Abstract
Self-assembly of electroactive molecules is a promising route to new types of functional semiconductors. Here we report a capsule-shaped molecule that assembles itself into a cellular semiconducting material. The interior space of the capsule with a volume of ~415 Å3 is a nanoenvironment that can accommodate a guest. To self-assemble these capsules into electronic materials, we functionalize the thiophene rings with bromines, which encode self-assembly into two-dimensional layers held together through halogen bonding interactions. In the solid state and in films, these two-dimensional layers assemble into the three-dimensional crystalline structure. This hollow material is able to form the active layer in field effect transistor devices. We find that the current of these devices has strong response to the guest’s interaction within the hollow spaces in the film. These devices are remarkable in their ability to distinguish, through their electrical response, between small differences in the guest.
Perylene diimide-bithiophene macrocycles are electroactive and shape-persistent hosts. Here, the authors describe their self-assembly into a cellular organic semiconducting film whose voids are electrically sensitive to different guests, and which can function as the active layer in a field-effect transistor device.
Introduction
There is a growing class of electroactive, conjugated cyclic molecules that are being applied in several areas of materials science1–27. These cyclic, conjugated organic semiconductors have interior spaces that should be useful as a locus for guest inclusion to tune the electronic and optoelectronic properties6,22,28–30. Conjugated, cyclic semiconductors that incorporate diphenyl perylene diimides (PDIs) have many benefits as the active elements in organic field-effect transistors (OFETs), organic photovoltaics31, and organic photodetectors32. In the cases we have studied until now, the diphenyl–PDI subunits provide open macrocycles with cavity diameters so large that they exhibit dynamic stereochemistry as the PDI subunits readily rotate through the interior of the ring20.
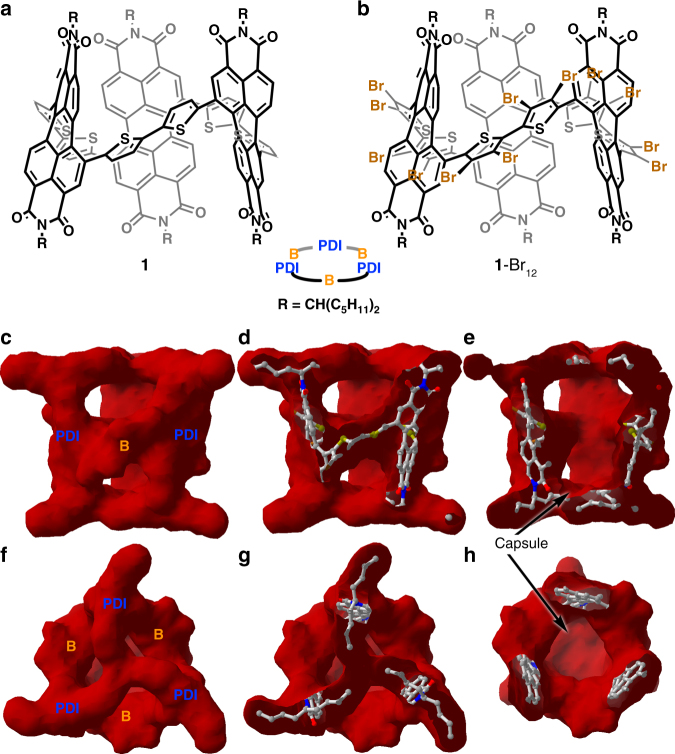
We describe here a new electronic material whose molecular components are shape persistent and can be functionalized so they self-assemble into semiconducting films. These films feature regular open spaces, cells, in them. We call this new material a cellular organic semiconductor. The molecular substructure results from the union of bithiophenes (B) and PDI into a macrocycle. We fully utilize the interior of the macrocycle in devices, and endow the materials with permanent, open voids, by rigidifying the macrocycle so they cannot collapse. We describe the syntheses and structures of the organic semiconductors shown in Fig. 1a, b: the trimeric macrocycle 1, (-PDI-B-)3 and its brominated version 1-Br12. These macrocycles exist as a single pair of enantiomers and are shape persistent to temperatures above 160 °C. Their capsular structure is capped on the ends by the alkyl sidechains and on the equator by the electronic components (the PDI and B subunits). This is shown in Fig. 1c–h. While 1 and 1-Br12 share the same overall shape and molecular structure, compound 1 does not organize well in films or in the solid state. However, the twelve bromines of 1-Br12 participate in halogen bonding interactions, facilitating self-assembly in films and crystals. 1-Br12’s self-organization creates films that have regular voids in them, forming the hollow organic semiconducting phase. The remarkable finding is that 1-Br12’s cellular films act as the active layer in OFETs, and the electrical response depends on the guest that occupies the interior space.
Fig. 1.
Structures of cellular semiconductors. a Trimer 1; and b 1-Br12. Van der Waals Surface of 1-Br12 seen from the side (c), and top (f). In the sequence c-e and f–h the molecule is trimmed down to expose its cavity or capsule
Results
Capsule construction
We developed a synthesis of 1 based on our own previous studies to make the diphenyl-PDI macrocycles20 that builds from the methodology originally pioneered for cyclothiophenes and later for cycloparaphenylenes20,31. The Supporting Information contains the details of the syntheses and characterization (Supplementary Methods and Supplementary Fig. 1–3) of 1 and 1-Br12. For each of the macrocycles, the 1,7-dithienyl-PDI subunit introduces an element of chirality because it can exist in either a R- or S-helical conformation. This allows for the possibility of two pairs of enantiomers (RRR/SSS and RRS/SSR)20. However, in the reaction to form 1, we only observe the RRR/SSS pair. We introduce bromine atoms in the thiophene rings of 1 to encourage self-assembly through halogen bonding interactions33.
To test the shape persistence of these macrocycles, we separate the two enantiomers of 1, using a chiral stationary phase for HPLC, and monitor their interconversion as we heat the samples. The two enantiomers of 1 exhibit an intense (and opposite) chiroptic response in their circular dichroism spectra (Supplementary Fig. 2). Remarkably, the enantiomers of 1 do not interconvert, even when heated up to 160 °C. Macrocycle 1 is the first PDI-based cyclic semiconductor that can be isolated in its optically active form and is shape-persistent.
Capsule structure
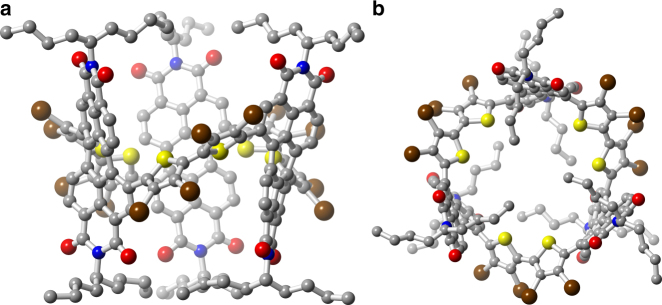
The crystals of 1, while sizable and faceted, do not diffract well enough to yield a structure from single-crystal X-ray diffraction (SCXRD), but we were able to grow single crystals of sufficient quality to yield the structures of 1-Br12. Figure 2a, b displays the structure of one of the two enantiomers of 1-Br12 [(SSS)-1-Br12]. Both enantiomers of 1-Br12 are present in the crystalline state. The structure of 1-Br12 is cylindrical with the three sets of bithiophenes and three PDIs forming the walls at the equator. The ends of the cylinder are capped with branched alkyl chains (Fig. 2 and highlighted in green in Fig. 3a). This creates windows on the side of the structure, displayed in Figs. 1c, f and 2a. We estimate the interior volume of the capsule in 1-Br12 (shown in Fig. 1e, h) to be approximately 415 Å334.
Fig. 2.
Molecular structure from SCXRD of 1-Br12. a Side and b top view of (SSS)-1-Br12. C, N, O, S, and Br atoms are colored in gray, blue, red, yellow, and brown, respectively. Hydrogen atoms have been removed to clarify the view. The alkyl chains on the imide are refined to only nine of the eleven carbon atoms due to disorder (see Supplementary Methods)
Fig. 3.
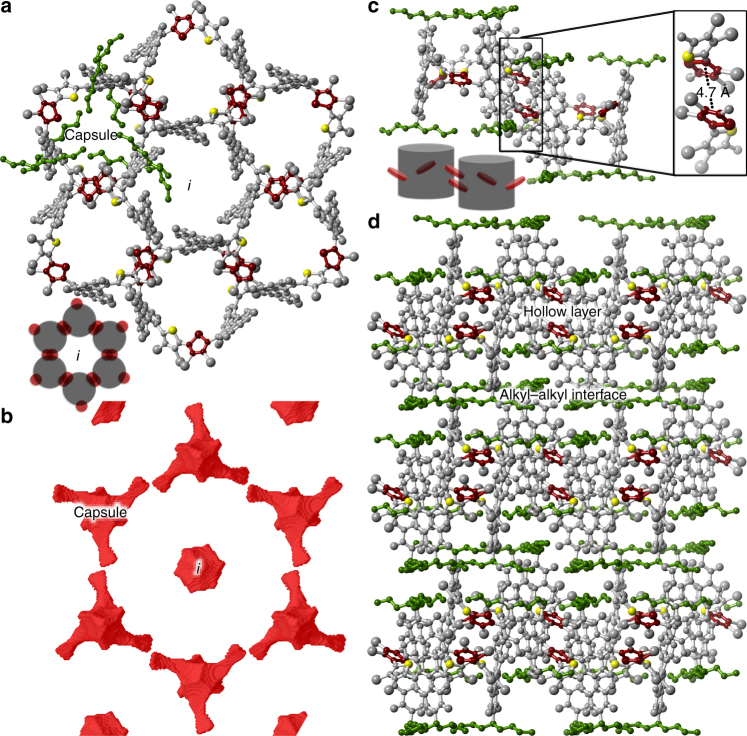
Structural packing of 1-Br12. a View of the honeycomb structure in the ab plane for 1-Br12. The capsule and i corresponds to the internal cavity of 1-Br12 and the cavity formed by the packing of 1-Br12, respectively. The remaining sulfur atoms are colored in yellow to provide a marker to identify the macrocycle cavities. See bottom left cartoon. Highlighted in green are the imide side chains (some of the sidechains have been removed to clarify the view of the cavity). In red are the thiophene rings likely involved in holding the macrocycles together. b Surface map of the void space in the ab plane of 1-Br12. c Two molecules of 1-Br12 where the thiophene-to-thiophene interaction is highlighted as an inset. Bottom left cartoon represents this interaction. d View of the packing of 1-Br12. As shown, the vertical stacking follows the c axis. The alkyl sidechains of the imide are shown in green. Hydrogen atoms have been removed from all structures to clarify the view
Cellular solids from capsular nanostructures
The packing structure in the solid state of 1-Br12 (Fig. 3) reveals why the bromines were necessary for long range crystallinity. The structure is composed of sheets of a honeycomb-like arrangement in the ab plane (Fig. 3a). The interactions that bind the cylinders within the ab plane are from neighboring brominated thiophene rings (4.7 Å apart, marked in red in Fig. 3a, c–d) and some secondary π–π contacts between those same rings (marked in blue in Supplementary Fig. 4). The PDIs do not π-stack with each other. Instead halogen bonding from the functionalized thiophenes drives the self-assembly process. The cavity of 1-Br12 (labeled “Capsule” in Fig. 3a, b) is ~11.4 Å in diameter and is a three-fold symmetric chiral nanoenvironment for guest incorporation within the two-dimensional layer. Due to the packing of the subunits of 1-Br12 into a hexameric cyclic structure, a second cavity forms at the center of each hexagon (labeled i in Fig. 3a, b). This cavity is not elongated compared to the capsule’s cavity, and each one accounts for ~110 Å3 (Supplementary Fig. 5). These honeycomb two-dimensional, cellular sheets then stack through the packing of the alkyl side chains of the imides, (shown in green in Fig. 3). This packing arrangement propagates along the c axis, hinting that these materials could likely be exfoliated to yield molecularly thin sheets of 1-Br12.
We find that 1-Br12 self-assembly in cast thin films and powder samples is analogous to what we described above for the single crystal. Supplementary Figs 6, 7 compare the thin film and powder diffraction data for 1-Br12 with the simulated pattern calculated from the SCXRD data. No other reflections are present in the films or powders indicating that the self-assembly motif using the halogen bonding is robust. In order to extract quantitative information about the long-range crystallinity of these new hollow semiconductors we performed pair distribution function (PDF) analyses on powders of 1 and 1-Br12 (Supplementary Figs. 8–12). Supplementary Fig. 12 displays the PDF of 1-Br12 at low (1.81 Å−1) and high (6.33 Å−1) Qmax to give a visualization of the coherence length. The lower and upper bounds of the coherence length of 1-Br12 are 335 and 385 Å, respectively (Supplementary Fig. 12). To put these values in perspective, this indicates that crystalline domains within 1-Br12 contain on average up to 23 to 27 capsules arranged in one direction. In contrast, 1, with poor crystallinity and an inability to self-organize (Supplementary Fig. 6), is essentially amorphous. The analysis shows coherence lengths ranging from 65 to 120 Å (Supplementary Fig. 11), which is only a few capsules in length.
Electron transport through cellular films
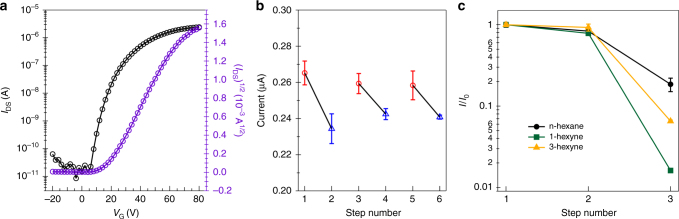
Figure 4a displays transfer curves from an OFET constructed using a self-assembled thin film of 1-Br12. Details for the device dimensions and its properties can be found in the Supplementary Methods. The device exhibits electron transporting character and has a mobility of ~1.5 × 10−2 cm2 V−1 s−1. The mobility of 1-Br12 is more than 20 times greater than that of 1 (~6.8 × 10−4 cm2 V−1 s−1) (Supplementary Fig. 13). We attribute this to the robust self-assembly process for 1-Br12. From atomic force microscope height images, films of 1 display a smooth surface with root mean square (RMS) roughness of 0.347 nm; in contrast 1-Br12 displays a larger RMS roughness of 3.2 nm, presumably due to its more crystalline nature and better self-assembly properties (Supplementary Fig. 14).
Fig. 4.
Electron transport for cellular films. a Transfer characteristics of OFET device for 1-Br12. b Device cycling response under vacuum (red circles) and N2 atmosphere (blue triangles). c Normalized behavior of the device response under vacuum (step 1), N2 (step 2), and different analytes atmosphere (step 3: n-hexane, 3-hexyne and 1-hexyne). Error bars represent the standard error obtained in three measurements
The exciting finding is that the devices show modulation in the drain current depending on what, if anything, is within the voids in the cellular film. Details of the experimental setup to measure the exposure to gases are given in the Supplementary Methods. Every potential guest tested had a measurable effect on the drain current in the device, but the absolute levels of the drain current varied depending on the guest. Figure 4b is a representative data set for a device measured sequentially in steps as the atmosphere is changed between vacuum and N2. In certain cases, traditional OFETs show a differential response to nitrogen and vacuum that arises from extrinsic effects (moisture, oxygen, or dielectric effects)35,36. We can test the importance of the cellular semiconductor’s response by making a control FET device from 1, which does not self-organize into cellular semiconducting films or crystals. We observe no response when comparing its response to nitrogen and vacuum (Supplementary Fig. 15a).
Incorporating more polarizable and functional guests causes more pronounced changes in the drain current. Supplementary Fig. 15b compares the effects of several different guests with a variety of functional groups such as ketones, alcohols, nitriles, alkynes, and alkanes. In each case we are able to differentiate the guest by the current in the device. We highlight here one striking example of how this material responds to a series of closely related hydrocarbons. These hydrocarbons were chosen so that their length, size, and polarity were roughly similar to that of n-hexane, while having an additional functional group in them. Fig. 4c compares the transistor output for three devices exposed to n-hexane, 3-hexyne, or 1-hexyne, carried by nitrogen under their saturated vapor pressure (148, 99, and 138 mmHg at 25 °C, respectively)37. Remarkably, the devices can easily detect these three materials and distinguish them from one another. There is also specificity towards particular analytes; the trend in the device responses does not simply follow the vapor pressure. For n-hexane and 3-hexyne the data was collected after the OFET was in contact with the vapor for ~70 min (Supplementary Fig. 16a). After this time, the drain current no longer decreased. The original current levels for n-hexane and 3-hexyne could be restored by placing the devices in vacuum (Supplementary Fig. 16b). During these long exposures, we speculate that the guests infiltrate the films to reside in the active part of the films at the gate dielectric interface38,39.
We tested the sensitivity of these hollow films and find that there is a linear response between vacuum and 20 part per thousand of the analyte in the atmosphere, after we observed a plateau region as the concentration reached saturation (Supplementary Fig. 17). Access to the porous network formed by the capsules interior (~415 Å3) and the i-sites (~110 Å3) is granted by the windows in the cellular structure as shown in Fig. 1c–h. Using BET, we find that powders of 1-Br12 have a surface area of 20 m2 g−1 (versus 1.2 m2 g−1 for 1). As n-hexane has a van der Waals molecular volume of ~113 Å340, the implication is that n-hexane would not fit into the i-site. In addition, most of the cellular nature of self-assembled 1-Br12 comes from the capsule interior which is present in a 2:1 numerical ratio relative to the i-site. Given the 8-fold difference in volume between these two cavities, we speculate that guests can be accommodated most feasibly at the capsules’ interior.
1-hexyne behaves differently than each of the other guests tested. For the devices in an atmosphere of 1-hexyne, the current continues to drop and does not reach a plateau even at times that exceed 2 h of exposure. In addition, the 1-hexyne devices do not recover to their original levels when placed in vacuum (Supplementary Fig. 18). We speculate that the terminal alkyne is undergoing a reaction under the device conditions that is not possible with the internal alkyne or the alkane. This offers the intriguing possibility that in addition to sensing, the nanoenvironments in these hollow semiconductors can be used as nanoreactors.
Discussion
We have described here a shape persistent, hollow macrocycle that self-assembles both in the solid state and thin films to form cellular organic semiconductors. The macrocycle is chiral and conformationally locked into a capsular structure, with ~415 Å3 volume within its interior. The self-assembly of a brominated derivative of the trimer into cellular films forms the active layer in an organic field-effect transistor device. Once assembled, these films have periodic, nanoscopic, cellular voids. Because the macrocyclic component in the film is conformationally locked, the self-assembled films maintain their interior, open spaces. The hollow films of 1-Br12 are responsive to the atmosphere in which the OFETs are measured. These studies chart a clear path to using the interior of the cellular organic semiconductors as gas sensors41,42 and nanoreactors.
Data availability
All data are available from the authors upon reasonable request. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number 1834626. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Electronic supplementary material
Acknowledgements
Primary support for this project was provided by the Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences, U.S. Department of Energy (DOE), under award number DE-FG02-01ER15264. PDF measurement and analysis (M.W.T. and S.J.L.B.) was supported by the NSF MRSEC program through Columbia in the Center for Precision Assembly of Superstratic and Superatomic Solids (DMR-1420634). The Columbia University Shared Materials Characterization Laboratory (SMCL) was used extensively for this research. We are grateful to Columbia University for the support of this facility. C.N. thanks Sheldon and Dorothea Buckler for their generous support. R.H.S. acknowledges the support from the Columbia Nano Initiative Postdoctoral Fellowship. This research used beamline 28-ID-2 of the National Synchrotron Light Source II, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DE-SC0012704.
Author contributions
F.N., M.L.S. and C.N. conceived the idea. B.Z. and R.H.S. designed the experiments. B.Z. performed device fabrication, characterization and data analysis. R.H.S. conducted PXRD, SCXRD measurements and data analysis. Y.Z. designed the setup and helped with device fabrication. M.B. performed DFT calculations. M.W.T. conducted PDF analysis. D.P. helped with SCXRD refinement. F.N. synthesized and purified compound 1 and 1-Br12. B.Z., R.H.S., and C.N. wrote the manuscript. Y.Z., M.B., M.W.T., D.P., S.J.L., F.N. and M.L.S. contributed to revision of the manuscript. All authors discussed the results and reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Boyuan Zhang, Raúl Hernández Sánchez.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-04246-0.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fay Ng, Email: fwn2@columbia.edu.
Michael L. Steigerwald, Email: mls2064@columbia.edu
Colin Nuckolls, Email: cn37@columbia.edu.
References
- 1.Krömer J, et al. Synthesis of the first fully α-conjugated macrocyclic oligothiophenes: cyclo[n]thiophenes with tunable cavities in the nanometer regime. Angew. Chem. Int. Ed. 2000;39:3481–3486. doi: 10.1002/1521-3773(20001002)39:19<3481::AID-ANIE3481>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 2.Nakao K, et al. Giant macrocycles composed of thiophene, acetylene, and ethylene building blocks. J. Am. Chem. Soc. 2006;128:16740–16747. doi: 10.1021/ja067077t. [DOI] [PubMed] [Google Scholar]
- 3.Jasti R, Bhattacharjee J, Neaton JB, Bertozzi CR. Synthesis, characterization, and theory of [9]-, [12]-, and [18]cycloparaphenylene: carbon nanohoop structures. J. Am. Chem. Soc. 2008;130:17646–17647. doi: 10.1021/ja807126u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang F, Götz G, Winkler HDF, Schalley CA, Bäuerle P. Giant cyclo[n]thiophenes with extended π conjugation. Angew. Chem., Int. Ed. 2009;48:6632–6635. doi: 10.1002/anie.200900101. [DOI] [PubMed] [Google Scholar]
- 5.Omachi H, Matsuura S, Segawa Y, Itami K. A modular and size-selective synthesis of [n]cycloparaphenylenes: a step toward the selective synthesis of [n, n] single-walled carbon nanotubes. Angew. Chem. Int. Ed. 2010;49:10202–10205. doi: 10.1002/anie.201005734. [DOI] [PubMed] [Google Scholar]
- 6.Iwamoto T, Watanabe Y, Sadahiro T, Haino T, Yamago S. Size-selective encapsulation of C60 by [10]cycloparaphenylene: formation of the shortest fullerene-peapod. Angew. Chem., Int. Ed. 2011;50:8342–8344. doi: 10.1002/anie.201102302. [DOI] [PubMed] [Google Scholar]
- 7.Iwamoto T, Watanabe Y, Sakamoto Y, Suzuki T, Yamago S. Selective and random syntheses of [n]cycloparaphenylenes (n = 8–13) and size dependence of their electronic properties. J. Am. Chem. Soc. 2011;133:8354–8361. doi: 10.1021/ja2020668. [DOI] [PubMed] [Google Scholar]
- 8.Segawa Y, et al. Concise synthesis and crystal structure of [12]cycloparaphenylene. Angew. Chem. Int. Ed. 2011;50:3244–3248. doi: 10.1002/anie.201007232. [DOI] [PubMed] [Google Scholar]
- 9.Yasutomo S, Petr Scaron, Sanae M, Haruka O, Kenichiro I. [9]Cycloparaphenylene: nickel-mediated synthesis and crystal structure. Chem. Lett. 2011;40:423–425. doi: 10.1246/cl.2011.423. [DOI] [Google Scholar]
- 10.Sprafke JK, et al. Belt-shaped π-systems: relating geometry to electronic structure in a six-porphyrin nanoring. J. Am. Chem. Soc. 2011;133:17262–17273. doi: 10.1021/ja2045919. [DOI] [PubMed] [Google Scholar]
- 11.Hitosugi S, Nakanishi W, Isobe H. Atropisomerism in a belt-persistent nanohoop molecule: rotational restriction forced by macrocyclic ring strain. Chem. Asian J. 2012;7:1550–1552. doi: 10.1002/asia.201200187. [DOI] [PubMed] [Google Scholar]
- 12.Hitosugi S, Yamasaki T, Isobe H. Bottom-up synthesis and thread-in-bead structures of finite (n,0)-zigzag single-wall carbon nanotubes. J. Am. Chem. Soc. 2012;134:12442–12445. doi: 10.1021/ja305723j. [DOI] [PubMed] [Google Scholar]
- 13.Ishii Y, et al. Size-selective synthesis of [9]-[11] and [13]cycloparaphenylenes. Chem. Sci. 2012;3:2340–2345. doi: 10.1039/c2sc20343j. [DOI] [Google Scholar]
- 14.Kayahara E, Sakamoto Y, Suzuki T, Yamago S. Selective synthesis and crystal structure of [10]cycloparaphenylene. Org. Lett. 2012;14:3284–3287. doi: 10.1021/ol301242t. [DOI] [PubMed] [Google Scholar]
- 15.Omachi H, Segawa Y, Itami K. Synthesis of cycloparaphenylenes and related carbon nanorings: a step toward the controlled synthesis of carbon nanotubes. Acc. Chem. Res. 2012;45:1378–1389. doi: 10.1021/ar300055x. [DOI] [PubMed] [Google Scholar]
- 16.Evans PJ, Darzi ER, Jasti R. Efficient room-temperature synthesis of a highly strained carbon nanohoop fragment of buckminsterfullerene. Nat. Chem. 2014;6:404–408. doi: 10.1038/nchem.1888. [DOI] [PubMed] [Google Scholar]
- 17.Kayahara E, Patel VK, Yamago S. Synthesis and characterization of [5]cycloparaphenylene. J. Am. Chem. Soc. 2014;136:2284–2287. doi: 10.1021/ja413214q. [DOI] [PubMed] [Google Scholar]
- 18.Yamago S, Kayahara E, Iwamoto T. Organoplatinum-mediated synthesis of cyclic π-conjugated molecules: towards a new era of three-dimensional aromatic compounds. Chem. Rec. 2014;14:84–100. doi: 10.1002/tcr.201300035. [DOI] [PubMed] [Google Scholar]
- 19.Asai K, Fukazawa A, Yamaguchi S. A cyclic octithiophene containing [small beta],[small beta][prime or minute]-linkages. Chem. Commun. (Camb., U. K.) 2015;51:6096–6099. doi: 10.1039/C5CC00570A. [DOI] [PubMed] [Google Scholar]
- 20.Ball M, et al. Chiral conjugated corrals. J. Am. Chem. Soc. 2015;137:9982–9987. doi: 10.1021/jacs.5b05698. [DOI] [PubMed] [Google Scholar]
- 21.Chang SW, Horie M. A donor-acceptor conjugated block copolymer of poly(arylenevinylene)s by ring-opening metathesis polymerization. Chem. Commun. (Camb., U. K.) 2015;51:9113–9116. doi: 10.1039/C5CC00498E. [DOI] [PubMed] [Google Scholar]
- 22.Chen Q, et al. Strain-induced stereoselective formation of blue-emitting cyclostilbenes. J. Am. Chem. Soc. 2015;137:12282–12288. doi: 10.1021/jacs.5b06258. [DOI] [PubMed] [Google Scholar]
- 23.Darzi ER, et al. Synthesis, properties, and design principles of donor–acceptor nanohoops. ACS Cent. Sci. 2015;1:335–342. doi: 10.1021/acscentsci.5b00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ito H, Mitamura Y, Segawa Y, Itami K. Thiophene-based, radial π-conjugation: synthesis, structure, and photophysical properties of cyclo-1,4-phenylene-2′,5′-thienylenes. Angew. Chem. Int. Ed. 2015;54:159–163. doi: 10.1002/anie.201409389. [DOI] [PubMed] [Google Scholar]
- 25.Jiang HW, et al. Cyclic 2,12-porphyrinylene nanorings as a porphyrin analogue of cycloparaphenylenes. J. Am. Chem. Soc. 2015;137:2219–2222. doi: 10.1021/ja513102m. [DOI] [PubMed] [Google Scholar]
- 26.Kuwabara T, Orii J, Segawa Y, Itami K. Curved oligophenylenes as donors in shape-persistent donor–acceptor macrocycles with solvatofluorochromic properties. Angew. Chem. Int. Ed. 2015;54:9646–9649. doi: 10.1002/anie.201503397. [DOI] [PubMed] [Google Scholar]
- 27.Van Raden JM, Darzi ER, Zakharov LN, Jasti R. Synthesis and characterization of a highly strained donor-acceptor nanohoop. Org. Biomol. Chem. 2016;14:5721–5727. doi: 10.1039/C6OB00133E. [DOI] [PubMed] [Google Scholar]
- 28.Iyoda M, Yamakawa J, Rahman MJ. Conjugated macrocycles: concepts and applications. Angew. Chem. Int. Ed. 2011;50:10522–10553. doi: 10.1002/anie.201006198. [DOI] [PubMed] [Google Scholar]
- 29.Iwamoto T, et al. Size- and orientation-selective encapsulation of C70 by cycloparaphenylenes. Chem. Eur. J. 2013;19:14061–14068. doi: 10.1002/chem.201302694. [DOI] [PubMed] [Google Scholar]
- 30.Nakanishi Y, et al. Size-selective complexation and extraction of endohedral metallofullerenes with cycloparaphenylene. Angew. Chem., Int. Ed. 2014;53:3102–3106. doi: 10.1002/anie.201311268. [DOI] [PubMed] [Google Scholar]
- 31.Ball M, et al. Macrocyclization in the design of organic n-type electronic materials. J. Am. Chem. Soc. 2016;138:12861–12867. doi: 10.1021/jacs.6b05474. [DOI] [PubMed] [Google Scholar]
- 32.Zhang B, et al. Rigid, conjugated macrocycles for high performance organic photodetectors. J. Am. Chem. Soc. 2016;138:16426–16431. doi: 10.1021/jacs.6b10276. [DOI] [PubMed] [Google Scholar]
- 33.Metrangolo, P., Resnati, G., Pilati, T. & Biella, S. In Halogen Bonding: Fundamentals and Applications (eds Metrangolo, P. & Resnati, G.) 105–136 (Springer, Berlin, Heidelberg, 2008).
- 34.Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009;42:339–341. doi: 10.1107/S0021889808042726. [DOI] [Google Scholar]
- 35.Pannemann, C., Diekmann, T. & Hilleringmann, U. On the degradation of organic field-effect transistors. Proc.16th International Conference on Microelectronics (ed. Masmoudi, M.) 76–79 (Nouha Editions, Tunis, 2004).
- 36.Wei-Cheng S, et al. Characterization of intrinsic hysteresis of pentacene-based organic thin-film transistor through in-situ real-time electrical measurement. Jpn. J. Appl. Phys. 2014;53:03CC03. doi: 10.7567/JJAP.53.03CC03. [DOI] [Google Scholar]
- 37.Yaws, C. L. & Satyro, M. A. In The Yaws Handbook of Vapor Pressure 2nd edn (ed. Yaws, C. L.) Ch. 1 (Gulf Professional Publishing, Houston, 2015).
- 38.Hsieh JC, Liu CJ, Ju YH. Response characteristics of lead phthalocyanine gas sensor: effects of film thickness and crystal morphology. Thin Solid Films. 1998;322:98–103. doi: 10.1016/S0040-6090(97)00964-4. [DOI] [Google Scholar]
- 39.Sakai G, Matsunaga N, Shimanoe K, Yamazoe N. Theory of gas-diffusion controlled sensitivity for thin film semiconductor gas sensor. Sens. Actuators B. 2001;80:125–131. doi: 10.1016/S0925-4005(01)00890-5. [DOI] [Google Scholar]
- 40.Zhao YH, Abraham MH, Zissimos AM. Fast calculation of van der waals volume as a sum of atomic and bond contributions and its application to drug compounds. J. Org. Chem. 2003;68:7368–7373. doi: 10.1021/jo034808o. [DOI] [PubMed] [Google Scholar]
- 41.Crone B, et al. Electronic sensing of vapors with organic transistors. Appl. Phys. Lett. 2001;78:2229–2231. doi: 10.1063/1.1360785. [DOI] [Google Scholar]
- 42.Huang W, et al. Highly sensitive NH3 detection based on organic field-effect transistors with tris(pentafluorophenyl)borane as receptor. J. Am. Chem. Soc. 2012;134:14650–14653. doi: 10.1021/ja305287p. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available from the authors upon reasonable request. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number 1834626. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.