Abstract
The N-methyl-D-aspartate receptor (NMDAR) has been implicated in the pathophysiology of neurological diseases, such as schizophrenia, autism spectrum disorders (ASD), and Alzheimer’s disease (AD), whose unique clinical hallmark is a constellation of impaired social and/or cognitive behaviors. GluN3A (NR3A) is a unique inhibitory subunit in the NMDAR complex. The role of GluN3A in social behavioral activities is obscure. In this study, we sought to evaluate altered social activities in adult GluN3A knockout (KO) mice. GluN3A KO mice spent less time in reciprocal social interaction in the social interaction test compared to wild type (WT) mice. A social approach test using a three-chamber system confirmed that mice lacking GluN3A had lower sociability and did not exhibit a preference for social novelty. GluN3A KO mice displayed abnormal food preference in the social transmission of food preference task and low social interaction activity in the five-trial social memory test, but without social memory deficits. Using a home cage monitoring system, we observed reduced social grooming behavior in GluN3A KO mice. Signaling genes that might mediate the altered social behaviors were examined in the prefrontal cortex, hippocampus, and thalamus. Among nine genes examined, the expression of the oxytocin receptor was significantly lower in the prefrontal cortex of GluN3A KO mice than that in WT mice. Oxytocin treatment rescued social activity deficits in GluN3A KO mice. These findings support a novel idea that a chronic state of moderate increases in NMDAR activities may lead to downregulation of the oxytocin signaling and impaired behavioral activities that are seen in psychiatric/neurodegenerative disorders.
Keywords: GluN3A, NR3A, Social behaviors, Oxytocin signaling
Introduction
Glutamate mediates the majority of excitatory synaptic transmission in the central nervous system (CNS) (Meldrum, 2000). It activates three distinct classes of ionotropic receptors: N-methyl-D-aspartate (NMDA) receptors (NMDARs), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (AMPARs), and kainic acid receptors (Hollmann and Heinemann, 1994; Karlsson et al., 2002; Lee et al., 1998). Many studies have focused on the NMDARs because of their widespread distribution in the CNS and broad functions involving brain development, neural plasticity, cognitive functions, excitotoxicity, and neurodegenerative diseases (Bettler and Mulle, 1995; Choi, 1992; Dingledine et al., 1999; Mota et al., 2014; Neill et al., 2014). NMDARs are hetero-multimers (with a tetrameric or pentameric structure) that are composed of at least two GluN1 subunits, and two or three GluN2A-D subunits (Nakanishi and Masu, 1994; Seeburg, 1993). GluN1 (NR1) is required for the fundamental formation of NMDARs, and the GluN2 (NR2) subunit is important in different functional properties, such as learning and memory (Lee et al., 1998; Seeburg, 1993). On the other hand, GluN3A (NR3A) and GluN3B (NR3B) were discovered later and its expression is relatively lower in the adult brain (Al-Hallaq et al., 2002; Ciabarra et al., 1995; Das et al., 1998). The GluN3 subunits are unique inhibitory NMDAR subunits; when a GluN3 subunit is assembled with GluN1 and GluN2, it reduces the Ca2+ permeability, affecting NMDAR-mediated synaptic currents and the overall NMDA responses (Nishi et al., 2001; Sucher et al., 1995; Tong et al., 2008). Demonstrated in patch clamp recordings, the expression of the GluN3 subunit substantially suppressed NMDA currents (Cavara and Hollmann, 2008; Ciabarra et al., 1995; Das et al., 1998; Sucher et al., 1995).
The GluN3A expression is widely observed in brain regions, such as the cortex, hippocampus, thalamus, and olfactory bulb, whereas GluN3B expression is identified in the brain stem, spinal cord, cortex, hippocampus, amygdala, cerebellum, and olfactory bulb (Bendel et al., 2005; Fukaya et al., 2005; Sucher et al., 1995). The GluN3A expression is identified in the brain of rodents, monkeys, and humans (Henson et al., 2010). GluN3A expression peaks during early postnatal life, and diminishes to lower levels in adulthood (Ciabarra et al., 1995; Perez-Otano et al., 2001). Therefore, most functional studies on the role of GluN3A/3B have focused on the developing stage; the functional role of these subunits in the adult brain remains incompletely defined. We recently demonstrated that even though the GluN3A expression level undergoes a downregulation after the neonatal period, GluN3A in the adult brain has profound impacts on locomotor activity, pain sensation, neuroprotection, olfactory, and cognitive functions (Lee et al., 2016a; Lee et al., 2015; Mohamad et al., 2013). Specifically, deletion or reduced level of GluN3 generates a chronic state of moderately increased activities of NMDAR in the brain. The consequence of this chronic regulation of NMDAR on behavior activities has rarely been investigated before.
NMDAR function has been implicated in the pathophysiology of neuropsychiatric and neurodegenerative diseases, such as schizophrenia, autism spectrum disorders (ASD), and Alzheimer’s disease (AD), whose unique hallmark is impaired social behaviors and/or cognitive function with impaired synaptic activities (Gladding and Raymond, 2011; Kocahan and Dogan, 2017; Neill et al., 2014; Rosenthal-Simons et al., 2013). For example, the glycine binding site in NMDARs can regulate social behavior in schizophrenia and ASD (Ghanizadeh, 2011; Labrie et al., 2008). GluN1-deficient mice have behavioral abnormalities, including reduced social interactions, hypo-locomotor activity, and deficits in cognitive inflexibility (Duncan et al., 2004; Mohn et al., 1999). NMDAR antagonists, such as ketamine and MK-801, have been shown to have inhibitory or regulatory effects in adult rodent sociability (Silvestre et al., 1997; Siviy et al., 1995). Basic and clinical evidence demonstrate antidepressant and anxiolytic effects of ketamine and other NMDAR antagonists (Murrough et al., 2017). Furthermore, GluN3B knockout (KO) mice showed markedly increased social interactions with their familiar cage mates in their home cage, but moderately increased anxiety-like behavior and decreased social interaction in a novel environment (Niemann et al., 2007). Recent studies have also linked sustained moderate activations of NMDA receptors to neurodegenerative changes in AD (Kocahan and Dogan, 2017). In the present investigation, we tested the hypothesis that chronic deletion of the GluN3A subunit might have a significant impact on the social behavior in adulthood. We also explored the alteration of social genes, such as oxytocin in the GluN3A knockout brain, as an underlying mechanism in the behavior changes.
Experimental Procedures
Wild type and GluN3A KO mice
Young adult (2–3-month-old) male GluN3A KO and littermate WT mice were tested in this investigation. All animal handling and experimental protocols were approved by the Emory University Institutional Animal Care and Use Committee (IACUC), in compliance with National Institutes of Health (NIH) guidelines. The GluN3A KO mice and WT counterparts were originally provided by Nobuki Nakanishi and Stuart A. Lipton at Sanford-Burnham Medical Research Institute (La Jolla, California, USA). Das and colleagues have described detailed information on the background of these mice (Das et al., 1998). Briefly, embryonic stem cells derived from 129/SvJ were electroporated with DNA carrying disrupted GluN3A gene, and then injected into blastocysts from C57BL/6 mice. The resulting chimeric males were crossed with BlackSwiss or 129SvEv females to produce F1 heterozygotes. GluN3A KO homozygote mice were then produced by cross-breeding F1 mice. In our lab, homozygote colonies of either WT or GluN3A KO mice were maintained under the same conditions with identical room temperature (22°C), same food and water supply, and animal care environment.
Genotyping of DNA sequence
The genotyping method used in this study was performed as described previously (Mohamad et al., 2013). DNA for genotyping was extracted from tail snips (approximately 2–4 mm). Two separate sets of primers were used for the GluN3A KO and WT mice, respectively. For the WT reaction, forward primer: 5’ – CCACGGTGAGCTTGGGGAAG-3’ and reverse primer: 5’-TTGGGGAGCGCCCTGCATGG-3’. For the KO reaction, forward primer: 5’-CCACGGTGAGCTTGGGGAAG-3’ and reverse primer: 5’-GCCTGAAGAACGAGATCAGG-3’. DNA (2 µl) was amplified on a thermal cycler (MJ minim, Personal Thermal Cycler, Bio-Rad, CA, USA) for 40 cycles (95°C for 60 sec, 58°C for 30 sec, 72°C for 60 sec). Afterwards, the samples were incubated for an additional 10 min at 72°C before being held at 4°C. PCR products were subjected to electrophoresis in 2% agarose gel with ethidium bromide. Relative intensity of PCR bands was analyzed using InGenius3 manual gel documentation system (Syngene, Frederick, MD, USA).
Oxytocin Treatment
WT and GluN3A KO mice were intraperitoneally (i.p.) administered 10 mg/kg oxytocin (Sigma-Aldrich, St. Louis, MO, USA) or saline vehicle treatment once daily for 7 days. This dosage was selected based on previous reports (Mooney et al., 2014).
Behavioral tests
In behavioral assays, all animals were tested at the same time of the day (early or late evening). An individual mouse was tested no more than twice a day.
Reciprocal social interaction test
This test was conducted with untreated, unfamiliar, weight-matched, age-matched, and sex-matched mice partners. Subject and stranger mice were put together in a clean empty home cage and recorded by the TopScan System (Clever Sys Inc., Reston, VA, USA). We scored time spent in social interaction of the animals for 10 min between 1 and 5 PM. The following social interaction categories were all blindly scored: social sniffing (both nose-to-nose sniffing and nose-to-anogenital sniffing), following, grooming, biting, and wrestling. Cages were washed with 70% ethanol solution and water before we performed the next test in order to prevent possible inter-subject cross-contamination.
Social approach test using the three-chamber system
The social approach test was utilized to test general sociability and response to social novelty. The test was performed in a three-chambered box with openings between the chambers. Glass panels were used to cover the openings during phase changes. First, the test subject was placed into the empty box and allowed to explore all chambers freely for 10 min. After the habituation period, a stranger (non-littermate) mouse contained in a wire cage was placed into the left chamber. The mouse was then allowed to explore all three chambers. Both the time spent with the stranger mouse (stranger #1) and the time in the empty chamber was recorded over a 10 min session. The test mouse was then returned to the center chamber and the openings were blocked. In the social novelty test, a second stranger mouse (stranger #2) was placed in the empty chamber. The central chamber door was opened, and the test mouse was again allowed to freely explore the chambers and interact with strangers #1 and #2. Since the test mouse already had contact with stranger #1 but not #2, the time spent with stranger #2 vs #1 tested the subject’s preference for novel social interaction.
Social transmission of food preference test
The social transmission of food preference test was used in rodents to assess social activity (Silverman et al., 2010). For this test, two demonstrator mice were removed from each test cage and individually housed overnight with water but without food (18 hrs). The demonstrator mice were then placed into clean cages containing almond-flavored food in small glass jars (3.9 cm diameter, 3.4 cm high). The glass jars were set in shallow Petri dishes so that food scattered by the digging of the mice was retained. Demonstrator mice were left to eat the cued food (almond) for 2 hrs. Dishes were weighed before and after to measure how much food was eaten. The demonstrator mouse was then placed in a clean experimental cage. One at a time, ‘observer’ mice from the same home cage of the demonstrator mice were placed in the cage containing the demonstrator mice and left there for 5 min. The observer mouse was then removed. After an interval of 15 min the sequence of interaction was repeated with each observer mouse being placed with the second demonstrator mouse from the home cage. All observer mice were then returned to the home cage, and demonstrator mice were individually housed. Six hours after the social interaction sessions, the observer mouse was food-deprived for 18 hrs (overnight). The following morning, each mouse was placed individually in a clean cage (45 cm × 28 cm × 12 cm) containing two ‘dishes’ in either corner at the back of the cage; one with almond-flavored food (cued) and the other containing normal diet (non-cued). Mice were allowed to eat for 2 hrs. Dishes were weighed before and after to determine the amount of food eaten. Food preference was calculated as the amount of cued food eaten, non-cued food eaten, and total food (non-cued food plus cued food) eaten.
Social direct interaction test
To measure social memory function, we performed the direct interaction test. In the first trial, the subject mouse was placed in a clean cage, and a novel mouse was introduced. Social interaction activity was quantified to examine the time spent in social sniffing, social following, and social grooming. After an inter-trial interval of 1 hr, either the previously encountered mouse or a novel mouse was introduced, and then the social interaction activity was measured for 5 min (Hitti and Siegelbaum, 2014).
Five-trial social memory test
The five-trial social memory test was performed to measure social memory ability. Briefly, subject mice were presented with four successive 1-min trials. On the last trial, we introduced a novel mouse. In each trial, we measured the social interaction activity (nose-to-nose sniffing, following, and grooming).
Western Blot analysis
Western blot analysis was used to analyze the expressions of social behavior-related proteins in the cortex, hippocampus, and thalamus from WT and GluN3A KO brains. Brain tissues were lysed in a lysis buffer containing 0.02 M Na4P2O7, 10 mM Tris-HCL (pH 7.4), 100 mM NaCl, 1 mM EDTA (pH 8.0), 1% Triton, 1 mM EGTA, 2 mM Na3VO4, and protease inhibitor cocktail (Sigma-Aldrich). The supernatant was collected after centrifugation at 15,000 g for 10 min at 4°C. Protein concentration was determined with bicinchoninic acid (Pierce Biothechnology, Rockford, IL, USA) assay. Protein was normalized, and equivalent amounts of total protein were separated by molecular weight on SDS-polyacrylamide gradient gel, and then transferred to PVDF membranes. The blot was incubated in 5% bovine serum albumin (Sigma-Aldrich) for 1 hr, and then reacted with primary antibodies at 4°C overnight.
The primary antibodies used and the dilutions for each were mouse anti-serotonin receptor (5-HTR; BD Bioscience, San Jose, CA, USA) at 1:2,500, rabbit anti-serotonin transporter (BD Bioscience) at 1:1,000, rabbit anti-oxytocin antibody (Fitzgerald Industries International, North Acton, MA, USA) at 1:1000, goat anti-oxytocin receptor antibody (Abcam, Cambridge, MA, USA) at 1:2,000, goat anti-CD73 antibody (Santa Cruz Biotech, Inc., Dallas, TX, USA) at 1:2,000, rabbit anti-BDNF antibody (Santa Cruz Biotech) at 1:2,000, mouse anti-TNFR1 antibody (Santa Cruz Biotech) at 1:2,000, rabbit anti-GluN3A antibody (Abcam) at 1:2,000, and mouse anti-actin (Sigma-Aldrich). After washing with TBST, membranes were incubated with AP-conjugated or HRP-conjugated secondary antibodies (GE Healthcare, Piscataway, NJ, USA) for 2 hrs at room temperature. After final washing with TBST, the signals were detected with bromochloroidolylphosphate/nitroblue tetrazolium (BCIP/NBP) solution (Sigma-Aldrich) or film. Signal intensity was measured by ImageJ and normalized to the actin signal intensity.
Immunohistochemistry
Frozen brain tissues were sliced into 10 µm-thick coronal sections using a cryostat (Leica CM 1950; Leica Microsystems, Buffalo Grove, IL, USA). Sections were dried on the slide warmer for ≥ 30 min, fixed with 10% formalin buffer, washed with −20°C precooled ethanol: acetic acid (2:1) solution for 10 min, and finally permeabilized with 0.2% Triton-X 100 solution for 5 min. All slides were washed three times with PBS (5 min each) after each step. Then, tissue sections were blocked with 1% fish gelatin (Sigma-Aldrich) in PBS for 1 hr at room temperature, and subsequently incubated with the primary antibodies against oxytocin (1:250; Fitzgerald Industries International), oxytocin receptor (1:200; Abcam), and NeuN (1:300; Millipore, Billerica, MA, USA) overnight at 4°C. The next day, slides were washed three times with PBS for 5 min, then reacted with the secondary antibodies Alexa Fluor®488 goat anti-mouse (1:300; Life Technologies, Grand Island, NY, USA), Cy3-conjugated donkey anti-rabbit (1:300; Jackson ImmunoResearch Laboratories, West Grove, PA, USA), or Cy5-conjugated donkey anti-mouse (1:400; Jackson ImmunoResearch Laboratories) for 90 min at room temperature. After three washes with PBS, nuclei were counterstained with Hoechst 33342 (1:20,000; Molecular Probes) for 5 min as a control. Then, all slides were fixed with Vectashield mounting medium (Vector Laboratory, Burliungame, CA) and finally covered by a coverslip for microscopy and image analysis.
Cell counting in brain sections
The counting of oxytocin receptor positive cells was performed following the principles of design-based stereology. Systematic random sampling was employed to ensure accurate and non-redundant cell counting. Every section under analysis was at least 90 µm apart away from the next. Three frozen 10-µm thick sections spanning the entire region of interest were selected for cell counting. Counting was performed on five non-overlapping randomly selected 20x fields per section in the cortex.
Isolation of total RNA and RT-PCR
Total RNA from tissues of WT and GluN3A KO mice was isolated as described before (Lee et al., 2016b). RNA integrity was confirmed by detection of 28S and 18S rRNA band. RNA was confirmed to be free of genomic DNA contamination by PCR in the absence of reverse transcriptase (RT). The RNA samples were reverse transcribed in 20 µl of a reaction mixture containing 2X RT buffer and 20X RT enzyme mix, according to the manufacturer’s instruction (Life Technologies) at 37°C for 60 min. The samples were then incubated at 95°C for 5 min and transferred to 4°C. One µl of RT product was subjected to PCR amplification with 10 pmol primer, 10X standard Taq reaction buffer, 10 mM dNTP, and 0.625 unit Taq polymerase in 20 µl buffer (New England Biolabs Inc., Ipswich, MA, USA). PCR primers were used as follows (5´-3´): for Vasopressin R1a, ATTGCTGGGCTACCTTCATCC (forward) and CCTTGGCGAATTTCTGCGCT (reverse); for Vasopressin R2, GACCCCCCTTTGTGTTGCTCA (forward) and TCAGGAGGGTGTATCCTTCAT (reverse); and for 18s, GACTCAACACGGGAAACCTC (forward) and ATGCCAGAGTCTCGTTCGTT (reverse). PCR mixtures were heated to 95°C for 10 min and cycled 30–37 times for each primer; cycles consisted of 95°C for 15 sec, 60°C for 1 min, and 72°C for 30 sec. After additional incubation at 72°C for 10 min, the PCR samples were transferred to 4°C. PCR products were subjected to electrophoresis in 2% agarose gel with ethidium bromide. Relative intensity of a PCR band was analyzed using InGenius3 manual gel documentation systems (Syngene).
Statistical analysis
GraphPad Prism 6 (GraphPad Software, San Diego, CA, USA) was used for statistical analysis and graphic presentation. Student’s two-tailed t-test was used for the comparison of two experimental groups, and One-way ANOVA followed by Bonferroni correction was used for multiple-group comparisons. Two-way ANOVA followed by Bonferroni correction was used for repeated measurements. Significant differences between groups were identified by a P value of <0.05. All data are presented as Mean ± SEM.
Results
Social interaction deficits of adult GluN3A KO mice
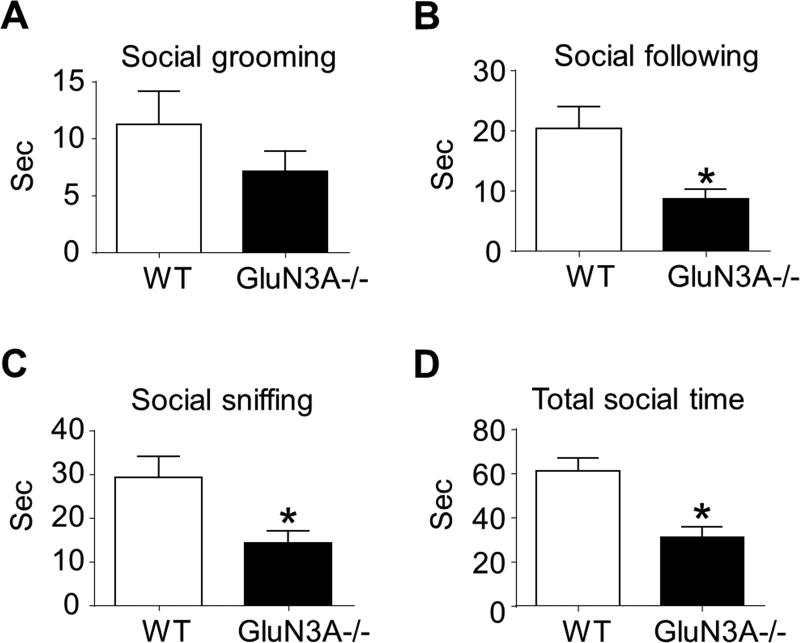
First, we examined whether deletion of GluN3A could influence reciprocal social interaction behaviors in adult mice. The times (durations) of animals spent in social sniffing (nose-to-nose sniffing and nose-to-anogenital sniffing), following, grooming, biting, and wrestling were measured using a TopScan Monitoring System in home cage environment. During 10 min surveillances, GluN3A KO mice spent significant less time in following and sniffing each other and showed a tendency of spending less time in social grooming compared to WT mice (Fig. 1A–1C). Overall, the total time of GluN3A KO mice spent in social behaviors was significantly shorter than that of WT mice (Fig. 1D). No allogrooming, biting, and wrestling behaviors were observed either in WT or GluN3A KO mice during the surveillance time (data not shown).
Figure 1. GluN3A KO mice show deficits in reciprocal social interaction.
A social interaction test was performed to reveal the effect of GluN3A on general social activity. A–D. We scored social sniffing, following, grooming, and total social time to quantify reciprocal social interaction. In this test, the trend of reduced social grooming in GluN3A mice was not statistically significant from WT mice. However, social following and social sniffing time, as well as the total social time, were significantly and markedly reduced in GluN3A KO mice compared to WT mice. Paired t test for A – C; Two-way ANOVA followed by Bonferroni correction: F(12,12)=1.516; *P<0.05 vs. WT; n=13 animals per group.
GluN3A KO mice showed low social approach
Next, we performed the social approach test in different groups using a three-chamber system. In the sociability test, when a stranger mouse was placed into one chamber (phase I), WT mice displayed a preference to the unfamiliar mouse, while GluN3A KO mice did not show any preference for either a familiar or an unfamiliar mouse in the chamber (Fig. 2A). In the social novelty test following the above phase I test, WT animals showed a preference to a novel stranger in the chamber, while GluN3A KO mice preferentially remained in the chamber with the familiar mice. Quantified analysis confirmed that GluN3A KO mice spent significantly less time in exploring the novel mouse chamber than that of WT animals (Fig. 2B).
Figure 2. GluN3A KO mice show differences in preference for social novelty in the social approach test and in the social activity test.
A social approach test using a three-chamber system was performed to determine whether the deletion of GluN3A could affect social approach, especially sociability and social novelty. A. In the first phase (sociability) of the social approach test, WT mice preferred to stay with the stranger mouse, while GluN3A KO mice spent significantly less time in a chamber containing a stranger mouse. *P<0.05 vs. WT; n=13 per group. B. In the second phase (social novelty) of the social approach test, again, WT mice spent most time with the novel stranger in the third chamber, but the time spent by GluN3A KO mice was significantly less compared to WT mice. *P<0.05 vs. WT; n=13 per group. C. The social transmission of food preference task was a measure of social activity. The carton diagrams on the left show the contact time of a demonstrator mouse (black) with cued food (black) and interaction with an observer mouse; the third diagram shows the observer mouse was placed with both cued and non-cued food in a cage 24 hours after staying with the demonstrator mouse. The bar graph summarized the consumed cued and non-cued food in the third cage. WT mice illustrated a substantial preference on cued food, apparently influenced by the demonstrator. On the contrary, GluN3A KO mice showed no significant difference in eating cued and non-cued food. One-way ANOVA followed by Bonferroni correction; F(1, 40)=15, 11, p<0.05, n=14 per group.
GluN3A KO mice showed aberrant social transmission behavior
The social transmission of food preference task was used to confirm social activity changes, taking the advantage that this test does not rely on motor function (Alvarez et al., 2002). After presentation of the cued food (almond) to the demonstrator mouse, the WT mice consumed a significantly greater amount of the cued food than non-cued food (normal diet) (Fig. 2C). However, the GluN3A KO mice showed no such preference between the cued and non-cued food (Fig. 2C). The total food consumed by WT and GluN3A KO mice was about the same. Our previous work showed that GluN3A KO mice exhibited deficits in olfactory functions (Lee et al., 2016a). It is possible that the reduced food preference could be a related consequence.
GluN3A KO mice exhibited normal social memory activity
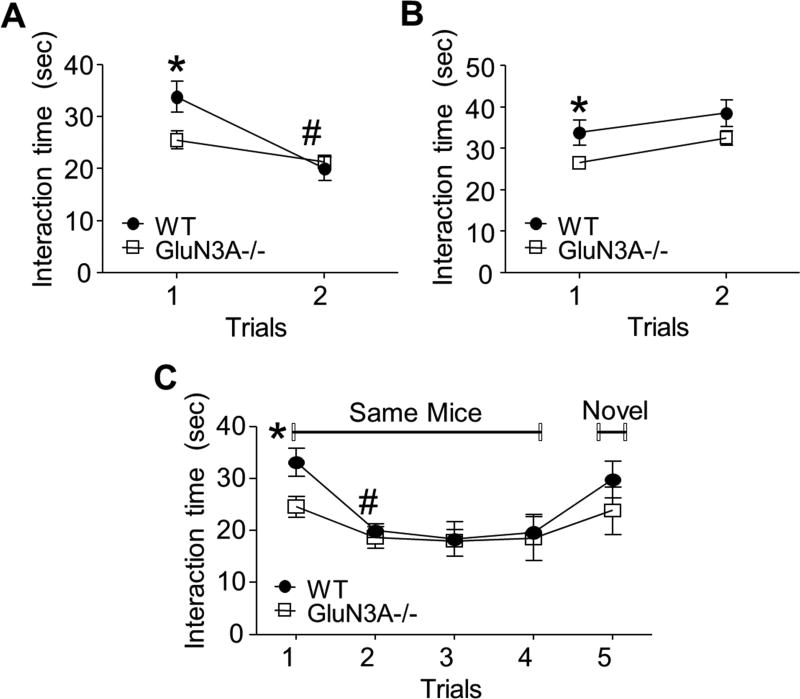
To further determine whether GluN3A could affect the behavior associated with social memory, we performed a social direct interaction test. When the subject mouse was exposed to the same mouse twice within a 1 hr interval, WT mice in trial 2 showed a decreased interaction time (Fig. 3A). GluN3A KO mice showed a trend of shorter interaction time during the second exposure, but the reduction in the interaction behavior was not significant (Fig. 3A). To ensure that the observed effects were directly related to social recognition and memory, not just decreased sociability in general, subject mice in both groups were also exposed to a novel mouse in trial 2. In this event, there were no significant differences between the two trials for either group (Fig. 3B). Comparing GluN3A KO mice to WT mice in this test, GluN3A KO mice had shorter total interaction time in trial 1, which corroborates our previous findings of decreased sociability in GluN3A mice (Fig. 1 and 3).
Figure 3. GluN3A KO mice exhibited normal social memory function.
Direct interaction and five-trial social memory tests were performed to investigate the effect of GluN3A on social memory. A and B. Setup of the social direct interaction test using the same (A) or different (B) mice in the two trials. A. WT mice showed proper habituation behavior with a decreased interaction time in trial 2, which is suggestive of intact social memory. Although GluN3A KO mice displayed relatively decreased habituation across two trials compared to WT mice, as indicated by their decreased interaction time during the second exposure, but the inter-trial difference was not significant (P=0.1157). One-way ANOVA; F(1,10)=5.725, *P<0.05 vs. WT, #P<0.05 vs. same group trial 1; n=7 animals per group. B. When subject mice were exposed to a novel mouse, both groups explored new stimulus mice similarly. One-way ANOVA; F(1.10)=0.06590, *P<0.05 vs. WT; n=7 per group. C. Five-trial social memory assay. Although GluN3A KO mice showed decreased interaction times, both WT and GluN3A KO mice habituated to the same mice during four trials and dishabituated to novel mice. Two-way ANOVA, F(4.40)=0.6176, *P<0.05 vs. WT, #P<0.05 vs. same group; n=7 per group. Student’s two-tailed t-test was applied.
Using new groups of animals, we performed a five-trial social memory test, a more sensitive test of social memory, to gain more conclusive results regarding the social memory behavior. WT mice exhibited an apparent habituation (decreased interacting time) during the first four trials with the same familiar mice and a dishabituation (increased interacting time) in trial 5 with a novel mouse, indicating normal social memory function (Fig. 3C). Like WT mice, GluN3A KO mice showed an apparent habituation (decreased interacting time) during the four exposures to the same mouse. Even though the total interaction time was relatively attenuated as compared to the WT mice, the inter-trial differences were significant, suggestive of relatively intact social memory (Fig. 3C). In trial 5 of exposure to a novel mouse, although there was no significant difference between WT and GluN3A KO mice, GluN3A KO mice had shorter social interaction time similar to that in trial 1 (Fig. 3C). This finding suggests low social activity without social memory deficit in GluN3A KO mice.
Expression of social behavior genes and downregulation of oxytocin receptor in GluN3A KO mice
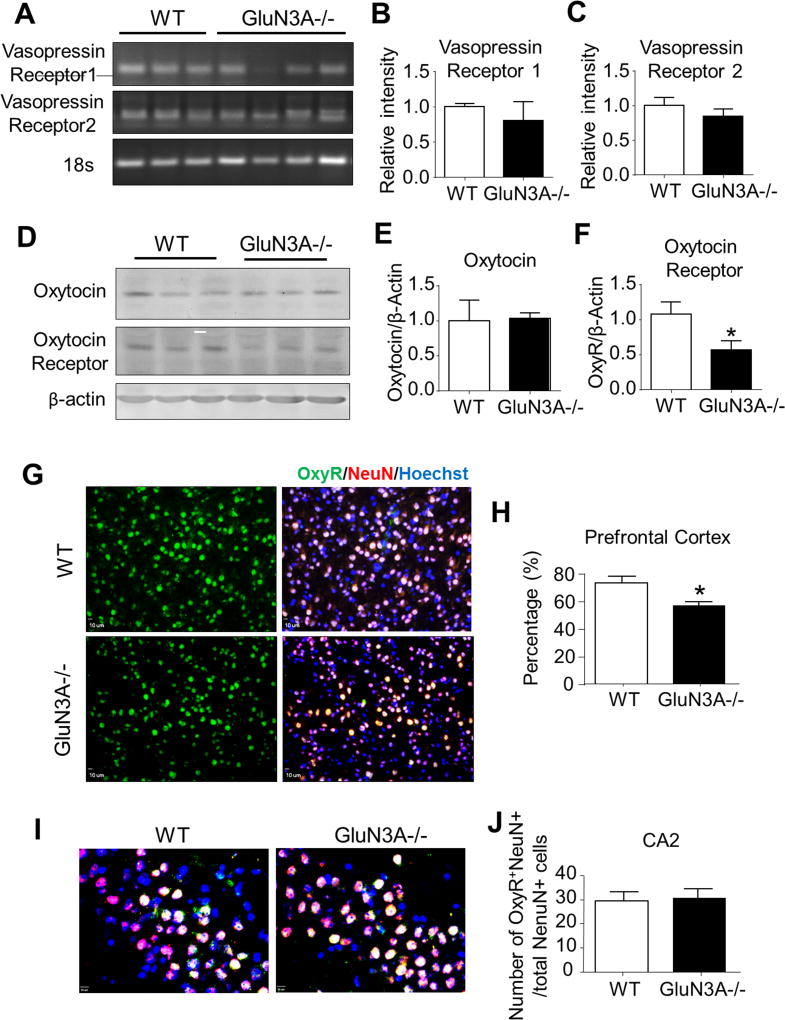
To understand the molecular mechanism as a consequence of deleting GluN3A in the brain, RT-PCR and Western blot analyses were applied to examine of mRNA and protein levels of vasopressin (AVP) receptors 1 and 2, oxytocin, and oxytocin receptor, which are key mediators/regulators of social behaviors and social recognition (Bielsky and Young, 2004; Heinrichs and Domes, 2008; Insel and Shapiro, 1992; Morrison and Melloni, 2014). RT-PCR assays showed no significant change of AVP receptors 1 and 2 in the prefrontal cortex of WT and GluN3A mice (Fig. 4A–4C). In Western blotting, the protein expression of oxytocin receptor in the prefrontal cortex was significantly downregulated in GluN3A KO mice (Fig. 4D and 4F); it’s level in the hippocampus and thalamus showed a trend of reduction (see Fig. 6A and 6B). Meanwhile, no difference was seen in the oxytocin level between WT and GluN3A mice (Fig. 4D and 4E).
Figure 4. Deletion of GluN3A leads to abnormal development of oxytocin receptor.
We next performed RT-PCR analysis, Western blotting, and immunohistochemistry to investigate the possibility that the deletion of GluN3A could alter the development of the oxytocin system and vasopressin system. A. RT-PCR analysis of vasopressin (AVP) receptors 1 and 2 in the prefrontal cortex of WT and GluN3A KO mice. B and C. Quantification of relative intensity (normalized to 18S ribosomal RNA control) of AVP receptors 1 and 2 in the prefrontal cortex. Student t test; n=6 per group. D. Western blotting of oxytocin and oxytocin receptor in the prefrontal cortex from WT and GluN3A KO mice. E and F. Quantification of the optic density (normalized to β-actin control) of oxytocin and oxytocin receptor in the prefrontal cortex. Student t test; *P<0.05 vs. WT; n=6 per group. G. Immunohistochemistry analysis revealed that oxytocin receptor-positive cells were completely co-labeled with NeuN positive cells in the prefrontal cortex. H. In the prefrontal cortex, the ratio of oxytocin receptor (green)-NeuN (red) double-positive cells to total NeuN-positive cells was lower in GluN3A KO mice as compared to WT mice. Student t test; *P<0.05 vs. WT; n=4–5 per group. I. Immunohistochemistry analysis revealed oxytocin receptor-positive cells in CA2 area of hippocampus. Scale bars = 50 µM. J. The number of oxytocin receptor-NeuN double-positive cells was similar in GluN3A KO mice as compared to WT mice. Student t test; *P<0.05 vs. WT; n=4–5 per group.
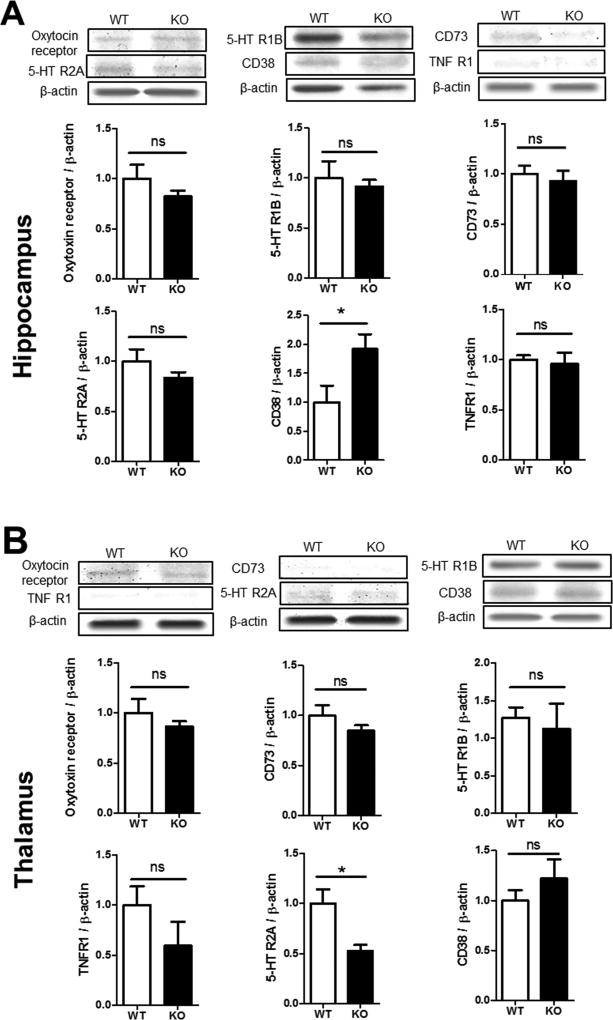
Figure 6. Expression of social behavior genes in the hippocampus and thalamus.
Western blot analysis was performed on brain tissues from the hippocampus and thalamus areas of WT and GluN3A KO mice. A. Western blot images and quantification of the band intensity compared to the basal level. The protein level of oxytocin receptor, serotonin receptors 5-HTR2A, 5-HTR1B, TNFR1 and CD73 showed no statistically difference between WT and KO mice. The level of CD38, however, was significantly higher in the KO hippocampus. N=6, Student t test; * p<0.05, F=1.299. B. The above assay was repeated in the thalamus. All measured protein expressions were similar between WT and KO mice, expect the 5-HTR2A level was significantly lower in this brain region. N=6; Student t test; * p<0.05, F=5.650.
In immunostaining analysis, the oxytocin receptor immunoreactivity was mainly colocalized with NeuN positive cells, (Fig. 4G), suggesting oxytocin receptors were primarily expressed in neuronal cells. Consistent with Western blot data, the oxytocin receptor level in the prefrontal cortex was particularly low in GluN3A KO mice compared to that in WT mice (Fig. 4H). No significant changes in oxytocin receptor in the hippocampal CA2 region (Fig. 4I and 4J).
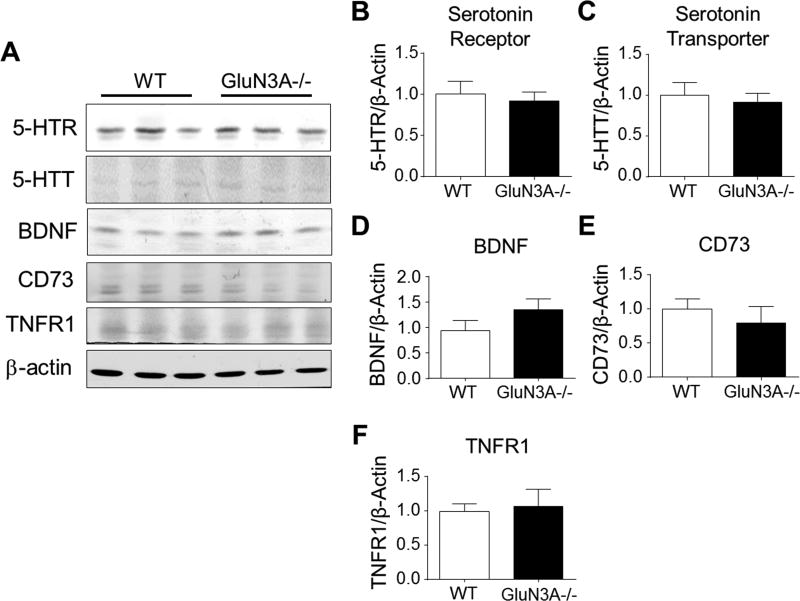
In Western blot assays, the levels of serotonin receptor (5-HTR) and serotonin transporter (5-HTT) in the cortex showed no difference between WT and GluN3A KO mice (5A-5C). Brain-derived neurotrophic factor (BDNF), cluster of differentiation 73 (CD73), and tumor necrosis factor receptor 1 (TNFR1) were also measured due to their potential roles in social activities (Fanous et al., 2011; Ito et al., 2011; Kulesskaya et al., 2013; Simen et al., 2006); no difference of their protein expression was detected in the prefrontal cortex (Fig, 5A and 5F).
Figure 5. GluN3A had no effect on the development of serotonin regulation, BDNF, CD73, and TNFR1.
We investigated the possibility that the deletion of GluN3A could altered social behavior-associated genes such as serotonin receptor (5-HTR), serotonin transporter (5-HTT), BDNF, CD73, and TNFR1. A. Western blotting of serotonin receptor, serotonin transporter, BDNF, CD73, and TNFR1 in the prefrontal cortex from WT and GluN3A KO mice. B-F. Quantification of the optic density (normalized to β-actin control) of 5-HTR, 5-HTT, BDNF, CD73, and TNFR1 in the prefrontal cortex. The expression of these genes was also unchanged in GluN3A KO mice. N=6 per group.
On the other hand, protein expression profile showed a different pattern in the hippocampus and thalamus. In the hippocampus, the levels of oxytocin receptor, 5-HTR1B/2A, CD73, and TNFR1 were no different between WT and KO mice (Fig. 6A). Interestingly, CD38, a multifunctional ectoenzyme shown to induce oxytocin release in the brain (Salmina et al., 2013; Young, 2007) was significantly higher in the hippocampus of GluN3A KO mice, presumably plays a compensatory role in the chronic absence of GluN3A (Fig. 6A). In the thalamus, the levels of oxytocin receptor, 5-HTR1B, TNFR1, and CD73/38 were similar between WT and GluN3A KO mice (Fig. 6B). However, 5-HTR2A was significantly lower in the thalamus (Fig. 6B), which is a brain region related to the regulation of mood/sleep, sensory processing and even cognitive functions (Barre et al., 2016; McGregor et al., 2003). This observation implies that a lower serotonin modulatory tone might contribute partly to altered psychological or social behaviors.
Oxytocin rescued the social activity deficits in GluN3A KO mice
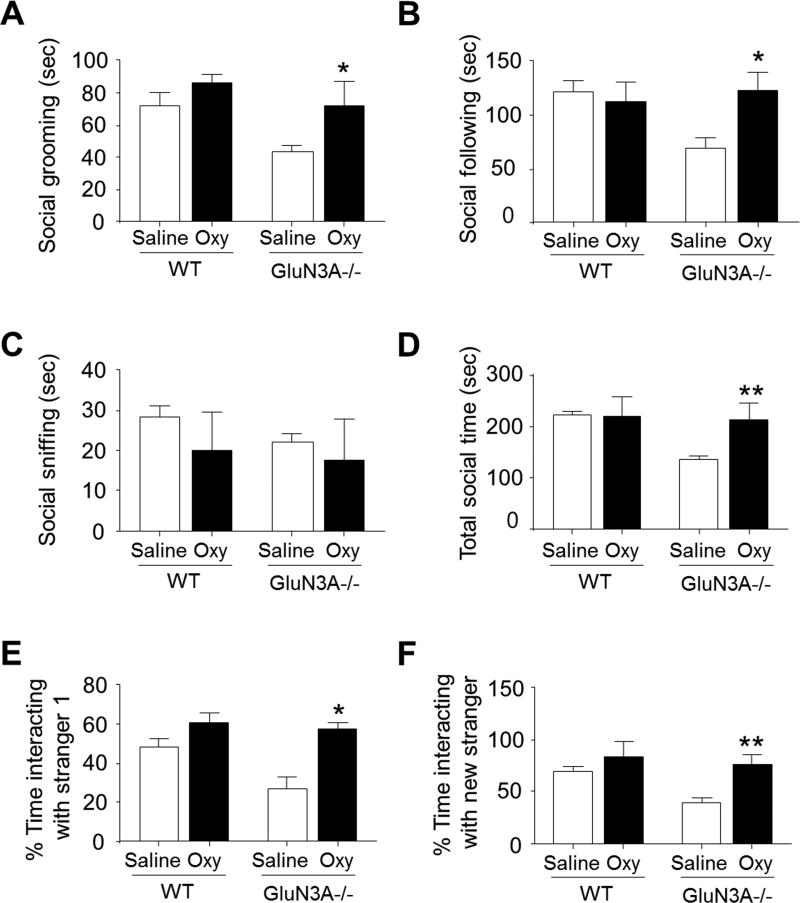
To determine whether the oxytocin system could contribute centrally to the impairment of social activity in GluN3A KO mice, we treated new groups of WT and GluN3A KO mice with oxytocin (10 mg/kg). All mice were administered oxytocin intraperitoneally (i.p.) for 7 days, and then social activity was examined using social interaction and social approach tests. In the social interaction test, although oxytocin did not show significant effect on social sniffing, oxytocin-treated GluN3A KO mice spent significant more time in social grooming and following compared to controls. Consistently, the total social time for oxytocin-treated GluN3A KO mice was significantly increased (Fig. 7A-7D). In the sociability test, oxytocin supplement significantly corrected the social behavioral deficit in GluN3A KO mice, and increased their preference for an unfamiliar mouse in the chamber (Fig. 7E). Moreover, in the social novelty test, oxytocin-treated GluN3A KO mice spent significantly more time in exploring the novel mouse chamber than that of saline-treated GluN3A KO mice (Fig.76F). In these two tests, oxytocin did not show significant effect on WT mice (Fig. 7).
Figure 7. Oxytocin treatment rescues impaired social behaviors in GluN3A KO mice.
Oxytocin (Oxy; 10 mg/kg) was intraperitoneally administered once per day for 7 days in adult WT and GluN3A KO mice. A–D. In the social interaction test, oxytocin treatment rescued the social deficits in social grooming, social following and, consequently, increased the total social time of GluN3A KO mice. One-way ANOVAP; F(1,16)=0.4797 (A), F(1,16)=5.010 (B), F(1,16)=0.2161 (C), F(1,16)=10.21 (D), *P<0.05 vs. saline-treated GluN3A KO mice, **P<0.01 vs. saline-treated GluN3A KO mice; n=5 per group. E. In the sociability test of three chamber system, oxytocin-treated GluN3A KO mice spent significantly more time in the chamber containing a stranger mouse. One-way ANOVA, F(1,8)=3.809, *P<0.05 vs. saline-treated GluN3A KO mice; n=5 per group. F. In the social novelty test, GluN3A KO mice showed social deficits of reduced interaction with a stranger while oxytocin treatment completely corrected this problem to the WT control level. One-way ANOVA, F(1,8)=0.8830; **P<0.01 vs. saline-treated GluN3A KO; n=5 per group.
Discussion
The present investigation focused on social behavioral activities, tested the hypothesis that under a chronic state of moderate increases in NMDAR activities such as removal of GluN3A from NMDARs, alterations of gene expression may lead to social deficits in adults. In a variety of social behavioral tests, we identified low social interactions and reduced social approach, suggesting impaired social behaviors. The impaired olfactory function revealed in our previous investigation (Lee et al., 2016a) may contribute to reduced social activities. In searching for a molecular mechanism mediating the observed effects, we identified low levels of the oxytocin receptor in the prefrontal cortex of GluN3A KO mice and showed that application of oxytocin rescues the GluN3A-associated social deficits. In addition, a reduced level of serotonin receptor 5-HTR2A in the thalamus may also play a role in the behavioral change. These results provide novel evidence for the signaling pathway linking the chronic NMDAR activity to the behavioral abnormality in adult animals. The expression of GluN3A, as a unique inhibitory NMDAR subunit, thus plays a regulatory role in the development and/or execution of normal social behavioral activities.
Recent progresses in basic and clinical research have identified critical periods of brain development that can have imperative impacts on behavioral and functional activities in adulthood (Ismail et al., 2017; Tucker et al., 2015; Tzanoulinou and Sandi, 2017). Among mechanisms that can be responsible for the long-term consequence of brain development, NMDA receptor expression and activities play important roles (Harris et al., 2003). Developmental changes in the composition of NMDA receptors can alter receptor physiology as well as intracellular signal pathways, potentially affecting thresholds for neural and behavioral plasticity. For example, neonatal ketamine exposure may cause NMDAR hypofunction, and led to altered NMDAR profile and long-term functional alterations persisting in adulthood (Akillioglu et al., 2012; Lecointre et al., 2015; Newcomer and Krystal, 2001). Results from monkey studies suggest that chronic developmental exposure to high doses of a NMDA antagonist has pronounced and long-lasting effects on learning (Haberny et al., 2002). There is evidence that developmental abnormalities occurring during the second trimester of pregnancy may play a role in the pathophysiology of schizophrenia (Marenco and Weinberger, 2000). GluN3A is highly expressed in the rat brain during development, peaking around postnatal day seven and subsequently decreasing over time (Ciabarra et al., 1995; Sucher et al., 1995). Similarly, GluN3A is robustly expressed in the human fetal brain during the second trimester (Mueller and Meador-Woodruff, 2003). The GluN3A subunit is expressed in regions associated with psychological disorders, including the prefrontal cortex, hippocampus, thalamus, hypothalamus, and fthe amygdaloid nuclei (Wong et al., 2002). It is likely that the level of GluN3A may show developmental impacts in a region-specific manner. Supporting this idea, in brains of people with schizophrenia, bipolar disorder, and depression, the GluN3A transcript level was elevated by 32% in schizophrenia relative to controls in the dorsolateral prefrontal cortex (DLPFC) restricted to gyral aspects of the DLPFC (Mueller and Meador-Woodruff, 2004). On the other hand, GluN3A mRNA significantly decreased by 12% in bipolar disorder relative to the comparison group in DLPFC (Mueller and Meador-Woodruff, 2004). Future studies may be needed to verify whether the absence or reduced levels of GluN3A in a specific brain region during development shows spatial specificity for changes in behavioral and psychological functions in adulthood.
Studies from human and animal models suggest that alterations of the expression, trafficking, and function of ionotropic glutamate receptors lead to distorted synapse development and plasticity in an ASD-specific manner (Uzunova et al., 2014). To date, there are hundreds of genes and factors speculated to be involved in normal social behavior and social disorders (Hoffmann and Spengler, 2014). Of these, the major groups include: neurohypophyseal peptides oxytocin and vasopressin (AVP) and their respective pathways, serotonin systems, and growth factors such as BDNF and inflammatory genes (Donaldson and Young, 2008; Insel et al., 1999). Serotonin has long been known to play a role in a variety of behaviors, including appetite, depression, emotion, and autonomic activity (Kaufman et al., 2016; Singh, 2014). Given these connections, we investigated the possible link between the social abnormalities observed with GluN3A KO mice and the underlying serotonin pathways. Interestingly, we 5-HTR2A is selectively reduced in the thalamus, a brain region relevant to emotional and psychological behaviors. This data is consistent with a previous report that NMDA treatment in rats decreased serotonin receptor densities in the cortex and/or thalamus, which could be responsible for emotional behavioral changes (McGregor et al., 2003). On the other hand, the levels of 5-HTR1B and 5-HTT showed no difference among the prefrontal cortex, hippocampus, and thalamus. Collectively, this is consistent with modest results in targeting serotoninergic pathways to reverse social deficits (Gordon et al., 1993; McBride et al., 1996; McDougle et al., 1996).
The two critical neuroendocrine peptides, AVP and oxytocin are frequently studied together, given their similarities in structure (9-amino acid peptides, or nonapeptides) and origin. Although their primary functions are different, both of these nonapeptides have been implicated in social behavior, and their dysregulations lead to abnormalities in social activity (Donaldson and Young, 2008; Insel et al., 1999; Silverman et al., 2007). In the present study, we investigated both the oxytocin and AVP systems as potential candidates for the etiology of the GluN3A-associated social deficits. We obtained the novel evidence showing significantly downregulated oxytocin receptor levels in the prefrontal cortex. The prefrontal cortex has been implicated as an integral region for processing social behaviors, and lesions in the prefrontal cortex lead to social-specific impairments, such as group dis-cohesiveness (Anderson et al., 1999; Myers, 1972). Therefore, a significantly low level of oxytocin receptor and its signaling activity in this region should have notable impacts on related functions. This idea is supported by the observation that oxytocin treatment in GluN3A KO mice largely ameliorated the social behavior deficits. The other region we focused on was CA2 of the hippocampus, and we did not observe any differences in oxytocin receptor in the area. This negative data is consistent with the observations that the CA2 region is most responsible for social memory (Hitti and Siegelbaum, 2014; Tirko et al., 2014). It was shown in a recent study that acutely administration of PEAQX, a GluN2A containing NMDA receptor antagonist, induced interruption on spatial working memory of 8-week old mice (Green et al., 2016). This acute effect of GluN2A blockade is consistent with the common notion that adequate NMDAR activity is essential for learning and memory. Without drug treatment, we did not detect significant differences in social memory between young adult WT and GluN3A KO mice. The memory interval (1 hour) in the test examined relatively short-term memory. Long-term memory changes in the absence of GluN3A remain to be verified.
Oxytocin has attracted more attention due to its direct social-related functions, given its effects on maternal events, such as parturition and lactation (Soloff et al., 1979). Oxytocin exposure leads to restoring social behavior in the mouse models of autism (Bales et al., 2014; Penagarikano et al., 2015). In a more recent report, oxytocin treatment of 1 mg/kg i.p. injections promotes hippocampal cell proliferation and dendritic maturation, and affects socio-emotional behavior (Sanchez-Vidana et al., 2016). In fact, intranasal delivery of oxytocin is already being used in pediatric populations as a way to restore and even augment sociability, and is effective for ameliorating the social deficits associated with post-traumatic stress disorder (PTSD) (Dadds et al., 2014; Koch et al., 2014). In the present investigation, oxytocin was administered systematically. A recent fMRI imaging study showed that peripheral injections of oxytocin (0.1 to 2.5 mg/kg) affected all subdivisions of the olfactory bulb, in addition to the cerebellum and several brainstem areas relevant to the autonomic nervous system (Ferris et al., 2015). The authors concluded that the patterns of brain activity suggest that either intranasally or systematic administered oxytocin can interact with central neurons. A high dosage of 10 mg/kg oxytocin was injected in our experiments compared with the previously reported dosage of 1 mg/kg (Sanchez-Vidana et al., 2016); presumably, more oxytocin could reach to brain tissues. A detail dose-response effect of oxytocin treatments should be performed in future translational studies on animal models and human subjects.
It is possible that the social behavior deficits are partly due to or related to sensorimotor dysfunction such as reduced olfactory sensitivity. In fact, patients with neurodegenerative diseases exhibit smell dysfunction ranging from severe loss, as seen in Alzheimer’s and Parkinson’s diseases, to relatively minor deficits, as shown in progressive supranuclear palsy (Doty, 2017; Umeda-Kameyama et al., 2017). It is speculated that differential disruption of a common primordial neuropathogenesis may cause these differences in olfactory function. Neurotransmitters and receptors may involve in this process, but so far there has been no clear clue for the pathophysiological basis. Moreover, some recent studies have linked sustained moderate activation of NMDA receptors to the neurodegenerative changes in AD (Kocahan and Dogan, 2017). The NMDAR related excitotoxicity hypothesis is supported by clinical evidence indicating that the NMDA receptor antagonist memantine slows AD progression (Parsons et al., 1998). Collectively, available data point to the idea that knocking out GluN3A results in a chronic state of moderate activation of the NMDAR containing GluN1 and GluN2, providing a potential causal relationship between glutamate chronic excitotoxicity, olfactory dysfunction, and neurodegenerative diseases.
CD73 (also known as ecto-5’-nucleotidase) is a cell surface enzyme that regulates purinergic signaling by dephosphorylating extracellular AMP to adenosine. A recent paper showed that CD73 deficient mice displayed altered social behavior, suggesting that CD73 can be involved in the process of social behaviors (Kulesskaya et al., 2013). In the present investigation, we did not observe significant alterations in CD73 expression in several brain regions. Interestingly and potential important, we observed that the CD38 expression was significantly higher in the hippocampus of GluN3A KO mice. CD38 is shown to promote oxytocin release, so this observation was somehow unexpected in animals of defective in social behaviors (Salmina et al., 2013; Young, 2007). We suspect that the increased CD38 is likely an endogenous compensatory mechanism under the condition of weakened oxytocin signaling as seen in the GluN3A KO mouse.
The link between GluN3A and the oxytocin system may provide another avenue for targeting social disorders. Since our result was obtained from mice lacking the GluN3A subunit throughout the life span, more experiments using a conditional knockout technique may be helpful to delineate whether there is a critical developmental period of the inhibitory regulation on NMDAR activities associated with the social activity deficits. Otherwise, the changes are simply due to absence of the inhibitory mechanism in adulthood. Clinically, the loss of olfactory function is chronically developed, but still precedes the appearance of cognitive deficits. Therefore, olfactory dysfunction has been suggested as a clinical marker for identifying vulnerable individuals for Alzheimer’s disease (Doty, 2017; Umeda-Kameyama et al., 2017). This is consistent with our observation that although young adult GluN3A KO mice show deficits in social behavior and olfactory function, their cognitive activities remain relatively normal. It will be important to delineate whether aged GluN3A KO mice are more vulnerable to cognitive deficits. The sex difference should be examined in future investigations. Our findings suggest that more attention on this NMDAR subunit is warranted, especially in the context of social abnormalities of neuropsychiatric and neurodegenerative diseases.
Acknowledgments
This work was supported by NIH grants NS073378, NS075338, NS091585, a VA National Merit grant RX000666, American Heart Association Postdoctoral Fellowship grants 15POST25680013, 15POST25710112, 14POST20130024, and a NIH Predoctoral Fellowship T32 007480-15.
Abbreviations
- GluN3A
Glutamate NMDA receptor subunit 3A or NR3A
- NMDA
N-Methyl-D-Aspartate
- NMDAR
N-Methyl-D-Aspartate receptor
- KO
Knockout
- WT
Wild type
- CNS
Central nervous system
- ASD
Autism spectrum disorders
- AVP
Vasopressin
- 5-HTR
Serotonin receptor
- 5-HTT
Serotonin transporter
- BDNF
Brain-derived neurotrophic factor
- CD38
Cluster of differentiation 38
- CD73
Cluster of differentiation 73
- TNFR1
Tumor necrosis factor receptor 1
Footnotes
Conflict of Interest
Authors declare no conflict of interest associated with this investigation.
References
- Akillioglu K, Binokay S, Kocahan S. The effect of neonatal N-methyl-D-aspartate receptor blockade on exploratory and anxiety-like behaviors in adult BALB/c and C57BL/6 mice. Behav Brain Res. 2012;233:157–161. doi: 10.1016/j.bbr.2012.04.041. [DOI] [PubMed] [Google Scholar]
- Al-Hallaq RA, Jarabek BR, Fu Z, Vicini S, Wolfe BB, Yasuda RP. Association of NR3A with the N-methyl-D-aspartate receptor NR1 and NR2 subunits. Mol Pharmacol. 2002;62:1119–1127. doi: 10.1124/mol.62.5.1119. [DOI] [PubMed] [Google Scholar]
- Alvarez P, Wendelken L, Eichenbaum H. Hippocampal formation lesions impair performance in an odor-odor association task independently of spatial context. Neurobiol Learn Mem. 2002;78:470–476. doi: 10.1006/nlme.2002.4068. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nature Neurosci. 1999;2:1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Bales KL, Solomon M, Jacob S, Crawley JN, Silverman JL, Larke RH, Sahagun E, Puhger KR, Pride MC, Mendoza SP. Long-term exposure to intranasal oxytocin in a mouse autism model. Transl Psychiatry. 2014;4:e480. doi: 10.1038/tp.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barre A, Berthoux C, De Bundel D, Valjent E, Bockaert J, Marin P, Becamel C. Presynaptic serotonin 2A receptors modulate thalamocortical plasticity and associative learning. Proc Natl Acad Sci U S A. 2016;113:E1382–1391. doi: 10.1073/pnas.1525586113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendel O, Prunell G, Stenqvist A, Mathiesen T, Holmin S, Svendgaard NA, Euler G. Experimental subarachnoid hemorrhage induces changes in the levels of hippocampal NMDA receptor subunit mRNA. Brain Res Mol Brain Res. 2005;137:119–125. doi: 10.1016/j.molbrainres.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Bettler B, Mulle C. Review: neurotransmitter receptors. II. AMPA and kainate receptors. Neuropharmacol. 1995;34:123–139. doi: 10.1016/0028-3908(94)00141-e. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Young LJ. Oxytocin, vasopressin, and social recognition in mammals. Peptides. 2004;25:1565–1574. doi: 10.1016/j.peptides.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Cavara NA, Hollmann M. Shuffling the deck anew: how NR3 tweaks NMDA receptor function. Mol Neurobiol. 2008;38:16–26. doi: 10.1007/s12035-008-8029-9. [DOI] [PubMed] [Google Scholar]
- Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- Ciabarra AM, Sullivan JM, Gahn LG, Pecht G, Heinemann S, Sevarino KA. Cloning and characterization of chi-1: a developmentally regulated member of a novel class of the ionotropic glutamate receptor family. J Neurosci. 1995;15:6498–6508. doi: 10.1523/JNEUROSCI.15-10-06498.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadds MR, MacDonald E, Cauchi A, Williams K, Levy F, Brennan J. Nasal oxytocin for social deficits in childhood autism: a randomized controlled trial. J Autism and Developmental Disorders. 2014;44:521–531. doi: 10.1007/s10803-013-1899-3. [DOI] [PubMed] [Google Scholar]
- Das S, Sasaki YF, Rothe T, Premkumar LS, Takasu M, Crandall JE, Dikkes P, Conner DA, Rayudu PV, Cheung W, Chen HS, Lipton SA, Nakanishi N. Increased NMDA current and spine density in mice lacking the NMDA receptor subunit NR3A. Nature. 1998;393:377–381. doi: 10.1038/30748. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science (New York, N.Y.) 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Doty RL. Olfactory dysfunction in neurodegenerative diseases: is there a common pathological substrate? Lancet Neurol. 2017;16:478–488. doi: 10.1016/S1474-4422(17)30123-0. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Moy SS, Perez A, Eddy DM, Zinzow WM, Lieberman JA, Snouwaert JN, Koller BH. Deficits in sensorimotor gating and tests of social behavior in a genetic model of reduced NMDA receptor function. Behav Brain Res. 2004;153:507–519. doi: 10.1016/j.bbr.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Fanous S, Terwilliger EF, Hammer RP, Jr, Nikulina EM. Viral depletion of VTA BDNF in rats modulates social behavior, consequences of intermittent social defeat stress, and long-term weight regulation. Neurosci Lett. 2011;502:192–196. doi: 10.1016/j.neulet.2011.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Yee JR, Kenkel WM, Dumais KM, Moore K, Veenema AH, Kulkarni P, Perkybile AM, Carter CS. Distinct BOLD Activation Profiles Following Central and Peripheral Oxytocin Administration in Awake Rats. Front Behav Neurosci. 2015;9:245. doi: 10.3389/fnbeh.2015.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaya M, Hayashi Y, Watanabe M. NR2 to NR3B subunit switchover of NMDA receptors in early postnatal motoneurons. Eur J Neurosci. 2005;21:1432–1436. doi: 10.1111/j.1460-9568.2005.03957.x. [DOI] [PubMed] [Google Scholar]
- Ghanizadeh A. Targeting of glycine site on NMDA receptor as a possible new strategy for autism treatment. Neurochem Res. 2011;36:922–923. doi: 10.1007/s11064-010-0381-2. [DOI] [PubMed] [Google Scholar]
- Gladding CM, Raymond LA. Mechanisms underlying NMDA receptor synaptic/extrasynaptic distribution and function. Mol Cell Neurosci. 2011;48:308–320. doi: 10.1016/j.mcn.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Gordon CT, State RC, Nelson JE, Hamburger SD, Rapoport JL. A double-blind comparison of clomipramine, desipramine, and placebo in the treatment of autistic disorder. Arch General Psychiatry. 1993;50:441–447. doi: 10.1001/archpsyc.1993.01820180039004. [DOI] [PubMed] [Google Scholar]
- Green TL, Burket JA, Deutsch SI. Age-dependent effects on social interaction of NMDA GluN2A receptor subtype-selective antagonism. Brain Res Bull. 2016;125:159–167. doi: 10.1016/j.brainresbull.2016.06.017. [DOI] [PubMed] [Google Scholar]
- Haberny KA, Paule MG, Scallet AC, Sistare FD, Lester DS, Hanig JP, Slikker W., Jr Ontogeny of the N-methyl-D-aspartate (NMDA) receptor system and susceptibility to neurotoxicity. Toxicol Sci. 2002;68:9–17. doi: 10.1093/toxsci/68.1.9. [DOI] [PubMed] [Google Scholar]
- Harris LW, Sharp T, Gartlon J, Jones DN, Harrison PJ. Long-term behavioural, molecular and morphological effects of neonatal NMDA receptor antagonism. Eur J Neurosci. 2003;18:1706–1710. doi: 10.1046/j.1460-9568.2003.02902.x. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Domes G. Neuropeptides and social behaviour: effects of oxytocin and vasopressin in humans. Prog Brain Res. 2008;170:337–350. doi: 10.1016/S0079-6123(08)00428-7. [DOI] [PubMed] [Google Scholar]
- Henson MA, Roberts AC, Perez-Otano I, Philpot BD. Influence of the NR3A subunit on NMDA receptor functions. Prog Neurobiol. 2010;91:23–37. doi: 10.1016/j.pneurobio.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitti FL, Siegelbaum SA. The hippocampal CA2 region is essential for social memory. Nature. 2014;508:88–92. doi: 10.1038/nature13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A, Spengler D. DNA memories of early social life. Neurosci. 2014;264:64–75. doi: 10.1016/j.neuroscience.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Insel TR, O’Brien DJ, Leckman JF. Oxytocin, vasopressin, and autism: is there a connection? Biological Psychiatry. 1999;45:145–157. doi: 10.1016/s0006-3223(98)00142-5. [DOI] [PubMed] [Google Scholar]
- Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci U S A. 1992;89:5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail FY, Fatemi A, Johnston MV. Cerebral plasticity: Windows of opportunity in the developing brain. Eur J Paediatr Neurol. 2017;21:23–48. doi: 10.1016/j.ejpn.2016.07.007. [DOI] [PubMed] [Google Scholar]
- Ito W, Chehab M, Thakur S, Li J, Morozov A. BDNF-restricted knockout mice as an animal model for aggression. Genes Brain Behav. 2011;10:365–374. doi: 10.1111/j.1601-183X.2010.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson U, Sjodin J, Angeby Moller K, Johansson S, Wikstrom L, Nasstrom J. Glutamate-induced currents reveal three functionally distinct NMDA receptor populations in rat dorsal horn - effects of peripheral nerve lesion and inflammation. Neurosci. 2002;112:861–868. doi: 10.1016/s0306-4522(02)00140-9. [DOI] [PubMed] [Google Scholar]
- Kaufman J, DeLorenzo C, Choudhury S, Parsey RV. The 5-HT1A receptor in Major Depressive Disorder. Eur Neuropsychopharmacol. 2016;26:397–410. doi: 10.1016/j.euroneuro.2015.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocahan S, Dogan Z. Mechanisms of Alzheimer’s Disease Pathogenesis and Prevention: The Brain, Neural Pathology, N-methyl-D-aspartate Receptors, Tau Protein and Other Risk Factors. Clin Psychopharmacol Neurosci. 2017;15:1–8. doi: 10.9758/cpn.2017.15.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch SBJ, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M. Intranasal oxytocin as strategy for medication-enhanced psychotherapy of PTSD: salience processing and fear inhibition processes. Psychoneuroendocrinol. 2014;40:242–256. doi: 10.1016/j.psyneuen.2013.11.018. [DOI] [PubMed] [Google Scholar]
- Kulesskaya N, Voikar V, Peltola M, Yegutkin GG, Salmi M, Jalkanen S, Rauvala H. CD73 is a major regulator of adenosinergic signalling in mouse brain. PLoS One. 2013;8:e66896. doi: 10.1371/journal.pone.0066896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie V, Lipina T, Roder JC. Mice with reduced NMDA receptor glycine affinity model some of the negative and cognitive symptoms of schizophrenia. Psychopharmacol (Berl) 2008;200:217–230. doi: 10.1007/s00213-008-1196-6. [DOI] [PubMed] [Google Scholar]
- Lecointre M, Vezier C, Benard M, Ramdani Y, Dupre N, Brasse-Lagnel C, Henry VJ, Roy V, Marret S, Gonzalez BJ, Jegou S, Leroux-Nicollet I. Age-dependent alterations of the NMDA receptor developmental profile and adult behavior in postnatally ketamine-treated mice. Dev Neurobiol. 2015;75:315–333. doi: 10.1002/dneu.22232. [DOI] [PubMed] [Google Scholar]
- Lee JC, Greig A, Ravindranathan A, Parks TN, Rao MS. Molecular analysis of AMPA-specific receptors: subunit composition, editing, and calcium influx determination in small amounts of tissue. Brain Res Brain Res Protoc. 1998;3:142–154. doi: 10.1016/s1385-299x(98)00035-x. [DOI] [PubMed] [Google Scholar]
- Lee JH, Wei L, Deveau TC, Gu X, Yu SP. Expression of the NMDA receptor subunit GluN3A (NR3A) in the olfactory system and its regulatory role on olfaction in the adult mouse. Brain Struct Funct. 2016a;221:3259–3273. doi: 10.1007/s00429-015-1099-3. [DOI] [PubMed] [Google Scholar]
- Lee JH, Wei ZZ, Cao W, Won S, Gu X, Winter M, Dix TA, Wei L, Yu SP. Regulation of therapeutic hypothermia on inflammatory cytokines, microglia polarization, migration and functional recovery after ischemic stroke in mice. Neurobiol Dis. 2016b;96:248–260. doi: 10.1016/j.nbd.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Wei ZZ, Chen D, Gu X, Wei L, Yu SP. A neuroprotective role of the NMDA receptor subunit GluN3A (NR3A) in ischemic stroke of the adult mouse. Am J Physiol Cell Physiol. 2015;308:C570–577. doi: 10.1152/ajpcell.00353.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marenco S, Weinberger DR. The neurodevelopmental hypothesis of schizophrenia: following a trail of evidence from cradle to grave. Dev Psychopathol. 2000;12:501–527. doi: 10.1017/s0954579400003138. [DOI] [PubMed] [Google Scholar]
- McBride PA, Anderson GM, Shapiro T. Autism research. Bringing together approaches to pull apart the disorder. Archiv General Psychiatry. 1996;53:980–983. doi: 10.1001/archpsyc.1996.01830110011002. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Naylor ST, Cohen DJ, Volkmar FR, Heninger GR, Price LH. A double-blind, placebo-controlled study of fluvoxamine in adults with autistic disorder. Archiv General Psychiatry. 1996;53:1001–1008. doi: 10.1001/archpsyc.1996.01830110037005. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Clemens KJ, Van der Plasse G, Li KM, Hunt GE, Chen F, Lawrence AJ. Increased anxiety 3 months after brief exposure to MDMA (“Ecstasy”) in rats: association with altered 5-HT transporter and receptor density. Neuropsychopharmacol. 2003;28:1472–1484. doi: 10.1038/sj.npp.1300185. [DOI] [PubMed] [Google Scholar]
- Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130:1007S–1015S. doi: 10.1093/jn/130.4.1007S. [DOI] [PubMed] [Google Scholar]
- Mohamad O, Song M, Wei L, Yu SP. Regulatory roles of the NMDA receptor GluN3A subunit in locomotion, pain perception and cognitive functions in adult mice. J Physiol. 2013;591:149–168. doi: 10.1113/jphysiol.2012.239251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- Mooney SJ, Douglas NR, Holmes MM. Peripheral administration of oxytocin increases social affiliation in the naked mole-rat (Heterocephalus glaber) Horm Behav. 2014;65:380–385. doi: 10.1016/j.yhbeh.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Morrison TR, Melloni RH., Jr The role of serotonin, vasopressin, and serotonin/vasopressin interactions in aggressive behavior. Curr Top Behav Neurosci. 2014;17:189–228. doi: 10.1007/7854_2014_283. [DOI] [PubMed] [Google Scholar]
- Mota SI, Ferreira IL, Valero J, Ferreiro E, Carvalho AL, Oliveira CR, Rego AC. Impaired Src signaling and post-synaptic actin polymerization in Alzheimer’s disease mice hippocampus--linking NMDA receptors and the reelin pathway. Exp Neurol. 2014;261:698–709. doi: 10.1016/j.expneurol.2014.07.023. [DOI] [PubMed] [Google Scholar]
- Mueller HT, Meador-Woodruff JH. Expression of the NR3A subunit of the NMDA receptor in human fetal brain. Ann N Y Acad Sci. 2003;1003:448–451. doi: 10.1196/annals.1300.049. [DOI] [PubMed] [Google Scholar]
- Mueller HT, Meador-Woodruff JH. NR3A NMDA receptor subunit mRNA expression in schizophrenia, depression and bipolar disorder. Schizophr Res. 2004;71:361–370. doi: 10.1016/j.schres.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Abdallah CG, Mathew SJ. Targeting glutamate signalling in depression: progress and prospects. Nat Rev Drug Discov. 2017;16:472–486. doi: 10.1038/nrd.2017.16. [DOI] [PubMed] [Google Scholar]
- Myers RE. Role of prefrontal and anterior temporal cortex in social behavior and affect in monkeys. Acta Neurobiologiae Experimentalis. 1972;32:567–579. [PubMed] [Google Scholar]
- Nakanishi S, Masu M. Molecular diversity and functions of glutamate receptors. Annu Rev Biophys Biomol Struct. 1994;23:319–348. doi: 10.1146/annurev.bb.23.060194.001535. [DOI] [PubMed] [Google Scholar]
- Neill JC, Harte MK, Haddad PM, Lydall ES, Dwyer DM. Acute and chronic effects of NMDA receptor antagonists in rodents, relevance to negative symptoms of schizophrenia: a translational link to humans. Eur Neuropsychopharmacol. 2014;24:822–835. doi: 10.1016/j.euroneuro.2013.09.011. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Krystal JH. NMDA receptor regulation of memory and behavior in humans. Hippocampus. 2001;11:529–542. doi: 10.1002/hipo.1069. [DOI] [PubMed] [Google Scholar]
- Niemann S, Kanki H, Fukui Y, Takao K, Fukaya M, Hynynen MN, Churchill MJ, Shefner JM, Bronson RT, Brown RH, Jr, Watanabe M, Miyakawa T, Itohara S, Hayashi Y. Genetic ablation of NMDA receptor subunit NR3B in mouse reveals motoneuronal and nonmotoneuronal phenotypes. Eur J Neurosci. 2007;26:1407–1420. doi: 10.1111/j.1460-9568.2007.05774.x. [DOI] [PubMed] [Google Scholar]
- Nishi M, Hinds H, Lu HP, Kawata M, Hayashi Y. Motoneuron-specific expression of NR3B, a novel NMDA-type glutamate receptor subunit that works in a dominant-negative manner. J Neurosci. 2001;21:RC185. doi: 10.1523/JNEUROSCI.21-23-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Quack G. Glutamate in CNS disorders as a target for drug development: an update. Drug News Perspect. 1998;11:523–569. doi: 10.1358/dnp.1998.11.9.863689. [DOI] [PubMed] [Google Scholar]
- Penagarikano O, Lazaro MT, Lu XH, Gordon A, Dong H, Lam HA, Peles E, Maidment NT, Murphy NP, Yang XW, Golshani P, Geschwind DH. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci Transl Med. 2015;7:271ra278. doi: 10.1126/scitranslmed.3010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Otano I, Schulteis CT, Contractor A, Lipton SA, Trimmer JS, Sucher NJ, Heinemann SF. Assembly with the NR1 subunit is required for surface expression of NR3A–containing NMDA receptors. J Neurosci. 2001;21:1228–1237. doi: 10.1523/JNEUROSCI.21-04-01228.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal-Simons A, Durrant AR, Heresco-Levy U. Autoimmune-induced glutamatergic receptor dysfunctions: conceptual and psychiatric practice implications. Eur Neuropsychopharmacol. 2013;23:1659–1671. doi: 10.1016/j.euroneuro.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Salmina AB, Lopatina O, Kuvacheva NV, Higashida H. Integrative neurochemistry and neurobiology of social recognition and behavior analyzed with respect to CD38-dependent brain oxytocin secretion. Curr Top Med Chem. 2013;13:2965–2977. doi: 10.2174/15680266113136660211. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vidana DI, Chan NM, Chan AH, Hui KK, Lee S, Chan HY, Law YS, Sze MY, Tsui WC, Fung TK, Lau BW, Lai CY. Repeated treatment with oxytocin promotes hippocampal cell proliferation, dendritic maturation and affects socio-emotional behavior. Neurosci. 2016;333:65–77. doi: 10.1016/j.neuroscience.2016.07.005. [DOI] [PubMed] [Google Scholar]
- Seeburg PH. The TiPS/TINS lecture: the molecular biology of mammalian glutamate receptor channels. Trends Pharmacol Sci. 1993;14:297–303. doi: 10.1016/0165-6147(93)90047-N. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman MN, Macdougall MG, Hu F, Pace TW, Raison CL, Miller AH. Endogenous glucocorticoids protect against TNF-alpha-induced increases in anxiety-like behavior in virally infected mice. Mol Psychiatry. 2007;12:408–417. doi: 10.1038/sj.mp.4001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestre JS, Nadal R, Pallares M, Ferre N. Acute effects of ketamine in the holeboard, the elevated-plus maze, and the social interaction test in Wistar rats. Depress Anxiety. 1997;5:29–33. [PubMed] [Google Scholar]
- Simen BB, Duman CH, Simen AA, Duman RS. TNFalpha signaling in depression and anxiety: behavioral consequences of individual receptor targeting. Biol Psychiatry. 2006;59:775–785. doi: 10.1016/j.biopsych.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Singh M. Mood, food, and obesity. Front Psychol. 2014;5:925. doi: 10.3389/fpsyg.2014.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siviy SM, Line BS, Darcy EA. Effects of MK-801 on rough-and-tumble play in juvenile rats. Physiol Behav. 1995;57:843–847. doi: 10.1016/0031-9384(94)00361-8. [DOI] [PubMed] [Google Scholar]
- Soloff M, Alexandrova M, Fernstrom M. Oxytocin receptors: triggers for parturition and lactation? Science. 1979;204:1313–1315. doi: 10.1126/science.221972. [DOI] [PubMed] [Google Scholar]
- Sucher NJ, Akbarian S, Chi CL, Leclerc CL, Awobuluyi M, Deitcher DL, Wu MK, Yuan JP, Jones EG, Lipton SA. Developmental and regional expression pattern of a novel NMDA receptor-like subunit (NMDAR-L) in the rodent brain. J Neurosci. 1995;15:6509–6520. doi: 10.1523/JNEUROSCI.15-10-06509.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirko NN, Mitre M, Marlin BJ, Froemke RC, Chao MV, Tsien RW. Neuroscience 2014 Abstracts. Washington, DC: Society for Neuroscience; 2014. Oxytocin receptor responses in mature hippocampus: Receptor expression and neuromodulation in CA2, a subfield that contributes to social memory. Program No. 300.03/B58. [Google Scholar]
- Tong G, Takahashi H, Tu S, Shin Y, Talantova M, Zago W, Xia P, Nie Z, Goetz T, Zhang D, Lipton SA, Nakanishi N. Modulation of NMDA receptor properties and synaptic transmission by the NR3A subunit in mouse hippocampal and cerebrocortical neurons. J Neurophysiol. 2008;99:122–132. doi: 10.1152/jn.01044.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker DM, Poulsen C, Luu P. Critical periods for the neurodevelopmental processes of externalizing and internalizing. Dev Psychopathol. 2015;27:321–346. doi: 10.1017/S0954579415000024. [DOI] [PubMed] [Google Scholar]
- Tzanoulinou S, Sandi C. The Programming of the Social Brain by Stress During Childhood and Adolescence: From Rodents to Humans. Curr Top Behav Neurosci. 2017;30:411–429. doi: 10.1007/7854_2015_430. [DOI] [PubMed] [Google Scholar]
- Umeda-Kameyama Y, Ishii S, Kameyama M, Kondo K, Ochi A, Yamasoba T, Ogawa S, Akishita M. Heterogeneity of odorant identification impairment in patients with Alzheimer’s Disease. Sci Rep. 2017;7:4798. doi: 10.1038/s41598-017-05201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova G, Hollander E, Shepherd J. The role of ionotropic glutamate receptors in childhood neurodevelopmental disorders: autism spectrum disorders and fragile x syndrome. Curr Neuropharmacol. 2014;12:71–98. doi: 10.2174/1570159X113116660046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HK, Liu XB, Matos MF, Chan SF, Perez-Otano I, Boysen M, Cui J, Nakanishi N, Trimmer JS, Jones EG, Lipton SA, Sucher NJ. Temporal and regional expression of NMDA receptor subunit NR3A in the mammalian brain. J Comp Neurol. 2002;450:303–317. doi: 10.1002/cne.10314. [DOI] [PubMed] [Google Scholar]
- Young LJ. Regulating the social brain: a new role for CD38. Neuron. 2007;54:353–356. doi: 10.1016/j.neuron.2007.04.011. [DOI] [PubMed] [Google Scholar]