Abstract
Research of the human brain metabolism in vivo has largely focused on total glucose use (via FDG PET) and, until recently, did not examine the use of glucose outside oxidative phosphorylation which is known as aerobic glycolysis (AG). AG supports important functions including biosynthesis and neuroprotection but decreases dramatically with aging. This multi-tracer PET study evaluated the relationship between AG, total glucose use (CMRGlc), oxygen metabolism (CMRO2), tau and amyloid deposition in 42 individuals, including those at preclinical and symptomatic stages of Alzheimer disease (AD). Our findings demonstrate that in individuals with amyloid burden, lower AG is associated with higher tau deposition. No such correlation was observed for CMRGlc or CMRO2. We suggest that aging related loss of AG leading to decreased synaptic plasticity and neuroprotection may accelerate tauopathy in individuals with amyloid burden. Longitudinal AG and AD pathology studies are needed to verify causality.
Keywords: Alzheimer disease, Aging, Positron emission tomography, Amyloid imaging, Tau imaging, Brain aerobic glycolysis, Cerebral metabolic rate of glucose, Cerebral metabolic rate of oxygen
INTRODUCTION
Alzheimer disease (AD) is characterized by a long (~20 years) preclinical period during which pathology accumulates in the absence of overt clinical symptoms (Price et al., 2009). The neuropathological hallmarks of AD are amyloid-β (Aβ) plaques and neurofibrillary tangles, which primarily are composed of hyperphosphorylated tau protein. Disruptions in the balance of Aβ production and clearance are considered to be initiating events in a biological cascade that leads to Aβ plaque formation and neurodegenerative tau pathology (Jack et al., 2013). Metabolic dysfunction is another pathological feature that occurs early in the disease in humans (Langbaum et al., 2009).
Research on glucose metabolism in the brain has focused on total glucose use (CMRGlc) as measured by fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) and, until recently (Vlassenko et al., 2010), did not examine the use of glucose outside of oxidative phosphorylation, known as aerobic glycolysis (AG). AG, a highly investigated feature of cancer metabolism (Lunt and Vander Heiden, 2011; Vlassenko et al., 2015), in the healthy brain may support metabolic functions which includes biosynthesis of glycogen, proteins, lipids and nucleic acids; neuroprotection by managing reactive oxygen species and apoptosis; production of lactate, a potential fuel and signaling molecule (Suzuki et al., 2011); and fast local generation of energy for membrane pumps (Vlassenko and Raichle, 2015). Recent studies indicate that AG supports synaptic and neurite formation and turnover (Goyal et al., 2014; Goyal et al., 2017; Shannon et al., 2016). However, total glucose use (CMRGlc) is largely driven by oxidative metabolism for synaptic activity and therefore may mask independent information provided by AG.
PET studies with multiple tracers allow measurements of AG, glucose (CMRGlc) and oxygen (CMRO2) metabolism in the same individual, and may deliver various important details otherwise missed by conventional 18F-FDG PET and MRI (Vlassenko et al., 2015). PET also affords in vivo measurements of Aβ and tau deposition, which, with quantitative estimates of CMRGlc, CMRO2 and AG, allows more explicit evaluation of the pathological progression in preclinical and symptomatic stages of AD. Here, we present preliminary findings with PET on the relationship between metabolism, including AG, and tau pathology. Specifically, we evaluate this relationship in a set of brain areas vulnerable to tau burden during preclinical AD.
MATERIALS AND METHODS
Participants
A total of 42 individuals (21 men) aged 53–88 years were recruited from the Washington University Knight Alzheimer Disease Research Center (ADRC). Forty participants were cognitively normal. Two participants demonstrated very mild dementia due to AD at the time of the study. The 2 symptomatic individuals were newly diagnosed with dementia (~1 year) but had demonstrated brain Aβ plaque burden for ~7 years.
All assessments and imaging procedures were approved by Human Research Protection Office and Radioactive Drug Research Committee at Washington University in St. Louis. Written consent was provided from each participant.
MRI
MRI scans were obtained on Biograph mMR or Trio 3T (Siemens, Erlangen, Germany) scanner using a 3D sagittal T1-weighted magnetization-prepared rapid gradient-echo (MPRAGE) sequence with 1 mm isotropic resolution.
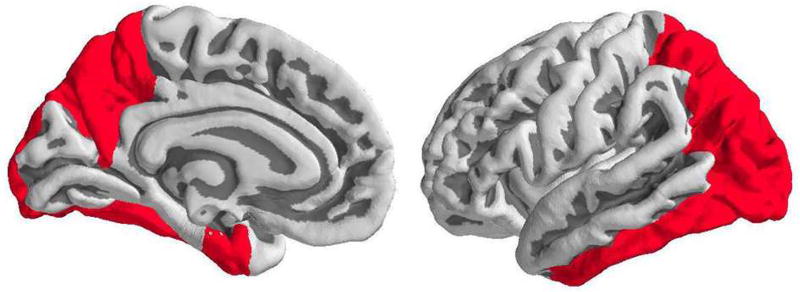
FreeSurfer 5.3 (http://freesurfer.net) was used to segment the MRI into cortical and subcortical regions of interest (ROIs)(Su et al., 2013). These ROIs were used for regional estimation of all PET measures, including those for brain metabolism, tau and amyloid deposition. An average for each of PET measures was defined for a composite ROI that combines regions known to accumulate high levels of tau, which include bilateral entorhinal, amygdala, precuneus, inferior temporal, inferior and superior parietal, fusiform and lateral occipital cortex (Gordon et al., 2016; Johnson et al., 2016; Scholl et al., 2016) (Figure 1).
Figure 1.
Region of interest combining FreeSurfer tau-prone regions, which includes precuneus, amygdala (not shown), entorhinal, inferior temporal, inferior and superior parietal, fusiform and lateral occipital cortex.
Partial volume correction (PVC) of PET data was performed for all PET measures using the regional spread function approach (Su et al., 2015).
AG, CMRGlc and CMRO2 PET imaging
In all individuals, 18F-FDG and 15O PET scans were performed on a Siemens model 962 ECAT EXACT HR+ PET scanner as described previously (Vaishnavi et al., 2010; Vlassenko et al., 2010). All subjects underwent one FDG scan and two sets of 15O-CO, 15O-H2O, and 15O-O2 scans.
FDG scans were performed after injection of ~5 mCi of FDG. Cerebral blood volume (CBV) was measured with a 5-min emission scan beginning 2 min after brief inhalation of ~75 mCi of [15O]CO in room air. Dynamic scans of 3 min were acquired after injection of ~50 mCi [15O]H2O in saline or inhalation of 60 mCi of [15O]O2 in room air. The CMRO2 parametric image was derived from these 15O scans and corrected for CBV.
The local-to-global images obtained as described above for CMRGlc, CMRO2, and CBF were summarized to the FreeSurfer ROIs. These were then multiplied by age-specific literature-based whole brain estimates for each of the metabolic parameters (Goyal et al., 2014; Goyal et al., 2017).
AG was defined by subtracting the oxidative fraction of CMRGlc, calculated by dividing molar CMRO2 by six, from total molar CMRGl (Goyal et al., 2017):
Tau PET Imaging
Tau PET imaging was performed using 18F-AV-1451. Participants received a bolus injection of between 7.2 and 10.8 mCi of AV-1451. Data were acquired on a Biograph 40 PET/CT scanner and converted to standardized uptake value ratios (SUVRs) using a cerebellar cortex reference.
Amyloid PET imaging
Amyloid-β PET imaging was performed using either florbetapir (n = 35) or 11C-Pittsburgh compound B (PIB, n = 7) on a Siemens Biograph mMR. For PIB and florbetapir scans, data were converted to SUVRs using a cerebellar cortex reference. A PIB mean cortical binding potential of 0.18 has been used to denote amyloid positivity (Mintun et al., 2006; Vlassenko et al., 2016), and PVC equivalent PIB SUVR (1.42) and florbetapir SUVR (1.22) was used (Gordon et al., 2016). Using these cutoffs the individuals were classified as either amyloid-positive (Aβ+, n=13) or amyloid-negative (Aβ−, n=29).
Statistical analysis
Demographic parameters and PET measures were evaluated with Student t tests or Chi-Square test as appropriate. Stratifying our cohort into Aβ+ and Aβ− groups, we used a linear regression model, adjusted for age, with metabolic parameters (AG, CMRGlc, CMRO2) predicting tau deposition. All analyses were performed using IBM SPSS Statistics v. 24 (IBM Corp., Armonk, NY). Probability values < 0.05 indicated statistical significance.
RESULTS
Participant demographics and PET results for all 42 participants are shown in the Table 1. The Aβ+ (n=13) group was older (t=2.612, p=0.013) and exhibited significantly higher tau deposition (t=3.246, p=0.006).
Table 1.
Demographics and PET data.
| Characteristic | Amyloid- positive |
Amyloid- negative |
P-value |
|---|---|---|---|
| No. | 13 | 29 | |
| Age, yrs (±SD) | 74.1 ± 7.8 | 67.4 ± 7.5 | 0.013 |
| Gender, M, % | 53.8 | 48.3 | 0.739 |
| APOE ε4, positive, % | 46.1 | 10.3 | 0.009 |
| MMSE (±SD) | 29.6 ± 0.7 | 29.2 ± 1.4 | 0.173 |
| Symptomatic AD, No. | 2 | 0 | |
| Interval, AG and tau PET, months (±SD) | 7.6 ± 11.7 | 11.3 ± 9.0 | 0.268 |
| Interval, AG and amyloid PET, months (±SD) | 4.6 ± 12.1 | 11.8 ± 11.7 | 0.072 |
| AG, µmol/100g/min | 2.5 ± 1.8 | 3.0 ± 1.2 | 0.341 |
| CMRGlc, µmol/100g/min | 34.3 ± 1.7 | 35.1 ± 1.6 | 0.192 |
| CMRO2, µmol/100g/min | 198.0 ± 7.3 | 203.0 ± 8.2 | 0.058 |
| Tau (AV-1451), SUVR | 1.4 ± 0.2 | 1.1 ± 0.1 | 0.006 |
| Amyloid (florbetapir), SUVR (No.) | 1.6 ± 0.3 (12) | 0.9 ± 0.1 (23) | <0.0001 |
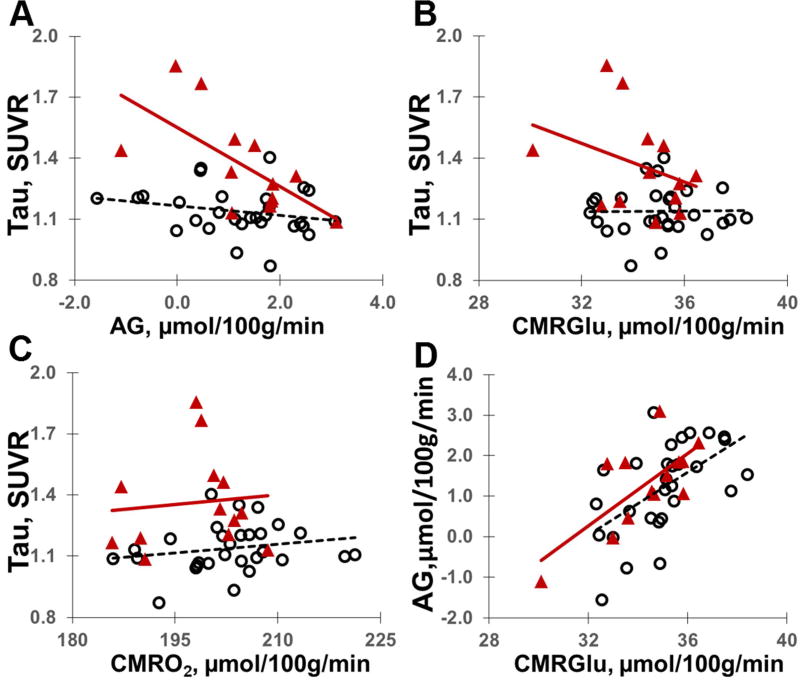
There was a significant interaction with Aβ status in the entire cohort, such that the relationship between AG and tau deposition was different between Aβ+ (n=13) and Aβ− (n=29) individuals (F1,37=7.062, p=0.012; Figure 2). Aβ+ and Aβ− individuals did not differ in the relationship between tau deposition and CMRGlc (F1,37=2.047, p=0.161) and CMRO2 (F1,37=0.146, p=0.704). In Aβ+ individuals (n=13), there was a significant relationship between tau deposition and AG (F1,10=6.402, p=0.030), but not CMRGlc (F1,10=0.615, p=0,.451) nor CMRO2 (F1,10=1.892, p=0.199) (Figure 2). No significant correlation between any of the three metabolic parameters and tau deposition was demonstrated in the Aβ− group.
Figure 2.
Relationship between AG (A), CMRGlc (B) and CMRO2 (C) and tau deposition defined for composite ROI (see Figure 1) in Aβ− (opened circles; n=29) and Aβ+ (triangles; n=13) individuals. Of note, whereas AG correlates with CMRGlu (D) because it is derived from total glucose consumption measurements, CMRGlu does not perfectly predict AG; thus, as shown in our study, AG may provide independent information on the relationship between the levels of AD biomarkers and brain physiology. There is no correlation between AG and CMRO2 (not shown).
When restricting analyses to the subset with florbetapir (n=35) to examine continuous levels of Aβ, no significant differences were found between Aβ+ and Aβ− groups in the relationship between AG, CMRGlc or CMRO2, and Aβ deposition; and no significant association was found between amyloid burden and metabolic PET measures in any group.
Similar findings were demonstrated after excluding the two symptomatic AD individuals from the analysis. There was a significant interaction between Aβ status and the relationship between AG (but not CMRGlc nor CMRO2) and tau deposition (F1,35=7.135, p=0.011). Preclinical AD (Aβ+ cognitively normal) individuals (n=11) demonstrated significant relationship between AG (F1,8=6.799, p=0.031) but not CMRGlc (F1,8=0.073, p=0.794) nor CMRO2 (F1,8=1.913, p=0.204) and tau deposition. After excluding the symptomatic individuals there were still no significant correlation between florbetapir uptake and AG, CMRGlc or CMRO2 in any group, and no significant differences were found between Aβ+ and Aβ− groups in these relationships.
DISCUSSION
Our findings demonstrate that in preclinical and very mildly symptomatic AD individuals, lower AG is associated with higher tau deposition in vulnerable regions of the brain. This finding is unaffected by adjusting for age (which itself correlates with AD pathology and lower AG) and by removing the 2 symptomatic AD individuals in our cohort. No such relationship was observed for CMRGlc nor CMRO2. While the relationship between tau and metabolism may be specific to AG in this cohort, our modest sample size may be insensitive to more subtle relationships between tau and CMRGlc or CMRO2. Further research in a larger cohort is needed to explore these relationships and to pursue more detailed regional analysis.
With aging, AG decreases dramatically (especially in functionally important areas, including those with high accumulation of Aβ and where AG is the highest in young adulthood) presumably resulting in loss of the biosynthetic and neuroprotective functions AG normally supports (Goyal et al., 2017). We believe that ongoing neuronal activity in the aging brain, deprived of proper AG-related synaptic maintenance and protection, might in part be responsible for the acceleration of neurodegeneration including tau pathology. However, it should be noted that aging-related decrease in AG can occur unaccompanied by AD pathology suggesting that other, presently unknown, elements may also be playing a role in the causal chain of events.
The relationship between AG and tau deposition in the presence of Aβ burden warrants more research in AD as well as in other primary tauopathies (not associated with accumulation of Aβ plaques). Our findings highlight the importance of measuring AG, as this relationship was not evident for total glucose use (CMRGlc) alone. One possible explanation for the relationship between AG and tau deposition is that tau, among its various toxic effects (Shi et al., 2017), might impair processes that demand AG, such as synaptic plasticity. Alternatively, AG loss could potentially accelerate tauopathy. Our findings suggest that longitudinal studies of AG and AD pathology will help to further define this relationship.
HIGHLIGHTS.
Aerobic glycolysis delivers important information missed by conventional FDG study
Aging related loss of aerobic glycolysis may accelerate AD pathology
Low aerobic glycolysis is associated with high tau deposition in preclinical AD
Acknowledgments
This work was supported by National Institutes of Health [P50 AG05681, P01 AG026276, P01 AG03991, R01 AG043434, R01 AG053503, R01 AG057536, UL1 TR000448, P30 NS098577, R01 EB009352]; the Dana Foundation, the Hope Center for Neurological Disorders, the Foundation for the American Society of Neuroradiology, Foundation for Barnes-Jewish Hospital, and the Charles and Joanne Knight Alzheimer Disease Initiative. Avid Radiopharmaceuticals (a wholly owned subsidiary of Eli Lilly) provided the technology transfer for production of AV-1451 and also the precursor.
We thank the Administration, Clinical, Biomarker, Imaging and Biostatistics Cores of the Knight ADRC; and our research participants and their families for their altruism.
TLSB receives research support from grants from NIH, and from Eli Lilly, Avid Radiopharmaceuticals, and Eli Lilly, Avid, Roche, Johnson & Johnson, and Biogen (clinical trials), outside the submitted work.
JCM is currently participating in clinical trials of antidementia drugs from Eli Lilly and Company, Biogen, and Janssen. JCM serves as a consultant for Lilly USA. He receives research support from Eli Lilly/Avid Radiopharmaceuticals and is funded by NIH grants # P50AG005681; P01AG003991; P01AG026276 and UF01AG032438.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
AGV, TLSB and MER were responsible for concept and design of the study, data acquisition and analysis, and drafting the manuscript and figures. BAG, MSG, YS, TMB, and JCM were responsible for data acquisition and drafting the manuscript and figures. TJD, LEC, JJC and HJ were responsible for data acquisition and analysis. All authors revised and approved the final version of the manuscript.
CONFLICTS OF INTEREST
AGV, BAG, MSG, YS, TMB, TJD, LEC, KMJ, HJ and MER have nothing to report.
All coauthors of this article have contributed significantly to and share in the responsibility for the release of all of the material contained within this article. They all have reviewed and approve the contents of the manuscript. The material submitted to the Neurobiology of Aging is new, original and has not been and is not under consideration elsewhere.
Human studies under-taken as part of the research, from which this manuscript was derived, are in compliance with regulations of our institution and with generally accepted guidelines governing such work.
References
- Gordon BA, Friedrichsen K, Brier M, Blazey T, Su Y, Christensen J, Aldea P, McConathy J, Holtzman DM, Cairns NJ, Morris JC, Fagan AM, Ances BM, Benzinger TL. The relationship between cerebrospinal fluid markers of Alzheimer pathology and positron emission tomography tau imaging. Brain. 2016;139(Pt 8):2249–2260. doi: 10.1093/brain/aww139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal MS, Hawrylycz M, Miller JA, Snyder AZ, Raichle ME. Aerobic glycolysis in the human brain is associated with development and neotenous gene expression. Cell metabolism. 2014;19(1):49–57. doi: 10.1016/j.cmet.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal MS, Vlassenko AG, Blazey TM, Su Y, Couture LE, Durbin TJ, Bateman RJ, Benzinger TL, Morris JC, Raichle ME. Loss of Brain Aerobic Glycolysis in Normal Human Aging. Cell metabolism. 2017;26(2):353–360. e353. doi: 10.1016/j.cmet.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Carrillo MC, Hendrix JA, Bain LJ, Catafau AM, Gault LM, Goedert M, Mandelkow E, Mandelkow EM, Miller DS, Ostrowitzki S, Polydoro M, Smith S, Wittmann M, Hutton M. Tau: From research to clinical development. Alzheimers Dement. 2016;12(10):1033–1039. doi: 10.1016/j.jalz.2016.03.018. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Schultz A, Betensky RA, Becker JA, Sepulcre J, Rentz D, Mormino E, Chhatwal J, Amariglio R, Papp K, Marshall G, Albers M, Mauro S, Pepin L, Alverio J, Judge K, Philiossaint M, Shoup T, Yokell D, Dickerson B, Gomez-Isla T, Hyman B, Vasdev N, Sperling R. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 2016;79(1):110–119. doi: 10.1002/ana.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langbaum JB, Chen K, Lee W, Reschke C, Bandy D, Fleisher AS, Alexander GE, Foster NL, Weiner MW, Koeppe RA, Jagust WJ, Reiman EM Alzheimer's Disease Neuroimaging, I. Categorical and correlational analyses of baseline fluorodeoxyglucose positron emission tomography images from the Alzheimer's Disease Neuroimaging Initiative (ADNI) Neuroimage. 2009;45(4):1107–1116. doi: 10.1016/j.neuroimage.2008.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annual review of cell and developmental biology. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Klunk WE, Mathis CA, DeKosky ST, Morris JC. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67(3):446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- Price JL, McKeel DW, Jr, Buckles VD, Roe CM, Xiong C, Grundman M, Hansen LA, Petersen RC, Parisi JE, Dickson DW, Smith CD, Davis DG, Schmitt FA, Markesbery WR, Kaye J, Kurlan R, Hulette C, Kurland BF, Higdon R, Kukull W, Morris JC. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging. 2009;30(7):1026–1036. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl M, Lockhart SN, Schonhaut DR, O'Neil JP, Janabi M, Ossenkoppele R, Baker SL, Vogel JW, Faria J, Schwimmer HD, Rabinovici GD, Jagust WJ. PET Imaging of Tau Deposition in the Aging Human Brain. Neuron. 2016;89(5):971–982. doi: 10.1016/j.neuron.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon BJ, Vaishnavi SN, Vlassenko AG, Shimony JS, Rutlin J, Raichle ME. Brain aerobic glycolysis and motor adaptation learning. Proc Natl Acad Sci U S A. 2016;113(26):E3782–3791. doi: 10.1073/pnas.1604977113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Yamada K, Liddelow SA, Smith ST, Zhao L, Luo W, Tsai RM, Spina S, Grinberg LT, Rojas JC, Gallardo G, Wang K, Roh J, Robinson G, Finn MB, Jiang H, Sullivan PM, Baufeld C, Wood MW, Sutphen C, McCue L, Xiong C, Del-Aguila JL, Morris JC, Cruchaga C, Alzheimer's Disease Neuroimaging, I. Fagan AM, Miller BL, Boxer AL, Seeley WW, Butovsky O, Barres BA, Paul SM, Holtzman DM. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature. 2017;549(7673):523–527. doi: 10.1038/nature24016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Blazey TM, Snyder AZ, Raichle ME, Marcus DS, Ances BM, Bateman RJ, Cairns NJ, Aldea P, Cash L, Christensen JJ, Friedrichsen K, Hornbeck RC, Farrar AM, Owen CJ, Mayeux R, Brickman AM, Klunk W, Price JC, Thompson PM, Ghetti B, Saykin AJ, Sperling RA, Johnson KA, Schofield PR, Buckles V, Morris JC, Benzinger TL Dominantly Inherited Alzheimer, N. Partial volume correction in quantitative amyloid imaging. Neuroimage. 2015;107:55–64. doi: 10.1016/j.neuroimage.2014.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, D'Angelo GM, Vlassenko AG, Zhou G, Snyder AZ, Marcus DS, Blazey TM, Christensen JJ, Vora S, Morris JC, Mintun MA, Benzinger TL. Quantitative analysis of PiB-PET with FreeSurfer ROIs. PLoS One. 2013;8(11):e73377. doi: 10.1371/journal.pone.0073377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144(5):810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnavi SN, Vlassenko AG, Rundle MM, Snyder AZ, Mintun MA, Raichle ME. Regional aerobic glycolysis in the human brain. Proc Natl Acad Sci U S A. 2010;107(41):17757–17762. doi: 10.1073/pnas.1010459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassenko AG, McConathy J, Couture LE, Su Y, Massoumzadeh P, Leeds HS, Chicoine MR, Tran DD, Huang J, Dahiya S, Marcus DS, Fouke SJ, Rich KM, Raichle ME, Benzinger TL. Aerobic Glycolysis as a Marker of Tumor Aggressiveness: Preliminary Data in High Grade Human Brain Tumors. Dis Markers. 2015;2015:874904. doi: 10.1155/2015/874904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassenko AG, McCue L, Jasielec MS, Su Y, Gordon BA, Xiong C, Holtzman DM, Benzinger TL, Morris JC, Fagan AM. Imaging and cerebrospinal fluid biomarkers in early preclinical alzheimer disease. Ann Neurol. 2016;80(3):379–387. doi: 10.1002/ana.24719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassenko AG, Raichle ME. Brain aerobic glycolysis functions and Alzheimer's disease. Clin Transl Imaging. 2015;3(1):27–37. doi: 10.1007/s40336-014-0094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassenko AG, Vaishnavi SN, Couture L, Sacco D, Shannon BJ, Mach RH, Morris JC, Raichle ME, Mintun MA. Spatial correlation between brain aerobic glycolysis and amyloid-beta (Abeta) deposition. Proc Natl Acad Sci U S A. 2010;107(41):17763–17767. doi: 10.1073/pnas.1010461107. [DOI] [PMC free article] [PubMed] [Google Scholar]