Abstract
Study Design
A cross-sectional clinical measurement study.
Introduction
Measuring intrinsic hand muscle strength helps evaluate hand function or therapeutic outcomes. However, there are no established normative values in adolescents and young adults between 13 and 20 years of age.
Purpose of the Study
To measure hand intrinsic muscle strength and identify associated factors that may influence such in adolescents and young adults through use of the Rotterdam intrinsic hand myometer.
Methods
A total of 131 participants (male: 63; female: 68) between 13 and 20 years of age completed the strength measurements of abductor pollicis brevis, first dorsal interosseus (FDI), deep head of FDI and lumbrical of second digit, flexor pollicis brevis (FPB), and abductor digiti minimi. Two trials of the measurements of each muscle were averaged for analyses. Self-reported demographic data were used to examine the influences of age, sex, and body mass index (BMI) on intrinsic hand muscle strength.
Results
Normative values of intrinsic hand muscle strength were presented by age groups (13, 14, 15–16, 17–18, 19–20 year olds) for each sex category (male, female). A main effect of sex, but not age, on all the muscles on both the dominant (FPB: P = .02, others: P < .001) and non-dominant (FDI: P = .005, FPB: P = .01, others: P < .001) sides was found. A significant effect of BMI was found on dominant (P = .009) and non-dominant abductor pollicis brevis (P = .002). In addition, FDI (P = .005) and FPB (P = .002) were stronger on the dominant side than the non-dominant side.
Discussion
Intrinsic hand muscle strength may be influenced by different factors including sex, BMI, and hand dominance. A larger sample is needed to rigorously investigate the influence of age on intrinsic strength in male and female adolescents and young adults.
Conclusion
The results provide reference values and suggest factors to be considered when evaluating hand function and therapeutic outcomes in both clinical and research settings. Further study is recommended.
Level of Evidence
VI.
Keywords: Intrinsic muscle, Rotterdam intrinsic hand myometer, Hand function
Introduction
Hand strength is a common outcome measure in hand and developmental therapy.1 Grip and pinch dynamometry reflect the combination of both the extrinsic and intrinsic muscle activity,2 whereas intrinsic myometry can provide more direct information regarding intrinsic muscle function.3 The strength of intrinsic muscles of digits and thumb contribute to important functional hand activities, especially for those requiring dexterous hand movements, such as handwriting.4,5 Moreover, measuring isolated hand intrinsic muscle strength can evaluate or monitor the progression or resolution of certain hand pathologies, hand therapy intervention effects, and research outcomes with greater specificity. For example, the first dorsal interosseous (FDI) and abductor digiti minimi are innervated by ulnar nerve, and thus the strength of these muscles may be clinical indicators to understand hand function and recovery in patients with ulnar nerve injuries.3 However, isolated intrinsic muscle strength of the hand is not commonly assessed in clinic. This might be due to manual muscle testing (MMT)’s lack of responsiveness to change,6 reduced awareness of the availability of clinically appropriate intrinsic myometers, and incomplete normative data for such instrumentation.
The Rotterdam intrinsic hand myometer (RIHM) has been found to be a valid and reliable tool used to measure strength of individual intrinsic hand muscles in children and adults aged 4–12 and 16–70 years.3,7–10 RIHM reference values are also available in younger children between 4 and 12 years; yet, there are no reports of similar normative values in adolescents and young adults between 13 and 20 years.11 This gap limits our understanding regarding the development of isolated intrinsic muscle strength, which further restricts the application of the RIHM among adolescents and young adults with hand dysfunction.
Measuring isolated intrinsic muscle strength of the hand has potential to be widely used in youth with genetic, developmental, neurologic, or orthopedic conditions. Decreased hand intrinsic strength may be experienced in youth with Charcot-Marie-Tooth disease, brachioplexopathies, juvenile rheumatoid arthritis, and hand overuse12–18 and may be associated with limitations in self-care skills (eg, opening a snack box) and academic performance (eg, handwriting).4 Many with hand dysfunction require surgeries such as tendon transfers or lengthening and nerve repair,19,20 hand therapy after acute injuries and operation, or developmental rehabilitation for hand use in those born with disabilities. The use of a responsive psychometrically sound tool with normative reference values can assist in evaluating the success of these therapy and surgical interventions.
However, to better monitor the success of interventions that target intrinsic hand function in children and youth, normative testing should be expanded to include adolescents and young adults. The primary purpose of this study was to establish normative strength values, using the RIHM, in selected intrinsic hand muscles in individuals between 13 and 20 years. A secondary purpose was to evaluate if factors known to influence gross grasp strength (ie, age, sex, handedness, and body mass index [BMI]) also influence intrinsic hand muscle strength in this age range.
Methods
Participants
Adolescents or young adults between 13 and 20 years were recruited in this study via stratified convenience sampling at an US state fair, a statewide gathering with exhibitions and recreational and university research activities. Exclusion criteria included self-reported hand dysfunction, any neurologic diseases that influenced their movement control and hand muscle strength, deformities or amputations of tested digits, pain that prohibited or was made worse by testing procedures, and the inability to follow standardized procedures.
General procedures
This study was approved by a Midwestern US institutional review board. Informed consent and assent were obtained from legal guardians and participants between 13 and 17 years; consent was obtained from participants between 18 and 20 years. Five testers, including 2 physical therapists, 1 occupational therapist, and 2 graduate physical therapy students, received a minimum of 2 hours of training in performing 5 intrinsic muscle strength measurements using the RIHM by a trained occupational therapy academician and certified hand therapist. Each tester was required to demonstrate competency on each measure (eg, consistency in positioning the device and participant for testing).7 Muscle strength measurement data were collected across 3 days on different participants at the state fair. Participants entered demographic information (ie, age, sex, race, ethnicity, and BMI) into a secured tablet device through use of the research electronic data capture electronic data capture tools before strength measurements,21 and the same system was used to record RIHM data.
Measurements
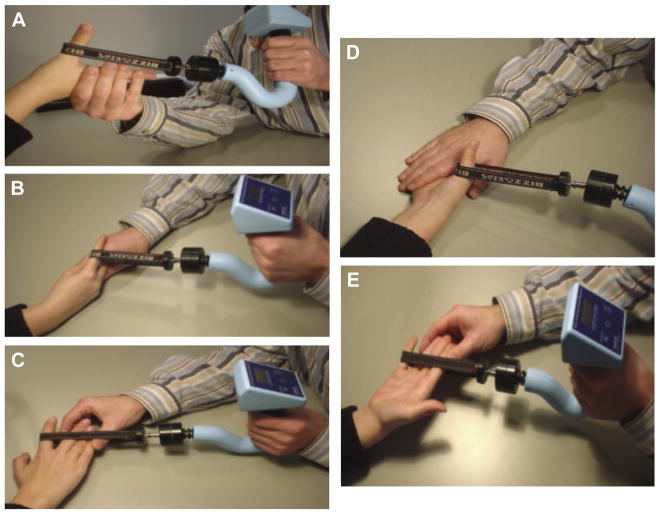
Three calibrated RIHM instruments, capable of accurately measuring up to 300 N (Fig. 1),22 were used to quantify the maximum voluntary strength of selected hand movements produced primarily by intrinsic musculatures. These movements included the thumb carpometacarpal palmar abduction (abductor pollicis brevis [APB]), index finger metacarpophalangeal (MP) abduction (superficial head of FDI), index finger MP flexion (deep head of FDI and lumbrical of second digit), thumb MP flexion (flexor pollicis brevis [FPB]), and small finger MP abduction (abductor digiti minimi). The aforementioned muscles are likely not the only muscles contracting during these assessments; however, as we presumed to be measuring the prime movers,11 we will henceforth be referring to the muscles and not the movements tested. In a manner consistent with MMT, the maximal voluntary contractile strength of the intrinsic muscles was quantified through the break test according to the standardized procedures described by Schreuders et al9,23 (Fig. 2). The testing sequence of the 5 muscle tests was randomized to control for an order effect; however, testing always began with the right hand. For our protocol, 2 trials of each intrinsic measure were performed by considering the feasible time that we asked from each volunteered participant and the existed protocols and acceptable reliability of muscle strength assessment from 2-trial averaged results.24–29 If a protocol deviation was observed during testing, such as slippage of the device sling, a third trial was recorded. The third trial measurement replaced the result of the failed attempt and was used for further analysis. A nonslip piece of Dycem Reel (Dycem, Bristol) was added under the sling by testers to reduce skin slippage if needed. Thirty-second rest breaks were offered between each testing trial to control for the influence of fatigue. The testing results were read and entered to the research electronic data capture system by the tester.
Fig. 1.
The Rotterdam intrinsic hand dynamometer.
Fig. 2.
Finger strength testing positions: (A) abductor pollicis brevis, (B) interossei and lumbrical of second digit, (C) first dorsal interosseous, (D) flexor pollicis brevis and (E) abductor digiti minimi.
Statistical analysis
For each participant and muscle tested, the mean of 2 trials was computed. BMI was calculated based on self-reported height and weight. Summary statistics (eg, means and standard deviations) were used to describe the characteristics of the sample. Two trials of peak forces across all 5 measures per hand were used to calculate the intrasession reliability of testers using intraclass correlation coefficient (ICC3,1). Analyses of covariance, a type of general linear modeling (GLM), were used to quantify the effects of independent variables (ie, known predictors of gross strength) on each measure of intrinsic strength. Sex (male and female) and 5 age groups (group 1: 13 year olds; group 2: 14 year olds; group 3: 15–16 year olds; group 4: 17–18 year olds; and group 5: 19–20 year olds) were treated as fixed factors (independent variables), and participants’ BMI was treated as the covariate in the models. Age grouping was based on the sample size of each age strata. The main effects of sex, age, and BMI and the interaction effect between sex and age were analyzed. Paired t tests were used for within-participant comparison on the 5 separate measures between dominant and nondominant hands. A P value less than .05 was defined as significant for paired t tests and the main and interaction effects of the GLM.
Results
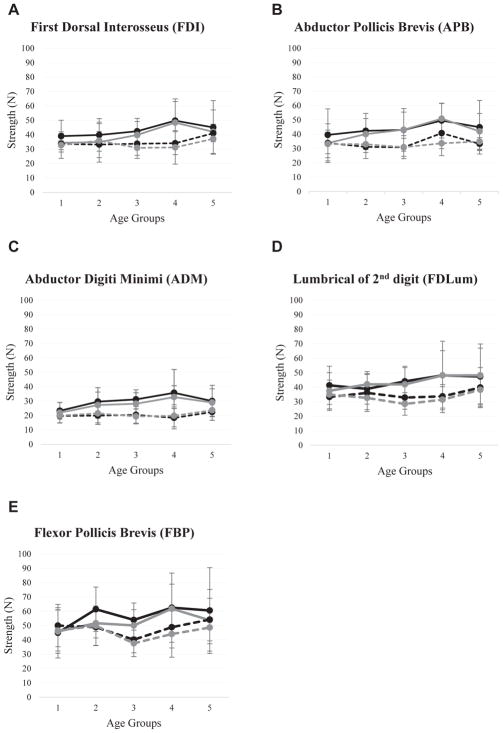
One hundred thirty-one participants completed intrinsic hand muscle strength testing (male: n = 63; female: n = 68). The sample’s demographic characteristics are presented in Table 1, and the summarized statistics of intrinsic strength according to age, sex, and hand dominance are presented in Figures 3A–3E and Tables 2 and 3. In each of the 131 participants, 10 muscles were tested resulting in a total of 1310 trials. In 243 trials (18.55%), a third trial was collected due to deviated testing trials (eg, slippage of the sling). The deviated data were excluded, and only 2 trials of the data of each muscle were averaged for statistical analyses. ICC values demonstrated excellent intrasession reliability (ICC3,1 = 0.874–0.946) for the pooled data of all testers across all measures (Table 4). According to the GLM analyses, there were main effects of sex on all 5 measures on both the dominant (FPB: P = .022; others: P < .001) and nondominant (FDI: P = .005; FPB: P = .011; others, P < .001) sides. There was no main effect of age on any measures; however, there was an interaction effect between sex and age on the nondominant APB (F = 2.68; P = .035). BMI also had a significant effect on dominant (F = 7.13; P = .009) and nondominant APB (F = 9.93; P = .002). Regardless of sex or age, the strength of FDI (P = .005) and FPB (P = .002) of the dominant side was stronger than the nondominant side.
Table 1.
Demographic data of participants
| Characteristic | N | % |
|---|---|---|
| Total | 131 | 100 |
| Gender | ||
| Male | 63 | 48.1 |
| Female | 68 | 51.9 |
| Age | ||
| Male (13) | 10 | 7.6 |
| Male (14) | 9 | 6.9 |
| Male (15–16) | 11 | 8.4 |
| Male (17–18) | 19 | 14.5 |
| Male (19–20) | 14 | 10.7 |
| Female (13) | 17 | 13.0 |
| Female (14) | 11 | 8.4 |
| Female (15–16) | 17 | 13.0 |
| Female (17–18) | 10 | 7.6 |
| Female (19–20) | 13 | 9.9 |
| Handedness | ||
| Right handed | 115 | 87.8 |
| Left handed | 16 | 12.2 |
| Ethnicity | ||
| Hispanic or Latino | 3 | 2.3 |
| Non-Hispanic or Latino | 128 | 97.7 |
| Race | ||
| Caucasian | 116 | 88.5 |
| Black or African American | 4 | 3.1 |
| Asian | 8 | 6.1 |
| American Indian or Alaska Native | 3 | 2.3 |
| Native Hawaiian or other Pacific Islander | 0 | 0 |
| BMI, Mean | ||
| Male | 23.1 | |
| Female | 22.8 | |
BMI = body mass index.
Fig. 3.
Strength as measured in Newton (N) of hand intrinsic muscles of different age groups separated by gender and dominant/non-dominant side. (A) Flexor Dorsal Interosseus; (B) Abductor Pollicis Brevis; (C) Abductor Digit Minimi; (D) Lumbrical of 2nd digit; (E) Flexor Pollicis Brevis. Age group 1: 13 year-old; age group 2: 14 year-old; age group 3: 15–16 year-old; age group 4: 17–18 year-old; age group 5: 1920 year-old. Black-solid and black-dashed lines indicate dominant side in males and females respectively; grey-solid and grey-dashed lines indicate non-dominant side of males and females respectively.
Table 2.
Descriptive data of intrinsic muscle strength in male participants
| Age (y) | Muscles | n | Dominant hand
|
Nondominant hand
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Minimum | Maximum | Mean | SD | Minimum | Maximum | Mean | SD | |||
| 13 | APB | 10 | 14.00 | 69.55 | 39.50 | 18.13 | 13.05 | 56.75 | 33.76 | 13.51 |
| FDI | 10 | 24.00 | 63.20 | 39.02 | 11.06 | 25.30 | 45.70 | 34.46 | 6.43 | |
| FPB | 10 | 25.30 | 85.70 | 44.93 | 17.56 | 27.35 | 79.25 | 45.87 | 15.20 | |
| ADM | 10 | 12.15 | 29.65 | 23.29 | 5.74 | 9.55 | 31.10 | 21.96 | 7.13 | |
| FDILum | 10 | 27.90 | 62.45 | 41.31 | 13.19 | 26.05 | 60.65 | 37.57 | 12.51 | |
| 14 | APB | 9 | 29.80 | 63.45 | 42.38 | 12.17 | 23.35 | 61.70 | 40.09 | 10.86 |
| FDI | 9 | 24.70 | 60.65 | 39.84 | 11.32 | 21.75 | 69.55 | 34.82 | 13.88 | |
| FPB | 9 | 44.12 | 80.10 | 61.37 | 15.53 | 40.70 | 75.50 | 51.67 | 10.26 | |
| ADM | 9 | 19.85 | 45.50 | 29.62 | 9.74 | 16.70 | 47.35 | 27.27 | 9.03 | |
| FDILum | 9 | 27.70 | 61.00 | 38.84 | 10.52 | 25.20 | 55.80 | 41.98 | 8.84 | |
| 15–16 | APB | 11 | 19.30 | 79.70 | 43.00 | 14.90 | 23.35 | 72.65 | 43.10 | 12.34 |
| FDI | 11 | 27.05 | 54.20 | 42.42 | 8.99 | 23.45 | 52.15 | 39.83 | 9.32 | |
| FPB | 11 | 40.70 | 78.70 | 53.92 | 11.83 | 30.50 | 73.30 | 50.09 | 10.97 | |
| ADM | 11 | 18.60 | 42.90 | 31.22 | 6.65 | 18.30 | 39.90 | 28.20 | 7.62 | |
| FDILum | 11 | 30.20 | 56.95 | 44.06 | 10.43 | 26.10 | 60.10 | 41.96 | 11.60 | |
| 17–18 | APB | 19 | 32.35 | 76.30 | 49.53 | 12.13 | 34.45 | 75.65 | 50.85 | 10.59 |
| FDI | 19 | 26.35 | 94.60 | 49.75 | 15.14 | 31.00 | 81.30 | 48.39 | 14.63 | |
| FPB | 19 | 32.55 | 49.45 | 62.50 | 24.17 | 41.95 | 110.75 | 61.79 | 17.16 | |
| ADM | 19 | 17.85 | 94.10 | 35.79 | 16.18 | 18.60 | 47.50 | 32.78 | 7.92 | |
| FDILum | 19 | 21.65 | 104.50 | 48.19 | 17.07 | 27.55 | 140.20 | 48.12 | 23.66 | |
| 19–20 | APB | 14 | 25.50 | 92.70 | 44.87 | 18.74 | 27.55 | 67.05 | 42.04 | 12.46 |
| FDI | 14 | 25.00 | 82.90 | 45.06 | 18.61 | 24.05 | 80.10 | 41.97 | 15.27 | |
| FPB | 14 | 21.30 | 140.45 | 60.50 | 29.90 | 29.40 | 104.50 | 53.67 | 21.50 | |
| ADM | 14 | 16.70 | 59.30 | 30.09 | 10.88 | 16.70 | 45.65 | 29.03 | 9.55 | |
| FDILum | 14 | 25.95 | 98.00 | 47.39 | 19.63 | 27.55 | 99.55 | 48.24 | 21.60 | |
| All | APB | 63 | 14.00 | 92.70 | 44.74 | 15.23 | 13.05 | 75.65 | 43.29 | 12.86 |
| FDI | 63 | 24.00 | 94.60 | 44.31 | 14.27 | 21.75 | 81.30 | 41.32 | 13.62 | |
| FPB | 63 | 21.30 | 149.45 | 57.61 | 22.25 | 27.35 | 110.75 | 53.97 | 16.78 | |
| ADM | 63 | 12.15 | 94.10 | 30.86 | 11.92 | 9.55 | 47.50 | 28.64 | 8.80 | |
| FDILum | 63 | 21.65 | 104.50 | 44.86 | 15.32 | 25.20 | 140.20 | 44.52 | 16.78 | |
SD = standard deviation; APB = abductor pollicis brevis; FDI = first dorsal interosseous; FPB = flexor pollicis brevis; ADM = abductor digiti minimi; FDILum = interossei and lumbrical of second digit.
Table 3.
Descriptive data of intrinsic muscle strength in female participants
| Age (y) | Muscles | n | Dominant hand
|
Nondominant hand
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Minimum | Maximum | Mean | SD | Minimum | Maximum | Mean | SD | |||
| 13 | APB | 17 | 24.60 | 49.35 | 33.83 | 7.49 | 15.45 | 50.50 | 33.18 | 9.62 |
| FDI | 17 | 28.30 | 43.65 | 33.58 | 3.96 | 15.30 | 47.55 | 32.77 | 9.18 | |
| FPB | 17 | 32.10 | 97.10 | 50.01 | 14.81 | 22.05 | 75.75 | 46.54 | 14.30 | |
| ADM | 17 | 11.95 | 30.70 | 19.76 | 4.83 | 13.25 | 30.10 | 19.96 | 4.99 | |
| FDILum | 17 | 12.50 | 51.75 | 33.24 | 9.26 | 11.40 | 58.23 | 34.95 | 9.98 | |
| 14 | APB | 11 | 17.50 | 49.90 | 31.16 | 8.18 | 20.30 | 39.95 | 32.95 | 5.71 |
| FDI | 11 | 14.40 | 49.80 | 33.18 | 9.13 | 18.15 | 51.60 | 35.86 | 11.75 | |
| FPB | 11 | 24.25 | 62.85 | 48.85 | 12.67 | 29.10 | 73.80 | 49.83 | 13.86 | |
| ADM | 11 | 10.00 | 26.75 | 19.96 | 6.05 | 10.45 | 31.25 | 21.48 | 6.57 | |
| FDILum | 11 | 21.90 | 64.30 | 36.09 | 12.79 | 21.60 | 48.35 | 32.62 | 8.08 | |
| 15–16 | APB | 17 | 19.70 | 45.10 | 30.82 | 6.28 | 19.55 | 42.55 | 30.94 | 8.03 |
| FDI | 17 | 17.70 | 46.30 | 33.74 | 8.04 | 20.85 | 43.95 | 30.89 | 7.30 | |
| FPB | 17 | 26.40 | 56.70 | 40.14 | 9.12 | 24.20 | 59.05 | 37.56 | 9.31 | |
| ADM | 17 | 11.45 | 30.85 | 20.48 | 5.76 | 11.55 | 30.65 | 19.35 | 5.14 | |
| FDILum | 17 | 18.60 | 47.55 | 32.84 | 7.99 | 14.75 | 40.25 | 28.47 | 7.77 | |
| 17–18 | APB | 10 | 27.20 | 55.65 | 40.76 | 10.83 | 22.20 | 47.35 | 33.64 | 8.65 |
| FDI | 10 | 23.30 | 44.20 | 34.06 | 7.95 | 13.55 | 49.75 | 31.20 | 11.61 | |
| FPB | 10 | 27.50 | 67.70 | 48.86 | 14.50 | 22.80 | 70.65 | 44.07 | 16.13 | |
| ADM | 10 | 10.00 | 34.75 | 18.36 | 7.61 | 9.50 | 32.90 | 19.86 | 7.68 | |
| FDILum | 10 | 25.50 | 52.00 | 33.73 | 8.11 | 20.95 | 46.95 | 31.43 | 8.96 | |
| 19–20 | APB | 13 | 28.15 | 41.05 | 33.24 | 4.34 | 27.15 | 47.35 | 34.85 | 6.28 |
| FDI | 13 | 31.80 | 50.10 | 40.98 | 5.85 | 21.60 | 53.30 | 37.14 | 10.17 | |
| FPB | 13 | 34.40 | 82.80 | 54.16 | 14.89 | 32.50 | 70.00 | 48.62 | 11.33 | |
| ADM | 13 | 13.30 | 33.45 | 22.42 | 5.67 | 13.05 | 36.60 | 23.73 | 7.04 | |
| FDILum | 13 | 25.65 | 75.00 | 39.78 | 13.81 | 26.40 | 63.60 | 38.24 | 9.77 | |
| All | APB | 68 | 17.50 | 55.65 | 33.55 | 7.91 | 15.45 | 50.50 | 32.97 | 7.83 |
| FDI | 68 | 14.40 | 50.10 | 35.04 | 7.40 | 13.55 | 53.30 | 33.41 | 9.81 | |
| FPB | 68 | 24.25 | 97.10 | 47.98 | 13.73 | 22.05 | 75.75 | 44.86 | 13.31 | |
| ADM | 68 | 10.00 | 34.75 | 20.28 | 5.83 | 9.50 | 36.60 | 20.76 | 6.17 | |
| FDILum | 68 | 12.50 | 75.00 | 34.92 | 10.49 | 11.40 | 63.60 | 33.06 | 9.37 | |
SD = standard deviation; APB = abductor pollicis brevis; FDI = first dorsal interosseous; FPB = flexor pollicis brevis; ADM = abductor digiti minimi; FDILum = interossei and lumbrical of second digit.
Table 4.
Intrasession ICCs for all the measurements on both the dominant and nondominant hands
| Muscles | n | Dominant hand
|
Nondominant hand
|
|---|---|---|---|
| ICC (95% CI) | |||
| APB | 131 | 0.930 (0.903 to 0.905) | 0.910 (0.975 to 0.936) |
| FDI | 131 | 0.927 (0.899 to 0.948) | 0.927 (0.898 to 0.948) |
| FPB | 131 | 0.940 (0.916 to 0.957) | 0.932 (0.905 to 0.951) |
| ADM | 131 | 0.937 (0.913 to 0.955) | 0.874 (0.826 to 0.909) |
| FDILum | 131 | 0.931 (−0.904 to 0.950) | 0.946 (−0.924 to 0.961) |
ICCs = intraclass correlation coefficients; 95% CI = 95% confidence interval; APB = abductor pollicis brevis; FDI = first dorsal interosseous; FPB = flexor pollicis brevis; ADM = abductor digiti minimi; FDILum = interossei and lumbrical of second digit.
Discussion
This study found that adolescents and young adults of the male sex demonstrated higher intrinsic hand muscle strength than females. There were no differences between age groups. In addition, higher BMI is associated with stronger APB on the dominant side; muscle strength of dominant APB and FDI is higher than the nondominant side. The intrasession reliabilities were excellent in this study, which suggests sufficient testing skills of testers to obtain consistent measures under the same testing conditions. The tester judgment on eliminating unsuccessful testing trials (eg, slippage of the sling caused by hand sweating of the participant) allowed for obtaining more accurate data for establishing normative data.
The study results demonstrate stronger intrinsic muscle strength of the hand in males compared with females. This finding is similar to previous studies on pinch and grip strength in both children and adults.30,31 Dianat et al32 compared 4 types of pinch strength between males and females between 7 and 30 years and found that males showed significantly higher strength compared with females in both the dominant and nondominant sides on all 4 strength tests. McQuiddy et al30 also found that sex is a factor influencing grip and pinch strength in children and adolescents between 6 and 19 years. Moreover, Xu et al33 tested intrinsic hand muscle strength in children and adolescents between 4 and 16 years using the Peg Restrained Intrinsic Muscle Evaluator. This study found that males demonstrated stronger strength than females, which is consistent with our results. Conversely, Molenaar et al8 investigated intrinsic strength of the hand in children between 4 and 12 years and did not find significant difference between males and females. We speculate that, because muscle strength has been associated with hormonal and lean muscle volume changes,34–36 the discrepancy of muscle strength between males and females may become more obvious in adolescents and adults than younger children. Although size and force differences between sexes in extrinsic muscles, such as forearm muscles that contribute to grip strength, may already exist in children before puberty,37 there is now evidence to support that the developmental changes in small intrinsic muscles may be different.
No significant effect of age on intrinsic hand muscle strength was observed in this study. This result is inconsistent with previous studies investigating grip and pinch strength in participants from preadolescent to adolescent age and intrinsic hand muscle strength in younger children.8,30,32,33,38 It is possible that the developmental trajectories of small intrinsic muscles are different from extrinsic muscles and lead to less increase in strength through age. Similar to our findings, Dianat et al32 found that the pinch strength was not associated with age in participants between 16 and 30 years. Therefore, less hand muscle strength changes from adolescents to adulthood may contribute to our result. The developmental trajectories of intrinsic muscles might be associated with many factors, such as growth curve of muscle volume, occupational demands as a student, and fine motor development plateaus. Another potential reason is because males and females have different developmental trajectories of muscle strength.36,37,39 We found an interaction effect of sex and age detected on nondominant APB but not on other muscles. A larger sample size of each strata might be needed to confirm the influence of age on intrinsic hand muscle strength in males and females separately.
Based on the GLM analyses, BMI noted was predictive of ABP strength but not other intrinsic muscles. Increased BMI may be, in part, attributed to increased muscle volume, including cross-sectional area and muscle thickness,40 resulting in increased muscle strength. A future study investigating the specific influence of the larger intrinsic muscles, the APB, on muscle volume during development may help to explain the current findings. When comparing muscle strength between dominant and nondominant hands, only the FDI and FPB showed stronger strength on the dominant hand. These 2 muscles play a critical role in tip-pinch movements.19 Tip pinch is important during daily life activities, such as turning a key and opening a package. Therefore, the muscles of the dominant side that contribute to tip-pinch movement may become stronger than the nondominant side because of the increased frequency of use.
There are limitations to this study. Although the standardized procedures were designed to, in principle, control for use of extrinsic muscles/other intrinsic muscles, the testing procedures have not been validated by electromyography. Resultantly, we cannot confirm that the muscle of interest has been optimally tested by RIHM. However, the same arguments can be made against MMT or pinch/grip dynamometry. Nonetheless, further electromyography validation is needed. Another limitation was that handedness, height, and weight were self/caregiver reported and not grounded in objective data assessed onsite by investigators. However, to control for concerns of subjectivity, hand dominance was operationalized as hand of preference for writing, and responses to this phrasing are known to be highly correlated with an objective measure of hand preference.41 Likewise, Spencer et al42 reported a high correlation between self-reported and measured height and weight data. Grip strength as measured by dynamometry can be impacted by hand size.43–46 Previous studies found that anthropometric measurements of the hand, such as thickness, length, and circumference, were associated with grip strength.44–46 Unlike dynamometry and its 2-handled design, the construction of RIHM interfaces with various hand sizes without adjustment. Nevertheless, future studies should include measures of hand anthropometry to further understand the association between such and intrinsic hand muscle strength. Finally, given the significant main effects of sex on strength measurements, results were reported according to age and sex strata, which further diminished sample sizes. Although Portney and Watkins47 have suggested that a sample size of 30+ participants is sufficient to estimate the population mean and these strata were approaching such, a larger sample size might enhance the potential to detect the effect of age on intrinsic hand muscle strength development, especially in different sexes.
Most participants in this study were right-handed Caucasians (Table 1) and, therefore, the normative values of intrinsic hand muscle strength from this homogenous group may not be representative of adolescents with different demographics. Increasing the heterogeneity of participants to improve external validity of the results or conducting similar studies in different countries will add valuable information. Moreover, the daily occupation (ie, hobbies and vocations) and occupational demands of these youth may help to explain strength differences and should be considered in future research.
Conclusions
Between the ages of 13 and 20 years, increasing age does not appear to have an independent effect on intrinsic hand muscle strength. Within this age range, males were found to have significantly greater intrinsic hand muscle strength in all measured muscles; however, the age of adolescent males appear to be a factor when considering only nondominant APB strength. Age and sex aside, BMI predicted only the nondominant APB, and the FDI and FPB appeared to have a greater force-generating capacity in the dominant hand. These findings suggest different capacity to generate force of hand intrinsic muscles between males and females, which may be associated with different lean muscle volume after puberty regardless BMI. The frequency of use and commonality of tasks to be accomplished during daily life are also potential factors that influence the force generation of specific intrinsic hand muscles; future study is required.
The normative values of different intrinsic muscles of the hand in adolescent and young adults between 13 and 20 years can be referenced in both the clinics and research. These values can be used as an important reference when evaluating impairment levels in adolescents with different types of hand dysfunction and to assess progress and response to interventions. Moreover, understanding the potential factors that contribute to changes and differences in strength of these intrinsic hand muscles can also inform our goal setting and assessment of responsiveness to our interventions. These factors can include, but are not limited to, the needs of considering developmental changes and altered body composition when evaluating therapeutic outcomes of intrinsic hand muscle strength.
Acknowledgments
We thank the families and children who participated in this study, and the graduate students Katelyn Kubat and Andrea Tobias for assisting with data collection at this Midwestern US state fair, 2015.
Footnotes
Conflict of interest: All named authors hereby declare that they have no conflicts of interest to disclose.
References
- 1.Shechtman O, Sindhu BS. ASHT’s Clinical Assessment Recommendations. Grip Strength. Chicago, IL: ASHT; 2015. [Google Scholar]
- 2.Kozin SH, Porter S, Clark P, Thoder JJ. The contribution of the intrinsic muscles to grip and pinch strength. J Hand Surg Am. 1999;24(1):64–72. doi: 10.1053/jhsu.1999.jhsu24a0064. [DOI] [PubMed] [Google Scholar]
- 3.Schreuders TA, Selles RW, Roebroeck ME, Stam HJ. Strength measurements of the intrinsic hand muscles: a review of the development and evaluation of the Rotterdam intrinsic hand myometer. J Hand Ther. 2006;19(4):393–401. doi: 10.1197/j.jht.2006.07.024. quiz 402. [DOI] [PubMed] [Google Scholar]
- 4.Alaniz ML, Galit E, Necesito CI, Rosario ER. Hand strength, handwriting, and functional skills in children with autism. Am J Occup Ther. 2015;69(4):6904220030. doi: 10.5014/ajot.2015.016022. p1-6904220030p9. [DOI] [PubMed] [Google Scholar]
- 5.Engel-Yeger B, Rosenblum S. The effects of protracted graphomotor tasks on tripod pinch strength and handwriting performance in children with dysgraphia. Disabil Rehabil. 2010;32(21):1749–1757. doi: 10.3109/09638281003734375. [DOI] [PubMed] [Google Scholar]
- 6.van der Ploeg RJ, Oosterhuis HJ, Reuvekamp J. Measuring muscle strength. J Neurol. 1984;231(4):200–203. doi: 10.1007/BF00313939. [DOI] [PubMed] [Google Scholar]
- 7.McGee CW, Garlough E, Gilbert J, Overlie A, Smurawa K. Measuring extrinsic and intrinsic hand strength in healthy adults: inter-rater reliability of the Rotterdam Intrinsic Hand Myometer. Hand Ther. 2014;27(3):e1–e2. doi: 10.1016/j.jht.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Molenaar HM, Selles RW, Schreuders TA, Hovius SE, Stam HJ. Reliability of hand strength measurements using the Rotterdam Intrinsic Hand Myometer in children. J Hand Surg Am. 2008;33(10):1796–1801. doi: 10.1016/j.jhsa.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 9.Schreuders TA, Roebroeck ME, Jaquet JB, Hovius SE, Stam HJ. Measuring the strength of the intrinsic muscles of the hand in patients with ulnar and median nerve injuries: reliability of the Rotterdam Intrinsic Hand Myometer (RIHM) J Hand Surg Am. 2004;29(2):318–324. doi: 10.1016/j.jhsa.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 10.McGee C. Measuring intrinsic hand strength in healthy adults: the accuracy, intra-rater and inter-rater reliability of the Rotterdam intrinsic hand myometer. J Hand Ther. 2017 doi: 10.1016/j.jht.2017.03.002. [Epub ahead of print] [DOI] [PubMed]
- 11.Molenaar HM, Selles RW, Willemsen SP, Hovius SE, Stam HJ. Growth diagrams for individual finger strength in children measured with the RIHM. Clin Orthop Relat Res. 2011;469(3):868–876. doi: 10.1007/s11999-010-1638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burns J, Bray P, Cross LA, North KN, Ryan MM, Ouvrier RA. Hand involvement in children with Charcot-Marie-Tooth disease type 1A. Neuromuscul Disord. 2008;18(12):970–973. doi: 10.1016/j.nmd.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Hoeksma AF, van Rossum MA, Zinger WG, Dolman KM, Dekker J, Roorda LD. High prevalence of hand- and wrist-related symptoms, impairments, activity limitations and participation restrictions in children with juvenile idiopathic arthritis. J Rehabil Med. 2014;46(10):991–996. doi: 10.2340/16501977-1879. [DOI] [PubMed] [Google Scholar]
- 14.Hulleberg G, Elvrum AK, Brandal M, Vik T. Outcome in adolescence of brachial plexus birth palsy. 69 individuals re-examined after 10–20 years. Acta Orthop. 2014;85(6):633–640. doi: 10.3109/17453674.2014.964614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inal EE, DemIrc IK, CetInturk A, Akgonul M, Savas S. Effects of smartphone overuse on hand function, pinch strength, and the median nerve. Muscle Nerve. 2015;52(2):183–188. doi: 10.1002/mus.24695. [DOI] [PubMed] [Google Scholar]
- 16.Lindehammar H. Hand strength in juvenile chronic arthritis: a two-year follow-up. Acta Paediatr. 2003;92(11):1291–1296. doi: 10.1080/08035250310006340. [DOI] [PubMed] [Google Scholar]
- 17.Selles RW, van Ginneken BT, Schreuders TA, Janssen WG, Stam HJ. Dynamometry of intrinsic hand muscles in patients with Charcot-Marie-Tooth disease. Neurology. 2006;67(11):2022–2027. doi: 10.1212/01.wnl.0000247272.96136.16. [DOI] [PubMed] [Google Scholar]
- 18.Wessel J, Kaup C, Fan J, et al. Isometric strength measurements in children with arthritis: reliability and relation to function. Arthritis Care Res. 1999;12(4):238–246. [PubMed] [Google Scholar]
- 19.Lee SK, Wisser JR. Restoration of pinch in intrinsic muscles of the hand. Hand Clin. 2012;28(1):45–51. doi: 10.1016/j.hcl.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Seu M, Pasqualetto M. Hand therapy for dysfunction of the intrinsic muscles. Hand Clin. 2012;28(1):87–100. doi: 10.1016/j.hcl.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Den Ouden AH, Den Ouden RH. User’s Manual RIHM Version 1.3. Last Mile Solutions; 2005. [Google Scholar]
- 23.Schreuders TA, Roebroeck ME, Jaquet JB, Hovius SE, Stam HJ. Long-term outcome of muscle strength in ulnar and median nerve injury: comparing manual muscle strength testing, grip and pinch strength dynamometers and a new intrinsic muscle strength dynamometer. J Rehabil Med. 2004;36(6):273–278. doi: 10.1080/16501970410033677. [DOI] [PubMed] [Google Scholar]
- 24.Lagerstrom C, Nordgren B. Methods for measuring maximal isometric grip strength during short and sustained contractions, including intra-rater reliability. Ups J Med Sci. 1996;101(3):273–285. doi: 10.3109/03009739609178926. [DOI] [PubMed] [Google Scholar]
- 25.Gagnon D, Nadeau S, Gravel D, Robert J, Belanger D, Hilsenrath M. Reliability and validity of static knee strength measurements obtained with a chair-fixed dynamometer in subjects with hip or knee arthroplasty. Arch Phys Med Rehabil. 2005;86(10):1998–2008. doi: 10.1016/j.apmr.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Aguiar LT, Lara EM, Martins JC, et al. Modified sphygmomanometer test for the assessment of strength of the trunk, upper and lower limbs muscles in subjects with subacute stroke: reliability and validity. Eur J Phys Rehabil Med. 2016;52(5):637–649. [PubMed] [Google Scholar]
- 27.Gerodimos V, Karatrantou K. Reliability of maximal handgrip strength test in pre-pubertal and pubertal wrestlers. Pediatr Exerc Sci. 2013;25(2):308–322. doi: 10.1123/pes.25.2.308. [DOI] [PubMed] [Google Scholar]
- 28.Wang CY, Chen LY. Grip strength in older adults: test-retest reliability and cutoff for subjective weakness of using the hands in heavy tasks. Arch Phys Med Rehabil. 2010;91(11):1747–1751. doi: 10.1016/j.apmr.2010.07.225. [DOI] [PubMed] [Google Scholar]
- 29.Brogardh C, Flansbjer UB, Carlsson H, Lexell J. Intra-rater reliability of arm and hand muscle strength measurements in persons with late effects of polio. PM R. 2015;7(10):1035–1041. doi: 10.1016/j.pmrj.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 30.McQuiddy VA, Scheerer CR, Lavalley R, McGrath T, Lin L. Normative values for grip and pinch strength for 6- to 19-year-olds. Arch Phys Med Rehabil. 2015;96(9):1627–1633. doi: 10.1016/j.apmr.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 31.Mullerpatan R, Karnik G, John R. Grip and pinch strength: normative data for healthy Indian adults. J Hand Ther. 2016;18(1):11–16. [Google Scholar]
- 32.Dianat I, Feizi H, Hasan-Khali K. Pinch strengths in healthy Iranian children and young adult population. Health Promot Perspect. 2015;5(1):52–58. doi: 10.15171/hpp.2015.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu S, Morse AM, Lacy B, Baggett LS, Gogola GR. Peg Restrained Intrinsic Muscle Evaluator (PRIME): development, reliability, and normative values of a device to quantify intrinsic hand muscle strength in children. J Hand Surg Am. 2011;36(5):894–903. doi: 10.1016/j.jhsa.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Dore E, Martin R, Ratel S, Duche P, Bedu M, Van Praagh E. Gender differences in peak muscle performance during growth. Int J Sports Med. 2005;26(4):274–280. doi: 10.1055/s-2004-821001. [DOI] [PubMed] [Google Scholar]
- 35.Henneberg M, Brush G, Harrison GA. Growth of specific muscle strength between 6 and 18 years in contrasting socioeconomic conditions. Am J Phys Anthropol. 2001;115(1):62–70. doi: 10.1002/ajpa.1057. [DOI] [PubMed] [Google Scholar]
- 36.Hogler W, Blimkie CJ, Cowell CT, et al. Sex-specific developmental changes in muscle size and bone geometry at the femoral shaft. Bone. 2008;42(5):982–989. doi: 10.1016/j.bone.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Neu CM, Rauch F, Rittweger J, Manz F, Schoenau E. Influence of puberty on muscle development at the forearm. Am J Physiol Endocrinol Metab. 2002;283(1):E103–107. doi: 10.1152/ajpendo.00445.2001. [DOI] [PubMed] [Google Scholar]
- 38.Mathiowetz V, Wiemer DM, Federman SM. Grip and pinch strength: norms for 6- to 19-year-olds. Am J Occup Ther. 1986;40(10):705–711. doi: 10.5014/ajot.40.10.705. [DOI] [PubMed] [Google Scholar]
- 39.Ploegmakers JJ, Hepping AM, Geertzen JH, Bulstra SK, Stevens M. Grip strength is strongly associated with height, weight and gender in childhood: a cross sectional study of 2241 children and adolescents providing reference values. J Physiother. 2013;59(4):255–261. doi: 10.1016/S1836-9553(13)70202-9. [DOI] [PubMed] [Google Scholar]
- 40.Simon NG, Ralph JW, Lomen-Hoerth C, et al. Quantitative ultrasound of denervated hand muscles. Muscle Nerve. 2015;52(2):221–230. doi: 10.1002/mus.24519. [DOI] [PubMed] [Google Scholar]
- 41.Corey DM, Hurley MM, Foundas AL. Right and left handedness defined: a multivariate approach using hand preference and hand performance measures. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14(3):144–152. [PubMed] [Google Scholar]
- 42.Spencer EA, Appleby PN, Davey GK, Key TJ. Validity of self-reported height and weight in 4808 EPIC-Oxford participants. Public Health Nutr. 2002;5(4):561–565. doi: 10.1079/PHN2001322. [DOI] [PubMed] [Google Scholar]
- 43.Lee SJ, Kong YK, Lowe BD, Song S. Handle grip span for optimising finger-specific force capability as a function of hand size. Ergonomics. 2009;52(5):601–608. doi: 10.1080/00140130802422481. [DOI] [PubMed] [Google Scholar]
- 44.Abe T, Counts BR, Barnett BE, Dankel SJ, Lee K, Loenneke JP. Associations between handgrip strength and ultrasound-measured muscle thickness of the hand and forearm in young men and women. Ultrasound Med Biol. 2015;41(8):2125–2130. doi: 10.1016/j.ultrasmedbio.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Bear-Lehman J, Kafko M, Mah L, Mosquera L, Reilly B. An exploratory look at hand strength and hand size among preschoolers. J Hand Ther. 2002;15(4):340–346. doi: 10.1016/s0894-1130(02)80005-9. [DOI] [PubMed] [Google Scholar]
- 46.Li K, Hewson DJ, Duchene J, Hogrel JY. Predicting maximal grip strength using hand circumference. Man Ther. 2010;15(6):579–585. doi: 10.1016/j.math.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 47.Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practice. 3. Upper Saddle River, NJ: Prentice Hall; 2009. [Google Scholar]