Abstract
Spinal cord epidural stimulation (SCS) represents a form of neuromodulation for the management of spasticity and pain. This technology has recently emerged as a new approach for potentially augmenting locomotion and voiding function in humans and rodents after spinal cord injury. However, the effect of SCS on micturition has not been studied extensively. Here, SCS was first applied as a direct stimulus onto individual segmental levels of the lumbar spinal cord in rats to map evoked external urethral sphincter (EUS) electromyography activity and SCS-induced voiding contractions. SCS of L2-3 inhibited EUS tonic activity, and SCS on L3 (L3/SCS) inhibited EUS tonic activity and elicited EUS bursting. In contrast, SCS of L1 and L4-6 evoked EUS tonic contractions, which resembled the urethral guarding reflex during bladder storage. Next, the effects of a bilateral pelvic nerve crush (PNC) injury on urodynamic function were examined at 14 days post-operatively. The PNC injury resulted in decreased voiding efficiency and maximum intravesical pressure, whereas the post-voiding residual volume was increased, suggestive of an underactive bladder. Finally, L3/SCS was performed to induce a voiding contraction and enable voiding in rats with a PNC injury. Voiding efficiency was significantly increased, and the residual volume was decreased by L3/SCS in rats after the PNC injury. We conclude that L3/SCS may be used to induce micturition reflexes in a partially filled bladder, reduce urethral resistance, and augment bladder emptying after PNC injury.
Keywords: underactive bladder, external urethral sphincter, electromyography, pelvic nerve
INTRODUCTION
Spinal cord stimulation (SCS) improves locomotion and lower urinary tract (LUT) function after spinal cord injury (SCI) (Harkema et al., 2011; Ichiyama et al., 2008; Ichiyama et al., 2005; Pettigrew et al., 2017). Voiding function seems to benefit from the combination of long-term locomotor training and SCS. However, the mechanisms by which SCS improves LUT function are not well understood.
LUT function depends on the coordinated activity of the bladder and external urethral sphincter (EUS). The rat exhibits EUS tonic contractions during the bladder filling phase, and the EUS switches to a bursting pattern, which consists of intermittent periods of relaxation and phasic activation, during voiding. EUS bursting represents rhythmic contractions and relaxations of the EUS that are necessary for efficient bladder emptying (Langdale and Grill, 2016; Maggi et al., 1986). The motoneurons of the pudendal nerve that innervate the EUS are located in dorsolateral nucleus of L6-S1. (McKenna et al., 1991; Schroder, 1980). Spinal interneurons are identified in the intermediate gray matter and medial gray of L2 to S2 following pseudorabies virus injections into the EUS (Marson, 1997). Other studies have indicated that both EUS tonic and bursting activity can be generated by circuitry in the lumbosacral spinal cord (Chang et al., 2007; Cheng and de Groat, 2004; Kruse and de Groat, 1993). In addition, a spinal EUS bursting generator located at the L3-4 segmental levels in rats was shown to produce bursting activity during voiding, an important feature for urine expulsion and bladder emptying (Abud et al., 2015; Chang et al., 2006). The use of experimental models for the mapping of spinal cord sites that promote voiding is an important and effective strategy to better understand neural reflex pathways and mechanisms underlying LUT function before extending the findings to clinical studies in patients with SCI (Pettigrew et al., 2017).
Prior studies in our laboratories have demonstrated that SCS of L3 (L3/SCS) produces EUS intermittent relaxation and phasic activation in neurologically intact animals and rats with a chronic spinal cord injury (Abud et al., 2015). However, the effects of SCS on the bladder-sphincter coordination have not been examined. The present study was performed to first determine the effects of SCS applied at different lumbar spinal cord levels on both detrusor contractions and EUS activation. Next, LUT function was investigated in control animals and in rats with a bilateral pelvic nerve crush (PNC) injury. PNC injuries are clinically relevant as they may be the consequences of pelvic floor surgeries and can result in an underactive bladder with decreased voiding efficiency and maximum bladder pressure during voiding (Tyagi et al., 2014). Finally, neuromodulation using L3/SCS was applied in an attempt to augment LUT function after a bilateral PNC injury in rats.
MATERIALS AND METHODS
All experiments were approved and carried out in accordance with the National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committees at the University of California, Irvine, and University of Southern California. Adult female Sprague-Dawley rats (227 ± 3 g, n=19) were purchased from Charles River Laboratories (Hollister, CA, USA), and maintained in the animal facility under controlled conditions (68 ± 2° F, equivalent to ~20 ± 1°C; humidity 30-70%), housed 2 animals per cage on a 12 h light-dark cycle, and fed with standard chow. After surgical procedures, rats were single housed for 5 days with enhanced enrichment, and then housed as 2 rats per cage until the urodynamic evaluations.
Preparation for mapping EUS activation by SCS in control rats
A total of 7 female spinally intact rats were anesthetized by urethane (1.2 g/kg, subcutaneously; sc) 1 hour prior to the surgical procedures. Each animal was placed on a water-circulating heating pad to maintain body temperature. A T11-L3 laminectomy was performed to expose the L1-6 spinal cord segments. Teflon-coated stainless steel wires (AS632; Cooner Wire, Chatsworth, CA, USA) were prepared by removing a small portion (∼1mm notch) of the Teflon coating to expose the stainless steel before placing the wire tips onto the dura overlying the dorsal surface of the spinal cord. The electrodes were secured in position by suturing each wire to the dura over the spinal segments of interest (Abud et al., 2015; Ichiyama et al., 2005). Bipolar differential electrodes were used, and the electrical current only circulated the area between the exposure sites. The wires were placed 2-3 mm apart from the 1-mm exposed tip of each wire. The estimated total area (rostro-caudally) receiving the electrical current was limited to a maximum of 5 mm2. The wires were connected to a stimulator (Grass S88, Grass Technologies, West Warwick, RI, USA) through a stimulus isolation unit (Grass SIU5, Grass Technologies, West Warwick, RI, USA).
Two 50-μm PFA-insulated platinum-iridium wire electrodes (A-M Systems, Everett, WA, USA) with 2-mm exposed tips were inserted into the EUS, aided by a 27-gauge needle bilaterally. The wire electrodes were subsequently connected to an amplifier (MP150; Biopac Systems Inc., Goleta, CA, USA) to record EUS EMG activity. For functional mapping of urethral activation, SCS was applied to the dorsal portion of the spinal cord at individual lumbar segmental levels, and the effects on EUS EMG activity were recorded.
Pelvic nerve crush (PNC) injury
Rats (n=6) were anesthetized with 2-4% isoflurane with oxygen. Each animal was placed on a water-circulating heating pad. A midline abdominal incision was made to expose the major pelvic ganglia and identify the bilateral pelvic nerves by established methods (Chang et al., 2007; Chang and Havton, 2010). The bilateral pelvic nerves were crushed by using a straight micro-mosquito clamp for 1 minute (Tyagi et al., 2014). The incision was closed, and all animals were allowed to recover. Enrofloxacin (5 mg/kg, sc, Putney Inc., Portland, ME, USA) and Ketoprofen (5 mg/kg, sc, Zoetis Inc., Parsippany, NJ, USA) were given once a day for 5 days post-operation. All animals were monitored post-operatively for 14 days before undergoing L3/SCS and urodynamic studies as a terminal procedure.
Preparation for L3/SCS and urodynamic recordings in control and PNC groups
A laminectomy to expose the dorsal surface of the lumbar spinal cord and place stimulation electrodes was performed under urethane anesthesia as described above. The stimulating wires were placed over the dura at the L3 segment level only to evaluate effect of L3/SCS on urodynamic recordings in control (n=6) and PNC injured (n=6) rats. All animals underwent placements of a bladder catheter and EUS EMG wires according to established methods (Abud et al., 2015; Chang et al., 2007). Briefly, a midline incision was made to expose the bladder. A flared tip of a PE-50 catheter (Instech Laboratories, Plymouth, MA, USA) was inserted through the top of the bladder dome and secured by a thread. The bladder catheter was connected to an infusion pump (KDS 100; KD Scientific, Holliston, MA, USA) and a pressure sensor (MP150; Biopac Systems Inc., Goleta, CA, USA) via a 3-way connector. For urodynamic studies, the infusion rate of saline was set at 0.1 ml/min. A collecting cup was positioned under the urethral outlet to measure the voided volume.
Two urodynamic experiments were conducted in the same animal to first determine the effects of continuous bladder infusion alone and next to determine the effects of L3/SCS when the bladder was partially filled. The threshold intensity for SCS was decided as the minimal intensity that produced inhibition of the EUS tonic activity. SCS was applied at 1-3 fold of the threshold intensity (1-5 Volts) at 40 Hz and 0.2 ms pulse width for 60-90 seconds. The intensity was adjusted to avoid and/or minimize hind limb movements and body tremors.
Morphological investigation
The bladder and urethra were harvested from animals in the control and PNC groups after the completion of the urodynamic recordings. The tissues were first placed in 10% Neutral Buffered Formalin (Fisher Scientific, Pittsburgh, PA, USA) for at least 24 hours. Next, the tissues were rinsed and stored in 70% ethanol before paraffin embedding by the histological service core at USC. Briefly, the tissue embedding process occurred overnight, using the pre-programmed Tissue Processor (ATP-1, Fisher Scientific, Pittsburgh, PA, USA), and then the tissue was embedded in paraffin wax (Surgipath Paraplast X-tra, LEICA, Buffalo Grove, IL, USA). The wax-embedded samples were next removed from the processor and longitudinally sectioned at a thickness of 5 μm. The tissue sections were placed on glass slides and heated at 60°C for 30 to 40 minutes to allow the samples to adhere to the glass.
Serial bladder and urethral longitudinal sections underwent hematoxylin–eosin (H&E) staining and were prepared for light microscopy (Zhang et al., 2016). A Leica (DM IRB, LEICA, Buffalo Grove, IL, USA) light microscope was used to measure the wall thickness of the middle portion of the bladder and the middle urethra containing the EUS using the SPOT camera and software program (Diagnostic Instruments Inc., Sterling Heights, MI, USA). The bladder wall thickness measurements included the urothelium, lamina propria and smooth muscle. The outermost layer, or serosa, which is external to the smooth muscle layer, was not included in these measurements.
Outcome measurements
During continuous bladder infusion with saline, three voiding contractions were recorded. Cystometry parameters included maximum intravesical pressure (IVPmax), voided volume after voiding, and contraction duration (CD). In addition, maximum amplitude and area under the curve (AUC) were determined from EUS EMG activity. The AUC of the EMG activity is the integral values identified by AcKnowledge 4.0 (Biopac Systems Inc., Goleta, CA, USA). Tonic AUC is measured during the period from the pressure threshold to the first peak of detrusor contraction. Bursting AUC is measured during the EUS bursting. The latency of the first void (LFV) was measured as the period from the start of filling of the empty bladder to the time of the onset of the first void. Bladder capacity was calculated as the volume (ml) based on [LFV (minute) × infusion rate (0.1 ml/minute)]. Residual volume (RV) was calculated as the value of (total infused volume – voided volume). Voiding efficiency (VE) was calculated as (voided volume / total infused volume) and expressed in percentage. To evaluate voiding contractions induced by L3/SCS, the bladder was partially filled to 50 - 95% of bladder capacity in each animal to facilitate bladder afferent activity before giving SCS (Abud et al., 2015; Chang et al., 2006, 2007; Tai et al., 2004). Three episodes were induced and separated by 5-minute resting periods.
Statistical analysis
Quantitative data were presented as mean ± standard error. Unpaired t-test was used to compare the urodynamic measurements between control and PNC rats. Paired t-test was used to compare urodynamic measurements with and without L3/SCS in the experimental groups. P < 0.05 was considered to reflect a significant change. Prism 6 (GraphPad Software, La Jolla, CA, USA) was used for the statistical analysis and graphs.
RESULTS
The present study examined the effects and specificity of neuromodulation applied to the lumbar spinal cord of rats on LUT function in intact rats and in a PNC injury model. The studies included three sets of experiments. First, urodynamic recordings were performed during SCS of individual lumbar segments to map LUT function in neurologically intact animals (n=7). Second, urodynamic recordings were performed to evaluate micturition reflex function 14 days after a bilateral PNC injury in rats (n=6). Third, SCS over the L3 spinal cord segment was combined with urodynamic studies of micturition reflexes in rats with PNC (n=6).
Mapping of LUT function using SCS and urodynamic studies
The effects of SCS were monitored using cystometry for bladder function and EUS EMG recordings for urethral function (Figure 1). All rats animal received 2-3 SCS episodes at intervals of 5 minutes for the testing of each individual spinal cord segment. The bladder capacity was kept below the voiding threshold. The stimulus intensity was adjusted to avoid voiding contractions. Therefore, the SCS-induced EUS bursting was clearly observed. The SCS of the L1 segment evoked EUS tonic contractions, which were similar to those present during the bladder storage phase (n=4 tested for L1). SCS of the L2 segment evoked inhibition of EUS activity corresponding to urethral relaxation (n=4 tested for L2). SCS over the L3 segment first evoked inhibition of EUS activity and subsequent EUS phasic activation, similar to the EUS bursting activity present during the voiding phase in rodents (n=3 tested for L3). SCS over the L4, L5, or L6 segments evoked EUS tonic contractions (n=3 tested for L4-6). In addition, concurrent cystometry performed during SCS showed small non-voiding contractions (Figure 1). However, during the initial testing of stimulation threshold, higher intensities that resulted in hind limb movement evoked small non-voiding contractions when SCS was provided at each segment from L1 to L6. The largest non-voiding contractions were evoked by SCS over L6 associated with the larger EUS tonic activity (Figure 1).
Figure 1.
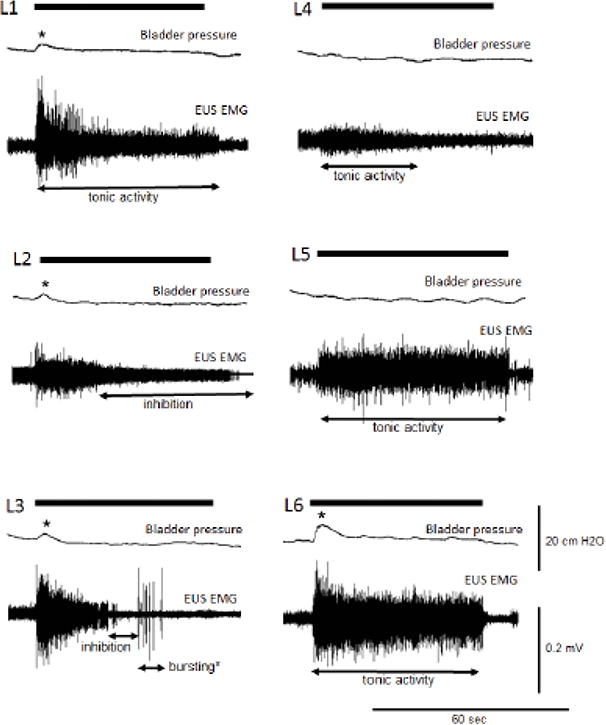
Mapping of the external urethral sphincter (EUS) spinal relaxation center. SCS was applied on the first lumbar spinal segment (L1) through the sixth lumbar spinal segment (L6) in rats (n=7). The bladder was filled at 50-80% of voiding capacity. SCS on L2 evoked inhibition of EUS tonic activity compared to the baseline without SCS in the same animal. SCS on L3 evoked not only inhibition of EUS tonic activity but also EUS bursting without bladder contraction. SCS applied to L1, L4-L6 elicited EUS tonic activity. The black thick bars indicate the duration of SCS. Asterisks indicate the non-voiding contractions during SCS.
Effects of a bilateral PNC injury on the LUT
Comprehensive urodynamic studies, including cystometry and EUS EMG studies showed significant changes in LUT reflex function after PNC injury in rats (Table 1; Figures 2A, B). At 14 days after PNC injury, VE and IVPmax were significantly reduced at 54 ± 5 % and 30 ± 3 cm H2O, n=6 compared to corresponding measurements of 78 ± 4 % and 45 ± 4 cm H2O, n=6 in the control rats (unpaired t-test, p<0.05). The RV was significantly increased at 0.18 ± 0.05 ml in the PNC group compared to 0.03 ± 0.01 ml in control animals (unpaired t-test, p<0.05). There was no statistical difference between the groups for bladder capacity (0.28 ± 0.04 and 0.29 ± 0.05 mL in control and PNC groups, respectively) and CD (Table 1).
Table 1.
Statistical analysis of voiding contractions induced by saline infusion and L3/SCS in the control and PNC rats.
| Cystometry | EUS EMG | |||||||
|---|---|---|---|---|---|---|---|---|
| IVPmax (cm H2O) | CD (sec) | RV (mL) | VE (%) | Tonic Amplitude (mV) | Tonic AUC (mV-sec) | Bursting Amplitude (mV) | Bursting AUC (mV-sec) | |
| Saline infusion | ||||||||
| controls (n=6) | 45 ± 4 | 26 ± 2 | 0.03 ± 0.01 | 78 ± 4 | 0.104 ± 0.012 | 0.094 ± 0.019 | 0.174 ± 0.027 | 0.106 ± 0.024 |
| PNC (n=6) | 30 ± 3* | 28 ± 3 | 0.18 ± 0.05* | 54 ± 5* | 0.151 ± 0.031 | 0.182 ± 0.029* | 0.192 ± 0.047 | 0.171 ± 0.059 |
| L3/SCS | ||||||||
| controls (n=6) | 30 ± 4 | 24 ± 1 | 0.03 ± 0.01 | 77 ± 4 | 0.117 ± 0.031 | 0.108 ± 0.057 | 0.191 ± 0.047 | 0.082 ± 0.029 |
| PNC (n=6) | 24 ± 1 | 27 ± 3 | 0.04 ± 0.01† | 74 ± 7† | 0.077 ± 0.017 | 0.088 ± 0.024† | 0.145 ± 0.037 | 0.105 ± 0.021† |
PNC: pelvic nerve crush. EUS: external urethral sphincter. EMG: electromyography. IVPmax: maximum intravesical pressure. RV: residual volume after voiding. CD: contraction duration. VE: voiding efficiency. AUC: area under the curve.
significant changes (p < 0.05) induced by saline infusion compared to controls.
significant changes (p < 0.05) between PNC rats with and without L3/SCS.
Figure 2.
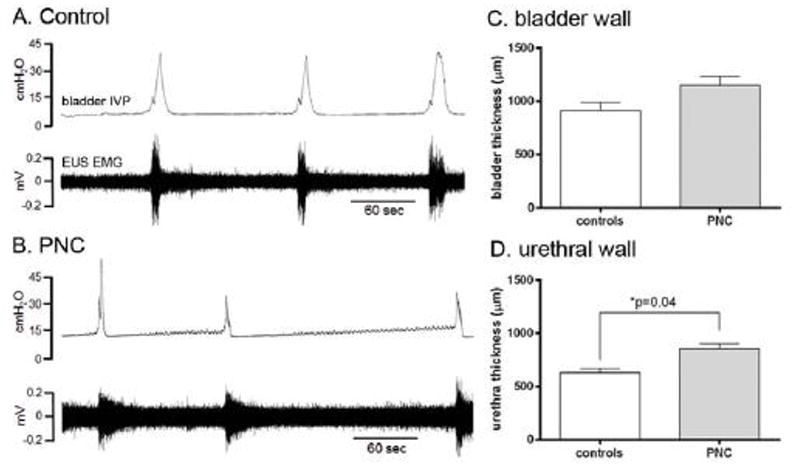
Urodynamic recordings and morphological bladder and urethral changes in control and PNC rats. Note that voiding contractions are associated with coordinated EUS EMG activity in both the control and PNC injury series (A, B). The PNC injury resulted in decreased amplitude of voiding contractions (B). The bladder wall thickness measurements showed a statistical trend for a possible increase (unpaired t-test, p=0.06; n=6) after the PNC injury (C), whereas the urethral thickness was significantly increased (unpaired t-test, p=0.04; n=6) after the PNC injury (D).
EUS EMG studies showed significantly increased tonic activity (AUC: 0.182 ± 0.029 mV-sec in the PNC group compared to corresponding measurement of 0.094 ± 0.019 mV-sec in the control series (unpaired t-test, p<0.05)). There was no statistical difference for the maximum tonic and bursting amplitudes or AUC for bursting period between groups (Table 1).
The wall thickness of the bladder and urethra was histologically examined by H&E staining. The morphological evaluation of the urothelium, lamina propria, and smooth muscle did not show any statistical difference between the control and injury groups for the individual layers. Therefore, the entire wall thickness of bladder and urethra was measured as a single measurement. The thickness of the bladder wall was 1150 ± 85 μm at 14 days after the bilateral PNC injury and 910 ± 81 μm in the control group, and statistical analysis showed a statistical trend for a possible injury-induced increase in thickness (unpaired t-test, p=0.06; Figure 2C). The urethral wall thickness was 857 ± 40 μm after the PNC injury and significantly increased compared to corresponding measurements of 631 ± 39 μm in the control group (unpaired t-test, p=0.04; Figure 2D).
Neuromodulation of LUT function using SCS at the L3 segment after PNC injury
To determine the effectiveness of SCS in improving LUT function after PNC injury, SCS was combined with urodynamic studies of reflex micturition in control rats (n=6; Figure 3A) and rats with a bilateral PNC injury (n=6; Figure 3B). The SCS was administered over the L3 segment of the spinal cord, as described in above mapping studies, and included EUS EMG recordings. The studies showed that electrical stimulation at the L3 segment inhibited EUS tonic activity followed by the onset of EUS phasic activity.
Figure 3.
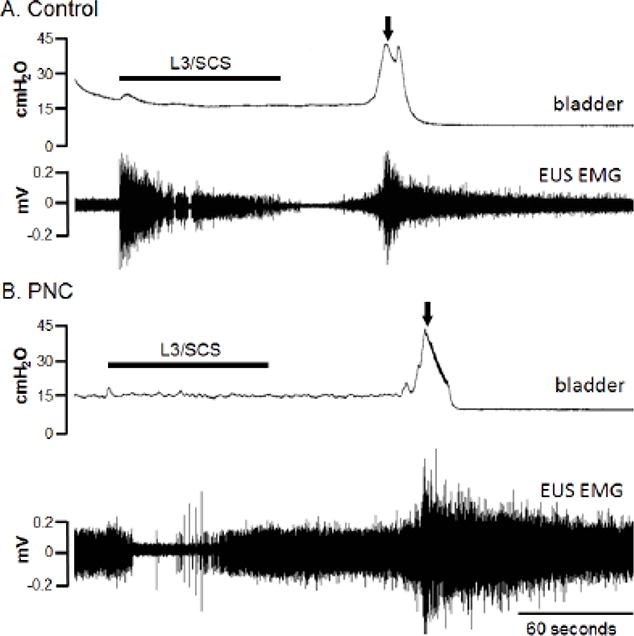
L3/SCS evoked voiding contractions in control and PNC rats. The control rat (A) received 55% of the bladder capacity by saline infusion at the rate of 0.1 mL/min. After stopping saline infusion, L3/SCS (40 Hz, 0.2 ms pulse width, 3.2 volts) was given for 82 s. The evoked voiding contractions happened at the latency of 48 s after the end of stimulation. The EUS EMG activity was suppressed before reaching the voiding threshold. In the PNC rat (B), the infusion volume was 72% of the bladder capacity. L3/SCS (40 Hz, 0.2 ms pulse width, 2.8 volts) was given for 84 s. The evoked voiding contractions happened at the latency of 60 s after the end of stimulation. The EUS EMG activity was suppressed during L3/SCS. The black thick bars indicate the duration of L3/SCS. Arrows indicate when the voiding occurred.
In control rats, the bladder was first infused with saline to 60 ± 9 % of calculated bladder capacity. At this partial bladder filling level, spontaneous EUS tonic activity was observed in all animals. After stopping the infusion, L3/SCS was given for 60-90 seconds. A bladder contraction and voiding occurred after a latency of 79 ±10 seconds after the start of electrical stimulation. Voiding contractions taking place over 90 seconds after the end of SCS were excluded for data analysis. Urodynamic parameters including VE, RV, and CD as well as EUS EMG amplitude and AUC measurements during the tonic and bursting phases of voiding contractions showed no statistical difference between the L3/SCS group and the control group without stimulation (Table 1).
In rats with bilateral PNC injury the bladder was infused with saline to 86 ± 7 % of the calculated bladder capacity and L3/SCS was given for 60-90 seconds. The L3/SCS-evoked detrusor contraction and voiding started at 68 ± 12 seconds after the onset of the electrical stimulation. There was no difference in the L3/SCS-to-voiding latency between the control and PNC injury groups. However, cystometry showed statistically improved VE of 74 ± 7 % and decreased RV of 0.04 ± 0.01 ml in the PNC group after L3/SCS compared to corresponding VE of 54 ± 5 % and RV of 0.18 ± 0.05 ml in the PNC only injury group (paired t-test, p<0.05). The CD in the PNC group was not different before and after L3/SCS. EUS EMG recordings showed statistically reduced tonic AUC of 0.088 ± 0.024 mv-sec and bursting AUC of 0.105 ± 0.021 mv- sec after L3/SCS in the PNC injury group compared to corresponding tonic AUC of 0.182 ± 0.029 mv-sec and bursting AUC of 0.171 ± 0.029 mv-sec in the PNC only injury group (paired t-test, p<0.05). The cystometry and EUS EMG outcome measures were not different between the L3/SCS treatment group after PNC injury and neurologically intact control animals. The IVPmax was not comparable between L3/SCS groups and experimental groups without stimulation, as the bladders were partially filled by saline before applying the stimulation as opposed to full capacity needed for physiologic voiding contractions.
DISCUSSION
The present study investigated the effects of SCS applied in the epidural space over individual lumbar spinal cord segments in female rats. Combined cystometry and EUS EMG recordings were performed. Initial functional mapping studies suggested that the EUS spinal relaxation center is located at the L2-3 spinal cord levels. Next, comprehensive urodynamic studies showed that a bilateral PNC crush injury resulted in an underactive bladder-like condition, which was significantly improved by SCS over the L3 spinal cord segment.
Neural control of micturition is complex and requires coordination between bladder and EUS functions (de Groat et al., 2015; Fowler et al., 2008). The LUT is innervated by both autonomic and somatic motoneurons with preganglionic parasympathetic neurons and EUS-innervating motoneurons residing within the L6 and S1 segments of the rat spinal cord (Hoang et al., 2003; Schroder, 1980). It is generally believed that these groups of spinal neurons receive input from the pontine micturition center (PMC), which is primarily responsible for coordinating the activity of the urinary bladder and urethral outlet during bladder filling and voiding (de Groat, 1998; de Groat and Yoshimura, 2015). However, our previous studies ((Abud et al., 2015; Chang et al., 2006, 2007)) revealed that the EUS bursting pattern generator is located in the upper lumbar spinal cord and is important for the generation of alternating periods of EUS intermittent relaxation and contractions, i.e., EUS bursting, an essential feature for efficient voiding in rodents (Langdale and Grill, 2016; Maggi et al., 1986).
Here, SCS was applied at each individual segment of the lumbar spinal cord to examine its effect on EUS EMG activity. The responses varied between segmental levels. L3/SCS consistently evoked EUS relaxation followed by a phasic activation response, thereby mimicking the specific voiding reflex in rodents (i.e. EUS bursting consisting of intermittent relaxation and phasic activation). L3/SCS-induced EUS bursting presented urethral relaxation. We conclude that the L3 spinal segment includes the interneurons and neuronal networks for controlling EUS relaxation in rats. In contrast, SCS over the L1, and L4-6 spinal cord segments resulted in EUS tonic contractions, which may instead increase the urethral resistance. Unlike L3/SCS-evoked EUS relaxation, L6/SCS augmented EUS tonic contractions in rats of the control group, a response similar to the EUS guarding reflex during the storage phase (D’Amico and Collins, 2012; D’Amico et al., 2011; de Groat and Yoshimura, 2015). Thus, SCS applied to L1, L4-L6 may provide an opportunity to increase urethral resistance during periods of increased bladder pressure to promote continence. The largest non-voiding contraction was evoked by L6/SCS and associated with the larger EUS tonic activity. This pattern of activation resembles detrusor-sphincter dyssynergia, and cannot produce efficient voiding.
Previous studies have characterized several electrophysiological properties of L3/SCS-evoked EUS phasic activation, including the optimal stimulation frequencies and pulse width as well as the peripheral pathways critical for EUS activation in spinally intact and SCI rats (Abud et al., 2015). The present study expanded the scope of our earlier investigations and showed the effects of functional modulation of micturition reflexes using an L3/SCS strategy in both control rats and rats with a bilateral PNC injury. There were no significant differences detected by cystometry and EUS EMG recordings between physiological voiding and L3/SCS-induced voiding in control rats. These findings suggest that micturition reflexes were not compromised by the SCS stimulation. In addition, the recordings in intact rats suggest the presence of a ceiling effect, and that improvement of LUT function beyond its normal physiological state may be challenging. Previous studies using electrical stimulation to elicit micturition reflexes or the pelvic-EUS reflex relied on partial bladder filling and distension as an additional sensory stimulus in cats and rats (Chang et al., 2007; Tai et al., 2004). The present study also performed a partial filling of the bladder prior to L3/SCS, and our findings are in agreement with prior reports suggesting that afferent input from the distended bladder has an important role for triggering micturition reflexes and voiding.
Preganglionic parasympathetic neurons innervate the major pelvic ganglia (MPG), and injuries to their peripheral axons may cause a neurogenic bladder condition in a variety of experimental models. A bilateral ventral root avulsion (VRA) injury at lumbosacral segmental levels followed by surgical root replantation resulted in an underactive bladder-like in rats (Chang and Havton, 2008; Hoang et al., 2006). A unilateral VRA injury at lumbosacral segments in rats also resulted in an underactive bladder with decreased bladder contractility and voiding efficiency (Chang and Havton, 2012, 2016). In the present study, the demonstration of decreased IVPmax, decreased VE, and increased PVR support the development of an underactive bladder also after a bilateral PNC injury. It is possible that the initial denervation of the MPG by the PNC injury may have been partially reversed from axonal regeneration by parasympathetic preganglionic fibers across the crush injury site. However, the MPG is innervated by both the pelvic and hypogastric nerves, and parasympathetic fibers have demonstrated sprouting in the pelvic ganglion after a hypogastric nerve injury (Keast, 2004). Therefore, it seems possible that sympathetic preganglionic fibers may undergo sprouting within the MPG and contribute to the LUT functional phenotype after a bilateral PNC injury. In future studies, we will assess nerve sprouting in the MPG, bladder and urethral wall in this model to correlate the morphological changes with functional assessments.
PNC injuries may be iatrogenic due to abdominal and pelvic surgery such as abdominoperineal resection, radical hysterectomy, and pelvic organ surgery (Miyazato et al., 2013; Tyagi et al., 2014). These injuries may lead to a neurogenic underactive bladder. The present animal model mimics the symptoms of the neurogenic underactive bladder. In the PNC injury series, the use of L3/SCS to activate micturition reflexes in the presence of a partially filled bladder in rats resulted in an improved VE and RV as well as improved tonic and bursting AUC measurements. In the presence of L3/SCS, the urodynamic recordings from PNC rats were similar to those obtained in neurologically intact rats. The filled bladder capacity (86%) of the PNC group was more than the capacity filled in the controls (60%) in order to induce the voiding contractions by L3/SCS. PNC-induced injury resulted in the damages to bladder afferent and efferent pathways. More mechanical distension with an increase of filled bladder capacity may be needed to evoke a voiding contraction combined with L3/SCS in the PNC group. These studies suggest that inhibition of the urethral guarding reflex by L3/SCS improves LUT function, in part by increasing VE and decreasing RV, in this neurogenic underactive bladder model.
In summary, functional mapping of the lumbar spinal cord in rats demonstrated that L3/SCS promoted urethral relaxation and could be the key feature for efficient voiding. SCS to the L1 and L4-6 segments resulted in tonic EUS contractions and showed the potentials of treating urinary incontinence. Bilateral PNC injury resulted in an underactive bladder, which was reversed to control levels by L3/SCS.
Highlights.
Spinal cord stimulation over the third lumbar segment produces urethral relaxation.
Prolonged urethral relaxation during detrusor underactivity promotes better voiding efficiency.
Acknowledgments
The authors thank Jackie Mao for excellent technical assistance and Dr. F. Aura Kullmann at University of Pittsburgh for critical and helpful comments on this manuscript. Histological services were provided by the Cell and Tissue Imaging Core of the USC Research Center for Liver Diseases (P30 DK048522).
Grants
The present study was supported by NIH NIDDK (R01 DK106181) to HH Chang, and by the University of California through a continuation of the Roman Reed Spinal Cord Injury Research Fund of California. LA Havton received support from the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abud EM, Ichiyama RM, Havton LA, Chang HH. Spinal stimulation of the upper lumbar spinal cord modulates urethral sphincter activity in rats after spinal cord injury. American journal of physiology. Renal physiology. 2015;308:F1032–1040. doi: 10.1152/ajprenal.00573.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HH, Havton LA. Modulation of the visceromotor reflex by a lumbosacral ventral root avulsion injury and repair in rats. American journal of physiology. Renal physiology. 2012;303:F641–647. doi: 10.1152/ajprenal.00094.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HH, Havton LA. A ventral root avulsion injury model for neurogenic underactive bladder studies. Experimental neurology. 2016;285:190–196. doi: 10.1016/j.expneurol.2016.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Cheng CL, Chen JJ, de Groat WC. Roles of glutamatergic and serotonergic mechanisms in reflex control of the external urethral sphincter in urethane-anesthetized female rats. American journal of physiology. Regulatory, integrative and comparative physiology. 2006;291:R224–234. doi: 10.1152/ajpregu.00780.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Cheng CL, Chen JJ, de Groat WC. Serotonergic drugs and spinal cord transections indicate that different spinal circuits are involved in external urethral sphincter activity in rats. American journal of physiology. Renal physiology. 2007;292:F1044–1053. doi: 10.1152/ajprenal.00175.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Havton LA. Surgical implantation of avulsed lumbosacral ventral roots promotes restoration of bladder morphology in rats. Experimental neurology. 2008;214:117–124. doi: 10.1016/j.expneurol.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Havton LA. Anatomical tracer injections into the lower urinary tract may compromise cystometry and external urethral sphincter electromyography in female rats. Neuroscience. 2010;166:212–219. doi: 10.1016/j.neuroscience.2009.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CL, de Groat WC. The role of capsaicin-sensitive afferent fibers in the lower urinary tract dysfunction induced by chronic spinal cord injury in rats. Experimental neurology. 2004;187:445–454. doi: 10.1016/j.expneurol.2004.02.014. [DOI] [PubMed] [Google Scholar]
- D’Amico SC, Collins WF., 3rd External urethral sphincter motor unit recruitment patterns during micturition in the spinally intact and transected adult rat. J Neurophysiol. 2012;108:2554–2567. doi: 10.1152/jn.00927.2011. [DOI] [PubMed] [Google Scholar]
- D’Amico SC, Schuster IP, Collins WF., 3rd Quantification of external urethral sphincter and bladder activity during micturition in the intact and spinally transected adult rat. Experimental neurology. 2011;228:59–68. doi: 10.1016/j.expneurol.2010.12.008. [DOI] [PubMed] [Google Scholar]
- de Groat WC. Anatomy of the central neural pathways controlling the lower urinary tract. Eur Urol. 1998;34:2–5. doi: 10.1159/000052265. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Griffiths D, Yoshimura N. Neural control of the lower urinary tract. Comprehensive Physiology. 2015;5:327–396. doi: 10.1002/cphy.c130056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Yoshimura N. Anatomy and physiology of the lower urinary tract. Handb Clin Neurol. 2015;130:61–108. doi: 10.1016/B978-0-444-63247-0.00005-5. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nature reviews. Neuroscience. 2008;9:453–466. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, Ferreira C, Willhite A, Rejc E, Grossman RG, Edgerton VR. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet. 2011;377:1938–1947. doi: 10.1016/S0140-6736(11)60547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang TX, Nieto JH, Tillakaratne NJ, Havton LA. Autonomic and motor neuron death is progressive and parallel in a lumbosacral ventral root avulsion model of cauda equina injury. J Comp Neurol. 2003;467:477–486. doi: 10.1002/cne.10928. [DOI] [PubMed] [Google Scholar]
- Hoang TX, Pikov V, Havton LA. Functional reinnervation of the rat lower urinary tract after cauda equina injury and repair. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:8672–8679. doi: 10.1523/JNEUROSCI.1259-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama RM, Gerasimenko Y, Jindrich DL, Zhong H, Roy RR, Edgerton VR. Dose dependence of the 5-HT agonist quipazine in facilitating spinal stepping in the rat with epidural stimulation. Neuroscience letters. 2008;438:281–285. doi: 10.1016/j.neulet.2008.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama RM, Gerasimenko YP, Zhong H, Roy RR, Edgerton VR. Hindlimb stepping movements in complete spinal rats induced by epidural spinal cord stimulation. Neuroscience letters. 2005;383:339–344. doi: 10.1016/j.neulet.2005.04.049. [DOI] [PubMed] [Google Scholar]
- Keast JR. Remodelling of connections in pelvic ganglia after hypogastric nerve crush. Neuroscience. 2004;126:405–414. doi: 10.1016/j.neuroscience.2004.03.059. [DOI] [PubMed] [Google Scholar]
- Kruse MN, de Groat WC. Spinal pathways mediate coordinated bladder/urethral sphincter activity during reflex micturition in decerebrate and spinalized neonatal rats. Neuroscience letters. 1993;152:141–144. doi: 10.1016/0304-3940(93)90503-d. [DOI] [PubMed] [Google Scholar]
- Langdale CL, Grill WM. Phasic activation of the external urethral sphincter increases voiding efficiency in the rat and the cat. Experimental neurology. 2016;285:173–181. doi: 10.1016/j.expneurol.2016.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi CA, Giuliani S, Santicioli P, Meli A. Analysis of factors involved in determining urinary bladder voiding cycle in urethan-anesthetized rats. The American journal of physiology. 1986;251:R250–257. doi: 10.1152/ajpregu.1986.251.2.R250. [DOI] [PubMed] [Google Scholar]
- Marson L. Identification of central nervous system neurons that innervate the bladder body, bladder base, or external urethral sphincter of female rats: a transneuronal tracing study using pseudorabies virus. J Comp Neurol. 1997;389:584–602. [PubMed] [Google Scholar]
- McKenna KE, Chung SK, McVary KT. A model for the study of sexual function in anesthetized male and female rats. The American journal of physiology. 1991;261:R1276–1285. doi: 10.1152/ajpregu.1991.261.5.R1276. [DOI] [PubMed] [Google Scholar]
- Miyazato M, Yoshimura N, Chancellor MB. The other bladder syndrome: underactive bladder. Rev Urol. 2013;15:11–22. [PMC free article] [PubMed] [Google Scholar]
- Pettigrew RI, Heetderks WJ, Kelley CA, Peng GC, Krosnick SH, Jakeman LB, Egan KD, Marge M. Epidural Spinal Stimulation to Improve Bladder, Bowel, and Sexual Function in Individuals With Spinal Cord Injuries: A Framework for Clinical Research. IEEE Trans Biomed Eng. 2017;64:253–262. doi: 10.1109/TBME.2016.2637301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder HD. Organization of the motoneurons innervating the pelvic muscles of the male rat. J Comp Neurol. 1980;192:567–587. doi: 10.1002/cne.901920313. [DOI] [PubMed] [Google Scholar]
- Tai C, Roppolo JR, de Groat WC. Block of external urethral sphincter contraction by high frequency electrical stimulation of pudendal nerve. The Journal of urology. 2004;172:2069–2072. doi: 10.1097/01.ju.0000140709.71932.f0. [DOI] [PubMed] [Google Scholar]
- Tyagi P, Smith PP, Kuchel GA, de Groat WC, Birder LA, Chermansky CJ, Adam RM, Tse V, Chancellor MB, Yoshimura N. Pathophysiology and animal modeling of underactive bladder. Int Urol Nephrol. 2014;46(Suppl 1):S11–21. doi: 10.1007/s11255-014-0808-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Alwaal A, Lin G, Li H, Zaid UB, Wang G, Wang L, Banie L, Ning H, Lin CS, Guo Y, Zhou L, Lue TF. Urethral musculature and innervation in the female rat. Neurourol Urodyn. 2016;35:382–389. doi: 10.1002/nau.22722. [DOI] [PMC free article] [PubMed] [Google Scholar]


