Abstract
Background and Aims
Esophageal food impaction (EFI) is a gastrointestinal emergency requiring immediate evaluation in the emergency room (ER) and an esophagogastroduodenoscopy (EGD) for disimpaction. EFI is also a distinct presenting feature of eosinophilic esophagitis (EoE). This study aimed at understanding the management of EFI among gastroenterologists (GIs), and estimated its impact on identification of EoE in United States (US).
Methods
GIs associated with three major gastroenterology societies based in US were invited to participate in a web-based survey. Information on the resources available and utilized, and the clinical decision-making process related to management of EFI cases was collected and analyzed.
Results
Of 428 responses, 49% were from pediatric GIs, 86% practiced in the US, and 78% practiced in an academic setting. Compared to the pediatric GIs, adult GIs were more likely to perform EGD in the emergency room [OR 87.96 (25.43–304.16)], and advance the food bolus into stomach [5.58 (3.08–10.12)]. Only 34% of respondents obtained esophageal biopsies during EGD, and pediatric GIs were more likely to obtain esophageal biopsies [3.49 (1.12–10.84)] compared to adult GIs. In US, by our conservative estimates, 10,494 patients presenting to ER with EFI and at risk for EoE are likely being missed each year.
Conclusions
EFI management varies substantially among GIs associated with three major gastroenterology societies in US. Based on their practice patterns, the GIs in US are likely to miss numerous EoE patients presenting to ER with EFI. Our findings highlight the need for developing and disseminating evidence-based EFI management practice guidelines.
Keywords: Esophageal food impaction, Eosinophilic esophagitis, Practice patterns, Gastroenterologists
Introduction
Esophageal food impaction (EFI) is a common gastrointestinal emergency requiring immediate evaluation in an emergency room (ER). EFI has been reported across a spectrum of ages and with a global distribution.[1,2] Management of EFI frequently involves an urgent flexible esophagogastroduodenoscopy (EGD) commonly performed by a gastroenterologist (GI) to relieve the impacted food bolus.[3] EFI is increasingly being recognized as a distinct presenting feature of eosinophilic esophagitis (EoE), and up to half of those requiring EGD for disimpaction may have EoE.[4–6] Therefore, EFI patients presenting to ERs and requiring EGD for disimpaction of food bolus offer a unique opportunity to identify potentially undetected latent EoE. EoE is an increasingly prevalent, clinicopathological condition affecting the esophagus, and has been reported in all age groups. It is clinically characterized by symptoms of esophageal dysfunction and is supported by histologic evidence of an intense eosinophilic inflammation in the esophageal epithelium that persists after an adequate proton-pump inhibitor (PPI) trial.[7–9]
There are minimal data and guidelines for management of EFIs, particularly in the context of identifying underlying esophageal mucosal diseases such as EoE. A recently published systematic review and meta-analysis examining the management of EFI and the association between EFI and EoE reported substantial variability in the management of EFI presenting to ERs.[10] In this study, the rate of esophageal biopsies obtained from patients presenting with EFI and undergoing EGD for disimpaction of food bolus was 54% (95% confidence interval [95% CI]: 40–68%), and the rate of EoE-attributable EFI among those who were biopsied was 54% (43–65%). However, the data on practice patterns for the management of EFI is limited, and the impact of practice patterns on identification of latent EoE among individuals presenting to ER with EFI and requiring EGD has not been approximated.
The aims of this study were to (1) investigate the real-world management of EFI presenting to an ER by GIs, and (2) based on our survey data and existing literature, to estimate the number of potentially missed EoE cases in the United States (US). We hypothesized that there would be substantial variability in practice among GIs managing EFI resulting in considerable number of missed latent EoE cases in the US.
Methods
Designing the survey
After a comprehensive review of the EFI literature published in PubMed, EMBASE and Scopus, guidelines to manage food impactions,[3,11,12] and consensus guidelines to identify and monitor EoE,[13,14] we developed a REDCap[15] survey consisting of 23 questions (Supplementary Appendix 1). The survey underwent multiple iterations of review and refinement by the authors, comprised of a group of pediatric GIs (GH, SKG and SA) and adult GIs (MFV, ESD) with expertise in esophageal disorders, EoE and survey design. The survey assessed resource availability and utilization [e.g., availability of Surgery, Ear, Nose & Throat (ENT), and Radiology services, radiology tests, location of EFI removal, time to EGD, support staff during EFI removal), and the clinical decision-making process (e.g., pharmacologic and non-pharmacologic interventions, removal devices utilized, esophageal mucosal biopsies obtained, immediate and long term care post-EFI removal). Data were collected without any personal identifiers. The questionnaire was designed to take less than 10 minutes to complete.
Survey distribution and data collection
The REDCap survey was distributed via electronic mail messages to the international community of pediatric and adult GIs accessible through the Pediatric Gastroenterology Bulletin board, the American Gastroenterological Association Online Community Forum, and to the program directors of gastroenterology fellowship programs listed on the American College of Gastroenterology website (who were asked to forward the survey to all faculty and trainees). Participation in this study was voluntary, and participants provided online consent. Electronic mail reminders were sent every 2 weeks for a total of 3 reminders, resulting in a data collection period of 8 weeks from September 2016 to October 2016. Survey responses were stored securely on the Vanderbilt University Medical Center server. The Institutional Review Board at Vanderbilt University (protocol # 161483) approved this study.
Statistical analysis
Data were exported to Stata version 14.0 (StataCorp, College Station, TX) for analyses. Descriptive statistics including counts and percentages [n (%)] for categorical variables, and means and standard deviations mean ± SD or medians and interquartile range [median (IQR)] for continuous variables were calculated.
Logistic regression was used to estimate unadjusted odds ratios (OR) with 95% CI for the probability of a response based on independent variables. Multivariate logistic regression was used to estimate adjusted odds ratio (aOR) with 95% CI for independent predictors of differences in practice patterns by (1) sub-specialty training (pediatric GIs vs. adult GIs), (2) practice setting (academic vs. private), or (3) location of practice (US vs. non-US), after adjusting for duration of practice, and estimated number of EFIs managed per year. Knowing that GI trainees work closely with their attendings and their practice is considerably informed by their attendings practice we combined the trainee and attending data in academic setting.
In order to estimate the number of potentially missed EoE cases in the US, we primarily relied on our survey data and when the data was not available from the survey we used information from published literature. First, we used the American Board of Pediatrics and American Board of Internal Medicine data to obtain the number of pediatric (1,372) and adult GIs (15,693) in the US. The analysis was restricted to pediatric GI or adult GI to avoid counting patients managed by both pediatric and adult GIs twice. We calculated the median (IQR) EFIs/ year managed by pediatric GI and adult GI in the US and the estimated number of EFI’s requiring EGD, knowing that between 68–92% of EFI patients presenting to ER require EGD.[10,16,17] Thereupon, using the meta-analysis finding that 54% (95% CI: 43–65%) of patients presenting to ER and undergoing EGD for EFI have EoE,[10] we calculated a conservative estimate of EoE-attributable EFIs/year in the US. Next, we calculated the proportion (95% CI) of GIs who do not routinely obtain esophageal biopsies during EFI removal (from question number 10 in our survey) and used it to estimate the median (IQR) number of EoE-attributable EFIs missed per year in US.
Next, in order to explore gaps within the broad categories of respondents, we grouped participants in our survey as those likely to adhere or not likely to adhere to the conceptual ‘best practices’ for management of EFI patients. We defined the ‘best practice’ as a combination of obtaining esophageal biopsies at the time of EGD for EFI removal irrespective of the mucosal appearance, initiating regular dose or high dose PPI therapy after EFI removal for cases of suspected EoE, scheduling a clinic follow-up, and scheduling a follow-up EGD. Statistical significance was determined at the P value (two-sided) of ≤ 0.05.
Results
Respondent characteristics
A total of 448 responses were received of which 428 (96%) were complete (Table 1). The number of pediatric GIs [210 (49%)] and adult GIs [200 (47%)] was comparable. Most of the respondents [334 (78%)] were practicing in an academic setting, with a quarter of whom them being trainees. A significantly higher proportion of respondents practiced in the US compared to those practicing in non-US countries (86% vs. 11%; P < 0.05). The average duration of practice post-fellowship training for GIs practicing in academic (excluding the trainees) and private practice setting was 15.71 ± 11.60 years, and the median (IQR) number of EFIs managed by them per year were 10 (6–14).
Table 1.
Characteristics of gastroenterologists participating in the survey [N=428]
| N (%) | |||
|---|---|---|---|
| Specialty | Pediatric | 210 (49) | |
| Adult | 200 (47) | ||
| Both | 18 (4) | ||
| Practice setting | Academic | 225 (53) | |
| Trainee | 109 (25) | ||
| Private | 94 (22) | ||
| Location | US | 379 (86) | |
| Non-US | 49 (11) | ||
| Region within U.S. | South | 109 (29) | |
| Northeast | 100 (26) | ||
| West | 106 (28) | ||
| Midwest | 64 (17) | ||
| Duration of practice* (years) | |||
| Practicing Gastroenterologists* | Mean ± SD | 15.71 ± 11.60 | |
| Median (IQR) | 13 (6–23) | ||
| Trainees | Mean ± SD | 1.16 ± 2.27 | |
| Median (IQR) | 0 (0–1) | ||
| Estimated number of esophageal food impactions (EFI) managed per year | |||
| Practicing Gastroenterologists* | Mean ± SD | 10.47 ± 7.03 | |
| Median (IQR) | 10 (6–14) | ||
| Trainees | Mean ± SD | 10.68 ± 6.78 | |
| Median (IQR) | 10 (6–14) | ||
Gastroenterologists in Academic and Private practice settings combined
Practice patterns for EFI
In all, [101 (23%)] of the respondents reported using services of Radiology, and chest x-ray [254 (42%)] was the most common radiologic test used to evaluate the EFI patient at presentation to the ER, and 91 respondents (21%) reported using an esophagogram (Table 2). Two hundred and thirteen (48%) indicated that they had access to an endoscopy nurse at the time of EGD. The proportion of GIs performing endoscopic EFI removal in a GI lab [260 (59%)] or in an operating room [240 (55%)] was similar. Over half of the respondents [230 (52%)] used smooth muscle relaxants (e.g., glucagon) as a pharmacologic intervention in an effort to relieve the EFI. The estimated time from presentation of an EFI patient to the ER to the start of endoscopy to relieve EFI was 5 ± 4 hours. A significantly higher proportion of GIs reported that they attempted to retrieve the impacted food bolus as opposed to pushing it distally into stomach [35% vs. 13%; P <0.001]. However, over half of the respondents [233 (54%)] indicated that they use both retrieve and push options to disimpact the food bolus.
Table 2.
Overall analysis of responses from gastroenterologists [N=428]
| N (%) | |
|---|---|
| Resource availability | |
| Sub-specialty services | |
| ENT | 70 (16) |
| Surgery | 57 (13) |
| Radiology | 101 (23) |
| Support staff | |
| Gastroenterology technician | 186 (42) |
| Endoscopy nurse | 213 (48) |
| Resource utilization | |
| Radiologic tests | |
| Chest x-ray | 254 (42) |
| Esophagogram | 91 (21) |
| Upper gastrointestinal (GI) series | 21 (5) |
| CT scan | 10 (2) |
| Location of EFI removal | |
| Emergency Room (ER) | 123 (28) |
| Gastroenterology lab | 260 (59) |
| Operating Room | 240 (55) |
| Clinical decision making | |
| Pharmacologic and non-pharmacologic interventions | |
| Peripherally acting agents | 6 (1) |
| Smooth muscle relaxants | 230 (52) |
| Benzodiazepines | 23 (5) |
| Calcium channel blockers | 12 (3) |
| Nitrates | 16 (4) |
| Gravity | 53 (12) |
| Effervescence | 15 (3) |
| Time from presentation to ER to start of upper endoscopy (EGD) (Hours)# | 5 ± 4 |
| Anesthesia | |
| General anesthesia with intubation | 305 (69) |
| Monitored anesthesia without intubation | 108 (25) |
| Conscious sedation | 105 (24) |
| Esophageal Food bolus disimpaction technique | |
| Retrieve | 148 (34) |
| Push | 55 (13) |
| Both | 233 (53) |
| Esophageal Food Impaction (EFI) removal devices | |
| Snare | 248 (56) |
| Forceps | 249 (57) |
| Net | 350 (76) |
| Graspers | 250 (57) |
| Baskets | 178 (40) |
| Suction cup | 121 (28) |
| Biopsy practice | |
| Yes, always obtain esophageal biopsies irrespective of endoscopic findings | 147 (34) |
| Only if I observe abnormal endoscopic findings | 220 (51) |
| No, I do not obtain biopsies | 61 (14) |
| Number of biopsies# | 5 ± 3 |
| Post EFI removal | |
| Wait for biopsy results without starting any medications | 196 (46) |
| Start medications irrespective of how the esophagus looks on endoscopy | 96 (23) |
| Start medications only if the esophagus looks abnormal on endoscopy | 130 (31) |
| Dilation during EFI removal | 197 (46) |
| Post-EFI removal immediate therapeutic management | |
| Carafate | 54 (12) |
| Proton-pump inhibitor (PPI) therapy – Regular dose | 200 (45) |
| PPI therapy - High dose | 175 (40) |
| Inhaled/swallowed steroids | 20 (5) |
| Swallowed steroids | 20 (5) |
| Post-EFI removal long term management | |
| Follow up | |
| No, I don’t follow up | 315 (72) |
| Only if I observe gross endoscopic abnormalities in the esophagus | 37 (8) |
| Only if the biopsies are abnormal | 74 (17) |
| Yes, always | 8 (2) |
| Duration to clinic follow up follow-up (Months) # | 1.52 ± 2.08 |
| Follow-up EGD | 176 (41) |
Mean ± SD.
About one in three [147 (34%)] respondents indicated that they always obtained esophageal biopsies at the time of EGD. Approximately half of the respondents [220 (51%)] indicated that they obtained biopsies only if they noted endoscopic abnormalities, and 61 (14%) indicated that they did not routinely obtain biopsies.
Respondents obtained 5 ± 3 esophageal biopsies at the time of EFI removal. Of those obtaining biopsies, almost half [196 (46%)] waited for histology results before starting any medication, and they would typically start either regular dose PPI therapy [200 (45%)] or high-dose PPI therapy [175 (40%)]. Only 8 (2%) of respondents reported that they routinely followed EFI patients, 37 (8%) indicated that they followed EFI patients only if mucosal abnormalities were observed during endoscopy, and 74 (17%) followed if the esophageal biopsies were abnormal [74 (17%)]. A total of 176 respondents (41%) indicated that they routinely perform a follow-up EGD.
Impact of sub-specialty training
Compared to pediatric GIs, adult GIs were less likely to utilize ENT [aOR (95% CI): 0.35 (0.16–0.77)] or Surgery [0.02 (0.003–0.21)] (Table 3). Adult GIs were more likely to consider pharmacological interventions such as smooth muscle relaxants [7.77 (4.38–13.77)] and benzodiazepines [6.70 (1.36–33.13)] prior to endoscopy to relieve the EFI. When compared to pediatric GIs, the adult GIs were more likely to perform EGD in the ER [87.96 (25.43–304.16)] or GI lab [2.43 (1.43–4.13)]. Adult GIs were less likely to retrieve the impacted food bolus [0.35 (0.17–0.74)] and more likely to push the impacted food bolus into the stomach [5.58 (3.08–10.12)] when compared to pediatric GIs. Adult GIs were less likely to obtain esophageal biopsies even if they observed abnormal endoscopic findings [0.11 (0.05–0.23)]. After EFI removal, adult GIs were less likely to initiate inhaled and swallowed steroids [0.20 (0.04–0.97)], and were more likely to schedule a follow-up EGD [2.86 (1.67–4.91)] compared to pediatric GIs.
Table 3.
Sub-group analysis and practice patterns among participating gastroenterologists [N=428]
| Adult Gastroenterologists$ Adjusted odds ratio (95% CI) |
Practicing in US¥ Adjusted odds ratio (95% CI) |
Private practice€ Adjusted odds ratio (95% CI) |
|
|---|---|---|---|
| Resource availability | |||
| Sub-specialty services | |||
| ENT | 0.35 (0.16 – 0.77)¶ | 0.35 (0.16 – 0.74)¶ | 0.34 (0.14–0.84)¶ |
| Surgery | 0.02 (0.003 – 0.21)¶ | 0.59 (0.26 – 1.35) | 0.81 (0.35–1.88) |
| Radiology | 1.03 (0.56–1.88) | 0.48 (0.23–1.00) | 0.63 (0.32–1.23) |
| Support staff | |||
| Gastroenterology technician | 1.18 (0.69–2.02) | 4.57 (1.85–11.30)¶ | 2.48 (1.45–4.25)¶ |
| Endoscopy nurse | 1.3 (0.82–2.31) | 1.63 (0.81–3.31) | 2.59 (1.15–4.69)¶ |
| Resource utilization | |||
| Radiologic tests | |||
| Chest x-ray | 0.60 (0.36–1.01) | 1.35 (0.68–2.64) | 0.51 (0.30–0.87)¶ |
| Esophagogram | 0.11 (0.04–0.27)¶ | 0.38 (0.18–0.82)¶ | 0.78 (0.38–1.63) |
| Upper Gastrointestinal (GI) series | 0.38 (0.07–1.84) | 0.29 (0.08–0.99)¶ | 0.66 (0.13–3.19) |
| CT scan | 3.5 (0.33–37.41) | 0.09 (0.01–0.75)¶ | 3.13 (0.47–20.86) |
| Location of EFI removal | |||
| Emergency Room | 87.96 (25.43–304.16)¶ | 5.24 (1.42–19.33)¶ | 0.70 (0.36–1.34) |
| Gastroenterology I lab | 2.43 (1.43–4.13)¶ | 1.12 (0.57–2.19) | 1.15 (0.62–2.89) |
| Operating Room | 0.17 (0.09–0.30)¶ | 2.40 (1.16–4.98)¶ | 0.39 (0.22–0.69)¶ |
| Clinical decision making | |||
| Pharmacologic and non-pharmacologic interventions | |||
| Peripherally acting agents | 0.87 (0.06–11.50) | 0.02 (0.01–0.30)¶ | 0.96 (0.12–7.23) |
| Smooth muscle relaxants | 7.77 (4.38–13.77)¶ | 1.57 (0.76–3.23) | 0.79 (0.45–1.38) |
| Benzodiazepines | 6.70 (1.36–33.13)¶ | 0.54 (0.13–2.28) | 1.58 (0.53–4.72) |
| Calcium channel blockers | 0.35 (0.03–3.46) | 0.02 (0.01–0.13)¶ | 1.85 (0.38–8.89) |
| Nitrates | 12.44 (1.44–106.95)¶ | 0.18 (0.04–0.86)¶ | 1.37 (0.38–4.89) |
| Gravity | 0.75 (0.32–1.76) | 0.39 (0.16–0.97)¶ | 1.46 (0.63–3.66) |
| Effervescence | 0.62 (0.10–3.68) | 0.12 (0.03–0.44)¶ | 0.12 (0.03–0.44)¶ |
| Time from presentation to ER to start of upper endoscopy (EGD) (Hours)# | 0.96 (0.90–1.03) | 0.90 (0.84–0.97)¶ | 0.92 (0.85–1.00) |
| Anesthesia | |||
| General anesthesia with intubation | 0.02 (0.009–0.06)¶ | 3.35 (1.35–8.31)¶ | 0.33 (0.18–0.62)¶ |
| Monitored anesthesia without intubation | 7.10 (3.57–14.09)¶ | 0.60 (0.26–1.37) | 1.70 (0.94–3.07) |
| Conscious sedation | 54.23 (17.42–168.82)¶ | 1.14 (0.43–2.99) | 0.98 (0.52–1.84) |
| Esophageal Food bolus disimpaction technique | |||
| Retrieve | 0.35 (0.17–0.74)¶ | 1.06 (0.41–2.73) | 1.03 (0.51–2.09) |
| Push | 5.58 (3.08–10.12)¶ | 0.97 (0.47–2.00) | 1.60 (0.87–2.92) |
| Both | 3.57 (2.30–5.56)¶ | 1.61 (0.37–6.95) | 1.00 (0.52–1.91) |
| Esophageal Food Impaction (EFI) removal devices | |||
| Snare | 1.41 (0.84–2.36) | 1.14 (0.58–2.22) | 1.88 (1.10–3.23)¶ |
| Forceps | 0.71 (0.43–1.18) | 0.94 (0.48–1.83) | 0.97 (0.57–1.62) |
| Net | 2.53 (1.23–5.22)¶ | 3.27 (1.56–6.83)¶ | 0.37 (0.19–0.71) |
| Graspers | 0.79 (0.47–1.32) | 1.70 (0.87–3.33) | 0.72 (0.42–1.22) |
| Baskets | 0.32 (0.18–0.56)¶ | 0.41 (0.20–0.83) | 1.29 (0.74–2.24) |
| Suction cup | 0.37 (0.20–0.67)¶ | 4.33 (1.61–16.11)¶ | 0.59 (0.32–1.08) |
| Biopsy practice | |||
| Yes, always obtain esophageal biopsies irrespective of endoscopic findings | Ref | Ref | Ref |
| Only if I observe abnormal endoscopic findings | 0.11 (0.05–0.23)¶ | 0.74 (0.31–1.78) | 0.80 (0.41–1.53) |
| No, I do not obtain biopsies | 3.49 (l.12–10.84)¶ | 4.20 (0.50–35.25) | 0.45 (0.18–1.09) |
| Number of biopsies | 0.93 (0.85–1.02) | 0.91 (0.80–1.03) | 1.03 (0.94–1.13) |
| Post EFI removal | |||
| Wait for biopsy results without starting any medications | Ref | Ref | Ref |
| Start medications irrespective of how the esophagus looks on endoscopy | 2.87 (1.42–5.78)¶ | 2.38 (0.88–6.43) | 0.74 (0.38–1.43) |
| Start medications only if the esophagus looks abnormal on endoscopy | 0.44 (0.23–0.84)¶ | 1.68 (0.77–3.66) | 0.40 (0.20–0.80)¶ |
| Dilation during EFI removal | 0.77 (0.46–1.27) | 0.72 (0.37–1.41) | 0.94 (0.56–1.58) |
| Post-EFI removal immediate therapeutic management | |||
| Carafate | 0.62 (0.28–1.37) | 1.03 (0.36–2.88) | 0.97 (0.43–2.17) |
| Proton-pump inhibitor (PPI) therapy – Regular dose | 1.27 (0.77–2.11) | 0.97 (0.50–1.89) | 1.39 (0.82–2.34) |
| PPI therapy - High dose | 1.63 (0.96–2.79) | 1.73 (0.81–3.68) | 0.72 (0.41–1.26) |
| Inhaled/swallowed steroids | 0.20 (0.04–0.97)¶ | 0.43 (0.12–1.52) | 1.24 (0.37–4.15) |
| Swallowed steroids | 0.50 (0.10–2.59) | 0.44 (0.10–1.89) | 0.58 (0.10–3.22) |
| Long term care Post-EFI removal long term management | |||
| No, I don’t follow up | Ref | Ref | Ref |
| Only if I observe gross endoscopic abnormalities in the esophagus | 1.30 (0.50–3.39) | 0.76 (0.23–2.48) | 0.84 (0.32–2.22) |
| Only if the biopsies are abnormal | 0.39 (0.18–0.82)¶ | 1.58 (0.57–4.35) | 1.22 (0.61–2.47) |
| Yes, always | 4.16 (0.39–43.81) | – | 0.99 (0.12–8.06) |
| Post-EFI removal follow-up | |||
| Duration to clinic follow up follow-up | 0.98 (0.88–1.10) | 0.86 (0.76–0.97)¶ | 1.07 (0.95–1.19) |
| Follow-up EGD | 2.86 (1.67–4.91)¶ | 0.62 (0.31–1.25) | 0.67 (0.39–1.16) |
Association between adult gastroenterologists and pediatric gastroenterologists after adjusting for practice setting, location of practice, duration of practice, and estimated number of EFIs managed per year
Association between gastroenterologists practicing in US and those practicing outside US after adjusting for sub-specialty training, practice setting, duration of practice, and estimated number of EFIs managed per year
Association between gastroenterologists in private practice and gastroenterologists practicing in academic setting after adjusting for sub-specialty training, location of practice, duration of practice, and estimated number of EFIs managed per year
Two-sided P value ≤ 0.05
Impact of country of practice
Compared to GIs practicing outside US, US-based GIs were less likely to utilize ENT services for EFI removal [0.35 (0.16–0.74)] and more likely to have access to a GI technician at the time of EFI removal [4.57 (1.85–11.30)] (Table 3). US-based GIs were less likely to use pharmacological agents such as peripherally acting agents [0.02 (0.01–0.30)], calcium channel blockers [0.02 (0.01–0.13)], nitrates [0.18 (0.04–0.86)], and non-pharmacological interventions such as gravity [0.39 (0.166–0.97)] and effervescence [0.12 (0.03–0.44)]. The US based GIs were more likely to use general anesthesia with intubation for EFI removal compared to non-US GIs.
Impact of practice setting
GIs in private practice were less likely to use ENT services [0.34 (0.14–0.84)], and were more likely to have GI technician [2.48 (1.45–4.25)] or endoscopy nurse support [2.59 (1.15–4.69] during the endoscopic EFI removal when compared to GIs practicing in academic settings (Table 3). The clinical decision-making was mostly similar between GIs in academic settings and those in private practice.
‘Missed opportunities’ to detect EoE in the US
Adult GIs indicated that they managed more EFI cases per year compared to pediatric GIs (median: 12 vs. 8). Based on the number of adult GIs and pediatric GIs practicing in the US and a conservative estimate (lower limit of 95% CI) of 43%[10] of EFIs being attributable to EoE, we estimated that 69,150 adult and 4,030 pediatric patients presenting to ERs with EFI were likely to have EoE. Using the ‘non-biopsy’ rate of 25% (95% CI: 19–31%) and 3% (1–6%) among adult GIs and pediatric GIs, respectively, we estimated that about 13,766 (95% CI: 10,462–17,070) adult and 96 (32–192) pediatric cases with EoE-attributable EFI presenting to ERs could be missed per year (Supplementary material Table 2).
Adherence to ‘best practice’
Only one in three [145 (33%)] respondents adhered to our conceptual ‘best practice’ (Figure 1). The pediatric GIs were likely to be 2-fold compliant compared to adult GIs [1.99 (1.23–3.22)] after adjusting for practice setting, location of practice, region within the US, duration of practice and estimated number of EFI’s managed per year. No other significant differences were noted between those who did and did not adhere to the ‘best practices’.
Figure 1. Characteristics of respondents adherent or non-adherent to the conceptual ‘best practice’ for EFI management.
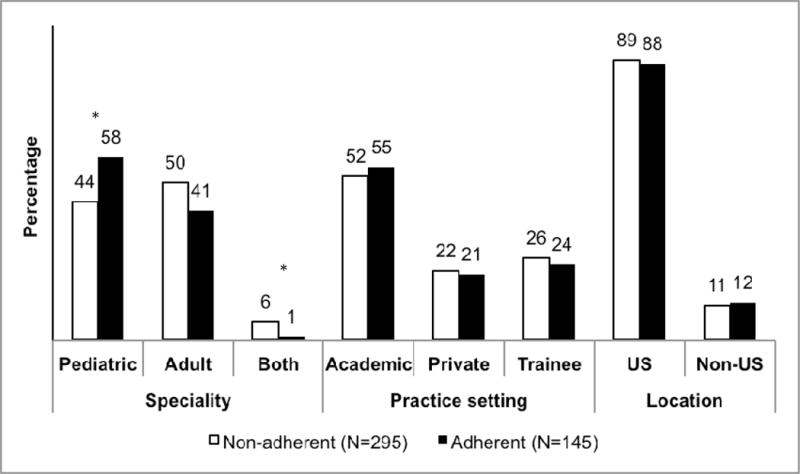
*P value < 0.05
Discussion
Esophageal malformations, strictures, surgery, dysmotility, EoE, lymphocytic esophagitis, injury and external compression are known risk factors for EFI. [2,18] The management of EFI in individuals with one or more of the risk factors can vary based on the complex analysis of patient’s condition and available therapeutic options. In order to assess EFI practices regardless of risk factors, we considered a scenario wherein the patient presenting to ER with EFI did not have any of the known risk factors. Our result suggests that there is substantial variability in the management of EFIs among GIs affiliated with three US based gastroenterology societies. These differences are related to the available resources, sub-specialty training, practice setting, and practice location.
The 2011 American Society for Gastrointestinal Endoscopy (ASGE) guidelines serve as an educational instrument for management of patients with ingested foreign bodies and food.[3] However factors related to patient’s clinical condition and those related to resources available guide a GIs course of action, and this might vary from the recommended guidelines. Despite esophageal narrowing being one of the primary contributors to EFI,[19] only 21% of respondents obtained an upper GI series for evaluation after EFI. The adult GIs reported performing EFI removal in the ER whereas pediatric GIs were more likely to perform EFI removal in an operating room. Adult GIs also indicated relatively more reliance on using pharmacologic and non-pharmacologic interventions to relieve the food impaction prior to performing endoscopy compared to the pediatric GIs. The ASGE guidelines advocate against advancing the bolus into stomach without first examining esophagus distal to obstruction by passing the endoscope around the food bolus. In our survey the majority of adult GIs indicated that they favor pushing the impacted food bolus distally whereas pediatric GIs preferred to retrieve the impacted food bolus.
EoE has emerged as a dominant cause of dysphagia and esophageal food impaction, especially in teenagers and adults. [20,21] Certain gross esophageal endoscopic features such as rings, linear furrows, and narrowing raise suspicion for EoE; however, these findings are neither universal nor diagnostic.[22] Therefore, the current recommendations advocate obtaining multiple esophageal mucosal biopsies safely and irrespective of endoscopic appearance of the esophagus for histologic confirmation EoE.[13] We found that esophageal biopsies were not routinely obtained during EGD for EFI and this is consistent with recently published reports.[23] Nearly two thirds of responders indicated that they would either obtain esophageal mucosal biopsies only if they observed abnormal endoscopic features during the EFI removal or would not obtain esophageal biopsies at all. This translated into potentially missing a considerable number of EoE-attributable EFI patients suggesting that the occurrence of EoE in EFI patients might be substantially underestimated.
At present, recommendations related to immediate and long-term care following EFI removal is unclear. In our study, pediatric GIs were more likely than adult GIs to initiate topical steroids (inhaled/swallowed) after EFI removal. While topical steroids have been shown to be effective in therapeutic management of EoE and may reduce the risk of recurrent EFIs, the implications of initiating topical steroids immediately after EFI removal and prior to establishing the diagnosis of EoE have not been well examined. It could be reasonably contended that an EFI patient with concerns for EoE should be initiated on PPI therapy prior to being re-assessed for EoE or proton-pump inhibitor therapy responsive esophageal eosinophilia (PPI-REE). A large majority (72%) of respondents indicated that they do not routinely follow their EFI patients, and only one in three respondents adhered to our conceptual definition of ‘best practice’. These observations underscore potential missed opportunities to detect EoE in this high-risk population, and may represent a non-trivial proportion of the currently diagnosed EoE cases as per the prevalence estimates.[24] The practice variability was also observed between GIs practicing within and outside of the US, and between GIs practicing in an academic setting and those in private practice. Most of the variability in these two subgroups was related to resource availability and utilization. However, the clinical decision-making by these subgroups was comparable except for differences in their use of pharmacological and non-pharmacological interventions.
This study has limitations. The first is the generalizability of our results. Our results cannot be extended past a small sample of GIs who participated in the survey. Additionally it cannot be generalized across GI sub-specialists as the adult GIs were underrepresented. We used a web-based approach and leveraged electronic mail to enroll participants. While this recruitment approach afforded us a distinct advantage of reaching out to a diverse and dispersed group of GIs, it is possible that the same approach also may have introduced selection bias as engaging GIs in an online survey could selectively represent practitioners who seek out on-line forums and web-based support. Additionally their reporting could have been influenced by clustering of responses based on management patterns by practice settings, institutions, regions or country of practice. As a result, their responses may not be an accurate reflection of their practice patterns. While the anonymity of participating in online surveys and self-reporting can be an asset for eliciting practice-related information, it did not allow us to confirm the demographic data provided by respondents. This approach was also susceptible to the Hawthorne effect in which responders might have artificially altered their response (or practice preferences) when they are part of a survey.[25] Lastly, we tested multiple associations and by chance alone some of the associations may be false. Despite these limitations, this study has address an important and clinically relevant question and has several strengths such as use of electronic media to disseminate the survey questionnaire to address a clinically relevant question with significant impact on clinical gastrointestinal practice, participation from GIs practicing in the US and outside of US, robust methodology, and a rigorous analytical approach.
In conclusion, this study highlights substantial variability among GIs managing EFI. While this variability could reflect multiple approaches to manage EFIs, it may also speak to a need for effectively disseminating existing guidelines. Future studies are warranted to explore the basis for the variability in management of EFIs. Finally, there is an unmet need to develop practice guidelines to optimize our ability to identify EoE in patients presenting to ER with EFI, and our conceptual ‘best practice’ could potentially lay the foundation.
Supplementary Material
Acknowledgments
GH, SKG and ESD are affiliated with the Consortium of Eosinophilic Gastrointestinal Disorders Researchers (CEGIR). CEGIR (U54 AI117804) is part of the Rare Disease Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), NCATS, and is funded through collaboration between NIAID, NIDDK, and NCATS. CEGIR is also supported by patient advocacy groups including APFED, CURED and EFC. GH is also supported by a grant from APFED. ESD has received research funding from Meritage, Miraca, Nutricia, Receptos/Celgene, Regeneron, and Shire.
ESD: Research funding from Meritage, Miraca, Nutricia, Receptos/Celgene, Regeneron, and Shire. Consultant for Adare, Alivio, Banner, Enumeral, GSK, Receptos/Celgene, Regeneron, and Shire. Educational grant from Banner.
Footnotes
Conflict of interest:
GH: None declared
MV: Consultant for Ironwood
SKG: Consultant for Abbott
SA: None declared
References
- 1.Longstreth GF, Longstreth KJ, Yao JF. Esophageal food impaction: epidemiology and therapy. A retrospective, observational study. Gastrointestinal Endoscopy. 2001 Feb;53:193–198. doi: 10.1067/mge.2001.112709. [DOI] [PubMed] [Google Scholar]
- 2.Ko HH, Enns R. Review of food bolus management. Canadian Journal of Gastroenterology. 2008 Oct;:805–808. doi: 10.1155/2008/682082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ikenberry SO, Jue TL, Anderson MA, Appalaneni V, Banerjee S, Ben-Menachem T, et al. Management of ingested foreign bodies and food impactions. Gastrointestinal Endoscopy. 2011 Jun 1;73:1085–1091. doi: 10.1016/j.gie.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Prasad GA, Talley NJ, Romero Y, Arora AS, Kryzer LA, Smyrk TC, et al. Prevalence and Predictive Factors of Eosinophilic Esophagitis in Patients Presenting With Dysphagia: A Prospective Study. The American Journal of Gastroenterology. 2007 Dec 1;102:2627–2632. doi: 10.1111/j.1572-0241.2007.01512.x. [DOI] [PubMed] [Google Scholar]
- 5.Byrne KR, Panagiotakis PH, Hilden K, Thomas KL, Peterson KA, Fang JC. Retrospective analysis of esophageal food impaction: differences in etiology by age and gender. Dig Dis Sci. 2007 Mar;52:717–721. doi: 10.1007/s10620-006-9499-0. [DOI] [PubMed] [Google Scholar]
- 6.Desai TK, Stecevic V, Chang C-H, Goldstein NS, Badizadegan K, Furuta GT. Association of eosinophilic inflammation with esophageal food impaction in adults. Gastrointestinal Endoscopy. 2005 Jun;61:795–801. doi: 10.1016/s0016-5107(05)00313-5. [DOI] [PubMed] [Google Scholar]
- 7.Furuta GT, Katzka DA. Eosinophilic Esophagitis. N Engl J Med. 2015 Oct 22;373:1640–1648. doi: 10.1056/NEJMra1502863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dellon ES. Epidemiology of Eosinophilic Esophagitis. Gastroenterol Clin North Am. 2014 Jun;43:201–218. doi: 10.1016/j.gtc.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dellon ES, Jensen ET, Martin CF, Shaheen NJ, Kappelman MD. Prevalence of eosinophilic esophagitis in the United States. Clin Gastroenterol Hepatol. 2014 Apr;12:589–96. doi: 10.1016/j.cgh.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiremath GS, Hameed F, Pacheco A, Olive A, Davis CM, Shulman RJ. Esophageal Food Impaction and Eosinophilic Esophagitis: A Retrospective Study, Systematic Review, and Meta-Analysis. Dig Dis Sci. 2015 Nov;60:3181–3193. doi: 10.1007/s10620-015-3723-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kramer RE, Lerner DG, Lin T, Manfredi M, Shah M, Stephen TC, et al. Management of Ingested Foreign Bodies in Children: A Clinical Report of the NASPGHAN Endoscopy Committee. Journal of Pediatric Gastroenterology and Nutrition. 2015 Apr 1;60:562–574. doi: 10.1097/MPG.0000000000000729. [DOI] [PubMed] [Google Scholar]
- 12.Georg Thieme Verlag KG, Birk M, Bauerfeind P, Deprez PH, Häfner M, Hartmann D, et al. Removal of foreign bodies in the upper gastrointestinal tract in adults: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2016 Feb 10;48:489–496. doi: 10.1055/s-0042-100456. [DOI] [PubMed] [Google Scholar]
- 13.Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011 Jul;128:3–20. doi: 10.1016/j.jaci.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 14.Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Katzka DA. ACG Clinical Guideline: Evidenced Based Approach to the Diagnosis and Management of Esophageal Eosinophilia and Eosinophilic Esophagitis (EoE) The American Journal of Gastroenterology. 2013 May 1;108:679–692. doi: 10.1038/ajg.2013.71. [DOI] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009 Apr;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sperry SLW, Crockett SD, Miller CB, Shaheen NJ, Dellon ES. Esophageal foreign-body impactions: epidemiology, time trends, and the impact of the increasing prevalence of eosinophilic esophagitis. Gastrointestinal Endoscopy. 2011 Nov;74:985–991. doi: 10.1016/j.gie.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lao J, Bostwick HE, Berezin S, Halata MS, Newman LJ, Medow MS. Esophageal food impaction in children. Pediatr Emerg Care. 2003 Dec;19:402–407. doi: 10.1097/01.pec.0000101581.65509.17. [DOI] [PubMed] [Google Scholar]
- 18.Truskaite K, Dlugosz A. Prevalence of Eosinophilic Esophagitis and Lymphocytic Esophagitis in Adults with Esophageal Food Bolus Impaction. Gastroenterology Research and Practice. 2016;2016:6. doi: 10.1155/2016/9303858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gentile N, Katzka D, Ravi K, Trenkner S, Enders F, Killian J, et al. Oesophageal narrowing is common and frequently under-appreciated at endoscopy in patients with oesophageal eosinophilia. Aliment Pharmacol Ther. 2014 Dec;40:1333–1340. doi: 10.1111/apt.12977. [DOI] [PubMed] [Google Scholar]
- 20.Kidambi T, Toto E, Ho N, Taft T, Hirano I. Temporal trends in the relative prevalence of dysphagia etiologies from 1999–2009. World Journal of Gastroenterology: WJG. 2012 Aug 28;18:4335–4341. doi: 10.3748/wjg.v18.i32.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sengupta N, Tapper EB, Corban C, Sommers T, Leffler DA, Lembo AJ. The clinical predictors of aetiology and complications among 173 patients presenting to the Emergency Department with oesophageal food bolus impaction from 2004–2014. Aliment Pharmacol Ther. 2015 Jul;42:91–98. doi: 10.1111/apt.13237. [DOI] [PubMed] [Google Scholar]
- 22.Kia L, Hirano I. Advances in the endoscopic evaluation of eosinophilic esophagitis. Curr Opin Gastroenterol. 2016 Jul 1;32:325–331. doi: 10.1097/MOG.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Philpott HL, Nandurkar S, Thien F, Bloom S, Lin E, Goldberg R, et al. Seasonal recurrence of food bolus obstruction in eosinophilic esophagitis. Internal Medicine Journal. 2015 Sep 1;45:939–943. doi: 10.1111/imj.12790. [DOI] [PubMed] [Google Scholar]
- 24.Arias A, Pérez-Martínez I, Tenias JM, Lucendo AJ. Systematic review with meta-analysis: the incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment Pharmacol Ther. 2016 Jan;43:3–15. doi: 10.1111/apt.13441. [DOI] [PubMed] [Google Scholar]
- 25.Fernald DH, Coombs L, DeAlleaume L, West D, Parnes B. An assessment of the Hawthorne Effect in practice-based research. J Am Board Fam Med. 2012 Jan;25:83–86. doi: 10.3122/jabfm.2012.01.110019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


