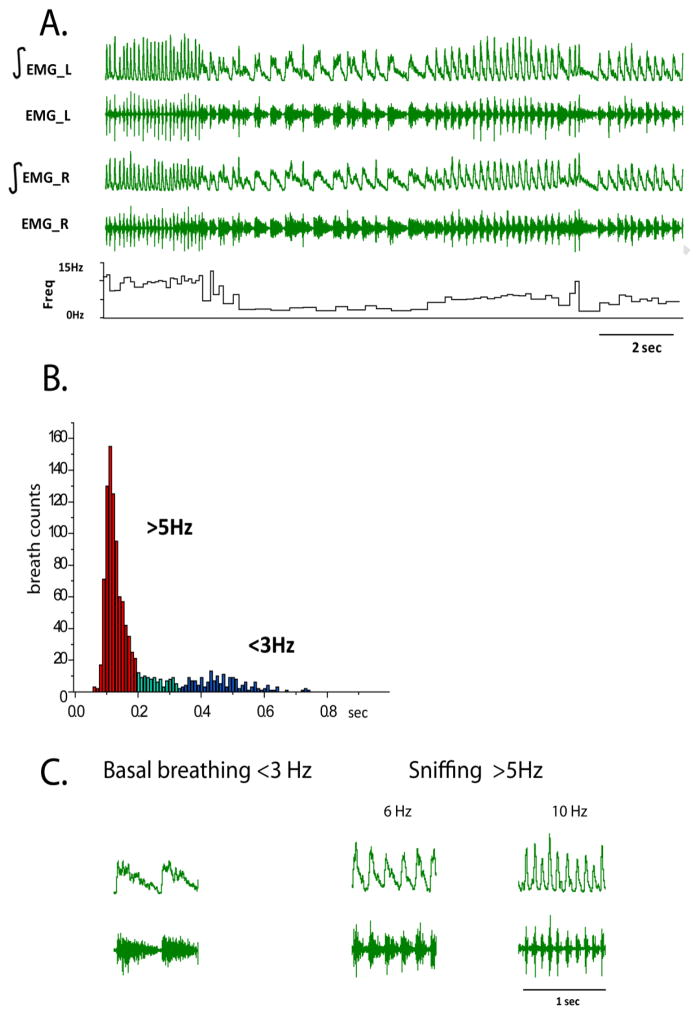
One week after implantation of diaphragm electrodes, bilateral diaphragm EMG was recorded in naïve, behaving rats. Live footage of the animal’s movements was taken during these recordings to identify and correlate diaphragm activity with behavior. During recording session (10–15 min), animals were moving freely around the cage, eating, grooming, standing and sitting quietly. Highly heterogeneous breathing patterns with long- and short- lasting inspiratory events were observed with a frequency that ranged from 1.3Hz to >10Hz (Figure 1A–B). Based on previous studies (Moore et al., 2013), two distinct breathing patterns can be identified in rats: low- and high-frequency. Low-frequency, or ‘basal’ breathing (<3Hz, Figure 1C), is reflected by rapid excitation followed by slow post-inspiratory inhibition and is not locked with whisker movements. Interestingly, it is detected not only during quiet behaving patterns but also when the animal is walking and actively exploring. Fast breathing, or sniffing (>5Hz, Figure 1C) is locked in 1:1 ratio with whisking. Sniffing breathing is presented by bell-shaped bursts with a large range of burst duration (30–100 ms).
Figure 1.
A. An example of bilateral diaphragm EMG recording in a behaving rat. EMG_L – left EMG, EMG_R – right EMG, ∫ -integrated EMG activity, Freq – instant frequency of breathing in Hz; B. Histogram of instant breath to breath intervals during 5 minutes of recording. Red color represents breathing frequency of 5 or more breaths per second (>5 Hz), which occurs during sniffing behavior, blue color represents breathing frequency of 3 or less breaths per second (<3 Hz), which is observed during basal breathing, and green color represents intermediate range of breathing frequencies. C. Examples of raw traces and integrated diaphragm activity during basal and breathing that was observed during sniffing behavior.
Functional and histological lesion analysis post-C2Hx
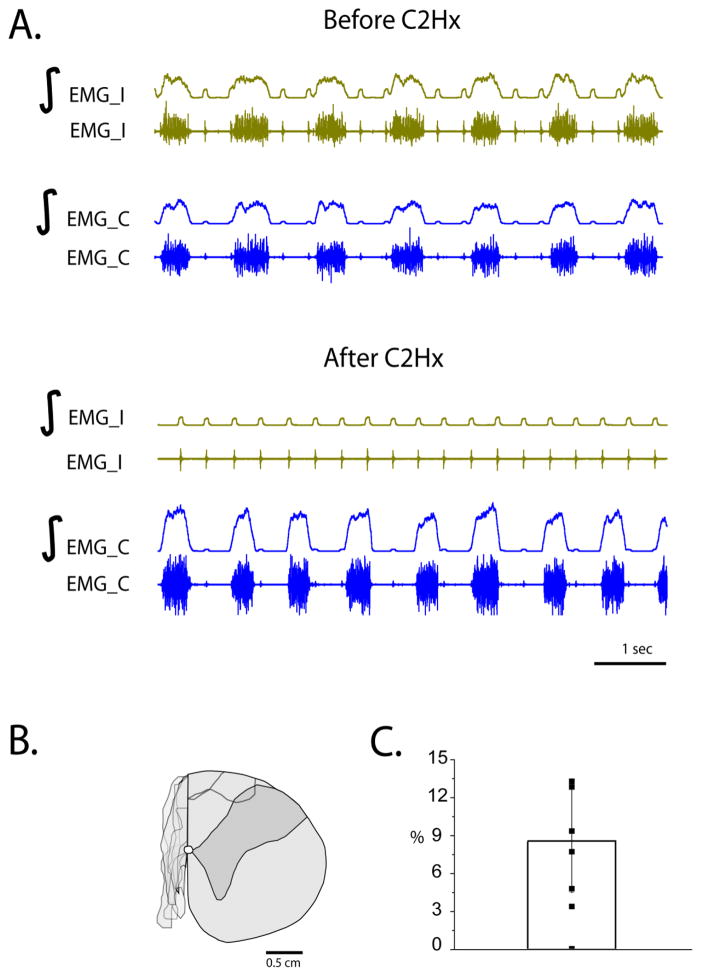
Functional confirmation of the extent of the injury was achieved using recording of bilateral diaphragm EMG activity before and immediately after injury in xylazine/ketamine anesthetized rats (Figure 2A). Complete loss of inspiratory activity ipsilateral to injury and increased amplitude on the contralateral side was seen immediately post-C2Hx in all animals (n=7). Anatomical assessment of the lesion extent was performed by post hoc histological analysis. Results revealed that only 1 of the 7 animals had no tissue sparing post-C2Hx. The remaining 6 animals had less than 15 % of tissue sparing (8.5±4.1%, n=6) with 2 animals less than 5% (Figure 2B,C). Moreover, two animals had minor tissue damage on the contralateral side in the dorsomedial funiculus.
Figure 2.
A. An example of bilateral diaphragm EMG recording in an anesthetized rat before and after C2Hx surgery, demonstrating complete abolishment of diaphragm activity on the side of the injury. EMG_I – ipsilateral EMG, EMG_C – contralateral EMG, ∫-integrated EMG activity; B. Reconstraction of lesions after C2Hx spinal cord cross-section through the lesion epicenter demonstrating minimal white matter sparing (<15 %; see the thin band of tissue at the medial-dorsal location). C. Bar graph represents mean, standard deviation and percentage of speared tissue for each animal (n=7).
Recovery of diaphragm EMG activity in awake animals post-C2Hx
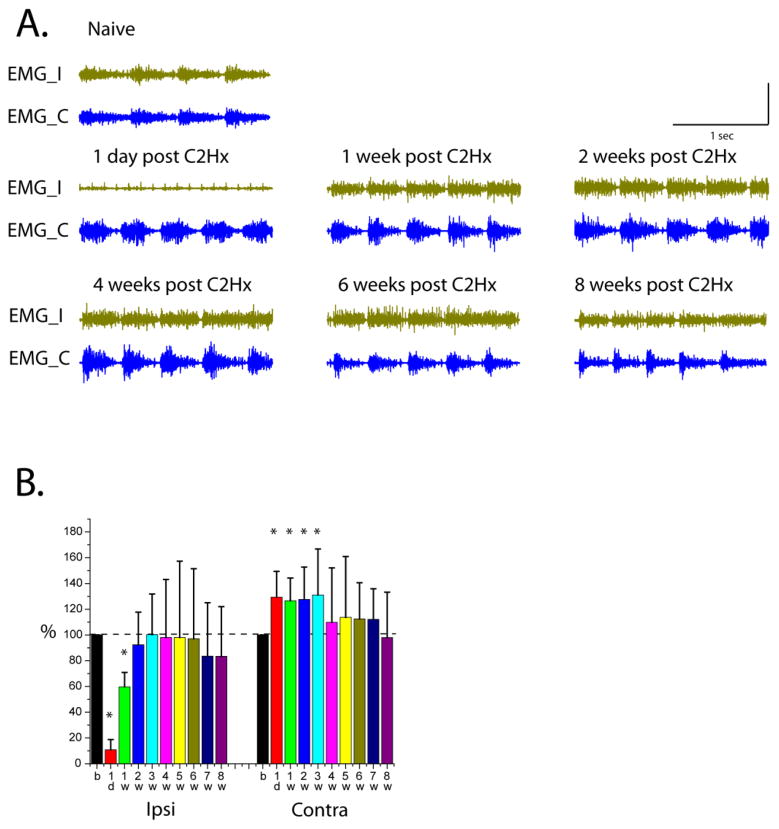
Recovery of diaphragm activity was assessed by analysis of EMGs during basal breathing (<3Hz) in 7 freely behaving rats. Diaphragm EMG data were collected 0–2 days pre-injury, 1 day, and weekly 1 to 8 weeks post-injury. Examples of raw traces through the weeks of recording are shown in Figure 3A. Diaphragm amplitudes were calculated for each week, normalized to pre-injury activity and shown as a percent (Figure 3B). In the first day post-injury, 5 out of 7 animals dropped their ipsilateral diaphragm EMG activity lower than 10% of the pre-injury level. The mean amplitude for all animals was 10.77±7.9 % (n=7) which was significantly (Z=2.28, P=0.016) less than pre-injury activity. One week post-injury ipsilateral diaphragm amplitude was still significantly lower (59.78±11.2 %, Z=2.28, P=0.016) than pre-injury level and improved further by 2 weeks (92.38±25.42%, Z=0.93, P=0.375). Contralateral hemidiaphragm amplitudes significantly increased immediately post-C2Hx (1d 129.4±20%, Z=−2.28, P=0.016; 1w 126.6±17.6%, Z=−2.11, P=0.0313; 2w 127.67±25%, Z=−2.28, P=0.016; 3w 131.1±35.6%, Z=−1.9, P=0.0468) and started to return to pre-injury levels at 4 weeks following injury.
Figure 3.
A. Examples of raw traces from bilateral diaphragm EMG recordings during basal breathing before (Naïve) and throughout experimental time points post-injury (1 day, 1, 2, 4, 6 and 8 weeks post-C2Hx). EMG_I – ipsilateral EMG, EMG_C – contralateral EMG; B. Averaged amplitudes of integrated diaphragm activity (EMGs) as a percent of pre-injury level in 7 animals recorded before (b) and 1 day (1d), 1 week (1w) through 8 weeks (8w) post-C2Hx for ipsi- and contra- sides
One day post-injury, ipsilateral diaphragm activity was unstable, inspiratory bursts not consistently detected, and breathing pattern was significantly changed. All inspiratory burs ts on the contralateral side of injury were bell-shaped (no basal breathing, see Figure 3, 1 d post-C2Hx) and breathing frequency remained relatively consistent throughout the recording. Often, the presence/absence of ipsilateral bursting appeared to correlate with the degree of the animal’s activity. In some cases, during limited movement or periods of rest, no inspiratory bursting was detected ipsilateral to injury. However, the activity could be induced when the animal was touched or forced to move.
Effect of anesthesia on spontaneous recovery post-C2Hx
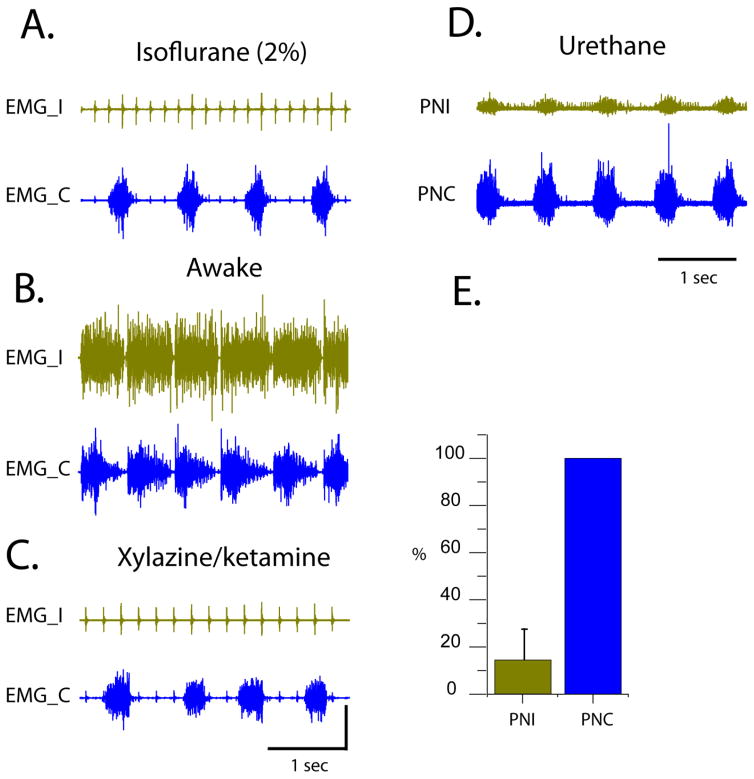
Diaphragm EMG activity was recorded at 6–8 weeks post-injury under anesthesia with either: isoflurane (2% in O2 delivered at 2 L/min) or xylazine/ketamine (xylazine −10m g/kg, s.q. and ketamine −120 mg/kg, i.p.). Anesthesia completely abolished ipsilateral hemidiaphragm activity in all animals. Figure 4 shows bilateral diaphragm EMG activity recorded during isoflurane (A), 10 min after the offset of anesthesia with isoflurane (B) and during xylazine/ketamine anesthesia (C) in the same animal at 8 weeks post-C2Hx, but a few days apart. Moreover, bilateral phrenic neurograms were recorded 8 weeks post-C2Hx using urethane (1.2 g/kg, i.v.) anesthesia in all animals. In contrast to other anesthetic agents, urethane did not completely abolish ipsilateral phrenic activity in the most animals. However, the difference between ipsi- (injured) and contralateral phrenic amplitudes was more robust (Figure 4D, the same rat as on Figure 4A–C) than seen in diaphragm activity in the awake animal. On average, the phrenic output on the ipsilateral to injury side was 5 times less of the contralateral output (Figure 4E; n=7).
Figure 4.
A. An example of a bilateral diaphragm EMG recording in a rat under isoflurane anesthesia (8 weeks post-C2Hx); B. An example of a bilateral diaphragm EMG recording 10 min post-isoflurane anesthesia (8 weeks post-C2Hx); C. An example of a bilateral diaphragm EMG recording in a rat under xylazine/ketamine anesthesia (8 weeks post-C2Hx); D. An example of a bilateral phrenic recording in a rat under urethane anesthesia (8 weeks post-C2Hx). Panels A–D represent recordings from the same rat. E. Summary histogram of averaged phrenic integrated amplitudes from the ipsilateral (PNI) and contralateral (PNC) sides. Ipsi- amplitude is normalized to the contrlateral side. EMG_I – ipsi EMG, EMG_C – contra EMG, PNI – ipsilateral phrenic neurogram, PNC – contralateral phrenic neurogram.
Diaphragm dysfunction in awake animals post-C2Hx
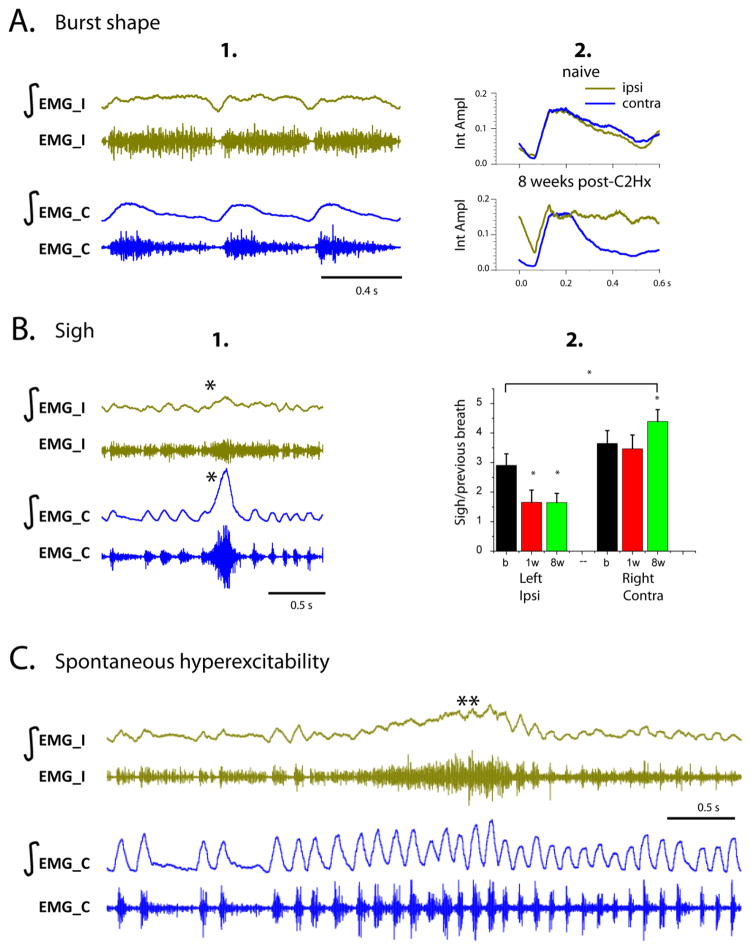
Despite the rapid recovery detected in awake animals post-C2Hx, functional deficits in diaphragm activity persisted. Both the shape of inspiratory bursts during basal (slow) breathing, and the amplitude of augmented breaths (or “sighs”) were altered in injured animals. Post-injury recordings revealed a prolonged, plateaued inspiratory bursts (Figure 5A), with no post-inspiratory inhibition. While the integrated EMG activity from ipsi- and contralateral sides of the diaphragm in naïve animals matched (overlap shown in Figure 5A2), there was a significant difference in the shape of the EMG post-injury. In addition, there was often little or no inhibition of activity between each inspiratory burst in injured animals.
Figure 5.
A.(1) An example of diaphragm activity (EMG) demonstrating different inspiratory burst shape on the ipsi and contralateral sides of the diaphragm during basal breathing 8 weeks post-C2Hx; (2) Overlapped averaged integrated diaphragm activity before injury (naïve) and 8 weeks post-C2Hx in the same animal. Note the plateau-like shape and increased background activity in the ipsilateral side of diaphragm. B. An example of diaphragm activity (EMG) during a sigh (*) at 8 weeks post-C2Hx (1) and quantification of diaphragm activity (2) presented as a ratio of activity during a sigh to the amplitude of the preceding breath, for ipsi and contralateral hemidiaphragms in n=7 animals. b = baseline, 1w = 1 week post C2Hx, 8w = 8weeks post C2Hx. Note the decrease in diaphragm amplitude during a sigh on the ipsilateral side of the diaphragm; C. An example of diaphragm activity (EMG) that occurs during a hyperexcitability event (**) on the ipsilateral (left) hemidiaphragm, representing spontaneous hyperexcitability seen on the injured side. In all panels, EMG_I – ipsi EMG, EMG_C – contra EMG, ∫-integrated EMG activity, Int Ampl – amplitudes of integrated activity.
The ratio of sigh amplitude to that of preceding breath on the left hemidiaphragm of uninjured animals was 2.9±0.4, which was significantly different than 3.6±0.43 on the right side (P=0.001). Following C2Hx, there was a significant change in sigh amplitudes ipsilateral (F(2,5)=232.3, P<0.001) and contralateral (F(2,5)=18.48, P=0.005) to injury. Tukey post hoc tests revealed that sigh amplitudes were significantly attenuated at 1 week (1.65±0.4, P<0.001) and 8 weeks (1.6±0.2, P<0.001) post-injury on the ipsilateral side (Figure 5B). Figure 5B presents an example of an attenuated sigh in the ipsilateral diaphragm at 8 weeks post-injury. Moreover, pairwise comparisons of sigh amplitudes on contralateral side showed a significant increase of sigh amplitudes at 8 weeks post C2Hx compared to pre-injury (4.39±0.4 vs 3.6±0.43, P=0.00274) and 1-week (4.39±0.4 vs 3.5±0.2, P<0.001) values.
Finally, the ipsilateral hemidiaphragm showed a spontaneous abnormal increase of diaphragm activity with no clear bursting pattern that is indicative of hyperexcitability (0.5–3 sec). Frequency and duration of episodes varied between animals and were observed up to 8 weeks post-injury. An example of spontaneous hyperexcitability on the ipsilateral hemidiaphragm at 2 weeks post-C2Hx is shown in Figure 5C. Spontaneous hyperexcitability appeared only on the ipsilateral side of the diaphragm and did not affect the contralateral hemidiaphragm significantly. Moreover, during these episodes, we did not observe any evidence of pain or discomfort in rats.
Discussion
Recovery of ipsilateral diaphragm activity post-C2Hx
In the current study, with chronic diaphragm EMG recordings in behaving animals, we show that ipsilateral hemidiaphragm activity is capable of recovering from the first days post-C2Hx and reaches its pre-injury amplitude in a few weeks post-injury (Figure 3). We attribute this fast recovery to recordings being done in awake (as opposed to anesthetized) rats. The majority of previous studies were performed using anesthetized animals and report ipsilateral diaphragm/phrenic recovery within weeks to months (Fuller et al., 2009; Golder et al., 2001a; Mantilla et al., 2013; Nantwi et al., 1999; Sieck and Mantilla, 2009). However, our previous data in decerebrate and non-anesthetized rats show phrenic recovery within hours post-C2Hx and -C1Hx (Bezdudnaya et al., 2017; Ghali and Marchenko, 2015).
Moreover, we have to acknowledge that time of recovery might also depend on the method of C2Hx surgery (one cut, multiple cuts, post-cut suction, dura suturing) and completeness of injury. In the current study, the estimated size of spared tissue post-injury was significantly less than minimum been systematically investigated in the previous research (23±4%; Fuller et al., 2009). Overall, our data show compete loss of ipsilateral diaphragm activity immediately post-injury (animals are still anesthetized) in rats with less than 15% of tissue sparing (Figure 2BC). Therefore, complete loss of ipsilateral diaphragm activity immediately post-injury does not correlate with a complete C2Hx. However, our observations show that animals with moderate white matter sparring (more 15%, not presented in this study) still have some residual activity in the ipsilateral hemidiaphragm immediately post-C2Hx. Recordings in unanesthetized animals with less than 5% of spared tissue post-C2Hx showed almost no activity on the ipsilateral hemidiaphragm within first days post-injury. As the animal becomes more active over the first few days post-injury, significant diaphragm activity is presented on the ipsilateral side during awake state. Accordingly, recovery of ipsilateral diaphragm post-C2Hx may be partially mediated by animal’s activity. We hypothesize that crossed-phrenic pathways are latent (suppressed) in anesthetized animals but active during eupneic breathing in awake rats.
Finally, an important consideration is that innervation of the diaphragm somatopically orgnaized. The ventral diaphragm is controlled by rostral phrenic motoneurons, and dorsal part of the muscle by more caudal motoneurons (Laskowsi & Sanes, 1987; Nicaise et al., 2012). Moreover, recovery of hemi-diaphragm might have some spatial organization too. Accordingly, the position of recording electrodes can affect the rate of diaphragm EMG recovery post-C2Hx and should be taken into consideration when comparing results. Therefore, the great care was taken in the present work to ensure electrodes were placed in the same location in each animal.
Effect of anesthesia on diaphragm recovery post-C2Hx
Our data show that commonly used anesthetics like isoflurane and combination of xylazine-ketamine completely abolish ipsilateral diaphragm activity, whereas urethane significantly attenuates ipsilateral phrenic activity (Figure 4) at 6–8 weeks post C2Hx. This result may partially explain differences in the extent of functional deficit and recovery that has been reported between experimental studies and research groups in the past where different anesthetics with various dosages have been used. In general, it is well known that anesthesia affects respiration and cardiovascular systems. Studies show that administration of isoflurane or xylazine-ketamine depresses respiratory function, decreases heart rate and arterial blood pressure (Fish, 2008; Sumitra et al., 2004; Teppema and Baby, 2011).
Ketamine is a well-known NMDA receptor antagonist (Zorumski et al., 2016). In addition, it inhibits nicotinic and muscarinic acetylcholine receptors but activates GABAa, AMPA and μ opioid receptors (Aleksandrova et al., 2017; Finck and Ngai, 1982; Garcia et al., 2010; Teppema and Baby, 2011). In the current study, ketamine was used in combination with xylazine – the alpha2 adrenergic agonist that leads to decrease in neurotransmission of norepinephrine.. It also affects μ opioid receptors inducing central antinociception (Romero et al., 2013).
Isoflurane (inhaled anesthetic) acts primarily through activation of GABAa and glycine neurotransmission (Grasshoff and Antkowiak, 2006), and inhibition of NMDA and nicotinic acetylcholine receptors (Brannigan et al., 2010; Brosnan, 2011; Petrenko et al., 2014; Solt et al., 2006). In contrast, the mechanism of urethane anesthesia is distinct from most anesthetics, and it is known for its minimal effect on respiratory and cardiovascular systems (Maggi and Meli, 1986a, b; Teppema and Baby, 2011).
Urethane exhibits a modest effect on multiple neurotransmitter systems including potentiation of inhibitory receptors (GABAa and glycine by 23% and 33% respectively), nicotinic acetylcholine receptors (by 15%), and suppression of excitatory glutamate (NMDA and AMPA by 10% and 18%, respectively) receptors (Hara and Harris, 2002). These small changes in various receptor systems are accountable for generating the anesthetic effect and make urethane the most frequently used in electrophysiological studies. Urethane affects NMDA receptors less than ketamine or isoflurane and shows a smaller reduction in activity of ipsilateral phrenic nerve at 8 weeks post-C2Hx. However, we cannot directly compare phrenic nerve and diaphragm EMG activities because a phrenic neurogram reflects all motor neuron output, whereas diaphragm EMG reflects activity in local clusters of motor units surrounding the recording electrodes. Moreover, animals are ventilated with a stable tidal volume and ventilation frequency during phrenic nerve recordings, whereas EMGs records are performed in spontaneously breathing animals.
Anesthesia used in the current study, and routinely in similar preclinical studies, affects multiple neurotransmitter systems, but with a substantial impact on NMDA receptors. The effect of anesthesia on phrenic motor function post-SCI is perhaps too often underappreciated. NMDA receptors on phrenic motoneurons have been demonstrated to play a significant role in respiratory recovery post-C2Hx (Alilain and Goshgarian, 2008; Gransee et al., 2017; Mantilla et al., 2012; Mantilla et al., 2017). The present data suggests that blocking NMDA receptors via anesthesia being used contributes to an attenuation of ipsilateral hemidiaphragm recovery post-SCI. However, NMDA receptors are widely distributed in the CNS. Systematic injections of anesthetics will affect activity not only spinal phrenic motor circuit but also supraspinal respiratory neurons (preBötzinger Complex, rostral Ventral Respiratory Group, etc.) that are responsible for generating and mediating respiratory rhythm to the phrenic motoneurons. Therefore, the effect of anesthesia on supraspinal circuits should be also considered.
Functional abnormalities post-C2Hx
Despite the fast recovery of the ipsilateral diaphragm seen in the present work, the pattern of diaphragm activity is impaired. The current study was focused on inspiratory bursts during basal breathing (Moore et al., 2013), augmented breaths (sighs) and spontaneous hemidiaphragm hyperexcitability post-C2Hx. The data show that C2Hx significantly alters the shape of inspiratory bursts. Recovering bursts become prolonged and plateau-shaped (Figure 5A1–2), which could indicate impaired post-inspiratory inhibition. In some cases, inhibition was absent between inspiratory bursts, and ipsilateral diaphragm activity did not follow the inspiration-expiration pattern seen on the contralateral side. Such impairment of post-inspiratory inhibition may reflect a loss of descending and/or intra-spinal inhibition that shapes inspiratory bursts on the side of the injury. Enhanced motoneuron excitability post-injury could also contribute to their abnormal activity during the inspiratory and expiratory phases (Li et al., 2004).
Another deficit observed in these animals is the presence episodic hyperexcitation in the ipsilateral diaphragm. These events are characterized by a sudden increase of muscle tone lasting at least 0.5–3 seconds (Figure 5C). Spontaneous hyperexcitability of ipsilateral hemidiaphragm was observed up to 8 weeks post-injury and may be related to muscle spasticity reported in patients with SCI. The main mechanism of spasticity that has been proposed is increased motoneuron excitability occurred as a sequel of reduced descending inhibition (Boulenguez et al., 2010; Cote et al., 2014; Li et al., 2004). As a result, impairment of post-inspiratory inhibition in inspiratory bursts and spontaneous hyperexcitability in ipsilateral hemidiaphragm can contribute to decreased tidal volume in injured animal shown in the previous studies (Fuller et al., 2008; Fuller et al., 2006; Golder et al., 2001b).
Animals in the present work also exhibited significant impairment in the amplitude of diaphragm activity during a sigh. A sigh – or ‘augmented breath’ - is typically comprised of a regular eupneic breath that triggers the high-amplitude inspiratory burst (the sigh) and followed by post-sigh inhibition. It has been proposed that eupnea and sighs are generated by different subsets of neurons of pre-Bötzinger complex assembled in one network (Toporikova et al., 2015). Sighs maintain normal lung function, prevent atelectasis and can reset the breathing pattern depending on physiological, psychological and behavioral requirements (see review Ramirez, 2014). Impaired sighs can lead to the progressive collapse of lung alveoli and decrease of oxygenation (Bendixen et al., 1964; Hartland et al., 2015). Analysis of EMG data from naive animals revealed that sigh amplitudes on the left side were significantly lower than sighs on the right hemidiaphragm indicating an asymmetry in diaphragm activity. Furthermore, there was a significant decrease in sigh amplitude ipsilaterally post-injury that does not recover within 8 weeks, and an increase of the sigh amplitude in the contralateral hemidiaphragm at 8 weeks post-injury (compensatory plasticity). This is consistent with previous studies (Golder et al., 2005; Golder et al., 2003) that reported decreased tidal volume during the sigh but increased their frequency following C2Hx in rats. Moreover, the lack of diaphragm amplitude recovery during sigh, as opposed to during eupneic breathing, post-C2Hx may suggest that these two breathing patterns are controlled by distinct spinal pathways.
Conclusions
Contrary to many reports of modest phrenic motor recovery following high cervical hemisection, the present work reveals a rapid and robust recovery of ipsilateral diaphragm activity in the awake, unrestrained animal post-C2Hx. The discovery that this recovery is extremely sensitive to anesthesia and can be completely abolished with common anesthetics like isoflurane and xylazine/ketamine, and significantly attenuated with urethane anesthesia, raises important considerations for future pre-clinical research in this field. Because most anesthetics affect NMDA transmission in the CNS, our data offer further support to the fact that recovery of ipsilateral diaphragm activity post-C2Hx is partially attributed to NMDA receptor activation.
Despite the extensive spontaneous recovery, ipsilateral hemidiaphragm dysfunction persists in the form of i) altered shape of basal breathing inspiratory bursts, ii) impaired sigh amplitude, and iii) spontaneous events of hyperexcitation. Different recovery of eupneic breathing and sighs suggests distinct spinal pathways driving these two respiratory events. The detailed mechanisms regarding receptor and anatomical changes during recovery of eupneic and sigh breathing patterns following high cervical SCI require further investigation.
Supplementary Material
Supplementary Figure 1. A. Schematic diagram of a recording electrode used in this study; B Schematic diagram of screw positions in the skull used to secure the head stage; C. Schematic diagram of a rat’s costal diaphragm showing the placement of implanted electrodes in the medial part. Recording electrodes were placed ~1cm apart on either side of the blood vessel generally running through the center of both hemidiaphragms; D. Schematic of a rat with implanted electrodes and with recording wires connected to the head stage.
Highlights.
Ipsilateral hemidiaphragm activity starts to recover from the first days post- C2Hx
It reaches pre-injury amplitude in a few weeks post-C2Hx
Inspiratory bursts on the side of injury showed altered shape
Spontaneous hyperexcitability and decreased sigh amplitudes were also observed
Recovery of ipsilateral diaphragm activity is greatly suppressed by anesthesia
Acknowledgments
Research reported in this publication was supported by the National Institute of Neurological Disorders And Stroke of the National Institutes of Health under Award Number R01NS081112 (Lane). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by Conquer Paralysis Now (Bezdudnaya) and the Edward Jekkal Muscular Dystrophy Association Fellowship (Bezdudnaya), the United States Department of Defense (CDMRP #SC140038; Marchenko), Craig H. Neilsen (#338432, Lane) and the Spinal Cord Research Center at Drexel University, College of Medicine (NIH, P01 NS 055976). The authors also offer special thanks to Dr. Suzanne L. Groah (Georgetown University Hospital, Washington, DC) for her advices and comments during this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aleksandrova LR, Phillips AG, Wang YT. Antidepressant effects of ketamine and the roles of AMPA glutamate receptors and other mechanisms beyond NMDA receptor antagonism. J Psychiatry & Neurosci. 2017;42:222–229. doi: 10.1503/jpn.160175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alheid GF, McCrimmon DR. The chemical neuroanatomy of breathing. Resp Physiol & Neurobiol. 2008;164:3–11. doi: 10.1016/j.resp.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alilain WJ, Goshgarian HG. MK-801 upregulates NR2A protein levels and induces functional recovery of the ipsilateral hemidiaphragm following acute C2 hemisection in adult rats. J Spinal Cord Med. 2008, 2007;30:346–354. doi: 10.1080/10790268.2007.11753950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alilain WJ, Goshgarian HG. Glutamate receptor plasticity and activity-regulated cytoskeletal associated protein regulation in the phrenic motor nucleus may mediate spontaneous recovery of the hemidiaphragm following chronic cervical spinal cord injury. Exp Neurol. 2008;212:348–357. doi: 10.1016/j.expneurol.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basura GJ, Zhou SY, Walker PD, Goshgarian HG. Distribution of serotonin 2A and 2C receptor mRNA expression in the cervical ventral horn and phrenic motoneurons following spinal cord hemisection. Exp Neurol. 2001;169:255–263. doi: 10.1006/exnr.2001.7682. [DOI] [PubMed] [Google Scholar]
- Bendixen HH, Smith GM, Mead J. Pattern of Ventilation in Young Adults. J Appl Physiol. 1964;19:195–198. doi: 10.1152/jappl.1964.19.2.195. [DOI] [PubMed] [Google Scholar]
- Bezdudnaya T, Marchenko V, Zholudeva LV, Spruance VM, Lane MA. Supraspinal respiratory plasticity following acute cervical spinal cord injury. Exp Neurol. 2017;293:181–189. doi: 10.1016/j.expneurol.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulenguez P, Liabeuf S, Bos R, Bras H, Jean-Xavier C, Brocard C, Stil A, Darbon P, Cattaert D, Delpire E, Marsala M, Vinay L. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat Med. 2010;16:302–307. doi: 10.1038/nm.2107. [DOI] [PubMed] [Google Scholar]
- Brannigan G, LeBard DN, Henin J, Eckenhoff RG, Klein ML. Multiple binding sites for the general anesthetic isoflurane identified in the nicotinic acetylcholine receptor transmembrane domain. Proc Nat Acad Sci USA. 2010;107:14122–14127. doi: 10.1073/pnas.1008534107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan RJ. GABA(A) receptor antagonism increases NMDA receptor inhibition by isoflurane at a minimum alveolar concentration. Vet Anaesth Analg. 2011;38:231–239. doi: 10.1111/j.1467-2995.2011.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitravanshi VC, Sapru HN. NMDA as well as non-NMDA receptors mediate the neurotransmission of inspiratory drive to phrenic motoneurons in the adult rat. Brain Res. 1996;715:104–112. doi: 10.1016/0006-8993(95)01565-5. [DOI] [PubMed] [Google Scholar]
- Cote MP, Gandhi S, Zambrotta M, Houle JD. Exercise modulates chloride homeostasis after spinal cord injury. J Neurosci. 2014;34:8976–8987. doi: 10.1523/JNEUROSCI.0678-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlot F, Cayetanot F, Gauthier P, Matarazzo V, Kastner A. Extensive respiratory plasticity after cervical spinal cord injury in rats: axonal sprouting and rerouting of ventrolateral bulbospinal pathways. Exp Neurol. 2012;236:88–102. doi: 10.1016/j.expneurol.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Dickinson R, Peterson BK, Banks P, Simillis C, Martin JC, Valenzuela CA, Maze M, Franks NP. Competitive inhibition at the glycine site of the N-methyl-D-aspartate receptor by the anesthetics xenon and isoflurane: evidence from molecular modeling and electrophysiology. Anesthesiol. 2007;107:756–767. doi: 10.1097/01.anes.0000287061.77674.71. [DOI] [PubMed] [Google Scholar]
- Dong XW, Feldman JL. Distinct subtypes of metabotropic glutamate receptors mediate differential actions on excitability of spinal respiratory motoneurons. J Neurosci. 1999;19:5173–5184. doi: 10.1523/JNEUROSCI.19-13-05173.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty BJ, Lee KZ, Lane MA, Reier PJ, Fuller DD. Contribution of the spontaneous crossed-phrenic phenomenon to inspiratory tidal volume in spontaneously breathing rats. J Appl Physiol. 2012;112:96–105. doi: 10.1152/japplphysiol.00690.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finck AD, Ngai SH. Opiate receptor mediation of ketamine analgesia. Anesthesiol. 1982;56:291–297. doi: 10.1097/00000542-198204000-00011. [DOI] [PubMed] [Google Scholar]
- Fish RE. Anesthesia and analgesia in laboratory animals. 2. Elsevier; 2008. [Google Scholar]
- Fuller DD, Baker-Herman TL, Golder FJ, Doperalski NJ, Watters JJ, Mitchell GS. Cervical spinal cord injury upregulates ventral spinal 5-HT2A receptors. J Neurotrauma. 2005;22:203–213. doi: 10.1089/neu.2005.22.203. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Doperalski NJ, Dougherty BJ, Sandhu MS, Bolser DC, Reier PJ. Modest spontaneous recovery of ventilation following chronic high cervical hemisection in rats. Exp Neurol. 2008;211:97–106. doi: 10.1016/j.expneurol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Golder FJ, Olson EB, Jr, Mitchell GS. Recovery of phrenic activity and ventilation after cervical spinal hemisection in rats. J Appl Physiol. 2006;100:800–806. doi: 10.1152/japplphysiol.00960.2005. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Sandhu MS, Doperalski NJ, Lane MA, White TE, Bishop MD, Reier PJ. Graded unilateral cervical spinal cord injury and respiratory motor recovery. Resp Physiol & Neurobiol. 2009;165:245–253. doi: 10.1016/j.resp.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia PS, Kolesky SE, Jenkins A. General anesthetic actions on GABA(A) receptors. Curr Neuropharmacol. 2010;8:2–9. doi: 10.2174/157015910790909502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghali MG, Marchenko V. Dynamic changes in phrenic motor output following high cervical hemisection in the decerebrate rat. Exp Neurol. 2015;271:379–389. doi: 10.1016/j.expneurol.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Golder FJ, Davenport PW, Johnson RD, Reier PJ, Bolser DC. Augmented breath phase volume and timing relationships in the anesthetized rat. Neurosci Lett. 2005;373:89–93. doi: 10.1016/j.neulet.2004.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Fuller DD, Davenport PW, Johnson RD, Reier PJ, Bolser DC. Respiratory motor recovery after unilateral spinal cord injury: eliminating crossed phrenic activity decreases tidal volume and increases contralateral respiratory motor output. J Neurosci. 2003;23:2494–2501. doi: 10.1523/JNEUROSCI.23-06-02494.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Ranganathan L, Satriotomo I, Hoffman M, Lovett-Barr MR, Watters JJ, Baker-Herman TL, Mitchell GS. Spinal adenosine A2a receptor activation elicits long-lasting phrenic motor facilitation. J Neurosci. 2008;28:2033–2042. doi: 10.1523/JNEUROSCI.3570-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Reier PJ, Bolser DC. Altered respiratory motor drive after spinal cord injury: supraspinal and bilateral effects of a unilateral lesion. J Neurosci. 2001a;21:8680–8689. doi: 10.1523/JNEUROSCI.21-21-08680.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Reier PJ, Davenport PW, Bolser DC. Cervical spinal cord injury alters the pattern of breathing in anesthetized rats. Journal of applied physiology. 2001b;91:2451–2458. doi: 10.1152/jappl.2001.91.6.2451. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG. Developmental plasticity in the respiratory pathway of the adult rat. Exp Neurol. 1979;66:547–555. doi: 10.1016/0014-4886(79)90201-2. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG. The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J Appl Physiol. 2003;94:795–810. doi: 10.1152/japplphysiol.00847.2002. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG. The crossed phrenic phenomenon and recovery of function following spinal cord injury. Resp Physiol & Neurobiol. 2009;169:85–93. doi: 10.1016/j.resp.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gransee HM, Gonzalez Porras MA, Zhan WZ, Sieck GC, Mantilla CB. Motoneuron glutamatergic receptor expression following recovery from cervical spinal hemisection. JComp Neurol. 2017;525:1192–1205. doi: 10.1002/cne.24125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasshoff C, Antkowiak B. Effects of isoflurane and enflurane on GABAA and glycine receptors contribute equally to depressant actions on spinal ventral horn neurones in rats. Br J Anaesth. 2006;97:687–694. doi: 10.1093/bja/ael239. [DOI] [PubMed] [Google Scholar]
- Hara K, Harris RA. The anesthetic mechanism of urethane: the effects on neurotransmitter-gated ion channels. Anesth Analg. 2002;94:313–318. doi: 10.1097/00000539-200202000-00015. [DOI] [PubMed] [Google Scholar]
- Hartland BL, Newell TJ, Damico N. Alveolar recruitment maneuvers under general anesthesia: a systematic review of the literature. Resp Care. 2015;60:609–620. doi: 10.4187/respcare.03488. [DOI] [PubMed] [Google Scholar]
- Hoh DJ, Mercier LM, Hussey SP, Lane MA. Respiration following spinal cord injury: evidence for human neuroplasticity. Resp Physiol & Neurobiol. 2013;189:450–464. doi: 10.1016/j.resp.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, Fuller DD, White TE, Reier PJ. Respiratory neuroplasticity and cervical spinal cord injury: translational perspectives. Trends in Neurosci. 2008a;31:538–547. doi: 10.1016/j.tins.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, Lee KZ, Fuller DD, Reier PJ. Spinal circuitry and respiratory recovery following spinal cord injury. Resp Physiol & Neurobiol. 2009;169:123–132. doi: 10.1016/j.resp.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, White TE, Coutts MA, Jones AL, Sandhu MS, Bloom DC, Bolser DC, Yates BJ, Fuller DD, Reier PJ. Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. J Comp Neurol. 2008b;511:692–709. doi: 10.1002/cne.21864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski MB, Sanes JR. Topographic mapping of motor pools onto skeletal muscles. J Neurosci. 1987;7:252–260. doi: 10.1523/JNEUROSCI.07-01-00252.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KZ, Gonzalez-Rothi EJ. Contribution of 5-HT2A receptors on diaphragmatic recovery after chronic cervical spinal cord injury. Resp Physiol & Neurobiol. 2017;244:51–55. doi: 10.1016/j.resp.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis LJ, Brookhart JM. Significance of the crossed phrenic phenomenon. The Americ J Physiol. 1951;166:241–254. doi: 10.1152/ajplegacy.1951.166.2.241. [DOI] [PubMed] [Google Scholar]
- Li Y, Gorassini MA, Bennett DJ. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol. 2004;91:767–783. doi: 10.1152/jn.00788.2003. [DOI] [PubMed] [Google Scholar]
- Liu G, Feldman JL, Smith JC. Excitatory amino acid-mediated transmission of inspiratory drive to phrenic motoneurons. J Neurophysiol. 1990;64:423–436. doi: 10.1152/jn.1990.64.2.423. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations in various systems. Part 2: Cardiovascular system. Experientia. 1986a;42:292–297. doi: 10.1007/BF01942510. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations. Part 3: Other systems and conclusions. Experientia. 1986b;42:531–537. doi: 10.1007/BF01946692. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Bailey JP, Zhan WZ, Sieck GC. Phrenic motoneuron expression of serotonergic and glutamatergic receptors following upper cervical spinal cord injury. Exp Neurol. 2012;234:191–199. doi: 10.1016/j.expneurol.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Gransee HM, Zhan WZ, Sieck GC. Impact of Glutamatergic and Serotonergic Neurotransmission on Diaphragm Muscle Activity after Cervical Spinal Hemisection. J Neurophysiol. 2017;118:1732–1738. doi: 10.1152/jn.00345.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Greising SM, Zhan WZ, Seven YB, Sieck GC. Prolonged C2 spinal hemisection-induced inactivity reduces diaphragm muscle specific force with modest, selective atrophy of type IIx and/or IIb fibers. J Appl Physiol. 2013;114:380–386. doi: 10.1152/japplphysiol.01122.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Seven YB, Hurtado-Palomino JN, Zhan WZ, Sieck GC. Chronic assessment of diaphragm muscle EMG activity across motor behaviors. Resp Physiol & Neurobiol. 2011;177:176–182. doi: 10.1016/j.resp.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrimmon DR, Smith JC, Feldman JL. Involvement of excitatory amino acids in neurotransmission of inspiratory drive to spinal respiratory motoneurons. JNeurosci. 1989;9:1910–1921. doi: 10.1523/JNEUROSCI.09-06-01910.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire M, Zhang Y, White DP, Ling L. Phrenic long-term facilitation requires NMDA receptors in the phrenic motonucleus in rats. JPhysiol. 2005;567:599–611. doi: 10.1113/jphysiol.2005.087650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minic Z, Wilson S, Liu F, Sankari A, Mao G, Goshgarian H. Nanoconjugate-bound adenosine A1 receptor antagonist enhances recovery of breathing following acute cervical spinal cord injury. Exp Neurol. 2017;292:56–62. doi: 10.1016/j.expneurol.2017.02.013. [DOI] [PubMed] [Google Scholar]
- Moore JD, Deschenes M, Furuta T, Huber D, Smear MC, Demers M, Kleinfeld D. Hierarchy of orofacial rhythms revealed through whisking and breathing. Nature. 2013;497:205–210. doi: 10.1038/nature12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nantwi KD. Recovery of respiratory activity after C2 hemisection (C2HS): involvement of adenosinergic mechanisms. Resp Physiol & Neurobiol. 2009;169:102–114. doi: 10.1016/j.resp.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nantwi KD, El-Bohy AA, Schrimsher GW, Reier PJ, Goshgarian HG. Spontaneous functional recovery in a paralyzed hemidiaphragm following upper cervical spinal cord injury in adult rats. Neororehab and Neural Repair. 1999;13:225–234. [Google Scholar]
- Nantwi KD, Goshgarian HG. Actions of specific adenosine receptor A1 and A2 agonists and antagonists in recovery of phrenic motor output following upper cervical spinal cord injury in adult rats. Clin Exp Pharmacol Physiol. 2002;29:915–923. doi: 10.1046/j.1440-1681.2002.03750.x. [DOI] [PubMed] [Google Scholar]
- Nicaise Ch, Hala TJ, Frank DM, Parker JL, Authelet M, Leroy K, Brion JP, Wright MC, Lepore AC. Phrenic motor neuron degeneration compromises phrenic axonal circuitry and diaphragm activity in a unilateral cervical contusion of spinal cord injury. Exp Neurol. 2012;235:539–552. doi: 10.1016/j.expneurol.2012.03.007. [DOI] [PubMed] [Google Scholar]
- O’Hara TE, Jr, Goshgarian HG. Quantitative assessment of phrenic nerve functional recovery mediated by the crossed phrenic reflex at various time intervals after spinal cord injury. Exp Neurol. 1991;111:244–250. doi: 10.1016/0014-4886(91)90012-2. [DOI] [PubMed] [Google Scholar]
- Petrenko AB, Yamakura T, Sakimura K, Baba H. Defining the role of NMDA receptors in anesthesia: are we there yet? Eur J Pharmacol. 2014;723:29–37. doi: 10.1016/j.ejphar.2013.11.039. [DOI] [PubMed] [Google Scholar]
- Porter WT. The Path of the Respiratory Impulse from the Bulb to the Phrenic Nuclei. J Physiol. 1895;17:455–485. doi: 10.1113/jphysiol.1895.sp000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM. The integrative role of the sigh in psychology, physiology, pathology, and neurobiology. Prog Brain Res. 2014;209:91–129. doi: 10.1016/B978-0-444-63274-6.00006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D, Ellenberger H. Distribution of N-methyl-D-aspartate and non-N-methyl-D-aspartate glutamate receptor subunits on respiratory motor and premotor neurons in the rat. J Comp Neurol. 1997;389:94–116. doi: 10.1002/(sici)1096-9861(19971208)389:1<94::aid-cne7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Romero TR, da Pacheco DF, Duarte ID. Xylazine induced central antinociception mediated by endogenous opioids and mu-opioid receptor, but not delta-or kappa-opioid receptors. Brain Res. 2013;1506:58–63. doi: 10.1016/j.brainres.2013.02.030. [DOI] [PubMed] [Google Scholar]
- Rosenbleuth A, Ortiz T. The crossed respiratory impulses to the phrenic. Americ J Physiol. 1936:495–513. [Google Scholar]
- Sandhu MS, Dougherty BJ, Lane MA, Bolser DC, Kirkwood PA, Reier PJ, Fuller DD. Respiratory recovery following high cervical hemisection. Resp Physiol & Neurobiol. 2009;169:94–101. doi: 10.1016/j.resp.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieck GC, Mantilla CB. Role of neurotrophins in recovery of phrenic motor function following spinal cord injury. Resp Physiol & Neurobiol. 2009;169:218–225. doi: 10.1016/j.resp.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solt K, Eger EI, 2nd, Raines DE. Differential modulation of human N-methyl-D-aspartate receptors by structurally diverse general anesthetics. Anesth Analg. 2006;102:1407–1411. doi: 10.1213/01.ane.0000204252.07406.9f. [DOI] [PubMed] [Google Scholar]
- Sumitra M, Manikandan P, Rao KV, Nayeem M, Manohar BM, Puvanakrishnan R. Cardiorespiratory effects of diazepam-ketamine, xylazine-ketamine and thiopentone anesthesia in male Wistar rats--a comparative analysis. Life Sci. 2004;75:1887–1896. doi: 10.1016/j.lfs.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Teppema LJ, Baby S. Anesthetics and control of breathing. Resp Physiol & Neurobiol. 2011;177:80–92. doi: 10.1016/j.resp.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Toporikova N, Chevalier M, Thoby-Brisson M. Sigh and Eupnea Rhythmogenesis Involve Distinct Interconnected Subpopulations: A Combined Computational and Experimental Study(1,2,3) eNeuro. 2015:2. doi: 10.1523/ENEURO.0074-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinit S, Boulenguez P, Efthimiadi L, Stamegna JC, Gauthier P, Kastner A. Axotomized bulbospinal neurons express c-Jun after cervical spinal cord injury. Neuroreport. 2005;16:1535–1539. doi: 10.1097/01.wnr.0000179075.32035.0f. [DOI] [PubMed] [Google Scholar]
- Vinit S, Darlot F, Aoulaiche H, Boulenguez P, Kastner A. Distinct expression of c -Jun and HSP27 in axotomized and spared bulbospinal neurons after cervical spinal cord injury. J Mol Neurosci. 2011;45(2):119–133. doi: 10.1007/s12031-010-9481-3. [DOI] [PubMed] [Google Scholar]
- Vinit S, Gauthier P, Stamegna JC, Kastner A. High cervical lateral spinal cord injury results in long-term ipsilateral hemidiaphragm paralysis. J Neurotrauma. 2006;23:1137–1146. doi: 10.1089/neu.2006.23.1137. [DOI] [PubMed] [Google Scholar]
- Zholudeva LV, Karliner JS, Dougherty KJ, Lane MA. Anatomical recruitment of spinal V2a interneurons into phrenic motor circuitry after high cervical spinal cord injury. J Neurotrauma. 2017;34:3058–3065. doi: 10.1089/neu.2017.5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski CF, Izumi Y, Mennerick S. Ketamine: NMDA receptors and beyond. J Neurosci. 2016;36:11158–11164. doi: 10.1523/JNEUROSCI.1547-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Shi-Yi, Castro-Moure F, Goahgarian HG. Activation of a latent respiratory motor pathway by stimulation of neurons in the medullary chemoreceptor area of the rat. Exp Neurol. 2001;171:176–184. doi: 10.1006/exnr.2001.7740. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. A. Schematic diagram of a recording electrode used in this study; B Schematic diagram of screw positions in the skull used to secure the head stage; C. Schematic diagram of a rat’s costal diaphragm showing the placement of implanted electrodes in the medial part. Recording electrodes were placed ~1cm apart on either side of the blood vessel generally running through the center of both hemidiaphragms; D. Schematic of a rat with implanted electrodes and with recording wires connected to the head stage.