Abstract
Purpose
To evaluate whether tumor uptake of [89Zr]trastuzumab can distinguish HER2-positive from HER2-negative breast cancer.
Methods
Women with HER2-positive (n=34) and HER2-negative (n=16) breast cancer underwent PET/CT 5 ± 2 days following [89Zr]trastuzumab administration. HER2 status was determined based on immunohistochemistry and/or fluorescence in situ hybridization of primary or metastatic/recurrent tumor. Tumor [89Zr]trastuzumab uptake was assessed qualitatively and semiquantitatively as maximum standardized uptake value (SUVmax), and correlated with HER2 status. Additionally, intrapatient heterogeneity of [89Zr]trastuzumab uptake was evaluated.
Results
On a per-patient basis, [89Zr]trastuzumab-PET/CT was positive in 30/34 (88.2%) HER2-positive and negative in 15/16 (93.7%) HER2-negative patients. Considering all lesions, the SUVmax was not significantly different in patients with HER2-positive versus HER2-negative disease (p=0.06). The same was true of when only hepatic lesions were evaluated (p=0.42). However, after excluding hepatic lesions, tumor SUVmax was significantly higher in HER2-positive compared to HER2-negative patients (p=0.003). A cutoff SUVmax of 3.2, determined by ROC analysis, demonstrated positive-predictive value of 83.3% (95% CI: 65.3%, 94.4%), sensitivity of 75.8% (57.7%, 88.9%), negative-predictive value of 50% (24.7%, 75.3%), and specificity of 61.5% (95% 31.6%, 86.1%) for differentiating HER2-positive from HER2-negative lesions. There was intrapatient heterogeneity of [89Zr]trastuzumab uptake in 20% of patients with multiple lesions.
Conclusions
[89Zr]trastuzumab has the potential to characterize the HER2 status of the complete tumor burden in patients with breast cancer, thus obviating repeat or multiple tissue sampling to assess intrapatient heterogeneity of HER2 status.
Keywords: HER2, zirconium-89, trastuzumab, positron emission tomography, breast cancer
Introduction
Breast cancer is the most common malignancy in women in the United States; one in eight women will develop this cancer during their lifetime. It is estimated that approximately 252,710 women will be diagnosed with breast cancer in 2017 and 40,610 will die from this disease (1). About 20–25% of breast cancers overexpress the human epidermal growth factor receptor 2 (HER2), by pathological evaluation of tumor tissue using immunohistochemistry (IHC) or by fluorescence in situ hybridization (FISH) (2). HER2 is a transmembrane protein that plays a vital role in the regulation of cell growth, survival and differentiation. HER2 overexpression is associated with aggressive biological behavior and poor clinical outcome. Patients with HER2-positive breast cancer often have significantly shorter disease-free survival and worse overall survival than those with other breast cancer subtypes (3). In breast cancer, HER2 and hormone receptor status remain the main predictive factors for the selection of targeted therapies. Therefore, HER2 is an important biomarker. It also is an ideal target for developing strategies for imaging and for treatment of HER2-positive breast cancer.
To date, the success of HER2-targeted therapy has depended on selecting patients for treatment based on pathological assessment of tumor biopsy materials. However, in vitro assays used for assessment of HER2 status are limited by tumor heterogeneity and the variability of assay results. Test-retest variability of IHC results of the same specimens has been attributed to non-uniform control of time, type of tissue fixation and temperature of paraffin embedding, and the absence of standards for processing tissue samples (3). FISH assays require extensive training and expertise to distinguish normal from malignant cells. In addition, the fluorescence may fade over time, and prior protein digestion may affect the morphology of tumor samples (3). Furthermore, in up to 25% of cases, HER2 status may be discordant between the primary tumor and metastatic lesions (4, 5), and among different metastatic lesions. This is why testing of metastatic lesions at relapse is recommended, given the importance of test results on choice of therapy. Thus, a noninvasive method for evaluation of HER2 expression throughout a patient’s entire tumor burden, including lesions not readily accessible to biopsy, would be very desirable.
We and others have shown that trastuzumab, a therapeutic monoclonal antibody directed against HER2, when labeled with 89Zr, can be used to detect HER2-positive breast cancer by positron emission tomography (PET) (6, 7). In this study, we planned to evaluate whether tumor uptake of [89Zr]trastuzumab can distinguish HER2-positive from HER2-negative breast cancer.
Materials and Methods
Patient Population
We studied 50 women with biopsy-proved breast cancer-17 HER2-negative and 34 HER2-positive. This study (Clinicaltrials.gov Identifier: NCT02065609) was approved by the Institutional Review Board and the Radioactive Drug Research Committee at Washington University School of Medicine and was conducted under an investigational new drug application (IND#118029) submitted to the U.S. Food and Drug Administration. All patients gave written informed consent before participation. The HER2 status was based on the clinical diagnosis from the pathological evaluation of the primary tumor and/or metastatic lesions at the time of relapse. HER2 positivity was defined per the 2013 American Society of Clinical Oncology/College of American Pathologists guidelines, which require tumors to be 3+ by HER2 IHC or have a FISH HER2:CEP17 ratio > 2 on primary, recurrent, or metastatic breast cancer tissue (2). All patients were required to have at least one lesion ≥ 1.5 cm, as determined by physical examination or imaging studies (mammography, ultrasonography, CT or MRI). Presence of bone metastasis was based on positive bone scintigraphy or FDG-PET/CT, with corresponding lytic or sclerotic changes on CT or radiographs or changes on MRI. In patients with several bone lesions on bone scintigraphy or FDG-PET/CT, not all lesions had corresponding anatomical findings, but at least one lesion with corresponding anatomical finding was needed to be eligible to participate. Patients were evaluated by various imaging studies (e.g., mammography, bone scintigraphy, CT, MRI or FDG-PET/CT), as clinically indicated. Patients who were receiving systemic therapy with or without trastuzumab therapy were eligible to participate. Patients with other invasive malignancies, with the exception of non-melanoma skin cancer, who had any evidence of the other cancer within the last 5 years were not eligible.
[89Zr]Trastuzumab Synthesis and Quality Control
[89Zr]trastuzumab was prepared on site in our Cyclotron Facility, as previously described by Vosjan et al. (8). As previously reported, the radiochemical yield was ≥ 95% by radio-ITLC using 50 mM DTPA as the developing solution. The antibody aggregation analysis was determined using size-exclusion chromatography (Superose 12 10/300 GL, GE Healthcare, Piscataway, NJ), which resulted in ≥ 80% [89Zr]trastuzumab monomer. In addition, we determined the radionuclide identity, visual appearance, pH, specific activity and bubble point filter membrane integrity prior to release of [89Zr]trastuzumab. The immunoreactivity assay was performed on HER2-positive SKBR3 human breast cancer cells following methods developed by Lindmo et al. (9); we achieved an average immunoreactive fraction of 89 ± 0.1% (acceptance criterion ≥ 65%). The immunoreactivity assay and sterility of the final product were determined after the release of [89Zr]trastuzumab.
PET Imaging Procedures
All PET imaging was performed with a Siemens Biograph 40HD PET/CT scanner. Trastuzumab-naive subjects received 50 mg “cold” trastuzumab, whereas those undergoing trastuzumab therapy received 10 mg, 30 min to 2 h before administration of [89Zr]trastuzumab (range 43.3 – 92.1 MBq, mean 76.6 MBq). We and others have shown these pre-doses of cold trastuzumab to be adequate for minimizing uptake of radiolabeled trastuzumab by normal organs (7, 10). Standard-body PET/CT was performed 5 ± 2 days following [89Zr]trastuzumab injection. All subjects were monitored for adverse reactions (e.g., dyspnea, chest tightness, fever, rigors or hypotension) during administration of trastuzumab and [89Zr]trastuzumab. Vital signs (blood pressure, heart rate, respiratory rate, and temperature), clinical laboratory testing (standard hematologic and comprehensive metabolic panels that included hemoglobin, white blood cells, neutrophils, lymphocytes, platelets, creatinine, blood urea nitrogen, calcium, sodium, potassium, carbon dioxide, alanine transaminase, aspartate aminotransferase, alkaline phosphatase, total bilirubin, and albumin), urinalysis, and electrocardiography were assessed in all patients prior to [89Zr]trastuzumab administration and at the imaging session.
PET/CT consisted of a spiral CT scan for attenuation correction (120 kVp, 50 effective mAs at 5-mm slice thickness) from the top of the skull through the upper thighs with the subject in the supine position. Immediately after the attenuation CT scan, emission images beginning at the top of the skull and proceeding down through the upper thighs were obtained (1–10 min per bed position depending on the time post-injection) over 6–7 bed positions with a total imaging duration of no more than 1 hour. Images were reconstructed with 3D-OSEM with 3 iterations, 24 subsets and a post-reconstruction Gaussian filter of 7 mm.
Image Analysis
[89Zr]trastuzumab-PET/CT images were correlated with physical examination and all available radiological images (mammography, bone scintigraphy, CT, MRI or FDG-PET/CT). Images were evaluated qualitatively for presence or absence of uptake in known lesions in comparison to the uptake within normal comparable tissue using standard clinical imaging criteria initially without the knowledge of HER2 status of the lesions. After the qualitative image analysis was completed, the HER2 status of the lesions was recorded for final analysis. The images also were evaluated semi-quantitatively using maximum standardized value (SUVmax) with the knowledge of the location of the lesion (based on all available imaging studies). Spherical volumes of interest (VOIs, ~ 1.5 cm) were drawn around the lesion. In patients with multiple lesions, the mean of SUVmax of up to 7 lesions was calculated and recorded. [89Zr]trastuzumab uptake was correlated with known HER2 status. In patients with multiple lesions confirmed on FDG-PET/CT, bone scintigraphy, MRI or biopsy, we evaluated intrapatient heterogeneity of [89Zr]trastuzumab uptake, defined as the presence of lesions with positive and negative [89Zr]trastuzumab uptake within an individual patient.
Statistical Analysis
Demographic and clinical characteristics were summarized by descriptive statistics. The PET imaging data were semi-quantified as SUVmax for each individual patient under three scenarios: (a) considering all lesions, (b) considering all lesions except for hepatic lesions, and (c) considering hepatic lesions only. For each scenario, the association between [89Zr]trastuzumab uptake values (i.e., SUVmax) and HER2 expression (i.e., positive vs. negative) were examined via Kruskal-Wallis test, which allowed analyses to be run on non-normally distributed imaging data. Median and interquartile range (IQR, i.e., 25th – 75th percentile) of SUVmax were reported by HER2 expression.
In addition, logistic regression was conducted to explore how well [89Zr]trastuzumab uptake values could classify HER2 positive and negative patients. The classification accuracy was evaluated via the area under a receiver operating characteristic (ROC) curve. An area of 1 suggests a perfect classification; an area of 0.5 represents that a classification is no better than random guessing. An optimum threshold of [89Zr]trastuzumab uptake values that could maximize the sum of the specificity and sensitivity was selected under the ROC curve. Positive-predictive value (PPV), negative-predictive value (NPV), sensitivity, and specificity were reported. All statistical analyses were considered as exploratory, even if statistical tests were used. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Patient Characteristics
Fifty women were enrolled in the study. Eighteen had locally-advanced breast cancer; 5 had not received neoadjuvant therapy prior to [89Zr]trastuzumab-PET/CT and the remaining 13 patients had received 1 to 3 cycles of neoadjuvant therapy (12 received taxotere®, carboplatin, herceptin®, and Perjeta® (TCHP) and 1 received epirubicin, fluorouracil, cyclophosphamide). Thirty-one of the 32 patients had metastatic/recurrent disease and had undergone 1 to 15 (median 5) prior regimens of systemic therapy (trastuzumab alone in 2 patients and in combination with chemotherapy in 10 patients; combination of trastuzumab and endocrine therapy in 3; HER2-targeted therapy with neratinib in 1; chemotherapy in 9; endocrine therapy in 4; and targeted therapy with mTOR and tyrosine kinase inhibitors in 3). The remaining patient with recurrent disease had not begun treatment before [89Zr]trastuzumab-PET/CT. Thirty-four patients had HER2-positive and 16 had HER2-negative breast cancer. The demographic characteristics of the patients are summarized in Table 1. Their mean age was 56 years (range 29 to 74 years). Thirty subjects were being treated with trastuzumab while participating in this trial and, thus, received the 10 mg pre-dose of trastuzumab, while the other 20, who were trastuzumab-naïve, received the 50 mg pre-dose of trastuzumab. There were no significant changes in vital signs or the results of laboratory studies or electrocardiograms.
Table 1.
Patient Profile.
| Total number of patients | 50 |
| Median age | 58 yr (range 29–74 yr) |
| Caucasian | 41 |
| African American | 9 |
| Disease Status | |
| Metastatic disease | 32 |
| Locally advanced/chest wall recurrence | 18 |
| Receptor status | |
| HER2-positive | 34 |
| HER2-negative | 16 |
| Prior systemic therapy | |
| None | 6 |
| Prior therapy for metastatic disease | 44 |
PET Results
The results of 12 of the 34 patients with HER2-positive breast cancer have been reported previously (6). On a per-patient basis, and based on visual assessment, the [89Zr]trastuzumab scans were positive in 30 of the 34 HER2-positive patients (88.2%; 4 patients did not have any lesions with increased [89Zr]trastuzumab uptake) and negative in 15 of the 16 HER2-negative patients (93.7%; 1 patient had only lesions with increased [89Zr]trastuzumab uptake). A total of 127 lesions were analyzed; 87 lesions were [89Zr]trastuzumab-positive and 40 were [89Zr]trastuzumab-negative. The distribution of lesions was as follows: 51 bone, 25 breast, 21 liver, 16 lymph nodes, 13 lung and 1 chest wall. Among 50 patients, 25 had one lesion, 7 had two lesions, and 18 had three or more lesions.
Table 2 and Figure 1 show the summary of PET findings by HER2 status. Considering all lesions, the tumor SUVmax was evaluated for 50 patients (34 HER2-positive and 16 HER2-negative). HER2-positive patients did not show significantly higher SUVmax (p = 0.06) values compared to HER2-negative patients. The median/IQR of SUVmax for HER2-positive vs. HER2-negative were 5.78/[3.6, 7.3] vs. 3.1/[2.03, 5.65].
Table 2.
Summary of PET findings.
| [89Zr]trastuzumab SUVmax | ||||
|---|---|---|---|---|
| N | Mean ± SD | Median/IQR | p | |
|
|
||||
| All Lesions | 0.06 | |||
| HER2-positive | 34 | 5.68 ± 2.64 | 5.78/[3.6, 7.3] | |
| HER2-negative | 16 | 4.46 ± 3.44 | 3.1/[2.03, 5.65] | |
| All Lesions Excluding Hepatic Lesions | 0.003 | |||
| HER2-positive | 33 | 5.4 ± 2.49 | 5.4/[3.3, 7.2] | |
| HER2-negative | 13 | 2.96 ± 1.55 | 2.8/[1.8, 3.2] | |
| Hepatic Lesions | 0.42 | |||
| HER2-positive | 6 | 10.03 ± 5.08 | 8/[6.4, 14.6] | |
| HER2-negative | 6 | 8.02 ± 4.24 | 7.85/[4.6, 10.8] | |
Note: N = number of patients; SD = standard deviation; IQR = interquartile range [25th Percentile, 75th Percentile]; SUVmax = maximum standardized uptake value; p = p-value from Kruskal-wallis test.
Figure 1.
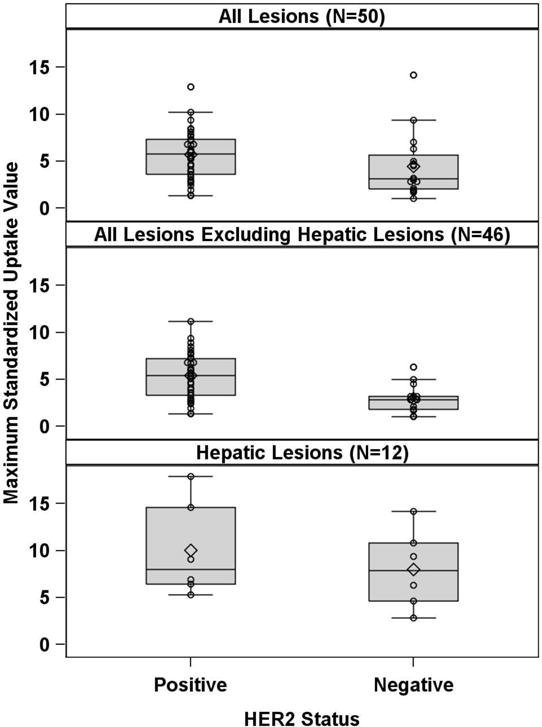
Boxplots of the relationship of HER2 status and [89Zr]trastuzumab uptake, expressed as SUVmax
After excluding hepatic lesions, the tumor SUVmax values were re-calculated for 46 patients (33 HER2-positvie and 13 HER2-negative). The SUVmax values were significantly higher (p = 0.003) in HER2-positive patients (median/IQR: 5.4/[3.3, 7.2]) compared to HER2-negative patients (median/IQR: 2.8/[1.8, 3.2]). Using logistic regression, it was found that SUVmax was significantly associated with HER2 status with an estimated odds ratio of 1.76 (95% CI: 1.17 – 2.65; p=0.007), indicating the larger the SUVmax, the more likely that HER2 status would be positive. An optimum SUVmax cutoff point of 3.2 was selected in a way that maximizes the sum of the specificity and sensitivity under the ROC curve (area under the curve =0.7879, Figure 2). 25 out of 33 HER2 positive patients had SUVmax greater or equal to 3.2; 8 out of 13 HER2-negative patients had SUVmax less than 3.2, with a PPV of 83.3% (95% CI: 65.3%, 94.4%), sensitivity of 75.8% (57.7%, 88.9%), NPV of 50% (24.7%, 75.3%), and specificity of 61.5% (31.6%, 86.1%) (Table 3).
Figure 2.
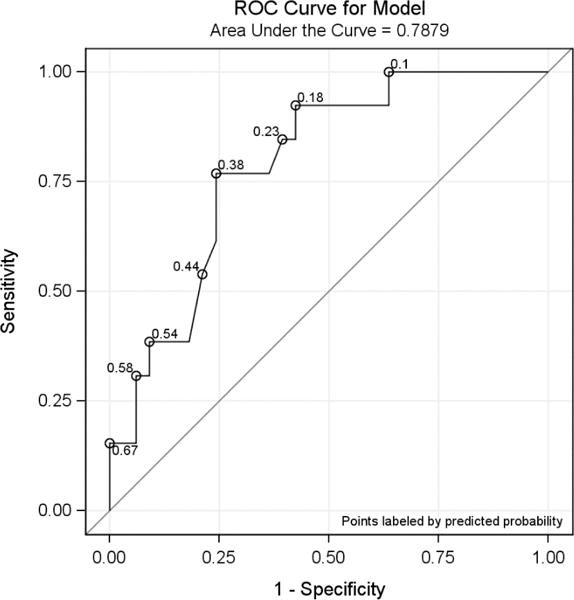
Area under the ROC curve of SUVmax for all lesions (excluding hepatic lesions).
Table 3.
Relationship of HER2 status and [89Zr]trastuzumab-PET/CT results (per-patient basis) using SUVmax cutoff point of 3.2.
| Type | Proportion (95% Exact Binomial Confidence Interval) |
|---|---|
| Sensitivity | 75.8% (57.7%, 88.9%) |
| Specificity | 61.5% (31.6%, 86.1%) |
| Positive Predictive Value | 83.3% (65.3%, 94.4%) |
| Negative Predictive Value | 50.0% (24.7%, 75.3%) |
There were 21 hepatic lesions, 10 in 6 patients with HER2-positive disease and 11 in 6 patients with HER2-negative disease. Four hepatic lesions in 1 patient with HER2-positive disease showed increased [89Zr]trastuzumab uptake, but uptake in the remaining 17 lesions in the other 11 patients was similar to the liver background. Considering only hepatic lesions, the tumor SUVmax was not significantly higher in patients with HER2-positive disease compared with HER2-negative disease (8/[6.4, 14.6] vs. 7.85/[4.6, 10.8]; p = 0.42).
We observed intrapatient heterogeneity of [89Zr]trastuzumab uptake in 10 of the 50 patients with multiple lesions. Five of these patients had HER2-positive disease in whom the liver lesions did not show increased [89Zr]trastuzumab uptake, while their other tumor sites were positive on [89Zr]trastuzumab-PET/CT. This result may represent true heterogeneity or be a limitation of this imaging agent in evaluating hepatic lesions. These patients received between 2 to 15 treatment regimens in the past. Two patients had no or very low [89Zr]trastuzumab uptake in lung metastases while their bone and liver metastases showed increased uptake (Figure 3). These patients received 9 and 14 treatment regimens, respectively, in the past. Another patient with lung metastasis with both positive and negative lung lesions, received 6 treatment regimens in the past. Similarly, 1 patient with osseous metastasis had both positive and negative osseous lesions, received 15 treatment regimens in the past. The remaining patient had metastatic breast cancer at diagnosis and was undergoing first-line systemic therapy; she had positive axillary lymph nodes, but negative breast lesion.
Figure 3.
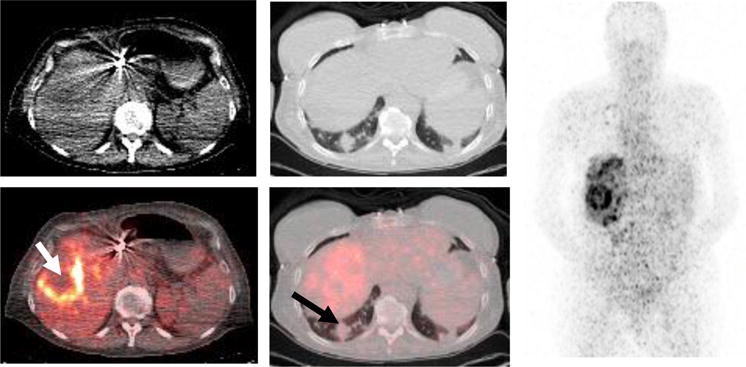
Intrapatient heterogeneity of [89Zr]trastuzumab uptake in a 64-year-old woman with metastatic estrogen-receptor-positive/HER2-positive breast cancer. She had received 10 prior regimens for metastatic disease. Axial CT (left top, soft tissue window), axial fused [89Zr]trastuzumab-CT (left lower), axial CT (middle top, lung window), axial fused [89Zr]trastuzumab-CT (middle lower), and anterior reprojection MIP image (right) demonstrate increased [89Zr]trastuzumab uptake (white arrow) within the hepatic lesions without increased uptake (black arrow) within the pulmonary metastases. These images were obtained 7 days after injection of [89Zr]trastuzumab.
Discussion
It has been shown that biomarkers, such as the estrogen receptor (ER), progesterone receptor (PR) and HER2, which are predictive of response to targeted therapy, continue to change throughout tumor progression. Based on tissue biopsy samples, Lindström et al. found that ER, PR and HER2 status changed over time when comparing the primary tumor and subsequent metastatic disease in 32.4%, 40.7% and 14.5%, respectively (11). Another study by Aiken et al. found a change in HER2 status in 9.9% of patients, 7.4% from negative to positive and 1.5% from positive to negative. Change of ER or HER2 status from negative to positive in the relapse setting would introduce additional therapeutic options, potentially leading to objective response and prolonged survival in some patients. The potential for changing biomarkers of therapeutic targets makes clinical decisions more difficult and necessitates repeated biopsies at recurrences to optimize treatment selection. In addition, many studies have demonstrated discordant expression of ER, PR and HER2 between primary breast cancer, synchronous axillary metastases, and distant metastatic sites (12). In a multicenter trial, discordance in HER2 status between primary tumor and synchronous axillary lymph nodes was noted in nearly 5% of patients (13). This discordance further indicates the importance of knowledge of the status of HER2 or other biomarkers of as many lesions as possible, both at initial diagnosis and recurrence, as this information will influence management and impact patient outcome. However, it is impractical or impossible to biopsy multiple lesions and, thus, a noninvasive method that can map the HER2 status of a patient’s entire tumor burden would be very useful.
Several PET radiopharmaceuticals have been proposed for imaging HER2; the most widely studied are [89Zr]trastuzumab and [64Cu]DOTA-trastuzumab. Mortimer et al. recently demonstrated significantly higher uptake of [64Cu]DOTA-trastuzumab in the lesions of 11 HER2-positive patients compared with those of 7 HER2-negative patients, with median average intrapatient SUVmax of 6.8 vs. 4.3 (p <0.005) on day 2 after tracer administration; there was overlap in the average intrapatient SUVmax in the two groups (14). They also found that 1 day after injection, the uptake of [64Cu]DOTA-trastuzumab in metastatic breast cancer is strongly associated with patient HER2 status and is indicative of binding to HER2. [89Zr]trastuzumab has been used primarily in Europe and demonstrated to be a sensitive tracer for detection of HER2-positive lesions by PET (7), and for prediction of response to T-DM1, a HER2-targeted therapy (15). More recently, Ulaner et al. studied 9 patients and demonstrated that [89Zr]trastuzumab-PET/CT detected unsuspected HER2-positive metastases in 5 patients with HER2-negative primary breast cancer (16). HER2 positivity was histologically confirmed in 2 of these 5 patients, who benefited from HER2-targeted therapy.
In our initial report of 12 HER2-positive patients, we found that [89Zr]trastuzumab was safe and yielded images of good quality at 4 days or later post-injection. The effective dose was 0.47 mSv/MBq, with the liver as the critical organ (average dose of 1.54 mGy/MBq). We have since evaluated an additional 39 patients in this current study, with HER2-positive or HER2-negative breast cancers. We found evaluation of hepatic lesions with [89Zr]trastuzumab to be limited, as the majority of hepatic lesions in both HER2-positive and HER2-negative patients had uptake similar to liver background activity. This finding has been reported by other investigators; Djikers et al. found that there was no increase in 89Zr-trastuzumab uptake in known hepatic metastases from 3 of the 7 patients with HER2-positive disease (7).
[89Zr]trastuzumab uptake, measured as SUVmax, was significantly higher in patients with HER2-positive disease versus HER2-negative disease, when hepatic metastatic lesions were excluded from the analysis. We found that an SUVmax cutoff of 3.2 has a high PPV of 83.3% and moderately high sensitivity of 75.8% in distinguishing HER2-positive and HER2-negative lesions. Intrapatient heterogeneity of [89Zr]trastuzumab uptake was observed in 10 patients with multiple lesions. All of these patients received prior therapy for metastatic disease; 7 of these patients had been treated with 6 or more metastatic regimens in the past. These results suggest that biopsy specimens may suffer from sampling error because of heterogeneous intratumoral HER2 expression or that a change in HER2 status occurred over time. Accordingly, the ability of [89Zr]trastuzumab-PET to characterize the HER2 status of all or most of a patient’s lesions may obviate repeat biopsy and has the potential to be an important tool for selecting those individuals likely to respond to HER2-targeted therapy.
One limitations of this study is the lack of recent biopsy of lesions of all patients; this could explain the only moderate sensitivity of the [89Zr]trastuzumab-PET for distinguishing HER2-positive and HER2-negative lesions, because the HER2status may have changed over time. Another limitation is the lack of biopsy in the majority of patients with discordant lesions. In addition, we did not study whether [89Zr]trastuzumab uptake is predictive of response to trastuzumab-targeted therapy. Trastuzumab is not typically used as a monotherapeutic agent and is often given in combination with various chemotherapeutic agents and, thus, its direct therapeutic effect cannot be easily evaluated.
Intrapatient heterogeneity of HER2 status may have an important impact in the selection of therapy and determining patient prognosis; however, this needs to be well documented before it can be applied to clinical practice. There is evidence that discordance in HER2 status occurs between primary breast cancer and synchronous axillary metastatic lymph nodes and metastatic/recurrent disease, as well as among metastatic/recurrent lesions. It has been shown by Niikura et al. that patients with discordant HER2 status had shorter overall survival than did patients with concordant HER2 status (4). An important issue to consider in patients with discordant lesions is the false-negative in vitro assays results for HER2 status, a problem that in vivo imaging may be able to address. Van Poznak et al. reviewed the literature to provide recommendations on the appropriate use of the results of biomarker assays to guide decisions on systemic therapy for metastatic breast cancer (17). They concluded that retesting of ER, PR, and HER2 status should be offered, but evidence is limited to determine whether changing cancer therapy on the basis of change in receptor status affects clinical outcomes. With discordance of results of the ER, PR, and HER2 status between primary and metastatic tissues, the Panel consensus was to use preferentially the receptor status of the metastasis to direct therapy if supported by the clinical scenario and the patient’s goals for care. However, simultaneous determination of HER2 in primary breast cancer and metastatic lymph nodes or among multiple metastatic lesions, while very important, is not clinically practical, as multiple biopsies would be required. Non-invasive imaging methods, such as [89Zr]trastuzumab-PET/CT, appear to have the potential for determining the availability of HER2 for binding to trastuzumab-targeted therapy, thus, guiding individualized treatment by identifying patients most likely (or unlikely) to benefit from such treatment. [89Zr]trastuzumab-PET/CT has the potential to identify patients with HER2-negative disease, who may not be candidates for trastuzumab-based therapy, without the need for additional biopsies. These patients can be treated with alternative approaches, such as oral tyrosine kinase inhibitors rather than continuing ineffective trastuzumab-based therapy.
Acknowledgments
This work was supported by National Cancer Institute Grant CA182945, U.S. Department of Energy Grant DE-SC0012737 and the Alvin J. Siteman Cancer Center Imaging and Response Assessment Core.
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 3.Lv Q, Meng Z, Yu Y, et al. Molecular Mechanisms and Translational Therapies for Human Epidermal Receptor 2 Positive Breast Cancer. Int J Mol Sci. 2016;17(12) doi: 10.3390/ijms17122095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niikura N, Liu J, Hayashi N, et al. Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. J Clin Oncol. 2012;30(6):593–599. doi: 10.1200/JCO.2010.33.8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niikura N, Tomotaki A, Miyata H, et al. Changes in tumor expression of HER2 and hormone receptors status after neoadjuvant chemotherapy in 21,755 patients from the Japanese breast cancer registry. Ann Oncol. 2016;27(3):480–487. doi: 10.1093/annonc/mdv611. [DOI] [PubMed] [Google Scholar]
- 6.Laforest R, Lapi SE, Oyama R, et al. [Zr]Trastuzumab: Evaluation of radiation dosimetry, safety, and optimal imaging parameters in women with HER2-positive breast cancer. Mol Imaging Biol. 2016;18(6):952–959. doi: 10.1007/s11307-016-0951-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dijkers EC, Oude Munnink TH, Kosterink JG, et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther. 2010;87(5):586–592. doi: 10.1038/clpt.2010.12. [DOI] [PubMed] [Google Scholar]
- 8.Vosjan MJ, Perk LR, Visser GW, et al. Conjugation and radiolabeling of monoclonal antibodies with zirconium-89 for PET imaging using the bifunctional chelate p-isothiocyanatobenzyl-desferrioxamine. Nat Protoc. 2010;5(4):739–743. doi: 10.1038/nprot.2010.13. [DOI] [PubMed] [Google Scholar]
- 9.Lindmo T, Boven E, Cuttitta F, Fedorko J, Bunn PA., Jr Determination of the immunoreactive fraction of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J Immunol Methods. 1984;72(1):77–89. doi: 10.1016/0022-1759(84)90435-6. [DOI] [PubMed] [Google Scholar]
- 10.Laforest R, Lapi SE, Oyama R, et al. [89Zr]Trastuzumab: Evaluation of Radiation Dosimetry, Safety, and Optimal Imaging Parameters in Women with HER2-Positive Breast Cancer. Mol Imaging Biol. 2016;18(6):952–959. doi: 10.1007/s11307-016-0951-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindstrom LS, Karlsson E, Wilking UM, et al. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J Clin Oncol. 2012;30(21):2601–2608. doi: 10.1200/JCO.2011.37.2482. [DOI] [PubMed] [Google Scholar]
- 12.Rossi S, Basso M, Strippoli A, et al. Hormone Receptor Status and HER2 Expression in Primary Breast Cancer Compared With Synchronous Axillary Metastases or Recurrent Metastatic Disease. Clin Breast Cancer. 2015;15(5):307–312. doi: 10.1016/j.clbc.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Ieni A, Barresi V, Caltabiano R, et al. Discordance rate of HER2 status in primary breast carcinomas versus synchronous axillary lymph node metastases: a multicenter retrospective investigation. Onco Targets Ther. 2014;7:1267–1272. doi: 10.2147/OTT.S65294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mortimer JE, Bading JR, Park JM, et al. Tumor Uptake of 64Cu-DOTA-Trastuzumab in Patients with Metastatic Breast Cancer. J Nucl Med. 2017 doi: 10.2967/jnumed.117.193888. ahead of print oi:10.2967/jnumed.117193888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gebhart G, Lamberts LE, Wimana Z, et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): the ZEPHIR trial. Ann Oncol. 2016;27(4):619–624. doi: 10.1093/annonc/mdv577. [DOI] [PubMed] [Google Scholar]
- 16.Ulaner GA, Hyman DM, Ross DS, et al. Detection of HER2-Positive Metastases in Patients with HER2-Negative Primary Breast Cancer Using 89Zr-Trastuzumab PET/CT. J Nucl Med. 2016;57(10):1523–1528. doi: 10.2967/jnumed.115.172031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Poznak C, Somerfield MR, Bast RC, et al. Use of Biomarkers to Guide Decisions on Systemic Therapy for Women With Metastatic Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2015;33(24):2695–2704. doi: 10.1200/JCO.2015.61.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]


