Abstract
Biological invasions have been associated with niche changes; however, their occurrence is still debated. We assess whether climatic niches between native and non-native ranges have changed during the invasion process using two globally spread mosquitoes as model species, Aedes albopictus and Aedes aegypti. Considering the different time spans since their invasions (>300 vs. 30–40 years), niche changes were expected to be more likely for Ae. aegypti than for Ae. albopictus. We used temperature and precipitation variables as descriptors for the realized climatic niches and different niche metrics to detect niche dynamics in the native and non-native ranges. High niche stability, therefore, no niche expansion but niche conservatism was revealed for both species. High niche unfilling for Ae. albopictus indicates a great potential for further expansion. Highest niche occupancies in non-native ranges occurred either under more temperate (North America, Europe) or tropical conditions (South America, Africa). Aedes aegypti has been able to fill its native climatic niche in the non-native ranges, with very low unfilling. Our results challenge the assumption of rapid evolutionary change of climatic niches as a requirement for global invasions but support the use of native range-based niche models to project future invasion risk on a large scale.
Introduction
Ongoing change in climatic conditions is expected to influence species’ distributions, which in turn will affect biodiversity patterns1,2. Biological invasions are promoted by climate change3 and are further enhanced by increasing global trade and tourism. More recently, they have also been associated with changes in the species’ realized climatic niches, with reports of niche changes between native and non-native populations4,5. However, the evidence of such niche changes during invasion processes is currently under debate6,7 and might be species-specific: whereas several authors argue for a climatic niche shift of different plant species in the invaded range8–10, a study by Petitpierre et al.11 suggests that climatic niches did not change substantially for most of the 50 investigated invasive terrestrial plant species. Strubbe et al.12 confirmed niche conservatism for non-native birds in Europe and Strubbe et al.5 proposed conserved niches in the introduced ranges (either Europe or North America) for most of the 29 vertebrate species studied.
Generally, climatic niche shifts contradict the assumption of a niche conservatism, which implies that species retain their niches in space and time13. Ecological niche modelling, which is the most commonly used approach to assess, firstly, the impact of climate change on biodiversity and secondly, invasion risk, strongly relies on this assumption. One way to assess whether the species’ niche is conserved over time and space is to investigate the distribution in the niche space (as an estimation of species’ realized niche) focusing on native and non-native ranges of the species. The investigation of species’ niches can thus be used to better assess and improve ecological niche modelling results (e.g. Aguirre-Gutiérrez et al.14). Furthermore, comparisons between species’ native and non-native range climatic niches may identify species that have undergone adaptive evolutionary changes during the invasion process (e.g. change of the fundamental climatic niche), but might generally benefit a better understanding of different niche dynamics6. Drivers other than climate can also be involved in niche shifts during invasions, e.g. ecological drivers such as biotic interactions. These should ideally not be considered separately15. However, data are usually available only on a different, much smaller scale and can therefore not be incorporated in the same models.
Here, Aedes albopictus and Aedes aegypti, two mosquito species that are listed among the world’s worst invasive alien species (Global Invasive Species Database, IUCN) and that are competent vectors for several diseases16, were used as model species to assess whether climatic niches between native and non-native ranges have changed. Aedes albopictus, native to Southeast Asia17, is regarded to be one of the fastest spreading invasive species worldwide18. Its invasion success has been highly promoted by increasing global trade and tourism. In addition to that, the area with suitable climatic conditions for the species is expected to expand under climate change (e.g. Cunze et al.19).
The closely related Ae. aegypti, native to Africa20,21, is similarly widespread but has a different history of invasion. While the global spread of Ae. albopictus took place mainly within the last 30–40 years22, the spread of Ae. aegypti into tropical and subtropical regions outside the African continent took place in conjunction with the increase of slave trade in the 16th and 17th century21. Today, Ae. aegypti is restricted to tropical and subtropical regions in which temperatures remain relatively warm throughout the year. Records from moderate climate zones are still missing or rare and may be the result of the species’ domesticity (feeding, mating, oviposition indoors) (e.g. Gloria-Soria et al.23).
The aim of this study was to assess whether the climatic niches of these two mosquito species have changed or remained stable (niche conservatism hypothesis) during the invasion process. Particularly regarding their different temporal invasion histories (30–40 years here refers to “short-term”, more than 300 years refers to “long-term”, i.e. time that has passed since the first observations outside their native range were made), we expected niche changes to be more likely for Ae. aegypti than for Ae. albopictus. The niches of these two closely related species were compared within a single continent (or range) where both species occur and were investigated for their overlap, but also for their similarity and equivalence. Following the applied niche comparison framework for native and non-native ranges by Guisan et al.24, three basic components were distinguished: niche unfilling, niche stability and niche expansion, accounting for the availability of environmental conditions within the respective range25.
Results
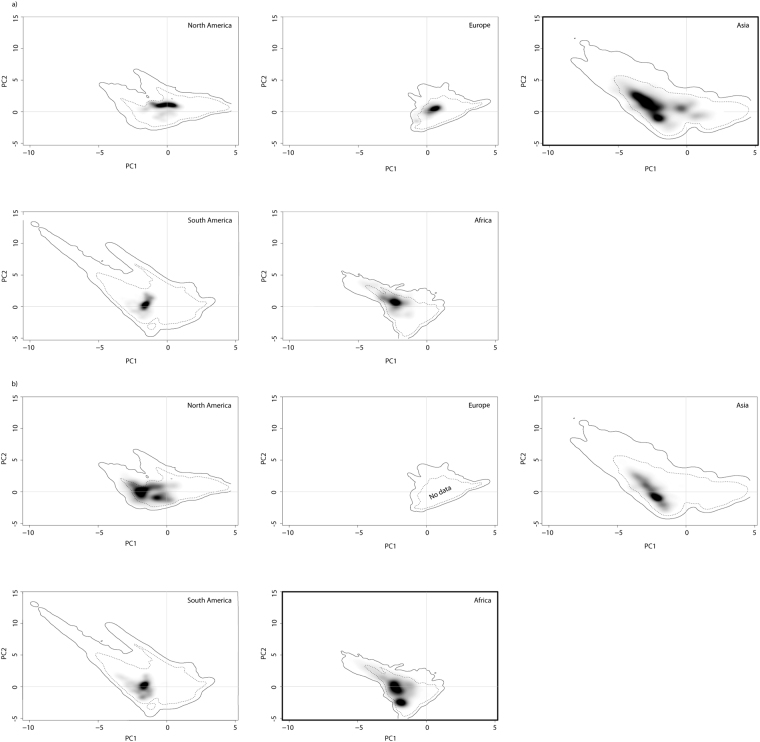
The different shapes of climatic niche space indicate some variation in climate backgrounds, i.e. the available environments, across the considered five geographical ranges (solid and dashed contour lines, Fig. 1). Furthermore, the occupied native and non-native range niches (dark shaded areas) of both mosquito species position differently along the two PCA axes. The first two PCA axes explain 77.5% of the variation in the data. The first PCA axis is positively related to temperature seasonality (bio04) and negatively related to mean temperature in the coldest quarter and to the annual mean temperature (bio11, bio01), whereas the second axis is positively related to the precipitation variables (Fig. 2).
Figure 1.
Climatic niches of Aedes albopictus and Aedes aegypti in the niche space spanned by two PCA-axes. The environment available and the environment occupied (realized niche) by (a) Aedes albopictus and (b) Aedes aegypti in the non-native ranges and in the native range (bold frame) are shown. Grey shading represents the density of the occurrences by cell, with darker shading indicating higher density of occurrences. Solid contour lines visualize 100% of the available environment; dashed contour lines indicate 50% of the most common background environment. Figures built using R Package ‘ecospat’ version 2.1.143,44 (www.unil.ch/ecospat/home/menuguid/ecospat-resources/tools.html).
Figure 2.
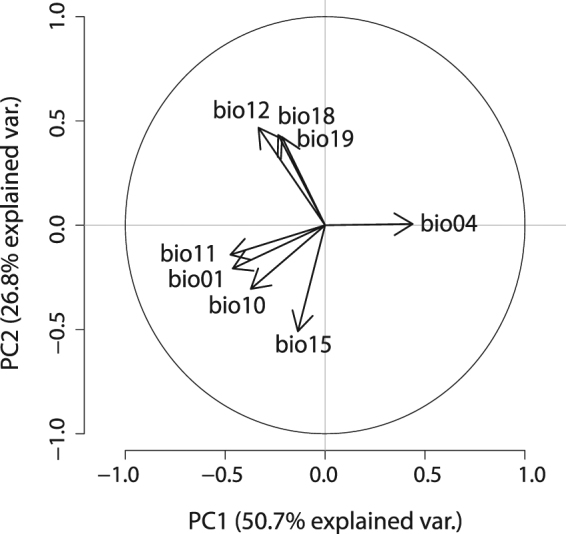
Principle component analysis (PCA) plot. The contribution of the eight bioclimatic variables - Annual Mean Temperature (bio01), Temperature Seasonality (bio04), Mean Temperature of Warmest Quarter (bio10), Mean Temperature of Coldest Quarter (bio11), Annual Precipitation (bio12), Precipitation Seasonality (bio15), Precipitation of Warmest Quarter (bio18) and Precipitation of Coldest Quarter (bio19) - on the two PCA-axes PC1 and PC2 and the percentage of variability explained by these axes are shown in the PCA-plot.
Niches of Aedes albopictus
Climatic niche occupancy of Ae. albopictus in the Asian native range exceeds the climatic niche occupancy in the non-native ranges; this pattern is evident for all considered non-native ranges, implicating a niche unfilling (Fig. 1a). In comparison to the Asian native range a rightward shift of the niche centroid can be observed, with the highest occupancy of Ae. albopictus under comparably lower temperatures within the non-native ranges (PC1 axis is negatively correlated to mean temperature in the coldest quarter and to the annual mean temperature), especially in Europe and North America.
In general, the native range niche for Ae. albopictus shows only little overlap with the non-native range niches (Table 1, Schoener’s D between 0.03 (minimum) for the comparison with the European niche and 0.16 (maximum) for the comparison with the African niche). The niche overlaps between the non-native range niches cover a broader range (Table 1, Schoener’s D between 0 for the overlap of the European and African niches and 0.36 for the overlap of the North and South American niches). For all pairs of ranges except for the pair North America and Europe, the niches of the respective ranges are significantly not equivalent (significance level α = 5%). According to the niche similarity test the North American and the European niche for Ae. albopictus are more similar than expected by chance (Table 1). The same is true for the Asian compared to the North American niche and the South American compared to the African niche but not vice versa (Table 1).
Table 1.
Comparison of the native and non-native niches for Aedes albopictus and Aedes aegypti. Niche overlap was calculated as Schoener’s D comparing the niches in range 1 (R1) and range 2 (R2).
| Range 1 | Range 2 | Niche overlap (D) | Niche similarity | Niche equivalency | |
|---|---|---|---|---|---|
| R1 → R2 | R2 → R1 | ||||
| Aedes albopictus | |||||
| Asia† | Europe | 0.03 | ns | ns | different* |
| Asia† | Africa | 0.16 | ns | ns | different* |
| Asia† | North America | 0.09 | similar* | ns | different* |
| Asia† | South America | 0.11 | ns | ns | different* |
| Europe | Africa | 0 | ns | ns | different* |
| Europe | North America | 0.25 | similar* | similar* | ns |
| Europe | South America | 0.02 | ns | ns | different* |
| Africa | North America | 0.01 | ns | ns | different* |
| Africa | South America | 0.16 | ns | similar* | different* |
| North America | South America | 0.36 | ns | ns | different* |
| Aedes aegypti | |||||
| Africa† | Asia | 0.27 | similar* | similar* | different* |
| Africa† | North America | 0.18 | ns | ns | ns |
| Africa† | South America | 0.18 | ns | ns | ns |
| Asia | North America | 0.31 | similar* | similar* | different* |
| Asia | South America | 0.28 | similar* | similar* | different* |
| North America | South America | 0.45 | ns | ns | ns |
†Native range.
ns = not significant.
*The ecological niches are significantly (α = 5%) more similar (similarity test) or different (equivalency test) than expected by chance.
Compared to the native range niche, no niche expansion but a full niche stability can be observed in the invaded ranges (niche expansion = 0, niche stability = 1, Table 2). Niche unfilling compared to the native range niche is relatively high for Ae. albopictus, ranging from 23% for the African niche up to 87% for the European niche (50.5% on average, Table 2).
Table 2.
Niche metrics. Comparison of the native ranges and the non-native ranges for Aedes albopictus and Aedes aegypti.
| Species (native range) | Non-native range | Expansion | Stability | Unfilling |
|---|---|---|---|---|
| Aedes albopictus (Asia) | Africa | 0.00 | 1.00 | 0.23 |
| Europe | 0.00 | 1.00 | 0.87 | |
| North America | 0.00 | 1.00 | 0.55 | |
| South America | 0.00 | 1.00 | 0.37 | |
| Aedes aegypti (Africa) | Asia | 0.04 | 0.96 | 0.00 |
| North America | 0.07 | 0.93 | 0.05 | |
| South America | 0.00 | 1.00 | 0.10 |
Niches of Aedes aegypti
The comparison of the non-native niches of Ae. aegypti in South America and Asia with its native range niche in Africa revealed no clear pattern of niche unfilling, expansion or niche shift (Fig. 1). However, in comparison to the African native range, the niche centroid seems slightly shifted to the right in North America towards comparably lower temperatures.
The native niche for Ae. aegypti shows a slight overlap with the non-native niches (Table 1, Schoener’s D between 0.18 for the comparison with the North and South America niche and 0.27 for the comparison with the Asian niche). The overlaps between the non-native range niches vary between 0.28 and 0.45 (Table 1). The non-native Asian range niche is significantly not equivalent to the native range niche in Africa (Table 1, α = 5%). According to the niche similarity test, the African native and the Asian niche, the Asian and the North American niche as well as the North American and the South American niche for Ae. aegypti are more similar to each other than expected by chance (Table 1). The Asian niche is more similar to the South American niche of Ae. aegypti than would be expected by chance, but not vice versa (Table 1).
The comparison of the African native range niche with the non-native range niches reveals a high niche stability (96.33%, averaged over three non-native ranges), a small niche expansion (3.67%), and a very small percentage of niche unfilling (0.05%) in the non-native ranges for Ae. aegypti (Table 2).
Comparison of niches between Aedes albopictus and Aedes aegypti within a single range
In contrast to the niche overlaps between ranges and within single species, a comparison of the niches of both mosquitos within the same range generally reveals higher niche overlaps (higher Schoener’s D values, see Table 3), with the lowest D value for the overlap of the niches of Ae. albopictus and Ae. aegypti in North America. In all considered ranges the niches of the two species are not equivalent and seem to be more similar than expected by chance only in Asia (Table 3). Within the considered climatic niche space, the niches of species in their respective native regions exceed the niches of the non-native species in that region, i.e. the niche of Ae. aegypti exceeds the niche of Ae. albopictus in Africa by 20% whereas the niche of Ae. albopictus exceeds the niche of Ae. aegypti in Asia by 7%.
Table 3.
Comparisons of Aedes albopictus and Aedes aegypti niches within a single range.
| Range | Niche overlap (D) | Niche similarity | Equivalency | |
|---|---|---|---|---|
| Ae. albopictus → Ae. aegypti | Ae. aegypti → Ae. albopictus | |||
| Asia | 0.61 | similar* | similar* | different* |
| Africa | 0.51 | ns | ns | different* |
| North America | 0.19 | ns | ns | different* |
| South America | 0.62 | ns | ns | different* |
ns = not significant.
*The ecological niches are significantly (α = 5%) more similar (similarity test) or different (equivalency test) than expected by random.
Discussion
The invasion of non-native species is considered a continuous process and sometimes happens within short time spans12,24,26, which can challenge a clear designation to the processes involved. According to Guisan et al.24 differences in realized niches between native and non-native ranges can be ascribed to either a) adaptive evolutionary changes in the physiological tolerance of the species during the invasion process, b) a broad fundamental niche and thus, preadaptation to conditions not available (anymore) within the native range but available within a non-native range, c) changes in biotic interaction and/or d) limitations in dispersal ability of the species. Here, we investigated whether the niches of the two competent vector mosquito species Ae. albopictus and Ae. aegypti, involving either a global short- or long-term invasion history, respectively, remained stable (niche conservatism) during the invasion process. In the following paragraphs, we discuss our results focusing more specifically on the different scenarios of niche expansion and niche unfilling for the two species. Possible interpretative approaches of the considered niche parameters and their likelihood are summarized for both species in Table 4.
Table 4.
Interpretative approach of niche scenarios for Ae. albopictus and Ae. aegypti.
| Indication | Mechanism | Aedes albopictus | Aedes aegypti | |
|---|---|---|---|---|
| niche expansion | evolutionary changes | unlikely (no observed expansion) | unlikely (no observed expansion) | |
| changes in biotic interaction | absence of neg. interaction/presence of new pos. interaction | unlikely | unlikely | |
| niche unfilling | dispersal limitation | likely (short time since invasion started but fast spreading velocity assumed) | unlikely (invasion process started long time ago) | |
| changes in biotic interaction | absence of pos. interaction/presence of new neg. interaction | unlikely | unlikely | |
Aedes albopictus is a recent global invader and it has been suggested that evolutionary changes through local adaptation might be possible, evidenced by changes in expression of diapause in different studies27–30. In concordance with the results of the study by Medley27, we also found that the niches of Ae. albopictus were not equivalent between native and non-native ranges. However, unlike Medley27, who assumed a niche change but did not differentiate between the different scenarios of niche changes, we found no evidence for a niche expansion in the non-native range. Rather, we found 100% niche stability for all considered non-native ranges compared to the Asian native range, which confirms niche conservatism according to Guisan et al.24.
Asia covers a broad range of environments, ranging from tropical to temperate conditions. Aedes albopictus, native to the Asian continent, occupies several of these environments and is therefore considered to have a broad native range niche (possibly due to two populations, one occurring under tropical conditions, and one occurring under more temperate conditions, see also discussion further below). The non-native ranges (except for South America) cover smaller ranges of environmental conditions with consequently smaller niches. An obvious shift in the centroids of occupancies compared to the native range niche can be seen towards more temperate conditions (North America and Europe), and towards more tropical conditions (South America and Africa) (Fig. 1). This corroborates the results of Kotsakiozi et al.31, who recently detected two major genetically differentiated population clusters in the native range.
As well as observing non-equivalent niches of Ae. albopictus, we found high values of niche unfilling. Generally, high values of niche unfilling indicate an imbalance between species occupied range (i.e. actual distribution) and potential range (i.e. all areas with suitable habitat conditions) and can be interpreted as an indication of the incomplete invasion process. For example and as indicated in Table 4, despite suitable habitat conditions, unoccupied parts might occur in the non-native range, particularly if the invasion process extends over only a short period of time and the species is not in equilibrium with its environment yet. This unfilling of the geographical space could then be reflected in an unfilling of the niche space.
Niche unfilling could, however, also arise from an altered situation of biotic interactions, e.g. the absence of an important symbiosis partners or the occurrence of predators or high competitive pressure in the non-native range. Aedes albopictus is considered a strong competitor23 (as is Ae. aegypti), showing a low specialization in its food spectrum as well as a broad spectrum of hosts for blood meal. The most relevant biotic interaction considering these two species would be interspecific competition. Although both species can interact with each other, the outcome seems to be population-specific and rapidly evolving32,33. On the other hand, small-scale spatial partitioning might lead to local co-existence of both species34,35.
Based on our results, there is no clear indication for a niche expansion that would require evolutionary adaptations of the species during its short-term invasion progress. However, more local adaptive evolutionary changes might still occur and have been observed such as those of photoperiodic response28,36 or resistance to satyrization33. These studies show evidence for trait evolution and not necessarily for niche evolution compared to native populations. With regard to Ae. albopictus, two “density-clouds” (depicted in the third graph of the top row in Fig. 1), associated to the more “tropical” or “temperate” populations in the native range in Asia, can be identified. If a “tropical” population was introduced and did subsequently evolve in the US, now showing signs of photoperiodic diapausing, this would not appear as a niche expansion in this approach as the ecological niche of the species would still fall into the extent of the native range niche.
Our results contradict a very recent study by Hill et al.37 who detected a niche expansion (0.28) and a lower unfilling (0.07) for Ae. albopictus. These differences might be ascribed to the different study designs, including differences in occurrence data and environmental backgrounds. Whereas we investigated the non-native range niches for each continent separately, Hill et al.37 considered a global non-native range niche, comprising North America, South America, Europe and Africa. Pooling our data of the non-native range niches we found similar values of low unfilling. Differences in the expansion index seem to underlie more complexity, but might be related to differences in background selection. Whereas we chose continent-wide ranges based on roughly the same number of pixels, ranges chosen by Hill et al.37 focused on species’ occurrence points within biomes. This more restrictive and narrow background might explain the higher expansion index. Valuable statements about niche expansion are only possible when analog environments are considered in both native and non-native ranges and studies that restricted their analyses to analog environments found niche conservatism to be dominant among invasive species24.
Aedes aegypti started its global expansion much earlier and consequently had much more time to invade suitable habitats; an equilibrium between potentially suitable niche space and occupied niche space was therefore expected. This was confirmed in our analysis with lower values of niche unfilling compared to Ae. albopictus. Thus, Ae. aegypti has been able to occupy its niche in the non-native range over the last four centuries, colonizing all continents except areas beyond 45° latitude including Europe. Furthermore, Ae. aegypti showed very high niche stability and a very low expansion with more pairs of similar or equivalent niches (native versus non-native niche). We would argue that also with this species, there is no indication for a violation of the assumption of niche conservatism.
Similarly to Ae. albopictus, Ae. aegypti has a wide geographical distribution and establishes populations in a variety of environments. The high values of overlap between the niches of both species’ niche in Asia, South America and Africa would suggest a high ecological similarity, however, the niches are not equivalent and only similar to each other in Asia. These differences as well as differences in the geographical ranges (Ae. aegypti does not occur or only rarely in more temperate areas) become apparent when considering eco-physiological adaptations of each mosquito38. Aedes albopictus is able to undergo egg diapause (in particular populations from temperate regions) allowing the species to persist during cold temperature periods that are unfavourable to adult survival. Aedes aegypti does not have this ability and thus, shows only limited adaptation to egg stage survival in unfavourable periods38. Furthermore, the two species have been found to segregate based on land use and human impacts on habitats, thus, Ae. albopictus tends to be associated with vegetated areas, with more suburban/rural land use, whereas A. aegypti tends to be associated with urban areas, with high human population density34,35,39.
The ability to undergo a diapause in the egg stage, a generally common characteristic of insects40, is largely considered an adaptation to temperate climatic conditions. Due to the wide latitudinal distribution in the ancestral native range in Asia, two distinct strains are proposed for Ae. albopictus: a tropical one that does not have to ability to undergo photoperiodic diapause when conditions are unfavourable, and a temperate one with a phenotypically plastic diapause response (see review by Armbruster28). It could be speculated that the pattern of the native Asian range niche of Ae. albopictus, with the two occurrence “density-clouds”, would be an indication for this theory. Furthermore, it seems that the North American and European niches match the more temperate occurrence density hotspot (near the point of origin in the considered niche space, Fig. 3). Strong evidence that the invasion history of North America was from a temperate origin has formerly been proposed by Armbruster28 and was recently confirmed using population genomics31. The South American and African niches are more similar in environmental conditions to the tropical occurrence density hotspot in the native range. Finally, assessing evolutionary niche changes during invasion relies on the knowledge of fundamental niches in the native and non-native ranges, which is very difficult to obtain and only exists for very few species. Future research of different invasive and native populations and their diapausing response in relation to latitude, also including reciprocal experiments, is still needed to further investigate differences between the aforementioned populations/strains (but see first attempts)29,30,41,42.
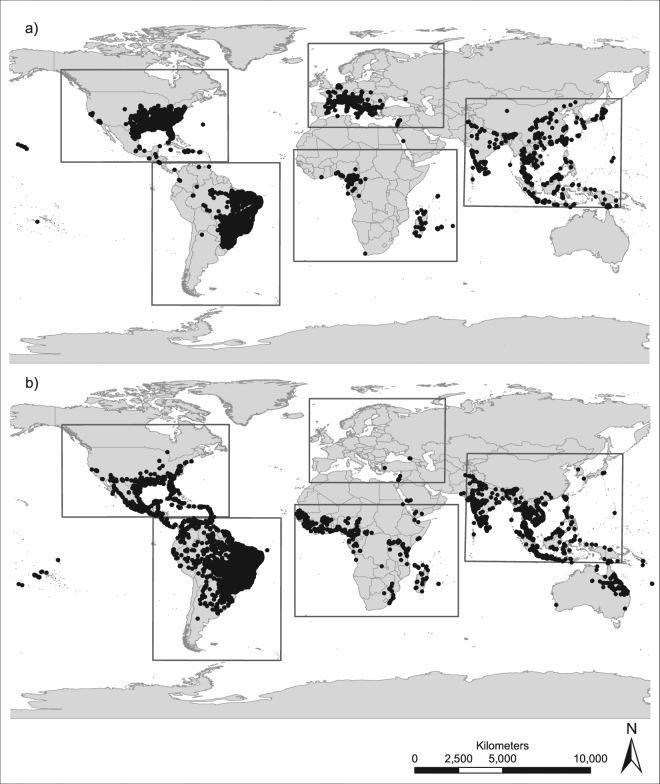
Figure 3.
Study areas and occurrence. Records for (a) Aedes albopictus and (b) Aedes aegypti. Grey boxes indicate the boundaries of the five considered ranges: North America, South America, Europe, Africa and Asia. Maps were built using ESRI ArcGIS 10.353 (www.esri.com/software/arcgis).
Considering the occurrence points used in this study (see Material and Methods section below), it was not possible to verify whether mosquito populations were established in each location. Thus, there could be locations that are re-colonized each year but don’t persist without immigration. Assuming that the mosquito species have not become established in some of these locations and the occurrence records have been just single findings, these records would fall outside their realized niche, i.e. the non-native niche would have been overestimated. However, as we did not find signs of a climatic niche expansion, wrongly taking these records into account does not change the main conclusion of our results. We consider our approach to be conservative and appropriate, especially for precautionary risk assessments or management of invasive species.
Due to current constraints on characterizations of the fundamental niche, most studies focus on realized niches (derived from distributional data) and apply metrics of niche unfilling, niche stability and niche expansion, while accounting for availability of environmental conditions in the respective ranges24. Applying an approach developed by Broennimann et al.25 implemented in the “ecospat” package43,44, we here confirmed the theory of niche conservatism for Ae. albopictus and Ae. aegypti without any indications for a niche expansion during their global route of invasion. For Ae. albopictus, a niche unfilling was shown, which explains the present differences between native and non-native range niches. The environmental space currently unoccupied by the species in the non-native ranges relates most likely to the short time span since the global spread of the species (Table 4). On the contrary, it appears that Ae. aegypti has been able to occupy its native niche in the non-native range over the last four centuries, colonizing all continents except areas beyond 45° latitude including Europe. In conclusion, differences in niche unfilling suggest two different stages of invasion for the two species; whereas Ae. aegypti might already be in equilibrium with the environment, this is not the case for Ae. albopictus. We would support the use of native-range based niche models to be used for projections of areas at a risk of invasion in the future, and confirm climatic niche conservatism as a valid underlying assumption, which does not exclude the potential of the species for evolutionary adaptations on a local scale.
Material and Methods
Data specifications and niche approach
We used occurrence records of both species from Kraemer et al.45,46. The coordinates of the records were adjusted to the raster of the environmental variables with a spatial resolution of 5 arc minutes (~10 km × 10 km) and accounted for only one occurrence record per grid cell in order to minimize spatial autocorrelation. The numbers of occurrences after adjusting to the 5 arc minutes resolution are given in Table 5. We considered five biogeographically separated regions with observed distribution for Ae. albopictus and Ae. aegypti: Asia (native range of Ae. albopictus), Africa (native range of Ae. aegypti), Europe, North America and South America (see Fig. 1). We chose the extents of these study areas so that they – if possible – cover all records in this region, and comprise approximately the same number of pixels. Despite the occurrence of Ae. aegypti in Australia, we decided not to consider Oceania in our study to keep the number of pixels for backgrounds equal for all considered continents. Established populations of Ae. albopictus were found in 2005 in Australia (on islands in the Torres Strait, between mainland Australia and Papua New Guinea). However, it was shown that due to effective control programmes, an expansion of Ae. albopictus was successfully prevented47. We would therefore expect a niche unfilling evoked by an anthropogenic dispersal limitation for Ae. albopictus in Australia. For Ae. aegypti the European range was not taken into consideration due to the small number of recorded occurrences in Europe (probably due to unsuitable climate). Eight out of 19 available bioclimatic variables provided by worldclim48 were selected to cover climatic variables that are considered ecologically relevant for both species. Those variables included annual mean, amplitude and seasonal variation for both temperature and precipitation, specifically: Annual Mean Temperature (bio01), Temperature Seasonality (bio04), Mean Temperature of Warmest Quarter (bio10), Mean Temperature of Coldest Quarter (bio11), Annual Precipitation (bio12), Precipitation Seasonality (bio15), Precipitation of Warmest Quarter (bio18) and Precipitation of Coldest Quarter (bio19). These eight bioclimatic variables are inter-correlated, but were transformed into non-correlated linear combinations (principal components) of the original variables45 by a principle component analysis (PCA). The first principal component accounts for as much of the variability in the data as possible, each succeeding component accounts for as much of the remaining variability as possible. To characterize the climatic niches of the two mosquito species, we applied the principal components analysis PCA-env approach developed by Broennimann et al.25. This approach works in the two-dimensional gridded environmental space (100 × 100 grid) spanned by the first two axes, which were derived from the PCA based on the above mentioned eight bioclimatic variables considering all five ranges together.
Table 5.
Number of occurrence points after adjusting to the 5 arc minutes resolution and extents of the considered ranges (N – latitude of the northern margin, E – longitude of the eastern margin, S – latitude of the southern margin and W – longitude of the western margin of the bounding box).
| Aedes albopictus | Aedes aegypti | N | E | S | W | |
|---|---|---|---|---|---|---|
| North America | 1453 | 600 | 60.50°N | −137.50°E | 13.17°N | −52.58°E |
| South America | 3324 | 4962 | 12.75°N | −90.25°E | −59.42°N | −26.17°E |
| Europe | 356 | (6)* | 76.50°N | −15.00°E | 29.58°N | 57.67°E |
| Africa | 806 | 2502 | 19.42°N | −17.50°E | −37.42°N | 63.50°E |
| Asia | 4204 | 6471 | 45.83°N | 70.00°E | −11.08°N | 149.33°E |
*Not included in the analysis.
Niche metrics
The niches were visualized and compared between native and non-native ranges accounting for niche overlap, niche similarity, niche equivalency, niche stability, niche expansion and niche unfilling, for Ae. albopictus and Ae. aegypti, respectively. In addition, we compared the niches of Ae. albopictus and Ae. aegypti within each of the four considered ranges where both species occur. The different niche metrics are described in the following paragraph.
The niche overlap corresponds to the intersection of two niches in the niche space. We considered Schoener’s D49,50, the most commonly used measure for niche overlap. Schoener’s D is an index ranging between 0 and 1, where 0 means no overlap between the considered niches and 1 means a total overlap between the considered niches, i.e. the two niches are identical.
In addition, we applied the niche equivalency test and the niche similarity test50. These are two frequently used statistical tests considering hypotheses of niche divergence or conservatism51. The niche equivalency test determines whether the niche overlap is constant when randomly reallocating the occurrences of the species among the two ranges25. More specifically, the equivalence test compares the overlap of both niches with the overlap of simulated niches; it pools the occurrences of both ranges and randomly splits the pooled data into two datasets, maintaining the number of occurrences as in the original datasets. On the other hand, the niche similarity test addresses whether the overlap between ecological niches in two ranges is different from the overlap between the niche in one range and niches selected at random from the other range25. The niche equivalency test is conservative as it only tests if the modelled niches of the two species are identical in their niche spaces accounting for the occurrences only and not for the background14,50. The niche similarity test assesses if the considered niches are more similar than expected by chance, accounting for the differences in the surrounding environmental conditions in the geographic areas where both species are distributed14,53. Significant differences according to the niche equivalency test together with significant similarity according to the niche similarity test means that niches are similar, but not equivalent.
As we aimed to test for niche conservatism we used the one-tailed test (alternative = “greater”, in the settings) and tested if niches are more similar (similarity test) or different (equivalency test) than expected by chance. For both tests we used 1000 permutations to evaluate the significance (α = 5%) of niche equivalency and niche similarity.
Furthermore, we evaluated niche stability (i.e. the proportion of the non-native range niche overlapping with the native range niche), niche expansion (i.e. the proportion of the non-native range niche not overlapping with the native range niche) and niche unfilling (proportion of the native range niche not occupied in the non-native range niche).
Niche expansion and niche stability always add up to 100%. The niche unfilling value would correspond to the niche expansion value when switching native range and non-native ranges.
Niche conservatism is defined as the tendency for species to retain their niche in space and time and is used synonymously with ‘niche stability’24. Niche conservatism is given when the niche stability is 100% and niche expansion is 0% - regardless of the niche unfilling.
All analyses were performed in R environment version 3.3.152. Niche comparisons were performed and visualized using “ecospat” package43,44.
Acknowledgements
The present study was funded by the ERA-Net BiodivERsA, with the national funding body DFG KL 2087/6-1, FWF I-1437 and ANR-13-EBID-0007-01 as part of the 2012–13 BiodivERsA call for research proposals and by the German Federal Ministry of Food and Agriculture (BMEL) through the Federal Office for Agriculture and Food (BLE), grant number 2819105115, the Graduate School IMPact-Vector funded by the Senate Competition Committee grant (SAW-2014-SGN-3) of the Leibniz-Association and the Uniscientia Stiftung.
Author Contributions
S.C. and S.K. designed the study. S.C. conducted the analyses and created the figures. S.C., J.K., L.K.K., and S.K. wrote the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Thomas CD, et al. Extinction risk fromclimate change. Nature. 2004;427:145–148. doi: 10.1038/nature02121. [DOI] [PubMed] [Google Scholar]
- 2.Chen I-C, Hill JK, Ohlemuller R, Roy DB, Thomas CD. Rapid range shifts of species associated with high levels of climate warming. Science (New York, N.Y.) 2011;333:1024–1026. doi: 10.1126/science.1206432. [DOI] [PubMed] [Google Scholar]
- 3.Walther G-R, et al. Alien species in a warmer world: risks and opportunities. Trends in Ecology & Evolution. 2009;24:686–693. doi: 10.1016/j.tree.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Tingley R, Vallinoto M, Sequeira F, Kearney MR. Realized niche shift during a global biological invasion. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:10233–10238. doi: 10.1073/pnas.1405766111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strubbe D, Beauchard O, Matthysen E. Niche conservatism among non-native vertebrates in Europe and North America. Ecography. 2015;38:321–329. doi: 10.1111/ecog.00632. [DOI] [Google Scholar]
- 6.Dellinger AS, et al. Niche dynamics of alien species do not differ among sexual and apomictic flowering plants. The New Phytologist. 2016;209:1313–1323. doi: 10.1111/nph.13694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiao H, Escobar LE, Peterson AT. Accessible areas in ecological niche comparisons of invasive species: Recognized but still overlooked. Scientific Reports. 2017;7:1213. doi: 10.1038/s41598-017-01313-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallagher RV, Beaumont LJ, Hughes L, Leishman MR. Evidence for climatic niche and biome shifts between native and novel ranges in plant species introduced to Australia. Journal of Ecology. 2010;98:790–799. doi: 10.1111/j.1365-2745.2010.01677.x. [DOI] [Google Scholar]
- 9.González-Moreno P, Diez JM, Richardson DM, Vilà M. Beyond climate. Disturbance niche shifts in invasive species. Global Ecology and Biogeography. 2015;24:360–370. doi: 10.1111/geb.12271. [DOI] [Google Scholar]
- 10.Camenen E, Porté AJ, Benito Garzón M. American trees shift their niches when invading Western Europe. Evaluating invasion risks in a changing climate. Ecology and Evolution. 2016;6:7263–7275. doi: 10.1002/ece3.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petitpierre B, et al. Climatic niche shifts are rare among terrestrial plant invaders. Science (New York, N.Y.) 2012;335:1344–1348. doi: 10.1126/science.1215933. [DOI] [PubMed] [Google Scholar]
- 12.Strubbe D, Broennimann O, Chiron F, Matthysen E. Niche conservatism in non-native birds in Europe. Niche unfilling rather than niche expansion. Global Ecology and Biogeography. 2013;22:962–970. doi: 10.1111/geb.12050. [DOI] [Google Scholar]
- 13.Wiens JJ, Graham CH. Niche Conservatism. Integrating evolution, ecology, and conservation biology. Annual Review of Ecology, Evolution, and Systematics. 2005;36:519–539. doi: 10.1146/annurev.ecolsys.36.102803.095431. [DOI] [Google Scholar]
- 14.Aguirre-Gutiérrez J, et al. Similar but not equivalent. Ecological niche comparison across closely-related Mexican white pines. Diversity and Distributions. 2015;21:245–257. doi: 10.1111/ddi.12268. [DOI] [Google Scholar]
- 15.Broennimann O, et al. Contrasting spatio-temporal climatic niche dynamics during the eastern and western invasions of spotted knapweed in North America. Journal of Biogeography. 2014;41:1126–1136. doi: 10.1111/jbi.12274. [DOI] [Google Scholar]
- 16.Medlock JM, et al. An entomological review of invasive mosquitoes in Europe. Bulletin of Entomological Research. 2015;105:637–663. doi: 10.1017/S0007485315000103. [DOI] [PubMed] [Google Scholar]
- 17.Medlock JM, et al. A review of the invasive mosquitoes in Europe: ecology, public health risks, and control options. Vector Borne and Zoonotic Diseases (Larchmont, N.Y.) 2012;12:435–447. doi: 10.1089/vbz.2011.0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benedict MQ, Levine RS, Hawley WA, Lounibos LP. Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector Borne and Zoonotic Diseases (Larchmont, N.Y.) 2007;7:76–85. doi: 10.1089/vbz.2006.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunze S, Koch LK, Kochmann J, Klimpel S. Aedes albopictus and Aedes japonicus - two invasive mosquito species with different temperature niches in Europe. Parasites and Vectors. 2016;9:573. doi: 10.1186/s13071-016-1853-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tabachnick WJ. Evolutionary genetics and arthropod-borne disease. The Yellow Fever Mosquito. American Entomologist. 1991;37:14–26. doi: 10.1093/ae/37.1.14. [DOI] [Google Scholar]
- 21.Kaplan L, Kendell D, Robertson D, Livdahl T, Khatchikian C. Aedes aegypti and Aedes albopictus in Bermuda. Extinction, invasion, invasion and extinction. Biological Invasions. 2010;12:3277–3288. doi: 10.1007/s10530-010-9721-z. [DOI] [Google Scholar]
- 22.Paupy C, Delatte H, Bagny L, Corbel V, Fontenille D. Aedes albopictus, an arbovirus vector: from the darkness to the light. Microbes and Infection. 2009;11:1177–1185. doi: 10.1016/j.micinf.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Gloria-Soria, A. et al. Origin of a high-latitude population of Aedes aegypti in Washington, DC. The American Journal of Tropical Medicine and Hygiene (2017). [DOI] [PMC free article] [PubMed]
- 24.Guisan A, Petitpierre B, Broennimann O, Daehler C, Kueffer C. Unifying niche shift studies: insights from biological invasions. Trends in Ecology & Evolution. 2014;29:260–269. doi: 10.1016/j.tree.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Broennimann O, et al. Measuring ecological niche overlap from occurrence and spatial environmental data. Global Ecology and Biogeography. 2012;21:481–497. doi: 10.1111/j.1466-8238.2011.00698.x. [DOI] [Google Scholar]
- 26.Liu X, et al. Realized climatic niches are conserved along maximum temperatures among herpetofaunal invaders. Journal of Biogeography. 2017;44:111–121. doi: 10.1111/jbi.12808. [DOI] [Google Scholar]
- 27.Medley KA. Niche shifts during the global invasion of the Asian tiger mosquito, Aedes albopictus Skuse (Culicidae), revealed by reciprocal distribution models. Global Ecology and Biogeography. 2010;19:122–133. doi: 10.1111/j.1466-8238.2009.00497.x. [DOI] [Google Scholar]
- 28.Armbruster PA. Photoperiodic diapause and the establishment of Aedes albopictus (Diptera: Culicidae) in North America. Journal of Medical Entomology. 2016;53:1013–1023. doi: 10.1093/jme/tjw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kreß A, Kuch U, Oehlmann J, Müller R. Effects of diapause and cold acclimation on egg ultrastructure: new insights into the cold hardiness mechanisms of the Asian tiger mosquito Aedes (Stegomyia) albopictus. Journal of Vector Ecology. 2016;41:142–150. doi: 10.1111/jvec.12206. [DOI] [PubMed] [Google Scholar]
- 30.Kreß A, Oppold A-M, Kuch U, Oehlmann J, Müller R. Cold tolerance of the Asian tiger mosquito Aedes albopictus and its response to epigenetic alterations. Journal of Insect Physiology. 2017;99:113–121. doi: 10.1016/j.jinsphys.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Kotsakiozi P, et al. Population genomics of the Asian tiger mosquito, Aedes albopictus: insights into the recent worldwide invasion. Ecology and Evolution. 2017;7:10143–10157. doi: 10.1002/ece3.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leisnham PT, Juliano SA. Interpopulation differences in competitive effect and response of the mosquito Aedes aegypti and resistance to invasion by a superior competitor. Oecologia. 2010;164:221–230. doi: 10.1007/s00442-010-1624-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bargielowski IE, Lounibos LP, Carrasquilla MC. Evolution of resistance to satyrization through reproductive character displacement in populations of invasive dengue vectors. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:2888–2892. doi: 10.1073/pnas.1219599110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rey JR, et al. Habitat segregation of mosquito arbovirus vectors in South Florida. Journal of Medical Entomology. 2006;43:1134–1141. doi: 10.1093/jmedent/43.6.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leisnham PT, Juliano SA. Spatial and temporal patterns of coexistence between competing Aedes mosquitoes in urban Florida. Oecologia. 2009;160:343–352. doi: 10.1007/s00442-009-1305-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urbanski J, et al. Rapid Adaptive Evolution of Photoperiodic Response during Invasion and Range Expansion across a Climatic Gradient. The American Naturalist. 2012;179:490–500. doi: 10.1086/664709. [DOI] [PubMed] [Google Scholar]
- 37.Hill MP, Gallardo B, Terblanche JS. A global assessment of climatic niche shifts and human influence in insect invasions. Global Ecology and Biogeography. 2017;26:679–689. doi: 10.1111/geb.12578. [DOI] [Google Scholar]
- 38.Brady OJ, et al. Modelling adult Aedes aegypti and Aedes albopictus survival at different temperatures in laboratory and field settings. Parasites and Vectors. 2013;6:351. doi: 10.1186/1756-3305-6-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leisnham PT, LaDeau SL, Juliano SA. Spatial and temporal habitat segregation of mosquitoes in urban Florida. PloS one. 2014;9:e91655. doi: 10.1371/journal.pone.0091655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zonato V, Collins L, Pegoraro M, Tauber E, Kyriacou CP. Is diapause an ancient adaptation in Drosophila? Journal of Insect Physiology. 2017;98:267–274. doi: 10.1016/j.jinsphys.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 41.Leisnham PT, Towler L, Juliano SA. Geographic variation of photoperiodic diapause but not adult survival or reproduction of the invasive mosquito Aedes albopictus (Diptera: Culicidae) in North America. Annals of the Entomological Society of America. 2011;104:1309–1318. doi: 10.1603/AN11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lounibos LP, Escher RL, Nishimura N. Retention and adaptiveness of photoperiodic egg diapause in Florida populations of invasive Aedes albopictus. Journal of the American Mosquito Control Association. 2011;27:433–436. doi: 10.2987/11-6164.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Broennimann, O. et al. Package ‘ecospat’ (2016).
- 44.Di Cola V, et al. ecospat: An R package to support spatial analyses and modeling of species niches and distributions. Ecography. 2017;40:774–787. doi: 10.1111/ecog.02671. [DOI] [Google Scholar]
- 45.Kraemer, M. U. G. et al. Data from: The global compendium of Aedes aegypti and Ae. albopictus occurrence. Scientific Data (2015). [DOI] [PMC free article] [PubMed]
- 46.Kraemer MUG, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife. 2015;4:e08347. doi: 10.7554/eLife.08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muzari MO, et al. Holding back the tiger: Successful control program protects Australia from Aedes albopictus expansion. PLoS neglected tropical diseases. 2017;11:e0005286. doi: 10.1371/journal.pntd.0005286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology. 2005;25:1965–1978. doi: 10.1002/joc.1276. [DOI] [Google Scholar]
- 49.Schoener TW. The anolis lizards of Bimini. Resource partitioning in a complex fauna. Ecology. 1968;49:704–726. doi: 10.2307/1935534. [DOI] [Google Scholar]
- 50.Warren DL, Glor RE, Turelli M. Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution. 2008;62:2868–2883. doi: 10.1111/j.1558-5646.2008.00482.x. [DOI] [PubMed] [Google Scholar]
- 51.Glennon KL, Ritchie ME, Segraves KA, Bardgett R. Evidence for shared broad-scale climatic niches of diploid and polyploid plants. Ecology letters. 2014;17:574–582. doi: 10.1111/ele.12259. [DOI] [PubMed] [Google Scholar]
- 52.R Core Team. R: A Language and Environment for Statistical Computing. 2014, R Foundation for Statistical Computing, Vienna, Austria, R Foundation for Statistical Computing (R Foundation for Statistical Computing, Vienna, 2014).
- 53.ESRI. Environmental Systems Research Institute (ESRI). ArcGIS Release 10. 3. Redlands, CA. (2014). http://www.esri.com/software/arcgi.