Abstract
One component of host defense at mucosal surfaces seems to be epithelium-derived antimicrobial peptides. Antimicrobial peptides are classified on the basis of their structure and amino acid motifs. Peptides of the defensin, cathelicidin, and histatin classes are found in humans. In the airways, α-defensins and the cathelicidin LL-37/hCAP-18 originate from neutrophils. β-Defensins and LL-37/hCAP-18 are produced by the respiratory epithelium and the alveolar macrophage and secreted into the airway surface fluid. Beside their direct antimicrobial function, antimicrobial peptides have multiple roles as mediators of inflammation with effects on epithelial and inflammatory cells, influencing such diverse processes as proliferation, immune induction, wound healing, cytokine release, chemotaxis, protease-antiprotease balance, and redox homeostasis. Further, antimicrobial peptides qualify as prototypes of innovative drugs that might be used as antibiotics, anti-lipopolysaccharide drugs, or modifiers of inflammation.
Keywords: host defense, defensin, cathelicidin, infection, chronic bronchitis
Introduction
The survival of a multicellular organism in a world laden with microorganisms depends on a network of host defense mechanisms, involving several levels of interacting systems. The initial contact of pathogenic microorganisms with the host usually takes place at inner or outer body surfaces. The surface of the airways is a primary site for the deposition and introduction of microorganisms, mainly through inspired air. Several possible consequences result from the contact of microorganisms with host tissue:
1. The invading microorganisms are eliminated by innate host defense mechanisms without an inflammatory response or the activation of adaptive immunity. This normally happens in the lower respiratory tract and occurs within a short time.
2. The microbe outgrows the innate host defense. As a consequence, effector mechanisms of the innate immune system are upregulated and have direct antimicrobial activity and mediator function to attract inflammatory cells and cells of the adaptive immune system. These mechanisms finally result in the elimination of the microorganisms. In this scenario, the innate host defense keeps the doubling time of the microorganisms long enough to avoid an overwhelming of the immune system.
3. The microorganism outgrows innate and adaptive immunity. Together with a strong inflammatory response this situation leads to the death of the host.
4. Microorganisms with specific physiological adaptations can colonize the airways for a long time. In this case the activities of the immune system are insufficient to eliminate the invader.
Recent studies have shown that antimicrobial peptides are expressed in human airways and are involved in the host defense. Antimicrobial peptides are effector molecules of innate immunity with direct antimicrobial and mediator function. They have an important role in all scenarios described above by providing an initial host defense mechanism. It is the aim of this review article to describe antimicrobial peptides as an endogenous part of the innate immune system, to summarize the structures of their genes and peptide molecules and to comment on their role in health and disease.
Antimicrobial peptides as effector substances of the innate immune system
A first line of defense against pathogenic insult is called the innate immune system, which is followed by acquired immune responses associated with the activation of T and B cells aimed against specific antigens [1,2]. In contrast to the clonal, acquired adaptive immunity, endogenous peptide antibiotics provide a fast and energy-effective mechanism as front-line defense. In the respiratory tract, the innate defense system of the host is complemented by several other mechanisms to protect the airways and the lung parenchyma from colonization and infection (Fig. 1). Components of the airway surface fluid (ASF) with antimicrobial activity are lysozyme, lactoferrin, secretory phospholipase A2, secretory leukocyte protease inhibitor (SLPI), and antimicrobial peptides. Other substances, such as complement, surfactant proteins, and Clara-cell protein (CC10, CCSP), contribute to the host defense [3]. Mucus secreted by mucous gland and goblet cells entraps particles that are then propelled by the movement of cilia and probably provides a microenvironment necessary for the activity of antimicrobial substances.
Figure 1.
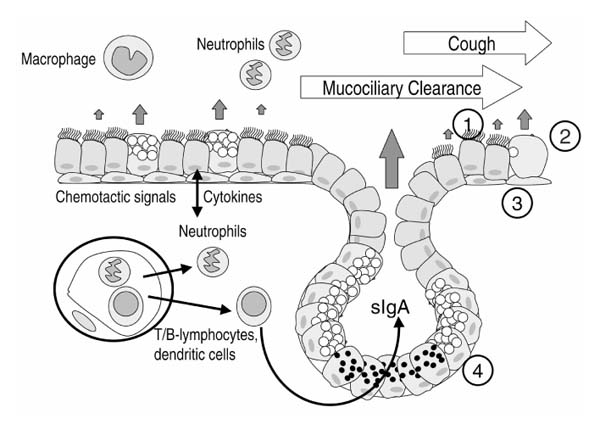
Host defense mechanisms of the respiratory epithelium. Coughing and cilia mechanically remove inhaled debris and microorganisms entrapped in mucus, a mechanism called mucociliary clearance. Multiple substances with proinflammatory and anti-inflammatory as well as antimicrobial activities are secreted by epithelial and inflammatory cells and function as effector substances of the innate immune system. Natural killer cells, dendritic cells, neutrophils, and macrophages represent the cellular components of innate immunity. Cells of the respiratory epithelium (1=ciliated cell, 2=goblet cell, 3=basal cell, 4=mucous and serous gland cells) are not passive bystanders of an inflammatory process but secrete effector molecules including antimicrobial peptides. The adaptive immune response with its T cells and B cells (sIgA=secretory immunoglobulin A) is triggered by innate mechanisms.
Essential to innate immunity are receptors that recognize colonizing or invading microorganisms and initiate a host defense reaction [1,2]. Homology studies of lipopolysaccharide (LPS)-binding receptors and signal transduction cascades between insects and mammals (Toll and Toll-like receptors, respectively) have revealed detailed insights into certain aspects of the host defense system and attracted a large amount of interest in innate immunity during recent years [4,5]. Antimicrobial peptides have emerged as potential participants in host defense at mucosal surfaces such as the airways.
Basic biology of antimicrobial peptides
Families of human antimicrobial peptides
Most antimicrobial peptides are cationic (polar) molecules with spatially separated hydrophobic and charged regions. These structural hallmarks are important for the proposed mechanisms of action of peptide antibiotics (see below). Beside these characteristics, antimicrobial peptides of various families differ in size, amino acid sequence and certain structural motifs (Fig. 2). Antimicrobial peptides are gene-encoded, meaning that one gene codes for one peptide. Families of antimicrobial peptide genes are located in clustered arrangements in the genome and map to syntenic chromosomal regions in different mammalian species, providing clues about the evolutionary development of this host defense system. The primary translational product is a prepropeptide consisting of an N-terminal signal sequence for targeting of the endoplasmic reticulum, a pro segment, and a C-terminal cationic peptide that has antimicrobial activity after cleavage (Fig. 3). The pro segment is often anionic in charge and might have several biological functions including the correct folding of the C-terminus, intracellular trafficking, or the inhibition of the activity of the mature peptide. The propeptide is cleaved off during later stages of intracellular processing or after secretion into the extracellular space.
Figure 2.
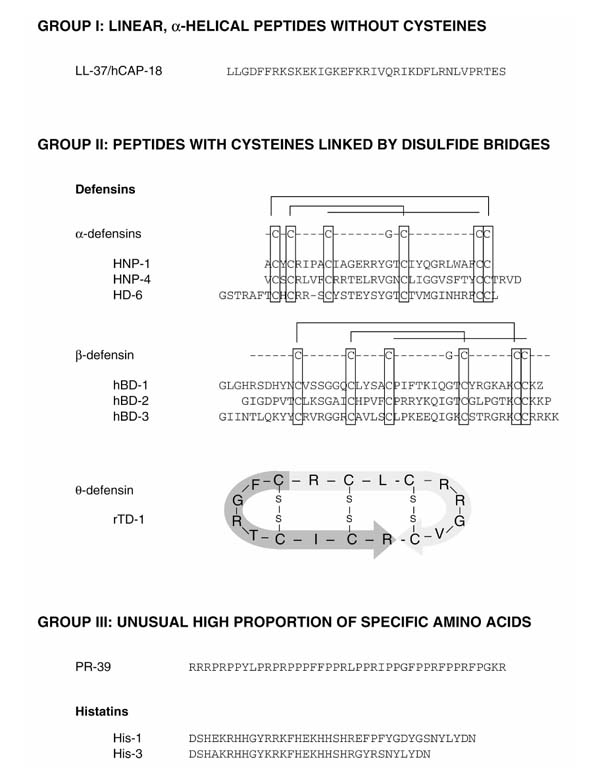
Classification of antimicrobial peptides based on motifs in the amino acid sequence similar to that described by Boman [63]. Antimicrobial peptides could also be grouped according to other principles. LL-37 and PR-39 belong to the cathelicidin family on the basis of their homologous propeptide. rTD-1=rhesus monkey θ-defensin 1; PR-39=cathelicidin from pig. Human β-defensin 3 (hBD-3) was identified by using bioinformatic screening procedures to search human chromosome 8p23 for the presence of sequence motifs typical of β-defensin (R Bals, unpublished data).
Figure 3.
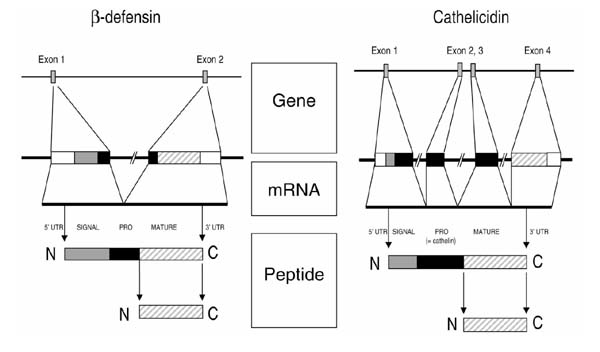
Structure of prototypical genes and peptides of the defensin and cathelicidin families. Both gene types have several exons whose primary translational product is a prepropeptide. The C-terminus is the part of the molecules that contains antimicrobial activity. The genes are represented schematically with the following individual components: 5' untranslated region (5' UTR), white box; signal sequence, shaded box; prosequence, black box; mature peptide, hatched box; 3' UTR, white box.
Antimicrobial peptides are stored as propeptides or mature C-terminal peptides. In addition to positively charged antimicrobial peptides, peptide antibiotics with a negative charge at neutral pH have also been identified [6]. These peptides seem to originate as cleavage products from larger proteins and are beyond the scope of this review. Antimicrobial peptides can be grouped according to their size, conformational structure, or predominant amino acid structure. On the basis of their gross composition and three-dimensional structure, peptide antibiotics are divided into three main classes (Fig. 2): group I, linear, α-helical peptides without cysteines, for instance cecropins from pig, magainins from frogs, LL-37/hCAP-18 from human; group II, peptides with an even number of cysteine residues linked by disulfide bridges, for instance defensins from mammals, protegrins (porcine cathelicidins); and group III, peptides with an unusually high proportion of one or two amino acids, for instance PR-39 from pig leukocytes.
On the basis of structural homology motifs, a different classification of families of antimicrobial peptides can be generated that reflects relationships between family members. In humans, peptide antibiotics of three families have been identified: the defensins, cathelicidins, and histatins.
Defensins
Mammalian defensins are cationic, relatively arginine-rich nonglycosylated peptides with a molecular mass of 3.5-4.5 kDa and contain six cysteine residues that form three characteristic intramolecular disulfide bridges [7]. According to the spacing of the cysteines, the alignment of the disulfide bridges, and the overall molecular structure, defensins can be divided into three classes: α-defensins, β-defensins, and θ-defensins.
α-Defensins. α-Defensins are 29–35 residues in length, contain three disulfide bridges in a 1–6, 2–4, 3–5 alignment, and reveal a triple-stranded β sheet structure with a β hairpin that contains cationic amino acids (Fig. 2). The first human α-defensin was isolated from neutrophils in 1985 [8]. So far, six α-defensins have been identified from humans. Human neutrophil peptides 1–4 (HNP-1 to HNP-4) are localized in azurophilic granules of neutrophil granulocytes, where they are the principal protein and contribute to the oxygen-independent killing of phagocytized microorganisms [8,9]. The two other α-defensins, human defensins 5 and 6, are primarily found in Paneth's cells of the small intestine and epithelial cells of the female urogenital tract [10].
The genes encoding α-defensins and β-defensins are located in a cluster on chromosome 8p23 [11]. The gene for HNP-2 has not been found at this location, indicating that HNP-2 is a proteolytic product of HNP-1 or HNP-3. α-defensins are produced as prepropeptides (Fig. 3). A recent report from Wilson et al [12] revealed insight into the processing of murine cryptins, α-defensins found in Paneth's cells of the small intestine. The disruption of the gene for matrilysin (MMP7), a tissue metalloprotease, in a knockout mouse model prevented the cleavage of the propeptide of cryptins, resulting in increased susceptibility of the animals to bacterial infection. Cleavage of the propeptide takes place in the lumen of the intestinal crypts where antimicrobial peptides are present in high concentrations together with the secreted MMP7. The source of peptides HNP-1 to HNP-4, which are found in abundance in the ASF of inflamed airways, are neutrophils that have migrated into the airway walls or lumen [13].
β-Defensins. In 1991 Diamond et al [14] isolated an antimicrobial peptide from cow tongue, called tracheal antimicrobial peptide (TAP), which contains six cysteine residues connected by three disulfide bridges but spaced differently from those in α-defensins. This new family of antimicrobial peptides was therefore named β-defensins; they are 36–42 residues in length, possess a disulfide alignment of 1–5, 2–4, 3–6, and have been isolated from several species (Fig. 2). The first human β-defensin, called human β-defensin-1 (hBD-1), was originally isolated from large volumes of hemofiltrate [15] and is expressed constitutively in epithelial cells of the urinary and respiratory tract [16,17,18]. Human β-defensin-2 (hBD-2) was isolated from psoriatic skin by using an affinity chromatography procedure applying columns coated with components of Escherichia coli [19]. hBD-2 was found to be expressed in epithelia of the inner or outer surfaces of the human body, such as skin and the respiratory and gastrointestinal tract [20,21].
Both human β-defensins have been isolated by means of bioscreening, which applies functional assays to the detection of the antimicrobial activity of candidate substances obtained from large amounts of biological material. hBD-1 and hBD-2 are expressed diffusely throughout the surface epithelium and the serous gland cells in the airway walls. Both peptides have been detected in airway secretions; concentrations are in the μg/ml range [20,21]. In contrast to hBD-2, which has been detected only in its 41-residue form, hBD-1 has been detected in urine in multiple forms ranging from 36 to 47 residues in length [18]. The genes encoding β-defensins are localized in the same chromosomal region as the α-defensins. The gene for hBD-1 is separated from that for HNP-1 by about 100–150 kilobases [11]. The processing of β-defensins is probably similar to that of α-defensins; however, no detailed analysis has yet been published.
θ-Defensins. Recently a novel class of defensins has been isolated from rhesus monkey neutrophils and named θ-defensins for their circular molecular structure (Fig. 2) [22]. The peptide rhesus θ-defensin-1 is produced by the post-translational ligation of two truncated α-defensins and demonstrates salt-independent antimicrobial activity. Levels of these peptides in the airways have not been determined. No data about the presence of these molecules in the airways are available at this time.
Cathelicidins
Peptide antibiotics of the cathelicidin family contain a highly conserved signal sequence and pro-region (`cathelin'=cathepsin L inhibitor) but show substantial heterogeneity in the C-terminal domain encoding the mature peptide, which can range in size from 12 to 80 or more amino acid residues [23] (Figs 2 and 3). The only human cathelicidin, LL-37/hCAP-18, was isolated from human bone marrow [24,25,26]. LL-37/hCAP-18 is expressed in myeloid cells where it resides in granules but is also found in inflamed skin. LL-37/hCAP-18 has been found to be regulated by inflammatory stimuli [27]. In the airways, the peptide is produced by the same cell types as the β-defensins and is secreted into the ASF [28]. Additionally, LL-37 originates from neutrophils that have invaded the airways. LL-37 has been detected in tissue-culture supernatants of respiratory epithelial cells as well as in lung washings from patients [28,29]. At present no details are known about the processing of LL-37/hCAP-18 in epithelial cells. In neutrophils, where LL-37/hCAP-18 is localized to specific granules, the peptide is stored in its propeptide form and cleaved during secretion, probably by the activity of an elastase (Fig. 3). Beside its antimicrobial activity, LL-37 binds and neutralizes LPS and protects against endotoxic shock in a murine model of septicemia [30]. The gene encoding LL-37/hCAP-18 consists of four exons and is localized on chromosome 3.
Histatins
Histatins are a family of histidine-rich peptides present in human saliva (Fig. 2) [31,32,33]. Their presence in airway secretions has not been investigated. The primary structures of the major family members (histatins 1 and 3) has been determined and revealed lengths of 38 and 32 amino acid residues. Smaller members of the histatin family, including histatin 5 (24 residues), originate from histatin 1 and 3 by post-translational processing. The genes that encode histatins 1 and 3 consist of several exons and have been mapped to human chromosome 4q13. The antimicrobial activity includes especially strong antifungal effects.
Gene regulation
Because peptide antibiotics are host defense substances with antimicrobial activity as well as a mediator function, their expression is tightly regulated. The regulation of antimicrobial peptides has been described in detail for murine, human, and bovine molecules. The expression of hBD-1 has been found to be constitutive [20,34]. In contrast, hBD-2 expression has been detected to be upregulated by infectious and inflammatory stimuli in cell cultures as well as in human studies. In human material, hBD-2 transcripts and peptide levels in the airways were upregulated during infection [35]. In cell culture experiments, hBD-2 expression was stimulated by interleukin (IL)-1α, IL-1β, tumor necrosis factor-α, microorganisms (Gram-positive and Gram-negative bacteria, and Candida albicans), and LPS [20,34,36]. These results are consistent with the detection of a consensus NF-κB binding site upstream of the hBD-2 gene. In addition, the expression of LL-37/hCAP-18 has been reported to be upregulated in inflamed skin [27], where the peptide is colocalized with IL-6 [37]. The gene encoding LL-37/hCAP-18 contains several potential binding sites for transcription factors [acute-phase response factor and nuclear factor for IL-6 expression (NF IL-6)] that are possibly involved in the regulation of gene expression.
The mechanism of induction of the antimicrobial peptide and the nature of the corresponding receptors and signalling pathways are speculative at present. Airway epithelial cells express several molecules that qualify as receptors to detect infection or inflammation (reviewed in [38]). It has been shown that these cells synthesize the receptor panel to detect bacterial LPS, including CD14 and Toll-like receptors 1–9 ([39], and R Bals, unpublished data). Intracellular signalling probably includes NF-κB, NF IL-6, and JAK/STAT pathways. The airway epithelium is an immune organ with the capabilities of detecting microorganisms and inducing an inflammatory and host defense response, including the secretion of antimicrobial peptides as effector molecules. Antimicrobial peptides of the respiratory tract might be induced by different stimuli and signalling pathways, and could have different functions as effector molecules. This concept of a large family of antimicrobial peptides with differential functions against diverse classes of microorganisms has been demonstrated for the host defense system of Drosophila [40] and should also be applicable to humans.
Activities of antimicrobial peptides
The antimicrobial activity of peptide antibiotics was deduced from tests in vitro assaying purified substances against microorganisms. Antimicrobial peptides, including defensins, cathelicidins and histatins, have a broad spectrum of activity against Gram-positive and Gram-negative bacteria as well as against fungi and enveloped viruses. The minimal inhibitory concentrations of the peptides are in the range 0.1–100 μg/ml. Antimicrobial peptides show synergistic activity with other host defense molecules such as lysozyme and lactoferrin. The microbicidal activity of defensins stems from the permeabilization of anionic lipid bilayers and the subsequent release of cellular contents and the destruction of the membrane's electrode potential (Fig. 4).
Figure 4.
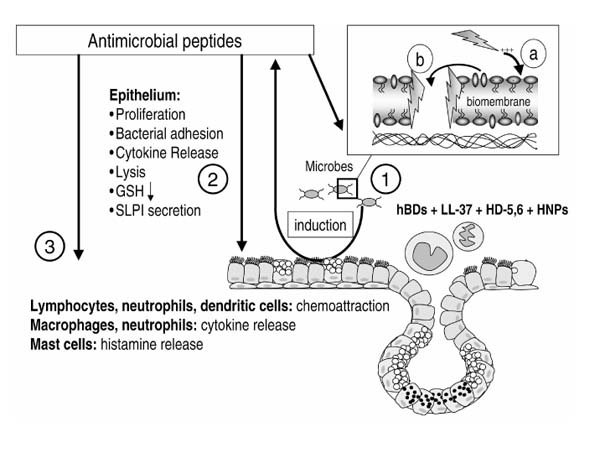
Activities of antimicrobial peptides. As well as their antimicrobial function (1), antimicrobial peptides have other potential roles in inflammation and infection (2,3). The mechanism of the antimicrobial activity is explained in the insert. After electrostatic interactions between the negatively charged bacterial wall and the positively charged peptides (a), the peptide associates with the membranes, either by insertion as pores (b) or by forming carpet-like structures that lead to a destabilization of the membrane. The sources (1) of antimicrobial peptides in the airways are epithelial cells and inflammatory cells. Defensins and LL-37 have a feedback mediator function that targets these cell types (2,3), influencing the release of mediators and other cellular processes such as proliferation and chemoattraction.
The first step of the interaction between the cationic peptide and the anionic microbial cell membrane is thought to involve electrostatic attraction, which is inhibited by high concentrations of salt in the solution. The second step is the permeabilization of the membrane. One mechanism of permeabilization is thought to involve the formation of ion pores. The existence of pores, their dimensions and electrical properties have been described for model bilayers and various cell types [41,42]. A second model, the so called `carpet' model, has been proposed for magainins, cecropine (antimicrobial peptides from frog and pig, respectively), and hBD-2 [43] and describes the aggregation of peptides into positively charged patches of the membrane [44], resulting in the formation of transient gaps. Additionally, defensin-related cell death has been related to interference with protein synthesis or DNA damage.
Functional studies on antimicrobial activity have primarily been restricted to experiments in vitro with purified components. In fact, the evidence that defensins actually contribute to innate immunity in vivo is largely indirect. Evidence for the host defense function of antimicrobial peptides came from the above-mentioned study involving MMP7 knockout mice with an increased susceptibility for infections, which was probably due to the defective cleavage of α-defensins in the small intestine [12]. In addition to their antimicrobial activity, defensins and cathelicidins can bind to lipopolysaccharide and inactivate the biological functions of this endotoxin. Bacterial resistance to antimicrobial peptides is a rare phenomenon. Mechanisms that result in the development of resistance involve modifications of outer cell wall components, such as lipopolysaccharide [45], teichoic acids [46], or phosphoicholine [47], and the modulation of efflux pumps [48].
Beside their role as endogenous antibiotics, antimicrobial peptides have functions in inflammation, wound repair, and regulation of the adaptive immune system (Fig. 4). Human neutrophil defensins have been described as being cytotoxic to various cell types [49], as inducing cytokine synthesis in airway epithelial cells [50], monocytes [51], and T lymphocytes [52], as increasing the release of SLPI from respiratory epithelial cells [53], and as decreasing intracellular glutathione concentration [54]. Further, they increase the binding of bacteria to epithelial cells [55] and induce the liberation of histamine from mast cells. Finally, α-defensins are involved in the chemotaxis of monocytes and T cells [56], the modulation of cell proliferation and an antibody response, and the inhibition of complement activation, of fibrinolysis and of the activity of serpin family members (reviewed in [57]). The human β-defensins have recently been identified as potent ligands for the chemokine receptor CCR6 that is present on dendritic and T cells, therefore providing a link between innate and adaptive immunity [58].
Role of antimicrobial peptides in health and disease
Host defense function
On the basis of their activity in vitro, their patterns of expression and gene regulation, and their involvement in pathways of innate immunity, there is strong suggestive evidence that antimicrobial peptides serve as host defense substances not only by direct antimicrobial activity but also as mediators. Animal experiments with the use of a human bronchial xenograft model with the genetic depletion of hBD-1 by antisense oligonucleotides [17], overexpression of antimicrobial peptides in animal models of infection [30], or the above-mentioned experiments with matrilysin knockout mice [12] support this view. An assessment of the relative contribution of individual proteins or peptides to the host defense is difficult to accomplish. The concentrations of antimicrobial peptides and proteins at the site of action (e.g. in the gel or sol layer of the ASF) is difficult to determine because of problems in sampling the ASF. A functional analysis of purified peptides and proteins in vitro does not reflect the complexity of component interactions, such as synergism and antagonism between multiple substances.
Role of antimicrobial peptides in inflammatory lung diseases
The story of cystic fibrosis (CF) research over the past decade has provided important lessons about the relation between a defect in an ion channel and a breach of the host defense system of the airways. An obvious defect of the host defense system of the respiratory tract is evident from clinical studies and was evaluated in several models in vitro or ex vivo [17,59]. The link between the defect in ion transport and decreased host defense is less obvious and remains speculative at present (reviewed in [60]). Antimicrobial peptides might have a role in this pathogenesis, either by being inactivated by increased salt concentration in secretions of CF airways or by being absent from the ASF owing to alteration of the secretory apparatus caused by the dysfunctional CF transmembrane conductance regulator (CFTR).
Beside their host defense function during infections, the proinflammatory activity of antimicrobial peptides is likely to have negative consequences too. In CF patients, high concentrations of α-defensins in lung washings have been detected that are in the range of cytotoxicity [13]. Chronic obstructive pulmonary disease (COPD) and other inflammatory lung diseases such as pulmonary fibrosis, α1-anti-trypsin deficiency, acute respiratory distress syndrome, or diffuse panbronchiolitis are often associated with the release of antimicrobial peptides and inflammatory mediators from neutrophils and other cell types.
On the basis of the activities of antimicrobial peptides, it is obvious that these substances affect the inflammatory process (Fig. 4). Owing to the lack of detailed knowledge of their functional spectrum in vivo, it is hard to decide which peptide antibiotic in which concentration results in a proinflammatory or anti-inflammatory activity. On the one hand, defensins attract inflammatory cells such as neutrophils, B-cells, and macrophages, and activate these and other cell types, including epithelial cells. They liberate inflammatory mediators such as IL-8, interferon-γ, IL-6, IL-10, and LTB4. Defensins might lead to an imbalance of the redox system by reducing glutathione levels in epithelial airway cells and might disturb the protease-antiprotease system by binding to proteinase inhibitor (serpin) family members. On the other hand, defensins might also exhibit anti-inflammatory activities by induction of the secretion of IL-10 [61] or SLPI [53], or by facilitating the binding of microorganisms to epithelia with subsequent clearance of the microorganisms by a bactericidal activity of the cell.
It is also likely that antimicrobial peptides in the airway secretions modulate the cytokine profile of lymphocytes towards T helper type 1 or 2 cells. This could have a direct effect on the development of bronchial hyper-responsiveness. Additionally, defensins have been shown to release histamine from mast cells and might induce hyper-responsiveness by their cationic charge [62]. These experimental results do not draw a complete picture of the functions that antimicrobial peptides have in inflammatory or infectious disease; however, they indicate that they fulfil not only an epiphenomenal bystander role but are linked to the underlying pathogenetic processes.
Antimicrobial peptides as drugs
The intriguing idea of developing antimicrobial peptides as innovative antibiotics has been followed up by several biotechnological companies. With the use of protein-biochemical methods and recombinant DNA technology, the structures of naturally occurring peptides serve as starting points for the development of new drugs. Several derivatives of antimicrobial peptides have been through the pharmaceutical process, including human phase I-III studies. The use of human antimicrobial peptides as drugs is restricted so far by the still unknown biological function of these molecules and the high costs of the generation of sufficient amounts. On the basis of their functions that have been elucidated so far, antimicrobial peptides might not serve only as antibiotics, but also as modulators of inflammation or anti-LPS medication.
Concluding remarks
Antimicrobial peptides have emerged as effector substances of the innate immune system involving activities not only as endogenous antibiotics but also as mediators of inflammation. Several important topics will have to be addressed in the future:
1. The identification of novel antimicrobial peptides. It is likely that human families of antimicrobial peptides consist of multiple molecules. Progress in the Human Genome Project will also reveal ways of shortcutting conventional bioscreening procedures for the identification of host defense substances.
2. Analysis of the biologically relevant functions of antimicrobial peptides. Beside experiments in vitro that give the first molecular insight into the function of peptide antibiotics, a broader approach involving genetic animal models is necessary to interpret results in vitro in the context of a whole organism.
3. Evaluation of the function of antimicrobial peptides in airway (and other) diseases will provide insights into the corresponding pathogenesis.
4. Development of antimicrobial peptides as drugs. Studying the biology of antimicrobial peptides might permit the development of novel therapeutics for infectious or inflammatory diseases.
Acknowledgments
Acknowledgement
I thank Dr DJ Weiner for helpful discussions, and Professor G Steinbeck, Professor JW Wilson, and Dr C Vogelmeier for their support. Studies in my laboratory related to innate immunity of the respiratory tract are supported by grants of the Deutsche Forschungsgemeinschaft (Ba1641/3-1) and the Friedrich-Baur-Stiftung.
References
- Fearon D, Locksley R. The instructive role of innate immunity in the aquired immune response. Science. 1996;272:50–54. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway CJ., Jr Advances in immunology: innate immunity. N Engl JMed. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- Wilmott R, Fiedler M, Stark J. Host defense mechanisms. . In Disorders of the Respiratory Tract in Children Edited by Chernick V, Boat TF Philadelphia: Saunders; 1998. pp. 238–264.
- Kopp E, Medzhitov R. The Toll-receptor family and control of innate immunity. Curr Opin Immunol. 1999;11:13–18. doi: 10.1016/S0952-7915(99)80003-X. [DOI] [PubMed] [Google Scholar]
- Modlin R, Brightbill H, Godowski P. The Toll of innate immunity on microbial pathogenesis. New Engl JMed. 1999;340:1834–1835. doi: 10.1056/NEJM199906103402312. [DOI] [PubMed] [Google Scholar]
- Brogden KA, Ackermann MR, McCray PB, Jr, Huttner KM. Differences in the concentrations of small, anionic, antimicrobial peptides in bronchoalveolar lavage fluid and in respiratory epithelia of patients with and without cystic fibrosis. Infect Immun. 1999;67:4256–4259. doi: 10.1128/iai.67.8.4256-4259.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R, Ganz T, Selsted M. Defesins: endogenous antibiotic peptides of animal cells. Cell. 1991;64:229–230. doi: 10.1016/0092-8674(91)90632-9. [DOI] [PubMed] [Google Scholar]
- Ganz T, Selsted ME, Szklarek D, Harwig SS, Daher K, Bainton DF, Lehrer RI. Defensins. Natural peptide antibiotics of human neuitrophils. . J Clin Invest. 1985;76:1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsted ME, Harwig SS, Ganz T, Schilling JW, Lehrer RI. Primary structures of three human neutrophil defensins. J Clin Invest. 1985;76:1436–1439. doi: 10.1172/JCI112121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quayle AJ, Porter EM, Nussbaum AA, Wang YM, Brabec C, Yip KP, Mok SC. Gene expression, immunolocalization, and secretion of human defensin-5 in human female reproductive tract. Am J Pathol. 1998;152:1247–1258. [PMC free article] [PubMed] [Google Scholar]
- Linzmeier R, Ho CH, Hoang BV, Ganz T. A 450-kb contig of defensin genes on human chromosome 8p23. Gene. 1999;233:205–211. doi: 10.1016/S0378-1119(99)00136-5. [DOI] [PubMed] [Google Scholar]
- Wilson CL, Ouellette AJ, Satchell DP, Ayabe T, Lopez-Boado YS, Stratman JL, Hultgren SJ, Matrisian LM, Parks WC. Regulation of intestinal α-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999;286:113–117. doi: 10.1016/S0009-2614(98)00088-8. [DOI] [PubMed] [Google Scholar]
- Soong L, Ganz T, Ellison A, Caughey G. Purification and characterization of defensins from cystic fibrosis sputum. Inflamm Res. 1997;46:98–102. doi: 10.1007/s000110050114. [DOI] [PubMed] [Google Scholar]
- Diamond G, Zasloff M, Eck H, Brasseur M, Maloy WL, Bevins CL. Tracheal antimicrobial peptide, a cysteine-rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proc Natl Acad Sci USA. 1991;88:3952–3956. doi: 10.1073/pnas.88.9.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensch K, Raida M, Magert H-J, Schulz-Knappe P, Forssmann W-G. hBD-1: a novel β-defensin from human plasma. . FEBS Lett. 1995;368:331–335. doi: 10.1016/0014-5793(95)00687-5. [DOI] [PubMed] [Google Scholar]
- McCray P, Jr, Bentley L. Human airway epithelia express a β-defensin. Am JRespir Cell Mol Biol. 1997;16:343–349. doi: 10.1165/ajrcmb.16.3.9070620. [DOI] [PubMed] [Google Scholar]
- Goldman MJ, Anderson GM, Stolzenberg ED, Kari UP, Zasloff M, Wilson JM. Human β-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell . 1997;88:553–560. doi: 10.1016/s0092-8674(00)81895-4. [DOI] [PubMed] [Google Scholar]
- Valore EV, Park CH, Quayle AJ, Wiles KR, McCray PB, Jr, Ganz T. Human β-defensin-1: an antimicrobial peptide of urogenital tissues. J Clin Invest. 1998;101:1633–1642. doi: 10.1172/JCI1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder J, Bartels J, Christophers E, Schroeder J-M. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- Singh P, Jia H, Wiles K, Hesselberth J, Liu L, Conway B, Greenberg E, Valore E, Welsh M, Ganz T, Tack B, McCray PJ. Production of β-defensins by human airway epithelia. Proc Natl Acad Sci USA. 1998;95:14961–14966. doi: 10.1073/pnas.95.25.14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bals R, Wang X, Wu Z, Freeman , Banfa V, Zasloff M, Wilson J. Human β-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J Clin Invest. 1998;102:874–880. doi: 10.1172/JCI2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y-Q, Yaun J, Osapay G, Osapay C, Tran D, Miller C, Quellette A, Selsted M. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated α-defensins. . Science. 1999;286:498–502. doi: 10.1126/science.286.5439.498. [DOI] [PubMed] [Google Scholar]
- Zanetti M, Gennaro R, Romeo D. Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 1995;374:1–5. doi: 10.1016/0014-5793(95)01050-O. [DOI] [PubMed] [Google Scholar]
- Gudmundsson GH, Agerberth B, Odeberg J, Bergman T, Olsson B, Salcedo R. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur J Biochem . 1996;238:325–332. doi: 10.1111/j.1432-1033.1996.0325z.x. [DOI] [PubMed] [Google Scholar]
- Cowland J, Johnsen A, Borregaard N. hCAP-18, a cathelin/probactenecin-like protein of human neutrophil specific granules. . FEBS Lett. 1995;368:173–176. doi: 10.1016/0014-5793(95)00634-L. [DOI] [PubMed] [Google Scholar]
- Agerberth B, Gunne H, Odeberg J, Kogner P, Boman HG, Gudmundsson GH. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc Natl Acad Sci USA. 1995;92:195–199. doi: 10.1073/pnas.92.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohm M, Agerberth B, Ahangari G, Stahle-Backdahl M, Liden S, Wigzell H, Gudmundsson GH. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997;272:15258–15263. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- Bals R, Wang X, Zasloff M, Wilson JM. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci US A. 1998;95:9541–9546. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agerberth B, Grunewald J, Castanos-Velez E, Olsson B, Jornvall H, Wigzell H, Eklund A, Gudmundsson GH. Antibacterial components in bronchoalveolar lavage fluid from healthy individuals and sarcoidosis patients. Am J Respir Crit Care Med. 1999;160:283–290. doi: 10.1164/ajrccm.160.1.9807041. [DOI] [PubMed] [Google Scholar]
- Bals R, Weiner D, Moscioni A, Meegalla R, Wilson J. Augmentation of innate host defense by expression of a cathelicidin antimicrobial peptide. Infect Immun. 1999;67:6084–6089. doi: 10.1128/iai.67.11.6084-6089.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim FG, Xu T, McMillian FM, Levitz SM, Diamond RD, Offner GD, Troxler RF. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J Biol Chem. 1988;263:7472–7477. [PubMed] [Google Scholar]
- vanderSpek JC, Wyandt HE, Skare JC, Milunsky A, Oppenheim FG, Troxler RF. Localization of the genes for histatins to human chromosome 4q13 and tissue distribution of the mRNAs. Am J Hum Genet. 1989;45:381–387. [PMC free article] [PubMed] [Google Scholar]
- vanderSpek JC, Offner GD, Troxler RF, Oppenheim FG. Molecular cloning of human submandibular histatins. Arch Oral Biol. 1990;35:137–143. doi: 10.1016/0003-9969(90)90175-a. [DOI] [PubMed] [Google Scholar]
- O'Neil DA, Porter EM, Elewaut D, Anderson GM, Eckmann L, Ganz T, Kagnoff MF. Expression and regulation of the human β-defensins hBD-1 and hBD-2 in intestinal epithelium. J Immunol. 1999;163:6718–6724. [PubMed] [Google Scholar]
- Hiratsuka T, Nakazato M, Date Y, Ashitani J, Minematsu T, Chino N, Matsukura S. Identification of human β-defensin-2 in respiratory tract and plasma and its increase in bacterial pneumonia. . Biochem Biophys Res Commun. 1998;249:943–947. doi: 10.1006/bbrc.1998.9239. [DOI] [PubMed] [Google Scholar]
- Harder J, Meyer-Hoffert U, Teran LM, Schwichtenberg L, Bartels J, Maune S, Schroder JM. Mucoid Pseudomonas aeruginosa, TNF-α, and IL-1β, but not IL-6, induce human β-defensin-2 in respiratory epithelia. Am J Respir Cell Mol Biol. 2000;22:714–721. doi: 10.1165/ajrcmb.22.6.4023. [DOI] [PubMed] [Google Scholar]
- Frohm Nilsson M, Sandstedt B, Sorensen O, Weber G, Borregaard N, Stahle-Backdahl M. The human cationic antimicrobial protein (hCAP18), a peptide antibiotic, is widely expressed in human squamous epithelia and colocalizes with interleukin-6. Infect Immun. 1999;67:2561–2566. doi: 10.1128/iai.67.5.2561-2566.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond G, Legarda D, Ryan LK. The innate immune response of the respiratory epithelium. Immunol Rev. 2000;173:27–38. doi: 10.1034/j.1600-065X.2000.917304.x. [DOI] [PubMed] [Google Scholar]
- Becker MN, Diamond G, Verghese MW, Randell SH. CD14-dependent LPS-induced β-defensin-2 expression in human tracheobronchial epithelium. J Biol Chem. 2000;275:29731–29736. doi: 10.1074/jbc.M000184200. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Reichhart JM, Hoffmann JA. Drosophila host defense: differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proc Natl Acad Sci U S A. 1997;94:14614–14619. doi: 10.1073/pnas.94.26.14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer RI, Barton A, Daher KA, Harwig SS, Ganz T, Selsted ME. Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J Clin Invest . 1989;84:553–561. doi: 10.1172/JCI114198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencer W, Cheung G, Strohmeier G, Currie M, Ouellette A, Selsted M, Madara J. Induction of epithelial chloride secretion by channel-forming cryptdins 2 and 3. Proc Natl Acad Sci USA. 1997;94:8585–8589. doi: 10.1073/pnas.94.16.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover DM, Rajashankar KR, Blumenthal R, Puri A, Oppenheim JJ, Chertov O, Lubkowski J. The structure of human β-defensin-2 shows evidence of higher-order oligomerization. J Biol Chem. 2000;275:32911–32918. doi: 10.1074/jbc.M006098200. [DOI] [PubMed] [Google Scholar]
- Ludtke SJ, He K, Heller WT, Harroun TA, Yang L, Huang HW. Membrane pores induced by magainin. Biochemistry. 1996;35:13723–13728. doi: 10.1021/bi9620621. [DOI] [PubMed] [Google Scholar]
- Ernst RK, Yi EC, Guo L, Lim KB, Burns JL, Hackett M, Miller SI. Specific lipopolysaccharide found in cystic fibrosis airway Pseudomonas aeruginosa. . Science. 1999;286:1561–1565. doi: 10.1126/science.286.5444.1561. [DOI] [PubMed] [Google Scholar]
- Peschel A, Otto M, Jack R, Kalbacher H, Jung G, Götz F. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J Biol Chem. 1999;274:8405–8410. doi: 10.1074/jbc.274.13.8405. [DOI] [PubMed] [Google Scholar]
- Lysenko ES, Gould J, Bals R, Wilson JM, Weiser JN. Bacterial phosphorylcholine decreases susceptibility to the antimicrobial peptide LL-37/hCAP18 expressed in the upper respiratory tract. Infect Immun . 2000;68:1664–1671. doi: 10.1128/IAI.68.3.1664-1671.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer WM, Qu X, Waring AJ, Lehrer RI. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc Natl Acad Sci USA. 1998;95:1829–1833. doi: 10.1073/pnas.95.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wetering S, Mannesse-Lazeroms SP, Dijkman JH, Hiemstra PS. Effect of neutrophil serine proteinases and defensins on lung epithelial cells: modulation of cytotoxicity and IL-8 production. J Leukoc Biol. 1997;62:217–226. doi: 10.1002/jlb.62.2.217. [DOI] [PubMed] [Google Scholar]
- Van Wetering S, Mannesse-Lazeroms SP, Van Sterkenburg MA, Daha MR, Dijkman JH, Hiemstra PS. Effect of defensins on interleukin-8 synthesis in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 1997;272:L888–L896. doi: 10.1152/ajplung.1997.272.5.L888. [DOI] [PubMed] [Google Scholar]
- Territo MC, Ganz T, Selsted ME, Lehrer R. Monocyte-chemotactic activity of defensins from human neutrophils. J Clin Invest. 1989;84:2017–2020. doi: 10.1172/JCI114394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chertov O, Michiel DF, Xu L, Wang JM, Tani K, Murphy WJ, Longo DL, Taub DD, Oppenheim JJ. Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J Biol Chem. 1996;271:2935–2940. doi: 10.1074/jbc.271.6.2935. [DOI] [PubMed] [Google Scholar]
- van Wetering S, van der Linden AC, van Sterkenburg MA, de Boer WI, Kuijpers AL, Schalkwijk J, Hiemstra PS. Regulation of SLPI and elafin release from bronchial epithelial cells by neutrophil defensins. . Am J Physiol Lung Cell Mol Physiol. 2000;278:L51–L58. doi: 10.1152/ajplung.2000.278.1.L51. [DOI] [PubMed] [Google Scholar]
- Van Wetering S, Rahman I, Hiemstra PS, MacNee W. Role of intra-cellular glutathione in neutrophil defensin-induced IL-8 synthesis and cytotoxicity in airway epithelial cells. Eur Respir J. 1998;12:420s. doi: 10.1183/09031936.98.12020420. [DOI] [Google Scholar]
- Gorter AD, Eijk PP, van Wetering S, Hiemstra PS, Dankert J, van Alphen L. Stimulation of the adherence of Haemophilus influenzae to human lung epithelial cells by antimicrobial neutrophil defensins. J Infect Dis. 1998;178:1067–1074. doi: 10.1086/515667. [DOI] [PubMed] [Google Scholar]
- Yang D, Chen Q, Chertov O, Oppenheim JJ. Human neutrophil defensins selectively chemoattract naive T and immature den-dritic cells. . J Leukoc Biol. 2000;68:9–14. [PubMed] [Google Scholar]
- van Wetering S, Sterk PJ, Rabe KF, Hiemstra PS. Defensins: key players or bystanders in infection, injury, and repair in the lung? . J Allergy Clin Immunol. 1999;104:1131–1138. doi: 10.1016/s0091-6749(99)70004-7. [DOI] [PubMed] [Google Scholar]
- Yang D, Chertov O, Bykovskaia S, Chen Q, Buffo M, Shogan J, Anderson M, Schroder J, Wang J, Howard O, Oppenheim J. β-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- Smith J, Travis S, Greenberg E, Welsh M. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- Bals R, Weiner D, Wilson J. The innate immune system in cystic fibrosis lung disease. J Clin Invest. 1999;103:303–307. doi: 10.1172/JCI6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillard JW, Jr, Boyaka PN, Chertov O, Oppenheim JJ, McGhee JR. Mechanisms for induction of acquired host immunity by neutrophil peptide defensins. Proc Natl Acad Sci USA. 1999;96:651–656. doi: 10.1073/pnas.96.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle AJ, Uchida D, Ackerman SJ, Mitzner W, Irvin CG. Role of cationic proteins in the airway. Hyperresponsiveness due to airway inflammation. Am J Respir Crit Care Med. 1994;150:S63–S71. doi: 10.1164/ajrccm/150.5_Pt_2.S63. [DOI] [PubMed] [Google Scholar]
- Boman HG. Peptide antibiotics and their role in innate immunity. . Annu Rev Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]


