Abstract
The mechanisms underlying superior cognitive performance in some older adults are poorly understood. We used a multimodal approach to characterize imaging and cognitive features of 26 successful agers (SA, defined by superior episodic memory ability) and 103 typical older adults (TOA). Cortical thickness was greater in multiple regions in SA, including right anterior cingulate and prefrontal cortex and was related to baseline memory performance. Similarly, hippocampal volume was greater in SA and associated with baseline memory. SA also had lower white matter hypointensity volumes and faster processing speed. While PiB DVR did not differ, there was a significant group interaction in the relationship between age and PiB DVR such that older SA individuals were less likely to have high brain Aβ. Over time, memory performance in TOA declined more rapidly than in SA, although there was limited evidence for different rates of brain atrophy. These findings indicate that superior memory in aging is related to greater cortical and white matter integrity as well as slower decline in memory performance.
Keywords: Superaging, superior memory, PET, MRI, cognitive reserve, cognitive resilience
1. Introduction
Cognitive decline is a common feature of normal aging (Hedden and Gabrieli, 2004). Aging trajectories, however, are inconsistent across individuals and variance of cognitive performance on neuropsychological tests is positively correlated with advancing age. Older adults who avoid cognitive decline and perform similarly to much younger people contribute to this age-related variance. There is great interest in these individuals and the underlying factors that mediate their unusually successful aging.
By definition, successful agers have not only avoided normal age-related cognitive decline, but also clinical expression of Alzheimer’s disease (AD) including both mild cognitive impairment (MCI) and dementia. AD, however, also has a prolonged asymptomatic prodrome that can be characterized by biomarkers of pathology. Thus, a crucial and tractable question is whether preserved cognition in successful aging reflects avoidance of AD-related pathology. There is evidence to suggest that successful agers defined by preserved episodic memory performance avoid age-related cortical thinning and have cortical morphology that more closely resembles middle-aged adults than their age-matched peers (Harrison et al., 2012). This could reflect greater brain reserve (e.g., larger cortex thinning at the same rate), or slower progression of age-related atrophy. In this regard, a recent study of “SuperAgers” showed that this unique group had a lower rate of whole cortex thinning over 18 months compared to a typical control group (Cook et al., 2017). Successful agers have also been shown to have greater hippocampal volume than their peers (Sun et al., 2016). These findings suggest a unique successful aging trajectory in which individuals resist changes in brain morphometry.
Two important pathological processes that are related to the development of cognitive decline and dementia in aging are β-amyloid (Aβ) deposition and cerebrovascular disease (CVD). Aβ can be quantified with PET tracers, and a widely reported measure of CVD is reflected in changes in white matter, often detected in hypointensities on T1 images. In contrast to differences in cortical thickness or hippocampal volume, Aβ accumulation and white matter signal abnormalities cannot be explained by higher lifetime brain reserve or slower age-related structural brain changes. Individuals with superior memory performance in the presence of age-related brain pathology like Aβ accumulation and white matter disease would be resilient to the effects of the pathology rather than resistant to the pathology itself (Latimer et al., 2017). It remains unclear whether successful agers also avoid Aβ accumulation and white matter disease, or if they are resilient to these pathologies. The factors underlying resistance to pathology (e.g, the avoidance of pathology itself) and resilience to pathology (e.g., the avoidance of typical cognitive consequences of pathology or coping with pathology) in cognitively superior older adults are underexplored.
In the present study we aimed to first replicate previous findings with cross-sectional structural MRI and second to expand the literature with investigations of Aβ burden, white matter hypointensities, longitudinal changes in cortical thickness as well as longitudinal cognition in unusually successful agers. We were further interested in how Aβ is related to age in superior memory performers and whether Aβ is predictive of cognitive changes. We examined cognitive trajectories in our cohort of successful agers using longitudinal cognitive follow-up data (mean total follow-up time = 5.2(2.5) years) to determine whether superior memory performance in older adults is predictive of future cognitive changes. Finally, we examined basic medical data and self-report questionnaires to search for factors underlying our successful aging cohort’s superior memory performance.
2. Methods
2.1 Participants
The current study included 150 participants enrolled in the Berkeley Aging Cohort Study (BACS), an ongoing longitudinal study of normal cognitive aging, who were aged 70 or older, and had structural MRI and PiB-PET data quantifying Aβ in the brain. Additional eligibility requirements included no imaging contraindications, baseline MMSE score ≥ 25, no neurological, psychiatric or major medical illness, no medications affecting cognition and that all participants were community-dwelling. Of the 150 participants, 26 met criteria for superior memory performance and were called successful agers (SA). Criteria for SA included a score of 14 or above (max score = 16) on the California Verbal Learning Test (CVLT) long delay free recall (LDFR) and normal for age performance on Trails B; the CVLT threshold of 14 reflects average performance for an individual aged 18–32 years old (Sun et al., 2016). Of the remaining subjects, 103 participants met criteria for typical older adults (TOA). TOA were required to score at or above one standard deviation below normal for age on the CVLT LDFR, making a score of 7 the inclusive cut-off. Thus, TOA scored in the range of 7–13 on the CVLT LDFR. A total of 21 subjects scored too low on the CLVT LDFR to be included in either experimental group. These criteria are based on previous studies and were designed to maximize interpretability in the context of the “SuperAging” literature and to allow for replication efforts within the present study (Harrison et al., 2012; Gefen et al., 2015; Sun et al., 2016). Baseline cognition was defined as the neuropsychological testing visit that was closest in time to each participant’s PiB-PET scan. Twenty five of 26 SA and 90 of 103 TOA had follow-up neuropsychological testing visits (Table 1). All older adult participants reported basic medical history including present or past history of hypertension, hypocholesteremia, arthritis, macular degeneration and diabetes. An unweighted linear co-morbidity index score was created based on these five age-related medical conditions to examine whether medical co-morbidity influences SA. Height and weight were measured for body mass index (BMI) calculation, and BMI differences between groups were also measured.
Table 1.
Cohort Characteristics
| SA | TOA | SA vs TOA p-value |
YA | |
|---|---|---|---|---|
| n | 26 | 103 | -- | 64 |
| Age (years) | 74.9±4.6 | 75.9±4.5 | 0.321 | 24.1±.29 |
| Sex (M/F) | 3/23 | 48/55 | 0.001 | 30/34 |
| Education (years) | 17.5±1.9 | 16.5±2.0 | 0.031 | 16.2±1.8 |
| APOE (% of ε4 carriers) | 30 | 27 (3N/A) | 0.810 | -- |
| Family History of Dementia (Y/N) | 12/13 (1N/A) | 21/80 (2N/A) | 0.010 | -- |
| BMI | 28.2±6 (2N/A) | 26.7±4.4 (2N/A) | 0.265 | -- |
| History of Hypertension (Y/N) | 8/18 | 37/66 | 0.818 | -- |
| Co-Morbidity Index Score | 1.54±1.2 | 1.45±.99 | 0.716 | -- |
|
| ||||
| Hippocampal Volume† (cm3) | 2.4±0.3 | 2.2±0.3 | <0.001 | 2.7±0.2 |
| WM Hypointensities† (cm3) | 1.5±1.0 | 2.1±1.8 | 0.029 | 0.6±0.3 |
| Whole Cortex Thickness (mm) | 2.3±0.2 | 2.3±0.1 | 0.432 | 2.5±0.1 |
| sMRI Follow-Up (years) | 4.5±2.7 | 3.5±1.9 | 0.145 | -- |
|
| ||||
| PiB DVR | 1.13±0.20 | 1.10±0.17 | 0.512 | -- |
|
| ||||
| CVLT LDFR | 14.8±0.8 | 10.2±1.9 | -- | 13.2±2.4‡ |
| Cognition Follow-Up (years) | 5.5±2.5 | 5.1±2.6 | 0.535 | -- |
|
| ||||
| LEQ | 123.6±18.5 (6N/A) | 117.1±19.8 (42N/A) | 0.187 | -- |
| Wilson Cognition: Present | 4.06±0.45 (1N/A) | 3.84±0.56 (7N/A) | 0.042 | -- |
SA = successful agers; TOA = typical older adults; YA = young adults; APOE = apolipoprotein E; AD = Alzheimer’s disease; BMI = body mass index; WM = white matter; PiB DVR = Pittsburgh Compound-B distribution volume ratio; CVLT LDFR = California Verbal Learning Test long delay free recall (16 words); LEQ = Lifetime Experiences Questionnaire; SA and TOA were compared using Welch’s t-tests for continuous variables and Fisher’s exact test for sex, APOE status, family history of AD and history of hypertension (significant differences at p<0.05 are bolded).
denotes volumes that have been adjusted by intracranial volume (ICV).
In raw CVLT LDFR score, SA performed better than YA while TOA performed worse than YA (both p< 0.0001).
Sixty-four participants aged 20–30 years old were also recruited using the same criteria (except age) to serve as a young control group. The young adult (YA) participants participated in neuropsychological testing and baseline structural MRI studies, but did not undergo PiB-PET or longitudinal MRI scanning.
The Institutional Review Board at Lawrence Berkeley National Laboratory and the University of California Berkeley approved the present study and written, informed consent was obtained from all participants.
2.2 Cognitive Assessment
All participants in the BACS, including YA, undergo neuropsychological testing to measure performance on specific cognitive tasks including those related to verbal and visual memory, working memory, processing speed, executive function, language and attention. Participants also complete questionnaires designed to assess lifetime cognitive activities, symptoms of depression and sleep quality. In the present study, composite scores were calculated to measure three cognitive domains: episodic memory (omitting all CVLT scores), working memory, and processing speed. Episodic memory tests were Visual Reproduction (VR) immediate recall total, VR delay recall total, VR recognition (hits + correct rejections), Logical Memory story A plus B1 and Visual Paired Associates Total Score. Working memory tests were Digit Span total score and Listening Span total recall. Processing speed tests were Trail Making Test B minus A, Stroop number correct in 1 minute and Digit Symbol total. Each composite was calculated by taking the average z-score of the constituent tests. For the older adults, z-scores for a specific test were calculated using the mean and standard deviation computed from older BACS participants with neuropsychological data regardless of imaging requirements (n=194). We chose to use this larger group as the z-score reference cohort instead of the study cohort for increased accuracy of the mean and variance estimates. The three composite scores were also calculated for all available follow-up neuropsychological sessions in addition to baseline. Of 129 older adult participants, 14 (1 SA) had no follow-up neuropsychological testing. The remaining participants had from 1 to 10 follow-up neuropsychological testing sessions.
For calculation of baseline composite z-scores for YA, mean and standard deviation were calculated from the 64 participants included in the present study.
Lifetime cognitive activity was measured using the Lifetime Cognitive Activity Questionnaire (Wilson et al., 2003). The Lifetime Experiences Questionnaire (LEQ) was also administered to a subset of the cohort (20 SA, 61 TOA) (Valenzuela and Sachdev, 2007).
2.3 APOE Genotyping
Determination of APOE alleles was performed using a TaqMan Allelic Discrimination Assay using a Real-Time PCR system (Applied Biosystems). This method has been previously described (Coppola et al., 2012). APOE genotyping was completed for all SA participants and 100 of 103 TOA participants.
2.4 Image Acquisition
PiB-PET data were acquired at the Lawrence Berkeley National Laboratory (LBNL). [11C]PiB was synthesized at the Biomedical Isotope Facility according to a previously published protocol (Mathis et al., 2003). Data collection was performed in 3D acquisition mode on either an ECAT EXACT HR scanner or a BIOGRAPH PET/CT Truepoint 6 scanner (both scanners Siemens Medical Systems, Erlangen, Germany). Previous work has demonstrated that PiB distribution volume ratio (DVR) values do not differ significantly between scanners (Elman et al., 2014). Immediately following injection of 10–15 mCi of PiB into an antecubital vein, thirty-five dynamic acquisition frames were recorded over a 90-minute session (4×15s, 8×30s, 9×60s, 2×180s, 10×300s and 2×600s). Each PiB-PET scan was accompanied by a transmission scan or computed tomography (CT) scan which was used for attenuation correction. Transmission scan or CT images were reconstructed using an ordered subset expectation maximization algorithm with weighted attenuation, scatter correction and smoothed with a 4mm Gaussian kernel.
A high-resolution T1-weighted magnetization prepared rapid gradient echo (MPRAGE) scan was acquired for each participant with the following parameters: TR=2110ms, TE=3.58ms, flip angle=15°, voxel size=1mm isotropic. These data were collected on a 1.5T Siemens Magnetom Avanto scanner at LBNL. A subset of the participants in the present study had at least one additional longitudinal 1.5T structural MRI (sMRI; 19 SA and 70 TOA). For 4 participants, their first longitudinal sMRI was collected more than 1 month (mean time 0.6(0.7) years) before the ‘baseline’ visit scans which included the PiB-PET scan and cross-sectional sMRI scan (used for group cortical thickness analyses shown in Figure 3). We chose to include these scans to use all available sMRI data.
2.5 PET Processing
PiB-PET data were processed using SPM12 (http://www.fil.ion.ucl.ac.uk/spm/). Data frames were realigned and frames corresponding to the first 20 minutes of the 90-minute scan were averaged and used for coregistration to each participant’s structural MRI. The resulting coregistration matrix was used to reslice the realigned PiB-PET frames into MRI space. DVR images in native MRI space were generated using Logan graphical analysis (35–90 min postinjection, cerebellar gray matter reference region; Logan et al., 1996). Global cortical PiB DVR values were calculated using FreeSurfer-derived gray matter ROIs as previously described (Mormino et al., 2011). A global PiB DVR threshold of 1.065, based on previous work in our lab, was used to dichotomize participants as PiB negative or positive (Villeneuve et al., 2015). After quality control, PiB-PET data from 5 participants (0 SA) were excluded from analyses in the present study due to participant placement and acquisition errors.
2.6 MRI Processing
T1-weighted MPRAGE scans were processed using FreeSurfer version 5.3 (http://freesurfer.net/). The main processing pipeline includes surface delineation for cortical thickness estimates, cortical parcellation based on anatomical landmarks and volumetric segmentation of subcortical structures (Dale et al., 1999; Fischl and Dale, 2000; Desikan et al., 2006). Results were manually checked for accuracy. The cortical parcellation was used to define the cortical ROIs used to calculate global PiB DVR for each participant (Mormino et al., 2011). The volumetric segmentation results were used to measure hippocampal volume and to define cerebellar gray matter which was used as the reference region in PiB-PET processing. The pial and white-gray matter boundary surface delineations were used to compare cortical thickness across experimental groups. Regions of interest (ROIs) identified in group comparisons between SA and TOA were registered from template space to each subject’s native space and mean thickness was extracted. In a separate analysis, the FreeSurfer longitudinal stream was used to process longitudinal sMRIs and linear mixed effects (LME) models were used to calculate unbiased, accurate slopes of change in hippocampal volume and cortical thickness in ROIs (Reuter et al., 2012). Finally, white matter hypointensities were labeled using a probabilistic procedure and total volume was calculated for each hemisphere (Fischl et al., 2002; Salat et al., 2009). We averaged these hemispheric values together to create a bilateral white matter hypointensity load measure.
2.7 Statistical Analysis
Two-sample, unpaired Welch’s t-tests were used to compare baseline cognition and sMRI measures across groups. Fisher’s Exact tests were used to test for differences in categorical variables like sex. Pearson correlation was used to probe associations between age and imaging metrics. Multiple linear regression models were used to examine the relationships between cognition, sMRI measures and PiB DVR at baseline. To interrogate how cognition and imaging biomarkers at baseline affect longitudinal cognition in older adults we used an LME model with fixed effects for confounding variables such as age, sex, years of education and the number of cognitive follow-up visits. Random effects for participant slope and intercept were included to account for our hypothesis that cognitive trajectories would be unique across participants. LME models including random effects for slope and intercept were also used to extract longitudinal slopes of change in hippocampal volume and cortical thickness and to test for group differences in rates of structural change.
Cortical thickness statistical surface maps were generated using a general linear model (GLM) framework in FreeSurfer. Sex was included as a binary covariate in the GLM comparing vertex-wise cortical thickness in SA versus TOA to account for the significant difference in sex composition between groups.
3. Results
3.1 Participants
Cohort characteristics are summarized in Table 1. There was no significant difference in age between SA and TOA. There were proportionally more women in the SA group than TOA (p=0.001). SA also had slightly higher years of education (p=0.03) and more neuropsychological follow-up visits (p=0.04) although there was no difference between groups in mean cognitive follow-up time (p=0.535). We took a conservative approach and controlled for age, sex, education and number of follow-up visits in all applicable multiple linear regression and linear mixed effects models. In cortical thickness GLM comparisons we included sex as a covariate, but did not include other covariates to conserve degrees of freedom. There was no difference in APOEε4 carriage rate between SA and TOA (SA: 8 carriers, 30% of group; TOA: 27 carriers, 27% of group; p=0.81). There was a higher incidence of family history of ‘dementia’ (self-reported, not just AD) in SA (p=0.010). Finally, there were no differences between groups in BMI, history of hypertension or in a co-morbidity index score that included hypertension, hypocholesteremia, arthritis, macular degeneration and diabetes (p>0.2).
3.2 Cognition
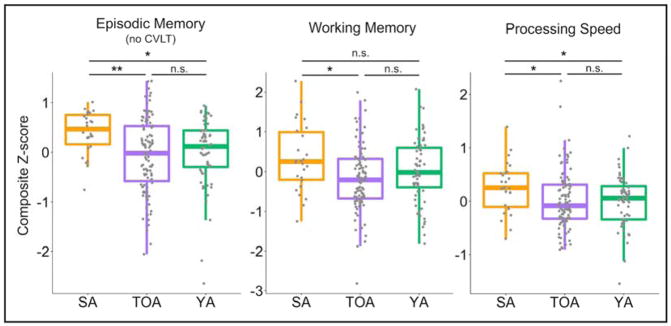
SA had better baseline episodic memory (p<0.001), working memory (p=0.009) and processing speed (p=0.025) than their typically-performing peers despite being defined as successful agers based on a single subscore of the CVLT, which was not included in any composite score (Figure 1). Compared to composite scores in YA, which were normalized to age-appropriate mean and variance, SA perform better in episodic memory (p=0.002), which was expected due to the SA memory performance criteria. SA also performed better for their age than YA in z-standardized processing speed (p=0.027) but not working memory (p=0.104). In contrast, there were no differences in performance in any composite score between TOA and YA (all p>0.16). Processing speed was correlated to episodic memory performance in SA (r=0.47, p=0.01) and in TOA (r=0.23, p=0.02). In raw CVLT LDFR score, SA performed better than YA while TOA performed worse than YA (both p<0.0001; Table 1). The LEQ and the Wilson Lifetime Cognitive Activities questionnaires both showed trending group differences in the expected direction, with higher scores in the SA group, but the only difference that reached significance was the “present” score for the Wilson measure (p=0.042, Table 1). The “present” score represents the level of engagement in cognitive activities reported by participants at their baseline visit.
Figure 1. SA have better baseline cognition across domains.
Domain-specific average z-scores revealed significantly higher performance in SA compared to TOA. The episodic memory domain score did not include any subscores of the CVLT because they would be highly correlated with CVLT LDFR which was used to define SA. Domain scores in the older adults (SA and TOA) and YA were z-normalized according to age-appropriate mean and standard deviations. Differences between older adults and YA are interpreted as differences in normal-for-age performance. SA=successful agers, TOA=typical older adults, YA= young adults, n.s.=not significant; *p<0.05; **p<0.001.
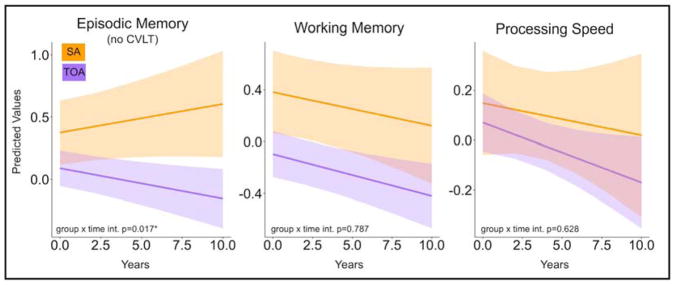
Analyses of longitudinal cognitive composite scores revealed that TOA decline in episodic memory more rapidly than SA over time (Figure 2). An LME model predicting episodic memory composite score uncovered a significant interaction of time and group status (SA or TOA; p=0.018) indicating that decline in TOA is greater than in SA. In fact, the SA group showed a trend toward increasing memory performance over time indicating the emergence of practice effects. The model, which controlled for fixed effects of age, sex, years of education and number of follow-up visits and included random effects for participant intercept and slope also revealed significant effects of age (p=0.001; older participants perform worse overall) and number of follow-up visits (p=0.001; participants with more follow-up visits perform better overall) across all older adults. There were no group differences in LME models predicting working memory or processing speed composite scores.
Figure 2. SA do not experience typical episodic memory decline.
Linear mixed effects (LME) models were used to extract predicted longitudinal trajectories for each cognitive domain in both experimental groups. Trajectories for episodic memory (left) domain scores showed a group by time interaction effect such that TOA declined while SA did not. For working memory (center) and processing speed (right) both groups declined at similar rates. SA=successful agers, TOA=typical older adults, *p<0.05.
3.3 Cortical Thickness
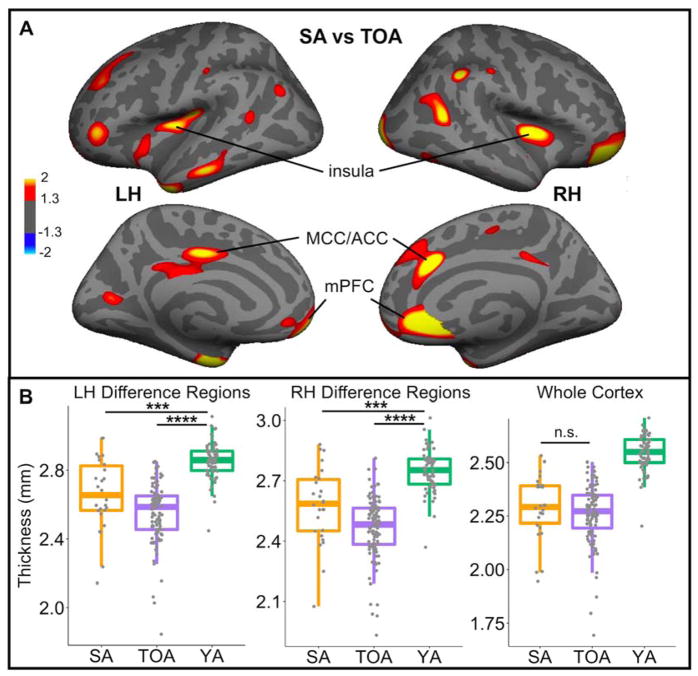
Cortical thickness analyses revealed several regions of greater cortical thickness in SA compared to TOA (p<0.05 vertexwise, uncorrected; Figure 3A), including right anterior cingulate and prefrontal cortex, which have been previously associated with SA (Harrison et al., 2012; Sun et al., 2016). There were no regions in either hemisphere that were thicker in TOA. A multiple regression model controlling for the effects of intracranial volume (ICV), age, sex, and years of education revealed that in those regions where cortical thickness is greater in SA, mean cortical thickness was related to baseline episodic memory performance across all participants (R2=0.12, p=0.02), while mean global cortical thickness was not (p=0.17). There was no difference between SA and TOA in bilateral mean cortical thickness (p=0.43), which highlights the regional specificity of cortical preservation in SA. In Figure 3B, cortical thickness values for SA and TOA are plotted alongside YA. Both older adult groups had significantly thinner cortex than YA, but the differences were less significant in SA (TOA vs YA p<1×10−29; SA vs YA p<0.0001).
Figure 3. Specific cortical regions are thicker in SA.
A) Whole brain cortical thickness analyses revealed regions of cortex that were significantly thicker in SA compared to TOA (p>0.05, uncorrected). There were no regions where cortex was thicker in TOA. B) Mean thickness values were extracted for difference regions in the LH and RH and plotted to illustrate the differences between groups. Bilateral mean thickness across the whole cortex was also extracted for each participant. There were no differences in whole cortex thickness between SA and TOA. SA=successful agers, TOA=typical older adults, YA=young adults, LH=left hemisphere, RH=right hemisphere, MCC=middle cingulate cortex, ACC=anterior cingulate cortex, mPFC=medial prefrontal cortex, ***p<0.0001; ****p<0.00001; n.s.=not significant
There were longitudinal sMRI data available for a subset of the older adult participants (19 SA and 70 TOA; mean number of additional longitudinal scans = 1.54(0.72); range = 1–4 longitudinal scans; Table 1). We examined longitudinal changes in cortical thickness of 1) the whole cortex and 2) regions that showed greater thickness in SA. There was no difference between SA and TOA in the longitudinal slope of cortical thickness for the whole cortex (p=0.660). Longitudinal thinning of left hemisphere (LH) regions with preserved cortical thickness in SA was more severe in TOA (p=0.01). There were no differences in longitudinal slope, however, in right hemisphere (RH) regions (p=0.43). Combined bilaterally, all the regions shown in Figure 3A were not thinning at statistically different rates in SA and TOA (p=0.23). Cortical thinning in LH or RH regions or across the whole cortex was not related to change in episodic memory over time in models that also accounted for age, sex and years of education (p>0.10).
3.4 Hippocampal Volume
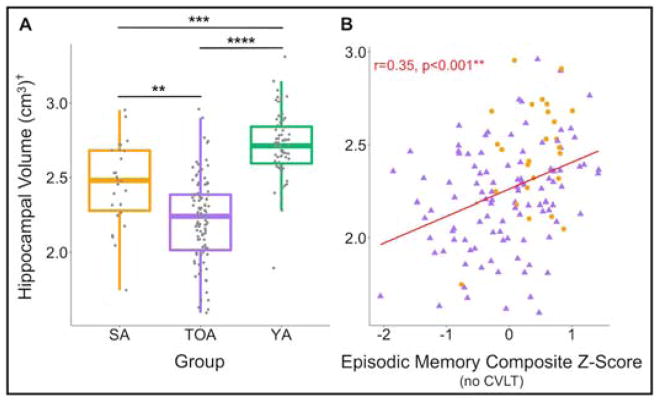
Baseline ICV-adjusted hippocampal volume was greater in SA (p<0.001) compared to TOA (Figure 4A). Both SA and TOA had significantly lower hippocampal volume compared to YA. Across all participants, hippocampal volume was associated with baseline episodic memory in a model controlling for ICV, age, sex and education (R2=0.13, p=0.01). Hippocampal volume was also related to episodic memory decline, measured by linearly fitted slopes, but this effect was driven by TOA (p=0.02). Figure 4B shows the relationship between ICV-adjusted hippocampal volume and baseline episodic memory in all older adult participants (r=0.35, p<0.001).
Figure 4. SA have greater hippocampal volume, which is related to their superior memory performance.
A) Hippocampal volume was greater in SA than TOA. Both older adult groups had significantly lower hippocampal volume than YA. B) ICV-adjusted, bilateral hippocampal volume was related to baseline episodic memory performance across all older adult participants. † denotes volumes that have been adjusted by intracranial volume (ICV). SA=successful agers, TOA=typical older adults, YA=young adults, **p<0.001; ***p<0.0001; ****p<0.00001
In the subset of older adults with longitudinal sMRI we examined longitudinal change in hippocampal volume. There was no difference between SA and TOA in hippocampal volume longitudinal change (p=0.715). In a model including hippocampal volume change (slope), age, sex and years of education we found that only change in hippocampal volume was a significant predictor (p=0.014) of change in episodic memory, but the model overall was not significant (R2=0.09, p=0.090).
3.5 White Matter Hypointensities
SA had lower bilateral volume of white matter hypointensities compared to TOA (p=0.020; Table 1). Both SA and TOA had a greater extent of white matter hypointensities compared to YA (p<0.001) but, similar to the findings in hippocampal volume and cortical thickness, the SA group were intermediate to YA and TOA. Across all older adult participants, the volume of white matter hypointensities was not associated with baseline episodic memory, working memory or processing speed in models controlling for ICV, age, sex and years of education (p>0.05). There was a trend (p=0.07) for the effect of white matter hypointensities on working memory but the model overall was not significant (R2=0.02, p=0.18).
3.6 PiB-PET
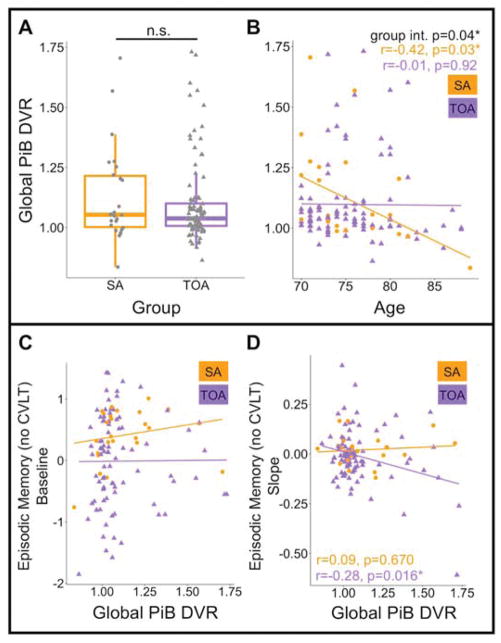
There were no differences in global PiB DVR between SA and TOA (p=0.512; Figure 5A), nor was there a significant difference in the proportions of PiB positivity (p=0.903, 38% of SA and 40% of TOA were PiB+). Across all participants, Pearson correlation showed no significant association between global PiB DVR and age (r=−0.11, p=0.22). There was, however, a significant group interaction effect (two-way ANOVA; p=0.045) driven by a significant negative correlation between age and global PiB DVR in SA (r=−0.42, p=0.03; Figure 5B) but not TOA (r=0.01, p=0.92). This association was driven in part by a single SA participant who was 89 years old and had very low PiB DVR. If this individual is removed, the relationship between age and global PiB DVR in SA is a trend (r=−0.31, p=0.14).
Figure 5. Aβ accumulation is similar in SA and TOA but advanced age, superior memory and PiB positivity appear to be incompatible.
A) There was no difference in global PiB DVR between SA and TOA. B) SA showed a significant negative relationship between global PiB DVR and age while TOA did not. There was a significant group interaction. C) Global PiB DVR was not predictive of episodic memory performance cross-sectionally in either SA or TOA. D) Global PiB DVR significantly predicted decline in episodic memory performance in TOA, but not SA. SA=successful agers, TOA=typical older adults, PiB DVR= Pittsburg Compound B distribution volume ratio, n.s.=not significant
Across all participants, global PiB DVR was not related to baseline episodic (Figure 5C) or working memory (p>0.10). There was, however, a significant positive relationship between global PiB DVR and baseline processing speed (p=0.037) in a model that also accounted for age, sex and years of education (overall model R2=0.12, p=0.002). We probed this relationship in each group separately and discovered that higher global PiB DVR is predictive of faster processing speed in SA (p=0.006) but not in TOA (p=0.384). The groupxPiB interaction was not significant (p=0.16).
Next, we examined relationships between global PiB DVR and subsequent changes in cognition. Fourteen participants (1 SA) included in PiB analyses did not have longitudinal cognitive data. In a model accounting for age, sex, years of education and number of follow-up visits, PiB DVR predicted decline in episodic memory performance in TOA (p=0.01), but not SA (p=0.38). The groupxPiB interaction was not significant but borderline at p=0.08. Figure 5D illustrates these findings by plotting the correlations between baseline global PiB DVR and change in episodic memory in SA and TOA. Global PiB DVR was not related to longitudinal change in working memory or processing speed (p>0.30).
In order to ensure PiB burden was not related to differences in brain structure, we examined the relationships between PiB burden and 1) cortical thickness in the regions with greater cortical thickness in SA, 2) cortical thickness across the entire cortex and 3) hippocampal volume. There were no significant relationships between PiB DVR and any of the morphological measures tested or in longitudinal change in these measures (p>0.05).
4. Discussion
Our unique dataset with an average of over 5 years of cognitive follow-up data allowed us to address a critical question: does being a successful ager mean that you are less likely to decline than your typically performing peers? Indeed our findings suggest that the superior memory performance in SA remains superior over time. We have shown that episodic memory performance slightly increased in our group of older adults with superior memory performance while declining in their typically performing peers. This positive slope can be interpreted as practice effects in the SA individuals who are avoiding age-related decline in episodic memory performance. In contrast, we did not observe differences between SA and TOA in working memory or processing speed performance trajectories. Rather, early changes in episodic memory performance, which along with processing speed is among the first cognitive processes to be negatively affected in normal aging, differentiated the SA and TOA groups (Salthouse, 2009). SA and TOA also differed in brain morphology, with SA showing regions of greater cortical thickness and greater hippocampal volume. These morphological features were related to memory suggesting that greater cortical thickness and hippocampal volume underlie, in part, the superior memory performance observed in SA. Past reports have found that gray matter volume mediates the effect of Aβ on cognition (Wirth et al., 2013). While Aβ burden did not differ between SA and TOA, more Aβ was associated with faster memory decline in the TOA but not SA. Together, this suggests that unique brain morphology in SA may contribute to the mitigation of cognitive decline in the presence of Aβ. We also discovered evidence that high Aβ was not compatible with superior cognition in the oldest participants, demonstrated by a negative relationship between PiB DVR and age in SA.
A previous study showed that cortical regions with greater thickness in successful agers are predominantly located within the default mode and salience networks (Sun et al., 2016). We observed a similar pattern in our SA group, with medial prefrontal and temporoparietal junction regions (default mode) and anterior/middle cingulate and insula (salience) showing evidence of preservation. Studies of “SuperAgers”, who are individuals aged 80 or older with memory performance equivalent to a 50-year-old, have implicated the anterior cingulate as a key region underlying superior memory performance in old age (Harrison et al., 2012; Gefen et al., 2015). Portions of both anterior and middle cingulate were implicated in our study despite the differences in how we defined our cohort compared to the “SuperAgers”. This provides further evidence that the cingulate is an important region involved in the circuitry underlying successful aging.
Further analyses with sMRI data revealed two additional features of SA: first, hippocampal volume, when accounting for age, sex, education and ICV, was greater in SA compared to TOA and second, the volume of white matter hypointensities in the brain was lower. The former finding is also consistent with previous work in SA, and underscores the strong link between hippocampal integrity and episodic memory performance (Sun et al., 2016). The latter finding, which suggests resistance to white matter disease in SA, is novel and may contribute, along with the morphological features of SA, to mitigating pathological effects of Aβ in SA. Previous studies have reported that white matter signal abnormalities increase with age, are related to cognitive decline and increased risk for clinical AD in the presence of Aβ, and are not correlated with Aβ accumulation (Abe et al., 2002; Burns et al., 2005; Hedden et al., 2012). Thus, lower white matter disease burden combined with typical Aβ levels in SA mean that they are relatively more protected from future cognitive decline resulting from Aβ-white matter disease interactions than TOA. Further in-depth studies of white matter using diffusion weighted imaging might reveal important associations between white matter microstructure and superior cognition.
Despite having greater baseline hippocampal volume, SA showed no difference from TOA in the change in hippocampal volume over time. Across both groups, change in hippocampal volume over time was related to decline in episodic memory. When we examined cortical regions with baseline differences in cortical thickness between SA and TOA, areas in the LH declined more slowly in SA compared to TOA although there were no differences when both hemispheres were examined together. The slope of cortical thinning in these LH regions was also not related to change in memory. Taken together, these findings define a relationship between brain structure (cortical thickness, hippocampal volume, white matter alterations) and cognition that supports a passive model in which brain reserve endows some individuals with greater capacity (e.g., larger brains or more neurons and synapses) so that they may escape age-related cognitive decline (Stern, 2002). While there is some evidence for different trajectories of brain volume loss in older SuperAgers ≥80 years old (Cook et al., 2017), the aggregate data in our cohort seem to suggest that TOA and SA both experience similar degrees of longitudinal atrophy across the whole cortex. This disagreement in findings may indicate that different processes underlie superior memory during different epochs of older adulthood, with slower longitudinal atrophy being important in the 9th and 10th decades of life.
Neither PiB positivity prevalence or global PiB DVR differed between SA and TOA. This is an important finding underscoring that SA do not avoid all pathological, age-related brain changes. Recent work with post-mortem tissue of highly successful agers is congruent with our findings, showing that Aβ pathology was observed in 5 of 10 individuals (Rogalski et al., 2018). The link between Aβ burden and cognition has been explored and the growing consensus is that the correlation, at least in cross-sectional comparisons, is weak (Hedden et al., 2013; Jansen et al., 2018). Thus, maintenance of superior memory performance even in PiB+ individuals is not surprising. Indeed another study recently found that older high memory performers do not differ from their typical peers in Aβ burden (Dekhtyar et al., 2017). It does, however, indicate that SA may be resilient to Aβ-triggered processes that negatively affect cognition. One clue in our results is that there is a negative relationship between age and PiB DVR in SA such that only the youngest members of the SA group have very high PiB DVR. In our data, very high Aβ burden, very old age and superior memory performance appear to be incompatible. It may be that SA are resilient to the high Aβ for a time, perhaps while preserved brain morphology remains protective, but eventually, combined with normal aging, this resilience begins to give way and cognitive impairment follows.
Initial resilience to elevated Aβ may also be related to faster processing speed, which we found was positively related to PiB DVR and also to better memory performance in SA. Processing speed has been previously suggested to support performance in other cognitive domains, such as memory (Salthouse, 2000). Youthful processing speed in older adults has been associated with greater volume of the corpus callosum, lower inflammatory markers and more physical activity, indicating that superior memory and processing speed in older adults are mediated by different morphometric and biological factors (Bott et al., 2017). The link between processing speed and Aβ in the present study may represent a unique feature of successful aging with Aβ, as a larger meta-analysis did not find a significant relationship between processing speed and Aβ deposition (Hedden et al., 2013).
The relationship between Aβ burden and longitudinal cognitive changes is stronger than to cross-sectional cognition (Landau et al., 2012; Wirth et al., 2013; Donohue et al., 2017). We observed this distinction in the present study by showing that baseline PiB DVR predicted decline in TOA, but not baseline performance. In fact, memory decline in TOA was associated with baseline hippocampal volume and with baseline PiB DVR, but these associations were not present in SA. These findings support the idea that SA is an aging trajectory with unique features, including slower age-associated decline in memory performance, with some protection conferred by differences in brain volumes.
Only 26 of 150 individuals that we screened for the present study met our rigorous criteria for successful aging. While the utilization of these criteria was essential to identify the SA group and facilitate reproducibility and interpretability of our results, the final cohort size was small and may have made effects within SA group difficult to detect. Our sample, however, was larger than SA groups in much of the previous work (Harrison et al., 2012; Gefen et al., 2014; Sun et al., 2016; Cook et al., 2017). Future studies will focus on screening a greater volume of subjects to identify a larger SA cohort. It should be noted that the TOA group, like the entire BACS cohort, is a highly educated sample that is likely not a “typical” aged group despite our designation as such. Thus, the differences in brain structure, white matter and cognition we have uncovered between SA and TOA are remarkable. A major limitation of the current study is the low number of men in the SA group. This could be related to the fact that we used a verbal memory task, the CVLT, to identify SA and women have been reported to perform better in tests of verbal memory (Andreano and Cahill, 2009; Munro et al., 2012). It is also likely related to the fact that about ~60% of the BACS cohort is female and the small overall size of the SA group. In a report by Sun and colleagues, that used SA inclusion criteria similar to the present study, 12 of 17 designated ‘superagers’ were female (Sun et al., 2016). We included sex as a covariate in all our analyses and it was not found to be a significant predictor in any of the results we report in the present study. Finally, we measured white matter abnormalities using T1-weighted images, which can result in lower volume estimates compared to those derived from T2-weighted or FLAIR images. Despite this, T1-weighted image derived estimates have been shown to be highly correlated (r>0.9) with T2-weighted or FLAIR estimates of white matter abnormalities in aging and disease (Coutu et al., 2015; Klistorner et al., 2016). FreeSurfer derived estimates of T1-weighted hypointense white matter have been validated against experienced raters at an intraclass correlation coefficient of 0.91 (Smith et al., 2011). In post mortem tissue, T1-derived estimates of white matter signal abnormality have been shown to reflect demyelination and axonal loss, perhaps capturing only more severe changes to white matter (Bitsch et al., 2001).
5. Conclusions
The present study provides new evidence for a model of successful aging in which brain morphology is crucially important. In addition, we found that AD-related pathology was not associated with superior memory performance in older adults, and occurred at similar rates in both high performing and typical groups. Our findings indicate that the concept of brain reserve plays a major role in the maintenance of superior memory performance in older adults. Longitudinal differences in atrophy rates are also likely important to maintaining healthy cognition, especially in older cohorts, but we found only limited evidence of this in our participants. Faster processing speed, a feature of our SA cohort that was not determined by criteria, and fewer white matter hypointensities also contribute to their superior memory performance. In contrast to the apparent advantages of SA in brain structure and cognition, there were no differences in Aβ accumulation between SA and TOA. An important future direction is the elucidation of the mechanisms of reserve and resilience that allow SA to maintain structural integrity and white matter health in the brain, supporting superior memory similar to that of an 18–32-year-old, while still experiencing typical-for-age Aβ accumulation and longitudinal, age-related atrophy. In terms of the detrimental effects of Aβ accumulation, our data suggest that SA may be resilient to these effects due to their greater brain volumes in key structures, reduced white matter abnormalities and faster processing speed. In our participants, a basic co-morbidity index score and lifetime cognition and activities questionnaires could not account for the imaging and cognitive differences we observed, although there were important trends in the self-report questionnaires. Taken together, our findings support a model in which a combination of protective effects including 1) brain reserve (e.g., hippocampal volume), 2) resistance to pathology (e.g., white matter hypointensities) and 3) resilience to pathology (e.g., Aβ accumulation) interact to support a unique successful aging trajectory defined by superior memory performance.
Highlights.
Older people with superior memory had less memory decline compared to typical peers
Hippocampal volume and regional cortical thickness was greater in successful agers
Successful agers had similar levels of brain amyloid compared to typical peers
Preserved brain morphology may help ameliorate the effects of amyloid in the brain
Successful agers show both resistance and resilience to age-related brain changes
Acknowledgments
This research was supported by the National Institutes of Health grants F32AG057107 (to T.M.H), T32AG000266 (to T.M.H), R01AG034570 (to W.J.J.) and P01-AG1972403 (to W.J.J.). Support was also provided by the Helmholtz Postdoc Program grant PD-306 (to A.M.).
Footnotes
Disclosure Statement
Dr. Jagust has served as a consultant to Banner Alzheimer Institute, Genentech, Novartis, Bioclinica and Merck. The other authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe O, Aoki S, Hayashi N, Yamada H, Kunimatsu A, Mori H, Yoshikawa T, Okubo T, Ohtomo K. Normal aging in the central nervous system: quantitative MR diffusion-tensor analysis. [Accessed October 2, 2017];Neurobiol Aging. 2002 23:433–441. doi: 10.1016/s0197-4580(01)00318-9. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11959406. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Sex influences on the neurobiology of learning and memory. [Accessed October 2, 2017];Learn Mem. 2009 16:248–266. doi: 10.1101/lm.918309. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19318467. [DOI] [PubMed] [Google Scholar]
- Bitsch A, Kuhlmann T, Stadelmann C, Lassmann H, Lucchinetti C, Brück W. A longitudinal MRI study of histopathologically defined hypointense multiple sclerosis lesions. [Accessed February 1, 2018];Ann Neurol. 2001 49:793–796. doi: 10.1002/ana.1053. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11409432. [DOI] [PubMed] [Google Scholar]
- Bott NT, Bettcher BM, Yokoyama JS, Frazier DT, Wynn M, Karydas A, Yaffe K, Kramer JH. Youthful Processing Speed in Older Adults: Genetic, Biological, and Behavioral Predictors of Cognitive Processing Speed Trajectories in Aging. [Accessed January 26, 2018];Front Aging Neurosci. 2017 9:55. doi: 10.3389/fnagi.2017.00055. Available at: http://www.ncbi.nlm.nih.gov/pubmed/28344553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JM, Church JA, Johnson DK, Xiong C, Marcus D, Fotenos AF, Snyder AZ, Morris JC, Buckner RL. White matter lesions are prevalent but differentially related with cognition in aging and early Alzheimer disease. [Accessed October 2, 2017];Arch Neurol. 2005 62:1870–1876. doi: 10.1001/archneur.62.12.1870. Available at: http://archneur.jamanetwork.com/article.aspx?doi=10.1001/archneur.62.12.1870. [DOI] [PubMed] [Google Scholar]
- Cook AH, Sridhar J, Ohm D, Rademaker A, Mesulam M-M, Weintraub S, Rogalski E. Rates of Cortical Atrophy in Adults 80 Years and Older With Superior vs Average Episodic Memory. [Accessed June 5, 2017];JAMA. 2017 317:1373. doi: 10.1001/jama.2017.0627. Available at: http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2017.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola G, et al. Evidence for a role of the rare p.A152T variant in MAPT in increasing the risk for FTD-spectrum and Alzheimer’s diseases. [Accessed June 5, 2017];Hum Mol Genet. 2012 21:3500–3512. doi: 10.1093/hmg/dds161. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22556362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutu J-P, Goldblatt A, Rosas HD, Salat DH Alzheimer’s Disease Neuroimaging Initiative (ADNI) White Matter Changes are Associated with Ventricular Expansion in Aging, Mild Cognitive Impairment, and Alzheimer’s Disease Gold B, ed. [Accessed February 1, 2018];J Alzheimer’s Dis. 2015 49:329–342. doi: 10.3233/JAD-150306. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26444767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical Surface-Based Analysis. [Accessed June 6, 2017];Neuroimage. 1999 9:179–194. doi: 10.1006/nimg.1998.0395. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9931268. [DOI] [PubMed] [Google Scholar]
- Dekhtyar M, Papp KV, Buckley R, Jacobs HIL, Schultz AP, Johnson KA, Sperling RA, Rentz DM. Neuroimaging markers associated with maintenance of optimal memory performance in late-life. [Accessed June 6, 2017];Neuropsychologia. 2017 100:164–170. doi: 10.1016/j.neuropsychologia.2017.04.037. Available at: http://www.sciencedirect.com/science/article/pii/S0028393217301653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. [Accessed July 14, 2014];Neuroimage. 2006 31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. Available at: http://www.sciencedirect.com/science/article/pii/S1053811906000437. [DOI] [PubMed] [Google Scholar]
- Donohue MC, Sperling RA, Petersen R, Sun C-K, Weiner MW, Aisen PS. Association Between Elevated Brain Amyloid and Subsequent Cognitive Decline Among Cognitively Normal Persons. [Accessed February 21, 2018];JAMA. 2017 317:2305. doi: 10.1001/jama.2017.6669. Available at: http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2017.6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman JA, Oh H, Madison CM, Baker SL, Vogel JW, Marks SM, Crowley S, O’Neil JP, Jagust WJ. Neural compensation in older people with brain amyloid-β deposition. [Accessed June 5, 2017];Nat Neurosci. 2014 17:1316–1318. doi: 10.1038/nn.3806. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25217827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. [Accessed January 16, 2015];Proc Natl Acad Sci U S A. 2000 97:11050–11055. doi: 10.1073/pnas.200033797. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=27146&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. [Accessed June 6, 2017];Neuron. 2002 33:341–355. doi: 10.1016/s0896-6273(02)00569-x. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11832223. [DOI] [PubMed] [Google Scholar]
- Gefen T, Peterson M, Papastefan ST, Martersteck A, Whitney K, Rademaker A, Bigio EH, Weintraub S, Rogalski E, Mesulam M-M, Geula C. Morphometric and Histologic Substrates of Cingulate Integrity in Elders with Exceptional Memory Capacity. [Accessed June 6, 2017];J Neurosci. 2015 :35. doi: 10.1523/JNEUROSCI.2998-14.2015. Available at: http://www.jneurosci.org/content/35/4/1781.long. [DOI] [PMC free article] [PubMed]
- Gefen T, Shaw E, Whitney K, Martersteck A, Stratton J, Rademaker A, Weintraub S, Mesulam M-M, Rogalski E. Longitudinal Neuropsychological Performance of Cognitive SuperAgers. [Accessed October 2, 2017];J Am Geriatr Soc. 2014 62:1598–1600. doi: 10.1111/jgs.12967. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25116988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison TM, Weintraub S, Mesulam M-M, Rogalski E. Superior memory and higher cortical volumes in unusually successful cognitive aging. [Accessed March 21, 2014];J Int Neuropsychol Soc. 2012 18:1081–1085. doi: 10.1017/S1355617712000847. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3547607&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JDE. Insights into the ageing mind: a view from cognitive neuroscience. [Accessed August 8, 2017];Nat Rev Neurosci. 2004 5:87–96. doi: 10.1038/nrn1323. Available at: http://www.nature.com/doifinder/10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Hedden T, Mormino EC, Amariglio RE, Younger AP, Schultz AP, Becker JA, Buckner RL, Johnson KA, Sperling RA, Rentz DM. Cognitive Profile of Amyloid Burden and White Matter Hyperintensities in Cognitively Normal Older Adults. [Accessed October 2, 2017];J Neurosci. 2012 32:16233–16242. doi: 10.1523/JNEUROSCI.2462-12.2012. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23152607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Oh H, Younger AP, Patel TA. Meta-analysis of amyloid-cognition relations in cognitively normal older adults. [Accessed August 8, 2017];Neurology. 2013 80:1341–1348. doi: 10.1212/WNL.0b013e31828ab35d. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23547267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen WJ, et al. Association of Cerebral Amyloid-β Aggregation With Cognitive Functioning in Persons Without Dementia. [Accessed February 21, 2018];JAMA Psychiatry. 2018 75:84. doi: 10.1001/jamapsychiatry.2017.3391. Available at: http://archpsyc.jamanetwork.com/article.aspx?doi=10.1001/jamapsychiatry.2017.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klistorner A, Wang C, Fofanova V, Barnett MH, Yiannikas C, Parratt J, You Y, Graham SL. Diffusivity in multiple sclerosis lesions: At the cutting edge? [Accessed February 1, 2018];NeuroImage Clin. 2016 12:219–226. doi: 10.1016/j.nicl.2016.07.003. Available at: http://www.ncbi.nlm.nih.gov/pubmed/27489769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Mintun MA, Joshi AD, Koeppe RA, Petersen RC, Aisen PS, Weiner MW, Jagust WJ Alzheimer’s Disease Neuroimaging Initiative. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. [Accessed August 8, 2017];Ann Neurol. 2012 72:578–586. doi: 10.1002/ana.23650. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23109153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latimer CS, Keene CD, Flanagan ME, Hemmy LS, Lim KO, White LR, Montine KS, Montine TJ. Resistance to Alzheimer Disease Neuropathologic Changes and Apparent Cognitive Resilience in the Nun and Honolulu-Asia Aging Studies. [Accessed January 26, 2018];J Neuropathol Exp Neurol. 2017 76:458–466. doi: 10.1093/jnen/nlx030. Available at: http://www.ncbi.nlm.nih.gov/pubmed/28499012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang G-J, Ding Y-S, Alexoff DL. Distribution Volume Ratios Without Blood Sampling from Graphical Analysis of PET Data. [Accessed June 5, 2017];J Cereb Blood Flow Metab. 1996 :834–840. doi: 10.1097/00004647-199609000-00008. Available at: http://jcb.sagepub.com/lookup/doi/10.1097/00004647-199609000-00008. [DOI] [PubMed]
- Mathis CA, Wang Y, Holt DP, Huang G-F, Debnath ML, Klunk WE. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. [Accessed June 5, 2017];J Med Chem. 2003 46:2740–2754. doi: 10.1021/jm030026b. Available at: http://pubs.acs.org/doi/abs/10.1021/jm030026b. [DOI] [PubMed] [Google Scholar]
- Mormino EC, Smiljic A, Hayenga AO, Onami SH, Greicius MD, Rabinovici GD, Janabi M, Baker SL, Yen IV, Madison CM, Miller BL, Jagust WJ. Relationships between β-amyloid and functional connectivity in different components of the default mode network in aging. [Accessed June 5, 2017];Cereb Cortex. 2011 21:2399–2407. doi: 10.1093/cercor/bhr025. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21383234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro CA, Winicki JM, Schretlen DJ, Gower EW, Turano KA, Muñoz B, Keay L, Bandeen-Roche K, West SK. Sex differences in cognition in healthy elderly individuals. [Accessed October 2, 2017];Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2012 19:759–768. doi: 10.1080/13825585.2012.690366. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22670852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. [Accessed September 26, 2017];Neuroimage. 2012 61:1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22430496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Gefen T, Mao Q, Connelly M, Weintraub S, Geula C, Bigio EH, Mesulam M-M. Cognitive trajectories and spectrum of neuropathology in SuperAgers: The first ten cases. [Accessed January 26, 2018];Hippocampus. 2018 doi: 10.1002/hipo.22828. Available at: http://www.ncbi.nlm.nih.gov/pubmed/29341318. [DOI] [PMC free article] [PubMed]
- Salat D, Greve D, Pacheco J, Quinn B, Helmer K, Buckner R, Fischl B. Regional white matter volume differences in nondemented aging and Alzheimer’s disease. [Accessed February 1, 2018];Neuroimage. 2009 44:1247–1258. doi: 10.1016/j.neuroimage.2008.10.030. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19027860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Aging and measures of processing speed. [Accessed October 12, 2017];Biol Psychol. 2000 54:35–54. doi: 10.1016/s0301-0511(00)00052-1. Available at: http://www.sciencedirect.com/science/article/pii/S0301051100000521?via%3Dihub. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. When does age-related cognitive decline begin? [Accessed January 25, 2018];Neurobiol Aging. 2009 30:507–514. doi: 10.1016/j.neurobiolaging.2008.09.023. Available at: https://www.sciencedirect.com/science/article/pii/S0197458009000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Salat DH, Jeng J, McCreary CR, Fischl B, Schmahmann JD, Dickerson BC, Viswanathan A, Albert MS, Blacker D, Greenberg SM. Correlations between MRI white matter lesion location and executive function and episodic memory. [Accessed February 1, 2018];Neurology. 2011 76:1492–1499. doi: 10.1212/WNL.0b013e318217e7c8. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21518999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. [Accessed August 7, 2017];J Int Neuropsychol Soc. 2002 8:448–460. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11939702. [PubMed] [Google Scholar]
- Sun FW, Stepanovic MR, Andreano J, Barrett LF, Touroutoglou A, Dickerson BC. Youthful Brains in Older Adults: Preserved Neuroanatomy in the Default Mode and Salience Networks Contributes to Youthful Memory in Superaging. [Accessed June 6, 2017];J Neurosci. 2016 36:9659–9668. doi: 10.1523/JNEUROSCI.1492-16.2016. Available at: http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.1492-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela MJ, Sachdev P. Assessment of complex mental activity across the lifespan: development of the Lifetime of Experiences Questionnaire (LEQ) [Accessed August 8, 2017];Psychol Med. 2007 37:1015. doi: 10.1017/S003329170600938X. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17112402. [DOI] [PubMed] [Google Scholar]
- Villeneuve S, et al. Existing Pittsburgh Compound-B positron emission tomography thresholds are too high: statistical and pathological evaluation. [Accessed November 1, 2016];Brain. 2015 138:2020–2033. doi: 10.1093/brain/awv112. Available at: http://www.brain.oxfordjournals.org/lookup/doi/10.1093/brain/awv112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Bennett DA. Assessment of Lifetime Participation in Cognitively Stimulating Activities. [Accessed June 5, 2017];J Clin Exp Neuropsychol (Neuropsychology, Dev Cogn Sect A) 2003 25:634–642. doi: 10.1076/jcen.25.5.634.14572. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12815501. [DOI] [PubMed] [Google Scholar]
- Wirth M, Oh H, Mormino EC, Markley C, Landau SM, Jagust WJ. The effect of amyloid β on cognitive decline is modulated by neural integrity in cognitively normal elderly. [Accessed October 2, 2017];Alzheimer’s Dement. 2013 9:687–698.e1. doi: 10.1016/j.jalz.2012.10.012. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23474040. [DOI] [PMC free article] [PubMed] [Google Scholar]