Abstract
Stroke results in enduring damage to the brain which is accompanied by innate neurorestorative processes, such as reorganization of surviving circuits. Nevertheless, patients are often left with permanent residual impairments. Cell based therapy is an emerging therapeutic that may function to enhance the innate neurorestorative capacity of the brain. We previously evaluated human umbilical tissue-derived cells (hUTC) in our non-human primate model of cortical injury limited to the hand area of primary motor cortex. Injection of hUTC 24 hours after injury resulted in significantly enhanced recovery of fine motor function compared to vehicle treated controls (Moore et al., 2013). These monkeys also received an injection of Bromodeoxyuridine (BrdU) 8 days after cortical injury to label cells undergoing replication. This was followed by 12 weeks of behavioral testing, which culminated 3 hours prior to perfusion in a final behavioral testing session using only the impaired hand. In this session, the neuronal activity initiating hand movements leads to the upregulation of the immediate early gene c-Fos in activated cells. Following perfusion-fixation of the brain, sections were processed using immunohistochemistry to label c-Fos activated cells, pre-synaptic vesicle protein synaptophysin, and BrdU labeled neuroprogenitor cells to investigate the hypothesis that hUTC treatment enhanced behavioral recovery by facilitating reorganization of surviving cortical tissues. Quantitative analysis revealed that c-Fos activated cells were significantly increased in the ipsi- and contra-lesional ventral premotor but not the dorsal premotor cortices in the hUTC treated monkeys compared to placebo controls. Furthermore, the increase in c-Fos activated cells in the ipsi- and contra-lesional ventral premotor cortex correlated with a decrease in recovery time and improved grasp topography. Interestingly, there was no difference between treatment groups in the number of synaptophysin positive puncta in either ipsi- or contra-lesional ventral or dorsal premotor cortices. Nor was there a significant difference in the density of BrdU labeled cells in the subgranular zone of the hippocampus or the subventricular zone of the lateral ventricle. These findings support the hypothesis that hUTC treatment enhances the capacity of the brain to reorganize after cortical injury and that bilateral plasticity in ventral premotor cortex is a critical locus for this recovery of function. This reorganization may be accomplished through enhanced activation of pre-existing circuits within ventral premotor, but it could also reflect ventral premotor projections to the brainstem or spinal cord.
Keywords: Reorganization, Cell Based Therapy, hUTC4, Cortical Damage, Functional Recovery, Macaca Mulatta, Ventral Premotor Cortex, Subgranular Zone
Introduction
Stroke is the leading cause of long-term disability in the United States and approximately 795,000 Americans experience a new or recurring stroke each year. The only current FDA-approved therapy for ischemic stroke is intravenous administration of tissue plasminogen activator (tPA) to return blood flow to the blocked artery, but this is only effective if administered within hours following onset of stroke (Ebinger et al., 2015; Wang et al., 2004). Many patients are unable to receive the tPA treatment due to the narrow therapeutic window, or other contraindications (Fugate and Rabinstein, 2015) and even those who do receive tPA often are left with varying amounts of irreversible brain damage and significant residual impairment. Accordingly, neurorestorative treatments are needed to enhance neuroplasticity and facilitate recovery of function following stroke or other traumatic events.
In both animal models and human stroke patients with motor impairments, physical therapy involving rehabilitative motor tasks can lead to significant improvement in motor function over a period of months following stroke (Calautti and Baron, 2003; Frost et al., 2003). Nevertheless, in humans, complete recovery to pre-stroke function is rare (Gowland, 1987). The central nervous system (CNS) has a limited ability to repair or regenerate neurons due to inhibitory factors released by CNS parenchyma and glial scarring (Yiu and He, 2006). As a result, functional recovery following CNS damage is not a result of neuronal regeneration, but more likely due to a variety of neuroplasticity mechanisms such as axonal sprouting, synaptic reorganization, and changes in myelination (Armstrong et al., 2016; Fields, 2015; Mensch et al., 2015; Pascual-Leone et al., 2012). In fact, there is evidence for cortical neuroplasticity and reorganization following CNS lesions in both humans and animal models (Kaas, 1991; Pons et al., 1988; Seitz et al., 1995). Specifically, there is evidence of increasing cortical reorganization of undamaged motor areas including premotor cortices (Frost et al., 2003; Nudo, 2007; Nudo and McNeal, 2013) and evidence of increased proliferation of neural progenitor cells (Anderson, 2001; Arvidsson et al., 2002; Jin et al., 2006; Li et al., 2002; Lindvall and Kokaia, 2015; Marlier et al., 2015; Minger et al., 2007; Zhang et al., 2013, 2011). Hence, targeting plasticity and reorganization mechanisms with new therapeutic agents may be one way to enhance more complete functional recovery after cortical injury.
Cell based therapies are an attractive avenue of neurorestorative treatments that have shown largely promising results in preclinical models of stroke. While the mechanism remains unclear, studies suggest that cell based therapies enhance endogenous repair mechanisms through increasing brain plasticity and synaptic remodeling (Savitz et al., 2014). While such neurorestorative treatments for stroke have been assessed preclinically, none have successfully translated to human patients. The Stroke Therapy Academic Industry Roundtable (STAIR) (Fisher et al., 2009) and Stem Cells as an Emerging Paradigm in Stroke (STEPS) (Savitz et al., 2011) committees assessed ways to enhance translation between preclinical and clinical studies. Both the STAIR (Fisher et al., 2009) and STEPS (Savitz et al., 2011) reports recommended the use of non-human primate models to validate and further assess efficacy and safety of promising therapies including cell based approaches. Further, STEPS recommends performing appropriate histological studies to examine the effects of the cell based therapy on the remodeling of surviving structures (Savitz et al., 2011).
To that end, this report is a histological follow-up to our earlier study that a cell based therapy of human umbilical tissue derived cells (hUTC) enhanced recovery of fine motor function in our reproducible non-human primate model of cortical injury (Moore et al., 2013). Specifically, it was demonstrated that intravenous administration of hUTC, 24 hours after cortical damage, significantly improved function and strength of the impaired hand in the first two weeks of recovery and improved finger-thumb grasp rating during the 12-week postoperative assessment as compared to placebo treated controls.
We hypothesized that the recovery of function observed with hUTC treatment may be due to reorganization of undamaged motor areas and increased proliferation of neural progenitor cells. To evaluate this hypothesis, we report here quantitative analysis in bilateral premotor cortices of c-Fos as a marker of cell activation and synaptophysin as a marker of synaptic density. We also report quantitative analysis of BrdU positive neural progenitor cells in the subventricular zone and subgranular zone as a marker for cell proliferation.
Materials and Methods
Subjects
Eight adult male rhesus monkeys (Macaca mulatta), ranging in age from 8.5 to 12.1 years, were used in this study. All were part of our previous study (Moore et al., 2013) that assessed the efficacy of hUTC therapy on recovery of motor function following cortical injury. Prior to entering the previous study, all monkeys received medical examinations and were screened to ensure that they did not have a history of malnutrition, diabetes, chronic illness, or any neurological diseases. All monkeys were given initial pre -operative MRI scans to ensure no occult brain abnormality. While enrolled in the study, the monkeys were housed in the Laboratory Animal Science Center of Boston University Medical Campus, which is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). Experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals from the National Institute of Health’s Office of Laboratory Animal Welfare and were approved by the Institutional Animal Care and Use Committee (IACUC) of the Boston University Medical Campus.
Fine Motor Testing and Lesion of the M1 Hand Area
All motor testing and surgical procedures were completed as part of the previous study (Moore et al., 2013) and have been previously described (Moore et al., 2010, 2013, 2012). In the following, the testing and surgical procedures are described briefly. Monkeys were trained on a fine motor task, modified version of a Klüver board (Klüver, 1935), to reach asymptotic performance and the preferred or dominant hand was determined using free choice trials in the testing apparatus. The lesion was then targeted to the hemisphere controlling the dominant hand to ensure that monkeys would be motivated to use the impaired hand during postoperative testing. All subjects then underwent an electrophysiologically guided lesion limited to the hand representation of primary motor cortex. Following exposure of the cortex, the lesion was created by inserting a small glass suction pipette under the pia and bluntly dissecting the small penetrating arterioles as they enter the underlying cortex, producing an ischemic lesion of the gray matter with preservation of underlying white matter (Moore et al., 2013). Twenty-four hours after the lesion, monkeys were given hUTC (CNTO 0007; Advanced Technologies and Regenerative Medicine, LLC – Johnson & Johnson, New Brunswick, NJ) or placebo via intravenous infusion at a dose of 10 million cells/kg and a rate of 0.5 mL per minute. Two weeks following surgery, monkeys were retested on the same fine motor task for 12 weeks with 70% of trials to the impaired hand and 30% to the unimpaired hand. Outcome measures included recovery time, the number of days to return to pre-operative performance, and grasp assessment, determined by a licensed Occupational Therapist (M.A.P.) using our Grasp Assessment Scale for non-human primates (NHP). The scale was adapted from scales used in human stroke patients (Carr et al., 1985; Fugl-Meyer et al., 1975; Whishaw et al., 2002) and consists of hierarchical categories from 0 (no movement) to 8 (normal grasp with accurate pinch between thumb and finger). All relevant subject information, including treatment and behavioral outcome measures, is summarized in Table 1. For all procedures, cognitive testers, surgeons and other research staff were blind to treatment condition throughout the experiment including tissue processing and data analysis reported here.
Table 1.
Subject Data
| Subject | Sex | Age (years) | Treatment Group | Lesion Hemisphere | BrdU Survival Time (days) | Recovery Time (days)* | Mean Grasp Assessment* | Supplier |
|---|---|---|---|---|---|---|---|---|
| SM014j | M | 8.5 | Placebo | L | 110 | 54 | 7.68 | CPRC |
| SM018j | M | 9.6 | Placebo | L | 92 | 96 | 6.46 | CPRC |
| SM020j | M | 9.3 | Placebo | R | 106 | 96 | 6.58 | CPRC |
| SM022j | M | 10.6 | Placebo | R | 138 | 96 | 6.46 | CPRC |
| SM016j | M | 10.1 | hUTC Treated | L | 116 | 56 | 7.88 | CPRC |
| SM017j | M | 8.6 | hUTC Treated | L | 99 | 35 | 7.72 | CPRC |
| SM021j | M | 11.5 | hUTC Treated | R | 111 | 51 | 7.80 | CPRC |
| SM024j | M | 12.1 | hUTC Treated | L | n/a | 48 | 7.86 | YNPRC |
Abbreviations: hUTC, human umbilical tissue derived cells (CNTO0007, Advanced Technologies and Regenerative Medicine, LLC, New Brunswick, NJ). CPRC, Caribbean Primate Research Center, University of Puerto Rico, San Juan, PR. YNPRC, Yerkes National Primate Research Center, Emory University, Atlanta, GA. BrdU, Bromodeoxyuridine.
Previously published data in Moore et al., 2013 (used in correlation studies in the current study)
BrdU Administration
Seven (3 treated and 4 placebo) monkeys received a single intraperitoneal injection of 200 mg/kg Bromodeoxyuridine (BrdU), prepared in a 15 mg/mL solution of warm, sterile Tris-buffered saline at pH 7.4. The BrdU solution was injected exactly eight days after surgically induced cortical injury to label cells in S-phase at that time. Since the BrdU dilutes in cells that remain in the cell cycle through many rounds of replication, the number of days between the BrdU injection and perfusion is listed as BrdU survival time in Table 1. BrdU survival time ranged from 92 to 138 days.
Pre-perfusion Testing and Immediate Early Gene Activation
Three hours prior to perfusion-fixation of the brain, monkeys were tested on the fine motor task for one hour. For this last testing session 100% of the trials were given to the impaired hand to maximize activation of neurons participating in function of the impaired hand. We hypothesized that testing only the impaired hand would activate the neurons supporting the recovery of function that may have occurred. After the testing session, the monkey was returned to the home cage for 2 hours to allow for expression of c-Fos protein resulting from neuronal activation before perfusion-fixation of the brain.
Perfusion and Tissue Acquisition
At the conclusion of the experiment, monkeys were sedated with ketamine (10 mg/kg IM) deeply anesthetized with sodium pentobarbital (25 mg/kg IV to effect) and were euthanized by exsanguination during transcardial perfusion-fixation of the brain. Perfusion with cold Krebs-Heinsleit buffer (4°C, pH 7.4) was followed immediately by fixation with 4% paraformaldehyde, (30°C, pH 7.4). The brain was blocked, in situ, in the coronal plane to ensure reproducible planes of section during later processing and then removed from the skull, weighed, and post-fixed overnight in 4% paraformaldehyde (no more than 18 hours). It was then transferred to cryoprotectant solution to eliminate freezing artifact (Rosene et al., 1986). Cryoprotected blocks were flash frozen at −75°C and stored at −80°C until they were cut on a microtome into interrupted series of coronal sections (eight series of 30 μm thick sections and one series of 60 μm thick sections) with a spacing between sections within a series of 300 μm. The 60 μm series was immediately mounted on microscope slides and stained with thionin for lesion reconstruction. The other series were collected in phosphate buffer with 15% glycerol and stored at −80°C for later histochemical processing (Estrada et al., 2017).
c-Fos and Synaptophysin Immunohistochemistry
Quarter series of 30 μm sections (i.e. sections spaced 1200 μm apart) through the premotor cortices were removed from storage and thawed for c-Fos and synaptophysin immunohistochemistry. All the sections for each marker were batch-processed (see Estrada et al, 2017 for discussion of batch processing) in the same reagents at the same time, according to the following steps. First, sections were rinsed in 0.05M Tris-buffered saline (TBS) to remove the glycerol. To quench endogenous peroxidases, sections were incubated for 30 minutes in TBS containing 1% sodium borohydride (c-Fos) or 3% H2O2 (synaptophysin) followed by washes in TBS. Sections were then incubated for 1 hour in a blocking solution of 10% Normal Goat Serum (NGS) and 0.4% Triton-X in TBS. The sections were then incubated for 48 hours at 4°C with gentle agitation in monoclonal primary antibodies to c-Fos (anti-c-Fos IgG in rabbit, 1:10000; Calbiochem, Billerica, MA) and synaptophysin (anti-synaptophysin IgG in mouse, 1:100000, Millipore, Billerica, MA) in a solution containing 2% NGS and 0.1% Triton-X in TBS. Several sections of brain tissue were left out of the primary antibody to control for immunoreactivity. Following incubation with the primary antibody, the sections were washed in a solution containing 2% NGS and 0.1% Triton-X in TBS, followed by a 2-hour incubation in biotinylated secondary antibody (1:600; Vector Laboratories, Burlingame, CA) in TBS containing 2% NGS, and 0.4% Triton-X. The sections were then washed in TBS and subsequently incubated with an avidin biotinylated horseradish peroxidase complex (ABC Elite; Vector Laboratories, Burlingame, CA) for 1 hour. The sections were then washed in TBS followed by washes in 0.175M sodium acetate solution. For visualization, all sections were incubated together in sodium acetate containing 0.55 mM 3-3′-diaminobenzidine (DAB; Sigma-Aldrich, St. Louis, MO), nickel sulfate (0.095M), and 0.0025% H2O2. The sections were then washed in sodium acetate to stop the DAB reaction, followed by rinses in TBS. Finally, sections were mounted on gelatin-coated slides, air dried, and cover-slipped using Permount mounting medium (Thermo Fisher Scientific, Waltham, MA).
BrdU Immunohistochemistry
A half series of 30 μm sections (i.e. sections spaced 600 μm apart) containing lateral ventricle and hippocampus was removed from storage and thawed for immunohistochemistry. All sections were batch-processed in the same reagents for BrdU according to the following steps. First, sections were washed in 0.05M Potassium Phosphate-Buffered Saline (KPBS) to remove the glycerol. For antigen retrieval, sections were incubated at 65°C for 2 hours in 50% formamide in 2× saline sodium citrate (SSC), followed by washes in SSC. Sections were then incubated in 2N Hydrochloric Acid (HCl) for 30 minutes at 37°C to create single-stranded DNA and expose the incorporated BrdU, then rinsed in a 0.1M Borate buffer (pH 8.5) for 10 minutes to neutralize the HCl. Sections were washed in KPBS and placed in SuperBlock (Thermo Fisher Scientific, Waltham, MA) for 30 minutes. Then, sections were incubated in the monoclonal primary antibody to BrdU (anti-BrdU IgG in rat; 1:500; Accurate Chemical & Scientific Corporation, Westbury, NY) in KPBS containing 0.4% Triton-X for 1 hour at room temperature, followed by 48 hours at 4°C with gentle agitation. Several sections of brain tissue were left out of the primary antibody to control for immunoreactivity. Next, the sections were washed in KPBS, then incubated in biotinylated secondary antibody (1:600, goat-anti-rat; Vector Laboratories, Burlingame, CA) in a solution containing 0.4% Triton-X in KPBS buffer for 1 hour at room temperature. The sections were washed in KPBS, incubated in an avidin-biotin peroxidase complex (ABC Elite; Vector Laboratories, Burlingame, CA) for 1 hour, then rinsed in KPBS. For visualization, all sections were incubated simultaneously for 8 minutes in KPBS containing DAB and 0.0025% H2O2, followed by washes in KPBS. Free floating sections were mounted on gelatin-coated slides and air dried. Sections were then lightly counterstained with a 0.05% thionin solution (pH 5.5), dehydrated through a graded series of alcohols, cleared with xylene, and coverslipped using Permount mounting medium (Thermo Fisher Scientific, Waltham, MA).
Regions of Interest for Stereology
Unbiased stereology was used to quantify c-Fos labeled neurons, synaptophysin puncta, and BrdU labeled cells visualized on a Nikon Eclipse E600 series light microscope (Nikon; Melville, NY, USA) equipped with a motorized stage integrated with StereoInvestigator 9 software (MicroBrightField Bioscience; Williston, VT). All sections were blinded and then regions of interest (ROI) were outlined using the 4X objective. For c-Fos and synaptophysin stereology, ROIs were created for the ipsilesional and contralesional dorsal premotor cortex (iPMd and cPMd) and the ipsilesional and contralesional ventral premotor cortex (iPMv and cPMv) on 6-10 equally spaced sections. ROIs were outlined as shown in Figure 1 using the Paxinos rhesus monkey stereotaxic atlas as a guide (Paxinos et al., 2008). Specifically, PMd was sampled from its first appearance near the rostral aspect of the superior limb of the arcuate sulcus, past the region of the arcuate sulcus to its disappearance at the rostral aspect of M1. PMv was sampled from its first appearance near the inferior arcuate sulcus to its disappearance at the rostral aspect of M1.
Figure 1. Regions of interest for stereological estimation of c-Fos and synaptophysin.

The entire extent of dorsal and ventral premotor cortices were evaluated. Schematic representations of rhesus monkey coronal sections adapted from Paxinos (2008) are shown from rostral to caudal (A–H) showing the boundaries of the dorsal and ventral premotor area regions of interest that were sampled for stereological analysis. The sagittal view (I) shows the rostral and caudal boundaries from which the sections sampled were taken.
Abbreviations: sar, superior arcuate sulcus. ps, principle sulcus. arsp, arcuate sulcus spur.
For BrdU analysis, ROIs were created for the ipsilesional and contralesional subventricular zone (iSVZ and cSVZ) on 7-8 equally spaced sections from the interventricular foramen to the rostral tip of the lateral ventricle. ROIs were also created on the ipsilesional and contralesional subgranular zone of the dentate gyrus (iSGZ and cSGZ) on 16-25 equally spaced sections through the hippocampus. The SVZ included an area extending 2500 μm dorsal to the most ventral tip of the ventricle and 250 μm distal from the ependymal layer bordering the lumen of the ventricle. Representative sections and ROI contours for SVZ are shown in Figure 2 (A–H). The SGZ, a subdivision of the dentate gyrus, was sampled through its rostral-caudal extent as shown in representative sections and contours in Figure 2 (I–P) and as previously described (Ngwenya et al., 2015). The hilus was identified as the polymorphic cell area within the blades of the granule cell layer. The SGZ was defined as the area within the hilus immediately beneath the granule cell layer but excluding the cell dense CA4 subfield that often extended into the hilus. BrdU positive cells were counted in the SGZ and adjacent deep layer of the granule cells.
Figure 2. Regions of interest for stereological estimation of BrdU.

The subventricular zone and granule cell layer of the dentate gyrus were evaluated. The boundaries of the subventricular zone ROI that was sampled for stereological analysis are shown as representative micrographs (A, C, E, G) and schematic illustrations (B, D, F, H). The boundaries of the dentate gyrus, including the granule cell layer that was sampled for stereological analysis, are shown as representative micrographs (I, K, M, O) and schematic illustrations (J, L, N, P).
Abbreviations: L, Lateral Ventricle. SVZ, Subventricular Zone. ECL, Ependymal Cell Layer. SGZ, Subgranular Zone. ML, molecular layer. H, Hilus.
Stereological Parameters and Inclusion Criteria
The Optical Fractionator Workflow was used to optimize and quantify all 3 markers and stereological parameters are summarized in Table 2. The Optical Fractionator Workflow places a sampling grid over the ROI using systematic random sampling. It then places a three-dimensional counting frame at a fixed corner of each grid square. Counting occurred within that 3-D frame. To ensure stereological accuracy, counting was performed with a minimum of 2 μm guard zones above and below the optical dissector box to ensure that lost caps could be identified and counted or excluded according to the respective face (West et al., 1991). c-Fos labeled cells were identified with the 40X objective based on intense nuclear staining which ranged from dense labeling of the entire nucleus to labeling of 40% of the nucleus compared to background. Labeling below 40% was regarded as background and not a positive cell. Inclusion criteria for Synaptophysin positive puncta were identified with the 100X objective as well-defined circular, dark profiles located within the counting frame. Since BrdU-positive cells are relatively rare, an exhaustive counting scheme was used such that the counting frame occupied 100% of the sampling grid (Table 2). BrdU positive cells were identified with the 60X objective and counted if more than one-third of the nucleus was darkly labeled and if the labeled nucleus was not part of the wall of a blood vessel. This counting method omitted very lightly stained cells and endothelial cells.
Table 2.
Stereological Parameters for Optical Fractionator.
| Regions of interest | Stereology Objective | Section Interval | Counting Frame (μm) | Grid Spacing (μm) | Dissector Height (μm) | Guard Zone (μm) Above | |
|---|---|---|---|---|---|---|---|
| c-Fos | Dorsal Premotor Cortex | 40X | 1/40 | 250 × 250 | 1200 × 1200 | 15 | 2 |
| Ventral Premotor Cortex | 950 × 950 | ||||||
| Synaptophysin | Dorsal Premotor Cortex Ventral Premotor Cortex |
100X oil immersion | 1/80 | 5 × 5 | 1000 × 1000 | 5 | 1 |
| Bromodeoxyuridine (BrdU) | Subventricular Zone Subgranular Zone |
60X oil immersion | 1/20 | 150 × 90 | 150 × 90 (exhaustive) | 15 | 1 |
Stereology Object Estimates
The estimated total number of c-Fos labeled cells, synaptophysin labeled puncta, and BrdU labeled cells in each ROI were calculated using the optical fractionator formula: N=Q−×1/ssf×1/asf×t/h (Sutula and West, 2002; West et al., 1991), where N is the estimate of the total number of objects, Q− is the number of objects counted, ssf is the section sampling fraction, asf is the area sampling fraction, and t/h is the actual section thickness (t) divided by the height (h) of the dissector. Parameters were optimized such that Coefficients of Error were less than 0.10 using a Smoothness Factor m=1 for biological tissue (Gundersen et al., 1999). Estimated activated cell (c-Fos) numbers were used for analysis. Estimated BrdU cell density and synaptophysin puncta density measures were used for analysis (estimated cell number divided by Cavalieri estimated volume in mm3).
Statistical Analysis
Statistical analyses were performed using R (RStudio Inc, Version 0.99.896, R foundation for Statistical Computing, Vienna, Austria). Two-way analysis of variance (ANOVA) with repeated measures was used to identify significant variables and interactions with treatment group (hUTC treated or placebo) as a between subject variable and hemisphere (ipsilesional and contralesional) as a within subject variable. Separate two-way ANOVAs with repeated measures (six total) were used to analyze c-Fos (PMv and PMd), synaptophysin (PMv and PMd), and BrdU (SVZ and SGZ). Significance level was set to p ≤ 0.05. Tukey Post-hoc tests were used to identify significant variables. Regression analyses were performed with subjects from both groups together. For correlations relating to the mean grasp (an ordinal scale, 0-8), Spearman’s Correlation was used, and all other correlations were assessed using a Pearson’s Correlation with an alpha level set at p ≤ 0.05.
Results
Effect of hUTC on c-Fos Activation to Localize Foci of Plasticity
To localize regions that were activated during recovered hand performance, 3 hours prior to perfusion monkeys were behaviorally tested using only the impaired hand to increase expression of the immediate early gene c-Fos. Subsequently, c-Fos immunohistochemistry, was used to identify activated cells and they were quantified with unbiased stereology. Estimated numbers c-Fos labeled cells were identified and counted as shown in Figure 3. Total estimated numbers of c-Fos labeled cells were used for comparisons between hUTC treated and placebo treated monkeys. Two-way ANOVAs were performed on c-Fos data from PMv and PMd with treatment (hUTC vs. placebo) as a between subject variable and hemisphere (ipsilesional vs. contralesional) as a within subject variable. Results showed that in PMv, the total number of c-Fos labeled cells was significantly increased in hUTC monkeys compared with the placebo monkeys in both hemispheres [F(1,6) = 12.82, p = 0.0116] but did not differ by hemisphere [F(1,6) = 0.162, p = 0.701]. The number of c-Fos labeled cells did not differ in PMd between groups [F(1,6) = 0.028, p = 0.873] or by hemisphere [F(1,6) = 4.742, p = 0.072]. As shown in Figure 4, these results demonstrate an upregulation of c-Fos activation in ventral premotor cortex of both the ipsilesional and contralesional hemispheres suggesting that this region may be an important substrate for hUTC stimulated neuroplasticity following damage to primary motor cortex.
Figure 3. Differential activation of c-Fos positive cells.

Representative micrographs of c-Fos activation in ipsilesional ventral premotor cortex in placebo (A) and hUTC treated (B) sections are shown. At a higher magnification, individual c-Fos positive cells of differential activation levels are discernable in placebo (C) and hUTC treated (D) sections. An example of a c-Fos labeled cell is shown with the black arrow in D. Scale bar: 100 μm
Figure 4. c-Fos positive cells in Ventral Premotor Cortex increase following hUTC treatment.
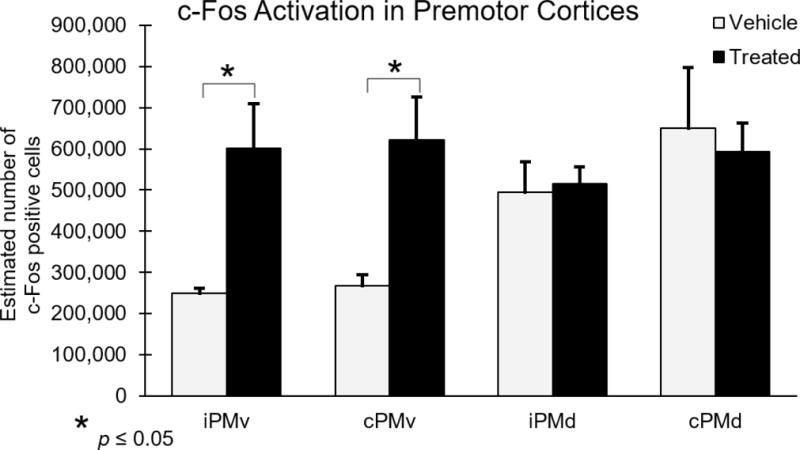
Numbers of c-Fos positive cells in the dorsal and ventral premotor cortex were estimated using unbiased stereology. There were significantly more c-Fos positive cells in the iPMv and cPMv in the hUTC treated monkeys compared to placebos [F(1,6) = 12.82, p = 0.0116].
Abbreviations: iPMv, ipsilesional ventral premotor cortex. cPMv, contralesional ventral premotor cortex. iPMd, ipsilesional dorsal premotor cortex. cPMd, contralesional dorsal premotor cortex.
Effect of hUTC on Synaptic Density
To investigate the changes in synaptic density in premotor cortices, synaptophysin immunohistochemistry and unbiased stereology was performed to quantify synaptophysin labeled puncta as shown in Figure 5 and density measures were calculated using Cavalieri estimated volume in mm3. Two-way ANOVAs were performed on synaptophysin data from PMv and PMd with treatment (hUTC vs. placebo) as a between subject variable and hemisphere (ipsilesional vs. contralesional) as a within subject variable. Results showed that the number of synaptophysin puncta did not differ significantly in PMv between groups [F(1,6) = 0.784, p = 0.41] or hemisphere [F(1,6) = 1.118, p = 0.331] (Figure 6). Similarly, synaptophysin puncta did not differ significantly in PMd between groups [F(1,6) = 0.436, p = 0.533], or hemisphere [F(1,6) = 2.709, p = 0.151] (Figure 6). Overall, these results suggest that synaptic density in the ventral and dorsal premotor cortices did not differ with hUTC treatment.
Figure 5. Uniform expression of synaptophysin puncta in the premotor cortices.

Representative micrograph of synaptophysin puncta in ipsilesional ventral premotor cortex are shown. Examples of a synaptophysin puncta are shown at the white arrows. The asterisks represent areas of background. Scale bar: 1 μm
Figure 6. Synaptophysin puncta in bilateral premotor cortices remain unchanged following hUTC treatment.
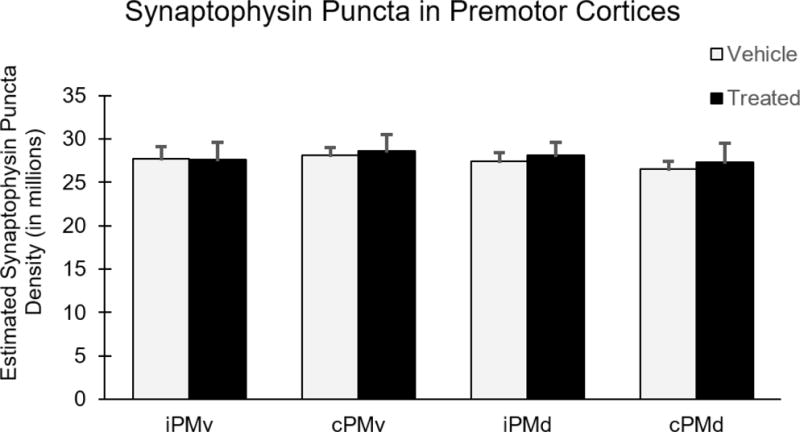
Numbers of synaptophysin puncta in the dorsal and ventral premotor cortex were estimated using unbiased stereology and are represented here (in millions). There was no significant difference between groups in any region.
Abbreviations: iPMv, ipsilesional ventral premotor cortex. cPMv, contralesional ventral premotor cortex. iPMd, ipsilesional dorsal premotor cortex. cPMd, contralesional dorsal premotor cortex.
Effect of hUTC on Progenitor Cell Proliferation
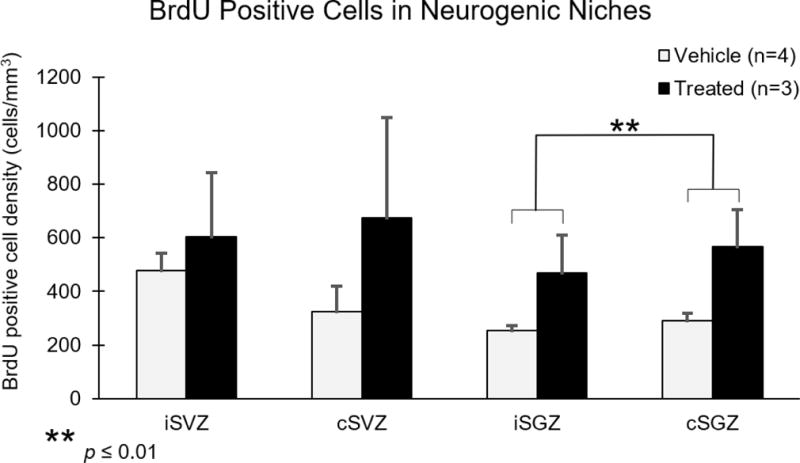
To investigate the effect of hUTC on neural progenitor cell proliferation, monkeys received an injection of the thymidine analog bromodeoxyuridine (BrdU) 8 days after injury. Following BrdU immunohistochemistry, unbiased stereology was used to obtain estimated numbers of BrdU labeled cells in frontal sections containing the subventricular zone (SVZ) and hippocampal sections containing the subgranular zone (SGZ) in both the ipsilesional and contralesional hemispheres as shown in Figure 7. Due to a variance in the volumes of the ROIs used for BrdU stereology, counts were normalized between subjects by using estimated BrdU cell density measures (estimated cell number divided by Cavalieri estimated volume in mm3). Two-way ANOVAs were performed on BrdU data from SVZ and SGZ with treatment (hUTC vs. placebo) as a between subject variable and hemisphere (ipsilesional vs. contralesional) as a within subject variable. BrdU labeled cells were detected in all ROI and all subjects. However, the density of BrdU labeled cells did not differ significantly in the SVZ between groups [F(1,5) = 0.803, p = 0.411] or hemispheres [F(1,5) = 0.450, p = 0.532] (Figure 8). However, the density of BrdU labeled cells approached a significant difference between groups in SGZ [F(1,5) = 4.259, p = 0.094] and was significantly different between hemispheres [F(1,5) = 18.63, p = 0.0076; Figure 8], with a greater density of BrdU labeled cells in cSGZ compared to iSGZ regardless of treatment group. Overall, these results suggest that progenitor cell proliferation did not differ in ipsilesional or contralesional SVZ with hUTC treatment. However, we observed a potential increase of BrdU labeled progenitor cell proliferation in the SGZ with hUTC treatment and an increase in proliferation in the cSGZ compared to the iSGZ. Overall, this suggests neural progenitor cell proliferation in ipsilesional and contralesional hemispheres after primary motor cortex damage.
Figure 7. BrdU positive cells in the subventricular zone and subgranular zone.
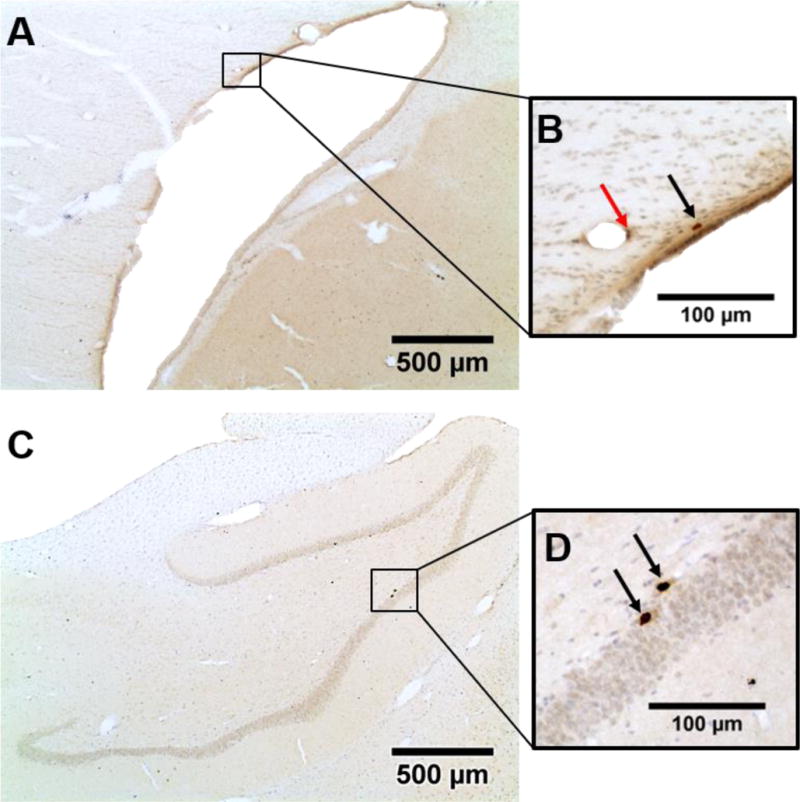
A representative micrograph of the iSVZ is shown (A). At a higher magnification (B), an example of a BrdU positive cell is shown at the black arrow and an endothelial cell (excluded from the current study) is shown at the red arrow. A representative micrograph of the ipsilesional dentate gyrus is shown (C). At a higher magnification (D), examples of two BrdU positive cells in the iSGZ are shown at the black arrows.
Figure 8. BrdU positive cells in Subgranular Zone increase following hUTC treatment.

The densities of BrdU positive cells in the subventricular zone and subgranular zone were estimated using unbiased stereology. There was no significant difference between groups in any region. However, there are significantly more BrdU positive cells in cSGZ compared to iSGZ. [F(1,5) = 18.63, p = 0.0076].
Abbreviations: iSVZ, ipsilesional subventricular zone. cSVZ, contralesional subventricular zone. iSGZ, ipsilesional subgranular zone. cSGZ, contralesional subgranular zone.
Relationship of Markers of Plasticity to Functional Recovery
As previously reported, hUTC treated monkeys returned to pre-operative levels of performance on fine motor tasks in significantly fewer days compared to monkeys that received placebo (Moore et al., 2013). Interestingly, reduced recovery time (in days) was predicted by increased numbers of c-Fos labeled cells in iPMv [Rp = −0.716, p = 0.046] and cPMv [Rp = −0.783, p = 0.022] (Figure 9). Thus, subjects with a higher number of c-Fos activated neurons in PMv at the end of testing had shown more rapid recovery of function. This relationship between fewer days to recovery and increased c-Fos activation was not observed in either the iPMd or cPMd (data not shown). Further, there was no relationship between recovery time and density of synaptophysin puncta or BrdU labeled cells in any region of interest (data not shown). These results suggest that increased c-Fos activation in iPMv and cPMv may be a significant predictor of improved recovery time following cortical injury.
Figure 9. Increased c-Fos activation is correlated with improved recovery time.
Recovery time is defined as days following cortical injury to return to asymptotic performance in a 120 day period. There is a significant negative correlation between recovery time and the level of c-Fos activation in the iPMv (A) and cPMv (B).
Another measure of recovery is the recovery of accurate pinch between finger and thumb. Mean grasp was rated over the post-operative period on a scale of 0 (no movement) to 8 (full recovery). As previously reported, recovery toward improved grasp topography was significantly enhanced in hUTC treated monkeys compared to placebo controls (Moore et al., 2013). Interestingly, enhanced grasp assessment (closer to full recovery) was predicted by increased numbers of c-Fos activated neurons in iPMv [Rs = 0.970, p = 0.00006] and cPMv [Rs = 0.946, p = 0.0004] (Figure 10). Thus, subjects with a higher number of c-Fos activated neurons in PMv at the end of testing had enhanced grasp topography over the recovery period. This relationship between enhanced grasp topography and more c-Fos activation was not observed in either the iPMd or cPMd (data not shown). This suggests increased numbers of c-Fos activated cells may be a significant predictor of grasp topography recovery.
Figure 10. Increased c-Fos activation is correlated with improved grasp topography.

The mean grasp assessment score is reported on a scale from 0 (no movement) to 8 (preoperative finger/thumb opposition). There is a significant positive correlation between the mean grasp assessment score and the level of c-Fos activation in the iPMv (A) and cPMv (B).
Discussion
Summary of Results
The overall findings of this study are that following injury to the primary motor cortex: (1) Cellular activation is increased bilaterally in the ventral premotor cortex in monkeys treated with hUTC compared to placebo treated controls, but is not altered in dorsal premotor cortex. (2) The number of activated cells in both the ipsi- and contra-lesional ventral premotor cortices is positively associated with improved recovery time and grasp topography, pointing to ventral premotor cortex as a potential locus of reorganization. (3) In contrast, the synaptic density, in both ventral and dorsal premotor cortices, does not differ between hUTC treated and placebo controls. (4) Proliferation of progenitor cells in the subventricular zone does not differ between hUTC treated and placebo controls, but proliferation in the subgranular zone bilaterally approached a significant increase with hUTC treatment. Overall, these results suggest that the effect of hUTC cell therapy on improved motor performance may be mediated in part by activating the ventral premotor cortex but that hUTC also produces a generalized facilitation of neuroplasticity that is reflected in enhancement of progenitor cell proliferation.
Previous Studies of Cell Based Therapy
Spontaneous recovery of motor function following stroke in the motor cortex has been shown to involve reorganization through activation of cell repair, axonal growth, dendritic remodeling, and synaptic plasticity leading to the formation of functional connections (Minger et al., 2007; Wieloch and Nikolich, 2006). This reorganization occurs most notably in the perilesional area, though changes also occur throughout the brain after stroke (Cotrina et al., 2016; Ward and Cohen, 2004). Recent studies in rodents have demonstrated enhanced motor recovery using human umbilical tissue derived cell (hUTC) infusion following stroke-induced motor impairment (Jiang et al., 2012; Shams Ara et al., 2015; Shehadah et al., 2013; Yang et al., 2012; Zhang et al., 2013, 2012, 2011). The enhanced motor recovery following hUTC treatment in rodents was accompanied by increased vascular and synaptic density in the perilesional area (Arbab et al., 2012; Jiang et al., 2012; Shehadah et al., 2013; Yang et al., 2012; Zhang et al., 2013, 2012, 2011) and an increase in progenitor cell proliferation in the subventricular zone (Yang et al., 2012; Zhang et al., 2013, 2012). Here we show that the same hUTC therapy used in rodents also facilitates recovery of function in monkeys with damage limited to the hand representation of motor cortex (Moore et al., 2013) but that upregulation of immediate early gene activation in ventral premotor cortex of these monkeys likely contributes to this effect.
Motor and Premotor Cortices
While the primary motor cortex is involved in the control and execution of motor movements, precision grasping of an object during goal directed hand movements is further mediated by the PMd and PMv. Specifically, the PMd is involved in the direction and amplitude of reaching toward an object to be grasped. In contrast, the PMv mediates the actual grasping component of goal directed hand movements including the appropriate shaping of the hand posture to grasp objects (Murata et al., 1997; Rizzolatti et al., 1996, 1990, 1988). Interestingly, PMv has been identified as a potential locus of reorganization to enable functional recovery following an insult to the primary motor cortex in both human patients and animal models. Specifically, through stimulation, studies in non-human primates observed an enlarged hand representation in ipsilesional PMv (iPMv) related to the size of M1 injury and that only stimulation in iPMv, but not M1, evoked a hand response following recovery (Dancause et al., 2006; Frost et al., 2003; Nudo and Milliken, 1996). Further, in imaging of human patients recovering from stroke affecting hand movements, studies observed bilateral recruitment of premotor cortices while performing fine motor functions with the impaired hand, which was not seen in performance with their unaffected hand or in healthy controls (Calautti and Baron, 2003; Cao et al., 1998). Therefore, functional recovery may depend on reorganization and plasticity of brain regions adjacent to the injury site, rather than a change in neuronal survival or regeneration of the lesion site.
Regions Supporting the Recovery of Function
To identify the role of premotor cortices involved in hUTC enhancement of recovery, we used motor testing and c-Fos immunohistochemistry. Activation of the c-Fos immediate early gene occurs because of influx through voltage gated calcium channels activated during neuronal depolarization. Activation of the c-Fos gene leads to production of the c-Fos nuclear protein, a transcription factor that initiates a cascade of other gene activations. The c-Fos protein production peaks at two to four hours after c-Fos gene activation (Harris, 1998; Morgan and Curran, 1991, 1989; Rosene et al., 2004). In the current study, subjects were perfused 3 hours after the start of motor testing with the impaired hand for one hour to activate the c-Fos protein in neurons functioning during testing. Subsequently, immunohistochemistry and stereology were used to quantify c-Fos expression as a marker for the location and number of neurons activated during the final testing session with the impaired hand.
We observed widespread c-Fos activation in dorsal and ventral premotor cortices bilaterally in all subjects but subjects that had hUTC treatment and demonstrated enhanced recovery showed increased activation in the ventral premotor cortex bilaterally. In contrast, there was no effect of hUTC treatment on dorsal premotor cortex acti vation, compared to placebo controls. Furthermore, results demonstrated that the number of activated neurons in bilateral PMv predicted a more successful grasp topography and a shorter time to recover preoperative levels of performance on fine motor tasks. This finding is consistent with studies, which have demonstrated reorganization in PMv after M1 lesions restricted to the digit representation (Frost et al, 2003). It is also consistent with the theory that the PMv is part of the parietofrontal circuit that mediates fine motor function of the hand and digits (Rizzolatti and Luppino, 2001). We conclude that hUTC treatment may have enhanced and facilitated the reorganization of the ventral premotor cortex of the hUTC treated animals and that this may underlie recovery of function in response to M1 injury.
Significance of c-Fos Cells for Recovery of Function
A close examination of Figures 9 and 10 shows that within the hUTC treated subjects, the number of c-Fos positive cells varies widely while the behavioral outcomes (lower number of days to recover and higher mean grasp rating) are relatively consistent. To further address this statistically, we performed an Analysis of Covariance that controlled for treatment group and found that only treatment group was significant with recovery time [F(1,6) = 10.887, p = 0.022] and grasp [F(1,6) = 10.153, p = 0.024]. When controlling for treatment group, c-Fos cell counts were not significant variables in relation to recovery time (iPMv [F(1,6) = 0.223, p = 0.656] and cPMv [F(1,6) = 0.896, p = 0.387]) or with grasp (iPMv [F(1,6) = 0.079, p = 0.789] or cPMv [F(1,6) = 0.218, p = 0.660]). This is not surprising considering that by visual inspection one can see that the number of c-Fos cells for subjects within each group was not a good predictor of recovery. It also is to be expected since each treatment group only had n=4 and one of the control subjects had cell number in each and behavioral outcome that overlapped the treatment group. While replicating this study with a much larger sample size might enable separate regression analyses within each treatment group to address this, it will likely be necessary to explore additional neurobiological variables that might underlie or contribute to recovery to understand better the processes stimulated by the hUTC. As an example, synaptogenesis in cervical spinal cord regions to which the c-Fos positive cells of ventral premotor project might identify differences in underlying mechanisms of plasticity that could account for better recovery in all treated subjects compared to the placebo controls.
Ipsilesional vs. Contralesional Activation
Recruitment of the intact contralesional primary motor cortex or premotor areas may also contribute to enhancing functional recovery. Animal models of stroke have shown increased reorganization of contralesional cortical areas with increased lesion size and involves changes in dendritic and synaptic plasticity (Kim and Jones, 2010). Increased activation of contralesional motor areas may contribute to the inhibition of damaged ipsilesional areas, which has been associated with a better outcome (Boroojerdi et al., 1996; Murase et al., 2004). In the current study, we observed an equivalent increased level of activation in both ipsilesional and contralesional premotor cortex with hUTC treatment. Further, the increase in both iPMv and cPMv predicted improved recovery time and grasp topography after cortical injury. Overall, while the exact role of reorganization in contralesional motor areas in recovery from M1 injury remains unclear, the contralesional hemisphere is a potential target for rehabilitation following cortical injury.
Synaptic Density and Neuroplasticity
Distinct changes in the premotor cortices with treatment observed through neuronal activation further raised the question of whether there was also change in innervation of these neurons. To explore synaptic remodeling in premotor cortices, we quantified synaptic density by calculating density of synaptophysin puncta. Synaptophysin is a glycoprotein found in pre-synaptic vesicles (Wiedenmann et al., 1986) that is commonly used to quantify synaptic density (Arbab et al., 2012; Jiang et al., 2012; Shehadah et al., 2013; Yang et al., 2012; Zhang et al., 2013, 2012, 2011). In the current study, there was no observable difference in synaptic density when comparing hUTC treated subjects to controls in bilateral ventral or dorsal premotor cortices. Studies assessing hUTC treatment have consistently found increased perilesional synaptic density with treatment (Arbab et al., 2012; Jiang et al., 2012; Shehadah et al., 2013; Yang et al., 2012; Zhang et al., 2013, 2012, 2011). Further, studies in vitro revealed that hUTC treatment enhanced synaptic formation and function through paracrine factors (Koh et al., 2015). Our finding that synaptic density remains stable does not preclude the possibility that synaptic efficacy has been altered by pre- or post-synaptic mechanisms such that the functional connections that compensated for the damaged cortex were strengthened or unmasked rather than completely rebuilt (Jacobs and Donoghue, 1991; Liguz-Lecznar et al., 2014; Pons et al., 1988).
Proliferation of Neural Progenitor Cells
In addition to remodeling of adjacent motor areas, we investigated the effect of hUTC treatment on progenitor cell proliferation. We speculate that neurotrophic factors released by the hUTCs may alter neural cell proliferation. Previous studies investigating cell based therapy for stroke in rats have shown that administration of human mesenchymal stem cells increased neurotrophic factor expression, which in turn augmented host brain plasticity (Li et al., 2002; Zhang et al., 2011). Here, we injected bromodeoxyuridine (BrdU) 8 days after cortical injury to label proliferating cells in the hippocampal SGZ and in the SVZ of the lateral ventricles during the S-phase of the cell cycle (Taupin, 2007). Studies have reported increased neural progenitor proliferation after cortical injury in the rodent brain in SGZ and SVZ (Anderson, 2001; Arvidsson et al., 2002; Jin et al., 2006; Lindvall and Kokaia, 2015; Marlier et al., 2015; Minger et al., 2007; Zhang et al., 2013, 2011).
Previous studies assessing hUTC (Yang et al., 2012; Zhang et al., 2013, 2012) and other cell based therapies (Bachstetter et al., 2008; Yoo et al., 2013) have reported an increase in neural progenitor proliferation in the iSVZ. In the current study, we found no significant difference in neural progenitor proliferation in the iSVZ and cSVZ between the treatment and placebo groups. However, neural progenitor proliferation in the SGZ bilaterally approached a significant increase with hUTC treatment. Due to our small sample size, it is possible that we observed a false negative in the iSVZ. Other possible explanations for this include the relatively small size of our cortical lesion in the hand representation compared to the widespread cortical and subcortical damage produced by middle cerebral artery occlusion (MCAO) in the rodent. Additionally, the rodent studies performed BrdU injections daily throughout the recovery period, while we performed one injection eight days after injury. Thus, our data on BrdU represent a snapshot of the capacity for plasticity in the injured brain on the eighth day of recovery. Finally, it is worth noting that our subjects survived for 3 months following BrdU injection and it’s possible that newly generated cells may have continued to divide, diluting the label.
Therapeutic Possibilities of Cell Based Therapy
Cell based therapies are a promising therapeutic option following stroke and our results add to the existing literature on the possible mechanisms by which the exogenous cell treatments might enhance recovery. Previous studies in rodents have corroborated that hUTC infusion after stroke results in enhanced motor recovery (Jiang et al., 2012; Moore et al., 2013; Shams Ara et al., 2015; Shehadah et al., 2013; Yang et al., 2012; Zhang et al., 2013, 2012, 2011), increased perilesional vascular and synaptic density (Arbab et al., 2012; Jiang et al., 2012; Shehadah et al., 2013; Yang et al., 2012; Zhang et al., 2013, 2012, 2011), increased progenitor cell proliferation (Yang et al., 2012; Zhang et al., 2013, 2012), but does not alter lesion volume (in rodents Shams Ara et al., 2015; Shehadah et al., 2013; Yang et al., 2012; Zhang et al., 2013, 2012, 2011 or in monkeys Moore et al., 2013;) or perilesional apoptosis (Zhang et al., 2012, 2011). Overall, our results suggest that cell based therapies function through enhancement of endogenous restorative mechanisms, some of which may include reorganization of intact adjacent motor areas and neural progenitor proliferation.
Conclusions
In this study, we provide for the first-time evidence that cell based therapy containing hUTC enhanced motor recovery after cortical injury by stimulating bilateral reorganization of ventral premotor cortex. These observations identify ventral premotor cortex as a target for therapeutic interventions. However, further studies are needed to determine whether cortical neurons activated were primarily excitatory or inhibitory as well as to assess reorganization in downstream pathways of the basal ganglia, brainstem, and spinal cord, which may also contribute to supporting recovery of function.
Supplementary Material
Highlights.
c-Fos activation increases with hUTC treatment in PMv but not PMd after M1 injury.
c-Fos activation is positively associated with improved recovery after M1 injury.
Density of synaptophysin in PM does not differ with hUTC treatment after M1 injury
Acknowledgments
This study was supported by a contract from Advanced Technologies and Regenerative Medicine (ATRM), LLC. [RR# 101115-PR] who provided the cell therapy product and the vehicle control and by the National Institutes of Health [NIH-NINDS R21NS081261 and NIH-NINDS R21NS102991-01]. We thank Megan McBurnie, Melissa Joblin, Reese Edwards, and Karen Slater for their expert technical assistance with all aspects of this study. On December 30, 2012, ATRM merged into DePuy Orthopaedics, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
hUTC: Human umbilical tissue derived cells
References
- Anderson DJ. Stem cells and pattern formation in the nervous system: The possible versus the actual. Neuron. 2001;30:19–35. doi: 10.1016/S0896-6273(01)00260-4. [DOI] [PubMed] [Google Scholar]
- Arbab AS, Thiffault C, Navia B, Victor SJ, Hong K, Zhang L, Jiang Q, Varma NR, Iskander A, Chopp M. Tracking of In-111-labeled human umbilical tissue-derived cells (hUTC) in a rat model of cerebral ischemia using SPECT imaging. BMC Med Imaging. 2012;12:33. doi: 10.1186/1471-2342-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RC, Mierzwa AJ, Marion CM, Sullivan GM. White matter involvement after TBI: Clues to axon and myelin repair capacity. Exp Neurol. 2016;275:328–333. doi: 10.1016/j.expneurol.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm. [DOI] [PubMed] [Google Scholar]
- Bachstetter AD, Pabon MM, Cole MJ, Hudson CE, Sanberg PR, Willing AE, Bickford PC, Gemma C. Peripheral injection of human umbilical cord blood stimulates neurogenesis in the aged rat brain. BMC Neurosci. 2008;9:22. doi: 10.1186/1471-2202-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroojerdi B, Diefenbach K, Ferbert A. Transcallosal inhibition in cortical and subcortical cerebral vascular lesions. J Neurol Sci. 1996;144:160–170. doi: 10.1016/S0022-510X(96)00222-5. [DOI] [PubMed] [Google Scholar]
- Calautti C, Baron JC. Functional Neuroimaging Studies of Motor Recovery After Stroke in Adults: A Review. Stroke. 2003;34:1553–1566. doi: 10.1161/01.STR.0000071761.36075.A6. [DOI] [PubMed] [Google Scholar]
- Cao Y, D’Olhaberriague L, Vikingstad EM, Levine SR, Welch KM. Pilot study of functional MRI to assess cerebral activation of motor function after poststroke hemiparesis. Stroke. 1998;29:112–22. doi: 10.1161/01.str.29.1.112. [DOI] [PubMed] [Google Scholar]
- Carr JH, Shepherd RB, Nordholm L, Lynne D. Investigation of a new motor assessment scale for stroke patients. Phys Ther. 1985;65:175–80. doi: 10.1093/ptj/65.2.175. [DOI] [PubMed] [Google Scholar]
- Cotrina ML, Lou N, Tome-Garcia J, Goldman J, Nedergaard M. Direct comparison of microglial dynamics and inflammatory profile in photothrombotic and arterial occlusion evoked stroke. Neuroscience. 2016;343:483–494. doi: 10.1016/j.neuroscience.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancause N, Barbay S, Frost SB, Plautz EJ, Popescu M, Dixon PM, Stowe AM, Friel KM, Nudo RJ. Topographically divergent and convergent connectivity between premotor and primary motor cortex. Cereb Cortex. 2006;16:1057–1068. doi: 10.1093/cercor/bhj049. [DOI] [PubMed] [Google Scholar]
- Ebinger M, Kunz A, Wendt M, Rozanski M, Winter B, Waldschmidt C, Weber J, Villringer K, Fiebach JB, Audebert HJ. Effects of Golden Hour Thrombolysis. JAMA Neurol. 2015;72:25–30. doi: 10.1001/jamaneurol.2014.3188. [DOI] [PubMed] [Google Scholar]
- Estrada LI, Robinson AA, Amaral AC, Giannaris EL, Heyworth NC, Mortazavi F, Ngwenya LB, Roberts DE, Cabral HJ, Killiany RJ, Rosene DL. Evaluation of Long-Term Cryostorage of Brain Tissue Sections for Quantitative Histochemistry. J Histochem Cytochem. 2017 doi: 10.1369/0022155416686934. 2215541668693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD. A new mechanism of nervous system plasticity: activity-dependent myelination. Nat Rev Neurosci. 2015;16:756–67. doi: 10.1038/nrn4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost SB, Barbay S, Friel KM, Plautz EJ, Nudo RJ. Reorganization of remote cortical regions after ischemic brain injury: a potential substrate for stroke recovery. J Neurophysiol. 2003;89:3205–14. doi: 10.1152/jn.01143.2002. [DOI] [PubMed] [Google Scholar]
- Fugate JE, Rabinstein AA. Absolute and Relative Contraindications to IV rt-PA for Acute Ischemic Stroke. The Neurohospitalist. 2015;5:110–121. doi: 10.1177/1941874415578532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugl-Meyer A, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- Gowland C. Management of hemiplegic upper limb. In: Brandstater M, Basmajian J, editors. Stroke Rehabilitation. Williams and Wilkens; Baltimore, MD: 1987. pp. 217–245. [Google Scholar]
- Gundersen H, Jensen E, Kieu K, Nielsen J. The efficiency of systematic sampling in stereology— reconsidered. J Microsc. 1999;193:199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- Harris JA. Using c-fos as a neural marker of pain. Brain Res Bull. 1998;45:1–8. doi: 10.1016/s0361-9230(97)00277-3. [DOI] [PubMed] [Google Scholar]
- Jacobs KM, Donoghue JP. Reshaping the Cortical Motor Map by Unmasking Latent Intracortical Connections. Science (80-) 1991;251:944–947. doi: 10.1126/science.2000496. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Thiffault C, Kramer BC, Ding GL, Zhang L, Nejad-Davarani SP, Li L, Arbab AS, Lu M, Navia B, Victor SJ, Hong K, Li QJ, Wang SY, Li Y, Chopp M. MRI detects brain reorganization after human umbilical tissue-derived cells (hUTC) treatment of stroke in rat. PLoS One. 2012;7:1–11. doi: 10.1371/journal.pone.0042845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, Shen J, Mao Y, Banwait S, Greenberg DA. Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci U S A. 2006;103:13198–202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH. Plasticity of Sensory and Motor Maps in Adult Mammals. Annu Rev Neurosci. 1991;14:137–167. doi: 10.1146/annurev.ne.14.030191.001033. [DOI] [PubMed] [Google Scholar]
- Kim SY, Jones TA. Lesion size-dependent synaptic and astrocytic responses in cortex contralateral to infarcts in middle-aged rats. Synapse. 2010;64:659–671. doi: 10.1002/syn.20777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klüver H. An Auto-Multi-Stimulation Reaction Board for Use with Sub-Human Primates. J Psychol. 1935;1:123–127. http://dx.doi.org/10.1080/00223980.1935.9917246. [Google Scholar]
- Koh S, Kim N, Yin HH, Harris IR, Dejneka NS, Eroglu C. Human Umbilical Tissue-Derived Cells Promote Synapse Formation and Neurite Outgrowth via Thrombospondin Family Proteins. J Neurosci. 2015;35:15649–65. doi: 10.1523/JNEUROSCI.1364-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N, Chopp M. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59:514–23. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- Liguz-Lecznar M, Zakrzewska R, Daniszewska K, Kossut M. Functional assessment of sensory functions after photothrombotic stroke in the barrel field of mice. Behav Brain Res. 2014;261:202–209. doi: 10.1016/j.bbr.2013.12.027. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z. Neurogenesis following Stroke Affecting the Adult Brain. Cold Spring Harb Perspect Biol. 2015;7 doi: 10.1101/cshperspect.a019034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlier Q, Verteneuil S, Vandenbosch R, Malgrange B. Mechanisms and Functional Significance of Stroke-Induced Neurogenesis. Front Neurosci. 2015;9:1–16. doi: 10.3389/fnins.2015.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensch S, Baraban M, Almeida R, Czopka T, Ausborn J, El Manira A, Lyons DA. Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat Neurosci. 2015;18:628–30. doi: 10.1038/nn.3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minger SL, Ekonomou A, Carta EM, Chinoy A, Perry RH, Ballard CG. Endogenous neurogenesis in the human brain following cerebral infarction. Regen Med. 2007;2:69–74. doi: 10.2217/17460751.2.1.69. [DOI] [PubMed] [Google Scholar]
- Moore T, Killiany R, Pessina M, Moss M, Rosene D. Assessment of motor function of the hand in aged rhesus monkeys. Somatosens Mot Res. 2010;27:121–130. doi: 10.3109/08990220.2010.485963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TL, Killiany RJ, Pessina MA, Moss MB, Finklestein SP, Rosene DL. Recovery from ischemia in the middle-aged brain: A nonhuman primate model. Neurobiol Aging. 2012;33:619.e9–619.e24. doi: 10.1016/j.neurobiolaging.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TL, Pessina MA, Finklestein SP, Kramer BC, Killiany RJ, Rosene DL. Recovery of fine motor performance after ischemic damage to motor cortex is facilitated by cell therapy in the rhesus monkey. Somatosens Mot Res. 2013;30:185–96. doi: 10.3109/08990220.2013.790806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–51. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Calcium and proto-oncogene involvement in the immediate-early response in the nervous system. Ann N Y Acad Sci. 1989;568:283–90. doi: 10.1111/j.1749-6632.1989.tb12518.x. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of Interhemispheric Interactions on Motor Function in Chronic Stroke. Ann Neurol. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Murata A, Fadiga L, Fogassi L, Gallese V, Raos V, Rizzolatti G. Object representation in the ventral premotor cortex (area F5) of the monkey. J Neurophysiol. 1997;78:2226–30. doi: 10.1152/jn.1997.78.4.2226. [DOI] [PubMed] [Google Scholar]
- Ngwenya LB, Heyworth NC, Shwe Y, Moore TL, Rosene DL. Age-related changes in dentate gyrus cell numbers, neurogenesis, and associations with cognitive impairments in the rhesus monkey. Front Syst Neurosci. 2015;9:102. doi: 10.3389/fnsys.2015.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ. Postinfarct cortical plasticity and behavioral recovery. Stroke. 2007;38:840–845. doi: 10.1161/01.STR.0000247943.12887.d2. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, McNeal D. Plasticity of cerebral functions. Handb Clin Neurol. 2013;110:13–21. doi: 10.1016/B978-0-444-52901-5.00002-2. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol. 1996;75:2144–9. doi: 10.1152/jn.1996.75.5.2144. doi:8551360. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Freitas C, Oberman L, Horvath JC, Halko M, Eldaief M, Bashir S, Vernet M, Shafi M, Westover B, Vahabzadeh-Hagh AM, Rotenberg A. Characterizing Brain Cortical Plasticity and Network Dynamics Across the Age-Span in Health and Disease with TME-EEG and TMS-fMRI. Brain Topogr. 2012;24:302–315. doi: 10.1007/s10548-011-0196-8.Characterizing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Huang XF, Petrides M, Toga AW. The Rhesus Monkey Brain in Stereotaxic Coordinates. 2nd. Academic Press; London: 2008. [Google Scholar]
- Pons TP, Garraghtyt PE, Mishkin AM, Mishkin M. Lesion-induced plasticity in the second somatosensory cortex of adult macaques. Neurobiology. 1988;85:5279–5281. doi: 10.1073/pnas.85.14.5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Camarda R, Fogassi L, Gentilucci M, Luppino G, Matelli M. Functional organization of inferior area 6 in the macaque monkey. II. Area F5 and the control of distal movements. Exp brain Res. 1988;71:491–507. doi: 10.1007/BF00248742. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Matelli M, Bettinardi V, Paulesu E, Perani D, Fazio F. Localization of grasp representations in humans by PET: 1. Observation versus execution. Exp brain Res. 1996;111:246–52. doi: 10.1007/BF00227301. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Gentilucci M, Camarda RM, Gallese V, Luppino G, Matelli M, Fogassi L. Neurons related to reaching-grasping arm movements in the rostral part of area 6 (area 6a beta) Exp brain Res. 1990;82:337–50. doi: 10.1007/BF00231253. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G. The cortical motor system. Neuron. 2001;31:889–901. doi: 10.1016/s0896-6273(01)00423-8. [DOI] [PubMed] [Google Scholar]
- Rosene DL, Lister JP, Schwagerl AL, Tonkiss J, McCormick CM, Galler JR. Prenatal Protein Malnutrition in Rats Alters the c-Fos Response of Neurons in the Anterior Cingulate and Medial Prefrontal Region to Behavioral Stress. Nutr Neurosci. 2004;7:281–289. doi: 10.1080/10284150400015573. [DOI] [PubMed] [Google Scholar]
- Rosene DL, Roy NJ, Davis BJ. A cryoprotection method that facilitates cutting frozen sections of whole monkey brains for histological and histochemical processing without freezing artifact. J Histochem Cytochem. 1986;34:1301–1315. doi: 10.1177/34.10.3745909. [DOI] [PubMed] [Google Scholar]
- Savitz SI, Chopp M, Deans R, Carmichael T, Phinney D, Wechsler L, STEPS Participants, Carmichael, S.T. Phinney D, Wechsler L. Stem Cell Therapy as an Emerging Paradigm for Stroke (STEPS) II. Stroke. 2011;42:825–9. doi: 10.1161/STROKEAHA.110.601914. [DOI] [PubMed] [Google Scholar]
- Savitz SI, Cramer SC, Wechsler L, STEPS 3 Consortium Stem cells as an emerging paradigm in stroke 3: enhancing the development of clinical trials. Stroke. 2014;45:634–9. doi: 10.1161/STROKEAHA.113.003379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz RJ, Huang Y, Knorr U, Tellmann L, Herzog H, Freund HJ. Large-scale plasticity of the human motor cortex. Neuroreport. 1995;6:742–4. doi: 10.1097/00001756-199503270-00009. [DOI] [PubMed] [Google Scholar]
- Shams Ara A, Sheibani V, Esmaeilpour K, Eslaminejad T, Nematollahi-Mahani SN. Coadministration of the Human Umbilical Cord Matrix-Derived Mesenchymal Cells and Aspirin Alters Postischemic Brain Injury in Rats. J Stroke Cerebrovasc Dis. 2015;24:2005–2016. doi: 10.1016/j.jstrokecerebrovasdis.2015.04.049. [DOI] [PubMed] [Google Scholar]
- Shehadah A, Chen J, Kramer B, Zacharek A, Cui Y, Roberts C, Lu M, Chopp M. Efficacy of Single and Multiple Injections of Human Umbilical Tissue-Derived Cells following Experimental Stroke in Rats. PLoS One. 2013;8 doi: 10.1371/journal.pone.0054083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutula T, West MJ. Design-based stereological methods for counting neurons. Prog Brain Res. 2002;135:43–51. doi: 10.1016/S0079-6123(02)35006-4. [DOI] [PubMed] [Google Scholar]
- Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res Rev. 2007;53:198–214. doi: 10.1016/j.brainresrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Wang X, Tsuji K, Lee S, Ning M, Furie KL, Buchan AM, Lo EH. Mechanisms of Hemorrhagic Transformation After Tissue Plasminogen Activator Reperfusion Therapy for Ischemic Stroke. Stroke. 2004;35:2726–2730. doi: 10.1161/01.STR.0000143219.16695.af. [DOI] [PubMed] [Google Scholar]
- Ward NS, Cohen LG. Mechanisms underlying recovery of motor function after stroke. Arch Neurol. 2004;61:1844–1848. doi: 10.1001/archneur.61.12.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJG. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Suchowersky O, Davis L, Sarna J, Metz GA, Pellis SM. Impairment of pronation, supination, and body co-ordination in reach-to-grasp tasks in human Parkinson’s disease (PD) reveals homology to deficits in animal models. Behav Brain Res. 2002;133:165–176. doi: 10.1016/S0166-4328(01)00479-X. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B, Franke WW, Kuhn C, Moll R, Gould VE. Synaptophysin: a marker protein for neuroendocrine cells and neoplasms. Proc Natl Acad Sci U S A. 1986;83:3500–3504. doi: 10.1073/pnas.83.10.3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieloch T, Nikolich K. Mechanisms of neural plasticity following brain injury. Curr Opin Neurobiol. 2006;16:258–64. doi: 10.1016/j.conb.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Yang D, Han Y, Zhang J, Seyda A, Chopp M, Seyfried DM. Therapeutic effect of human umbilical tissue-derived cell treatment in Rats with experimental intracerebral hemorrhage. Brain Res. 2012;1444:1–10. doi: 10.1016/j.brainres.2012.01.024. [DOI] [PubMed] [Google Scholar]
- Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo J, Seo JJ, Eom JH, Hwang DY. Enhanced recovery from chronic ischemic injury by bone marrow cells in a rat model of ischemic stroke. Cell Transplant. 2013;9447:1–46. doi: 10.3727/096368913X674666. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li Y, Romanko M, Kramer BC, Gosiewska A, Chopp M, Hong K. Different routes of administration of human umbilical tissue-derived cells improve functional recovery in the rat after focal cerebral ischemia. Brain Res. 2012;1489:104–112. doi: 10.1016/j.brainres.2012.10.017. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li Y, Zhang C, Chopp M, Gosiewska A, Hong K. Delayed administration of human umbilical tissue-derived cells improved neurological functional recovery in a rodent model of focal ischemia. Stroke. 2011;42:1437–1444. doi: 10.1161/STROKEAHA.110.593129. [DOI] [PubMed] [Google Scholar]
- Zhang L, Yi L, Chopp M, Kramer BC, Romanko M, Gosiewska A, Hong K. Intravenous administration of human umbilical tissue-derived cells improves neurological function in aged rats after embolic stroke. Cell Transplant. 2013;22:1569–1576. doi: 10.3727/096368912X658674. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


