Abstract
In spondyloarthritis, little is known about the relation between circulating cytokines and patient phenotype. We have quantified serum levels of T helper type 1 cell (Th1), Th2 and Th17 cytokines in patients with recent-onset axial spondyloarthritis (AxSpA) from the DESIR cohort, a prospective, multicenter French cohort consisting of 708 patients with recent-onset inflammatory back pain (duration >3 months but <3 years) suggestive of AxSpA. Serum levels of Th1, Th2, and Th17 cytokines were assessed at baseline in patients from the DESIR cohort fulfilling the ASAS criteria (ASAS+) and were compared with age- and sex-matched healthy controls. At baseline, ASAS+ patients (n = 443) and healthy controls (n = 79) did not differ in levels of most of the Th1, Th2 and Th17 cytokines except for IL-31, and sCD40L, which were significantly higher for ASAS+ patients than controls (p < 0.001 and p = 0.012, respectively). On multivariable analysis of ASAS+ patients, IL-31 level was associated with sCD40L level (p < 0.0001), modified Stoke AS Spine Score (mSASSS) < 1 (p = 0.035). The multivariable analyses showed that IL-31 was an independent factor associated with mSASSS < 1 (p = 0.001) and low bone mineral density (p = 0.01). Increased level of IL-31 might protect against structural damage but is also related to low BMD.
Introduction
Axial spondyloarthritis (AxSpA) is characterized by inflammation, followed by bone erosion and syndesmophyte formation in the more severe forms of the disease. These structural damages in AxSpA remain a major challenge in clinical practice. Indeed, no treatment used in daily practice have been reproductibly shown to stop syndesmophyte formation despite their capacity to significantly reduce inflammation and the painful manifestations of the disease1–3. Bone formation involves cellular actors, growth factors (bone morphogenetic protein), and signaling pathways (Wnt and its inhibitors DKK-1 and sclerostin), whose activity is modulated by pro-inflammatory cytokines, in particular tumor necrosis factor (TNF). The involvement of these different actors and activators can vary depending on the evolutionary stages of the disease.
The T helper type 1 (Th1) group of cytokines including interferon γ (IFNγ) cells have been implicated in the pathogenesis of SpA4. However, this key role of Th1 cytokines has been challenged by the identification of interleukin-23 (IL-23), a member of the IL-12 family of heterodimeric cytokines, and the IL-17 family cytokines as key players in SpA pathogenesis. The finding is supported by studies showing higher frequency of IL-17–producing cells in peripheral blood from patients than healthy donors5–10. However, Th1 and Th17 cytokines may not be the only cytokine subsets to promote inflammation during the course of the disease.
Some data have shown that the Th2 pathway may be involved in the pathophysiology of SpA. To gain further insight into the role of the Th1/Th2 cytokine balance in patients with AxSpA, Rudwaleit et al. studied the impact of Th2-driven atopy in AxSpA versus rheumatoid arthritis (RA) patients11. Atopy was associated with decreased disease severity in RA but this was not seen in AxSpA.
To gain more insight into the role of several cytokines in the disease phenotype and their potential role in the structural severity of AxSpA, we measured a broad range of Th1, Th2 and Th17 cytokines in patients at an early stage of the disease.
Patients and Methods
Study population: The DESIR cohort
DESIR is a longitudinal prospective cohort of patients with inflammatory back pain (IBP) suggestive of axSpA of recent onset recruited from 25 regional centres in France12. All participants in the study gave their written informed consent and all methods were carried out in accordance with relevant guidelines and regulation. A detailed description of the centres, organisation of the cohort and full detailed protocol are available at http://www.lacohortedesir.fr (ClinicalTrials.gov: NCT01648907 approved by Comité de Protection des Personnes Île de France III reference CPP file N°Am5975-5-2457). The participants included in the DESIR cohort were >18 and <50 years old. IBP was defined according to the Calin and/or Berlin criteria13,14, lasting for >3 months but <3 years, with symptoms suggestive of AxSpA according to the local rheumatologist’s assessment (i.e., score ≥5 on a numerical rating scale of 0–10, with 0, not suggestive, and 10, very suggestive of AxSpA). The exclusion criteria were other clearly defined spinal disease (e.g., discarthrosis), history of any biologic treatment and history or current disorders that might interfere with the validity of the informed consent and/or prevent optimal compliance with the cohort. Corticosteroid intake was permitted only with doses of <10 mg prednisone per day and had to be stable for at least 4 weeks before baseline. A total of 708 patients with IBP were included between October 2007 and April 2010. Patients were evaluated every 6 months during the first 2 years and then on a yearly basis for an expected total follow-up duration of 10 years.
The following data were collected at the baseline visit:
Clinical data were axial involvement, peripheral joint involvement, uveitis, inflammatory bowel disease, psoriasis, enthesitis, dactylitis, synovitis, duration of symptoms (defined as the time between the fist axial symptom and the initial interview), activity and severity features of the disease. Other data collected were Bath Ankylosing Spondylitis Global Assessment (BAS-G) (0–100); Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) (0–100); Bath Ankylosing Spondylitis Functional Index (BASFI) (0–100); spinal mobility as measured by the Bath Ankylosing Spondylitis Metrology Index (BASMI) (0–10); Medical Outcomes Survey short form 36 score (SF-36); Health Assessment Questionnaire (HAQ); and the use of non-steroidal anti-inflammatory drugs (NSAIDs) or disease-modifying antirheumatic drugs (DMARDs) (yes/no).
To have a homogeneous population, patients were classified by the Assessment of Spondyloarthritis International Society (ASAS) criteria15, the B. Amor (AMOR) or European Spondylarthropathy Study Group (ESSG) criteria. In our study, only those patients who fulfilled these classification criteria were assessed within the DESIR cohort. Risk factors for osteoporosis collected were age, gender, menopause, tobacco use, excess alcohol consumption, height, weight, body mass index (BMI; kg/m2), and the presence of inflammatory bowel disease.
Biological parameters: erythrocyte sedimentation rate (ESR), levels of C-reactive protein (CRP) and high-sensivity CRP (hsCRP) and presence of human leukocyte antigen B27 (HLA-B27). The ankylosing spondylitis disease activity score (ASDAS)–CRP was calculated by using CRP level15.
Imaging modalities and central reading of x-ray and MRI images were described elsewhere16,17. In brief, pairs of trained central readers independently evaluated x-ray and MRI images of both the sacroiliac joints and spine, with blinding to clinical data and the other imaging modalities. For dichotomus scores, if the 2 readers disagreed, an adjudicator’s score was used. Scores for 2 of 3 agreeing readers were used. For continuous scores, the mean of the 2 agreeing readers was used.
Based on the modified New York (mNY) criteria, sacroiliitis was defined as at least grade >2 bilaterally or grade 3–4 unilaterally by central reading (pos-X-SI)18. Lumbar and cervical spine radiographs were scored by using the modified Stoke AS Spine Score (mSASSS)19. T1-weighted fast spin echo and short τ inversion recovery 1–1.5 tesla MRI of the whole spine (MRI-spine) and sacroiliac joints (MRI-SI) was performed to assess inflammatory and structural changes at baseline. MRI-SI images were considered positive according to the ASAS definition (i.e., presence of bone marrow edema [BME] lesions highly suggestive of SpA (with ≥1 BME lesion on ≥2 consecutive slices or several BME lesions visible on a single slice)20. The MRI inflammation in the spine was defined according to the ASAS criteria21.
Bone mineral density (BMD) measurements: BMD was measured by dual-energy x-ray absorptiometry at baseline for all included patients in 12 centres (i.e., half of the participating centres) with investigators having expertise in BMD measurements. Experienced investigators obtained BMD measurements with Hologic, Inc. or Lunar (GE Healthcare) devices. BMD of the lumbar spine (second to fourth vertebrae) and the upper part of the left femur (total femur and femoral neck) was determined. The results are given as BMD (g/cm2), Z- and T-scores. The definition of low BMD in young adults lacks consensus. The International Society of Clinical Densitometry recommends the threshold of Z-score −2 SD for defining low BMD22; the WHO definition, based on T-scores, cannot be used for non-menopausal women and men <50 years old22. Therefore, we used Z-score ≤ −2 SD (at least one site) as low BMD. One site was defined by total lumbar spine (L1–L4), or total hip, or femoral neck. Z-scores were determined according to references provided by the manufacturers. Gender-specific Z-scores were based on female and male reference curves. All examinations were performed according to the manufacturer’s recommendations. Devices were controlled by measuring a spine phantom at least 3 times a week throughout the study.
Healthy controls: Variété cohort
Patients were compared to 79 age - and sex-matched healthy controls from the “Variété cohort”.
Variété is an open, prospective, French national, multicenter, non-randomized study of healthy volunteers23 (ClinicalTrials.gov: NCT01831648). Subjects included in this cohort were a large random selection from the general population that included representation from all age groups (about 100 participants for each decade age range). Participants with medical conditions and receiving medications that may affect cytokines measurement were excluded. A total of 974 healthy participants were recruited in 10 centers in France. Each participant underwent clinical examination23. Personal medical history was recorded and gonadal status evaluated. Patients underwent biological standard workup, and 80 ml blood was sampled; serum and plasma samples were aliquoted and frozen and stored at −80 °C before hormone measurements. All patients gave their informed consent to participate in the study, which was approved by the local ethics committee.
Serum assays
The biological resources centre (Paris, Bichat Hospital Claude Bernard–CRB BCB, Certificate no. 34457, S. Tubiana) was in charge of centralising and managing biological data collection.
Cytokines in serum samples were measured by using the Bio-Plex Pro Human Th17 Cytokine Assays 1 (Bio-Rad Laboratories, Inc.). The samples were diluted 4-fold with the diluting solution and centrifuged at 10,000 g for 5 min. An amount of 50 μL of the supernatant was used for the cytokine assay in accordance with the manufacturer’s instructions. The following cytokines were measured: IL-1β, IL-4, IL-6, IL-10, IL-17A, IL-17F, IL-21, IL-22, IL-23, IL-25, IL-31, IL-33, IFNγ, soluble CD40 ligand (sCD40L), and TNFα. The lower limits of detection according to our standard curves were 0.24 pg/ml for IL-1β, 1.33 pg/ml for IL-4, 1.65 pg/ml for IL-6, 1.99 pg/ml for IL-10, 1.20 pg/ml for IL-17A, 3.04 pg/ml for IL-17F, 8.97 pg/ml for IL-21, 3.88 pg/ml for IL-22, 7.35 pg/ml for IL-23, 1.00 pg/ml for IL-25, 3.87 pg/ml for IL-31, 4.18 pg/ml for IL-33, 2.54 pg/ml for IFNγ, 2.41 pg/ml for sCD40L, and 0.57 pg/ml for TNFα.
To define the normal range of cytokine measurements, 50 μL of serum from the 79 healthy controls was provided.
DKK-1 level was quantified by sandwich ELISA (BiomedicaMedizinprodukte, Vienna, Austria). Quality controls for DKK-1 measurements have been provided elsewhere24.
Statistical analysis
Statistical analyses were performed on the database locked on July 2014. Cytokines in serum are presented in percentages of samples with detectable cytokine (percent detectable, %) and as cytokine level (mean ± SD, pg/ml) for each group. Cytokines with values below the limits of detection were graded as negative and the levels were assigned a numerical value of 0 pg/ml for statistical analysis. Spearman correlation was used to examine the relation between IL-31 level and that of other cytokines. To evaluate the characteristics of the axSpA patients associated with IL-31 serum level, we performed a first-step univariate analyses including the variables age, disease duration, sex, axial and peripheral involvement, tobacco use, CRP and hsCRP levels, ESR, BASDAI, BASFI, ASDAS-CRP, ASDAS-ESR, HAQ, SF-36 physical and mental component scores (PCS and MCS), uveitis, inflammatory bowel disease, psoriasis, current use of NSAIDs and DMARDs, HLA-B27-positive, mNY-SI, MRI-SI, MRI-spine, low BMD, and DKK-1 level. Thereafter, we used multivariable analysis including in the model the variables with p < 0.15 on univariate analysis.
We conducted 2 analyses aiming to evaluate bone-related parameters: first the characteristics of the AxSpA patients associated with mSASSS score ≥ 1 and then with low BMD, including IL-31 serum level in the model. We performed as a first step univariate analysis including age, disease duration, sex, axial and peripheral involvement, tobacco use, CRP and hsCRP levels, ESR, BASDAI, BASFI, ASDAS-CRP, ASDAS-ESR, HAQ, SF36 PCS and MCS, uveitis, inflammatory bowel disease, psoriasis, current use of NSAIDs and DMARDs, HLA-B27-positive, mNY-SI, MRI-SI, MRI-spine, low BMD, DKK-1, sCD40L and IL-31. Thereafter, we used multivariable analysis including in the model only the variables with p < 0.15 on univariate analysis. Because IL-31 and sCD40L levels were highly correlated, we analyzed them separately in the multivariable models. All statistical analyses involved use of R software. Results were considered significantly different at p < 0.05.
Results
Characteristics of the population
The 708 patients included in the DESIR cohort had a mean age of 33.8 years; 51.5% were women, and 62.6% fulfilled the ASAS criteria. Serum levels were assessed in 2 subgroups of individuals at baseline: 1) patients from the DESIR cohort fulfilling the ASAS criteria (ASAS-positive, n = 443) and 2) healthy controls (51% men, mean age 32 ± 9.1 years) from the Variété cohort, age and sex-matched to a random sample of the DESIR cohort patients (control group, n = 79). Baseline characteristics of DESIR cohort ASAS+ patients are in Table 1.
Table 1.
Characteristics of the study population (DESIR cohort ASAS+ patients).
| Variables | ASAS-positive (n = 443) |
|---|---|
| Clinics parameters | |
| Age (mean ± SD) | 31.43 ± 7.33 (n = 443) |
| Male, n (%) | 215 (48.53%) (n = 443) |
| Disease duration (months) (mean ± SD) | 18.85 ± 10.76 (n = 443) |
| Axial disease, n (%) | 237 (53.50%) (n = 443) |
| Uveitis, n (%) | 40 (33.06%) (n = 121) |
| IBD, n (%) | 7 (1.58%) (n = 443) |
| Psoriasis, n (%) | 72 (16.25%) (n = 443) |
| Enthesitis, n (%) | 200 (45.15%) (n = 443) |
| Dactylitis, n (%) | 58 (13.09%) (N = 443) |
| Synovitis, n (%) | 13 (4.91%) (n = 442) |
| Tobacco use (ever vs never), n (%) | 175 (39.59%) (n = 442) |
| Biologic variables | |
| HLA B27-positive, n (%) | 373 (84.20%) (n = 443) |
| CRP (mean ± SD) | 8.91 ± 13.70 (n = 428) |
| ESR (mean ± SD) | 14.83 ± 16.70 (n = 427) |
| hsCRP (mean ± SD) | 8.08 ± 14.05 (n = 434) |
| Radiologic variables, n (%) | |
| mSASSS ≥1 | 65 (15.37%) (n = 423) |
| mNY-SI-positive | 121 (27.63%) (n = 438) |
| MRI-SI-positive | 195 (46.54%) (n = 419) |
| MRI-spine-positive | 86 (20.33%) (n = 423) |
| BMD, n (%) | |
| Low bone density | 28 (13.08%) (n = 225) |
| Current treatments, n (%) | |
| NSAIDs | 315 (71.11%) (n = 443) |
| Steroids | 10 (2.26%) (n = 443) |
| DMARDs | 43 (9.71%) (n = 443) |
|
Disease activity and handicap, mean±SD | |
| BASDAI | 42.53 ± 20.30 (n = 442) |
| BASFI | 2.66 ± 1.02 (n = 363) |
| ASDAS-CRP | 2.66 ± 1.02 (n = 363) |
IBD, inflammatory bowel disease; HLA-B27, human leukocyte antigen B27; CRP, C-reactive protein; hsCRP, high-sensitivity CRP; ESR, erythrocyte sedimentation rate; BASDI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; BASMI, Bath Ankylosing Spondylitis Metrology Index; BMD, bone mineral density; mSASSS, modified Stoke Ankylosing Spondylitis Spine Score; NSAIDs, nonsteroidal anti-inflammatory drugs; DMARDs, disease-modifying anti-rheumatic drugs.
Increased serum levels of IL-31 and sCD40L in SpA
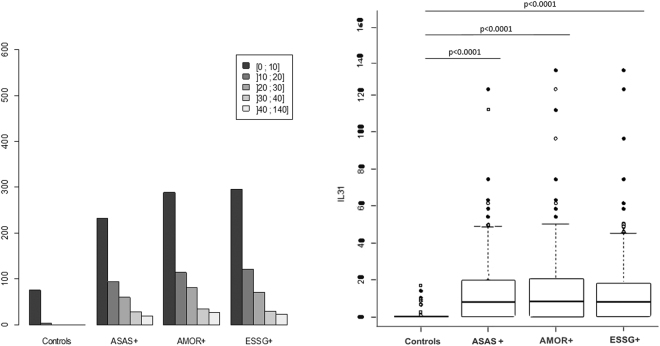
IL-31 serum levels distribution among controls and SpA patients according to the different classification criteria is presented in Fig. 1A. IL-31 was detectable among 60.9% of ASAS+ patients (n = 270) as compared with 18% of healthy controls (p < 0.0001) (Table 2). Quantification of the other cytokines is detailed in Table 2.
Figure 1.
Distribution (left) and serum levels (right) of interleukin 31 (IL-31) among patients from the DESIR cohort who were positive for Assessment of Spondyloarthritis (ASAS), Amor (AMOR) and European Spondylarthropathy Study Group (ESSG) criteria and among healthy controls. (Right) Horizontal bar is median, box edges are Q1–Q3 and whiskers are ranges.
Table 2.
Quantification of cytokines in ASAS+ patients and healthy control groups.
| ASAS+ (n = 443) | Controls (n = 79) | |||||
|---|---|---|---|---|---|---|
| Detectable samples (%) | Range (pg/ml) | Median detectable (pg/ml) | Detectable samples (%) | Range (pg/ml) | Median detectable (pg/ml) | |
| IL-31 | 270 (60.94%) | [0–122.88] | 7.67 | 14 (17.72%) | [0–16.85] | 0.00 |
| sCD40L | 419 (94.58%) | [0–1867.54] | 463.33 | 75 (94.94%) | [0–1337.77] | 318.70 |
| TNFα | 307 (69.30%) | [0–24.54] | 1.88 | 76 (96.20%) | [0–10.41] | 2.12 |
| IL-6 | 268 (60.50%) | [0–124.20] | 5.84 | 55 (69.62%) | [0–10.18] | 0.99 |
| IL-4 | 64 (14.44%) | [0–304.20] | 12.00 | 12 (15.19%) | [0–114.41] | 6.92 |
| IL-33 | 30 (6.77%) | [0–860.50] | 24.33 | 23 (29.11%) | [0–894.29] | 84.07 |
| DKK-1 | 434 (97.96%) | [0–134.48] | 27.57 | 79 (100.00%) | [2.46–21.52] | 11.27 |
| IL-1β | 112 (25.28%) | [0–30.62] | 0.82 | 29 (36.71%) | [0–2.42] | 0.16 |
| IL-10 | 8 (1.81%) | [0–40.36] | 16.49 | 7 (8.86%) | [0–37.64] | 13.76 |
| IL17-A | 3 (0.67%) | [0–4.20] | 2.73 | 4 (5.06%) | [0–2.34] | 1.78 |
| IL-17F | 26 (5.86%) | [0–126.60] | 34.16 | 5 (6.33%) | [0–119.75] | 42.79 |
| IL-21 | 7 (1.58%) | [0–184.80] | 89.30 | 1 (1.27%) | [0–70.05] | 70.05 |
| IL-22 | 2 (0.45%) | [0–87.39] | 46.70 | 2 (2.53%) | [0–9.37] | 7.23 |
| IL-23 | 5 (1.13%) | [0–267.3] | 61.37 | 0 (0.00%) | — | — |
| IL-25 | 10 (2.26%) | [0–6.46] | 1.71 | 3 (3.80) | [0–1.71] | 1.71 |
| IFNγ | 6 (1.35%) | [0–44.35] | 13.34 | 1 (1.27%) | [0–15.75] | 15.75 |
The median value among quantifiable samples is provided as is the percentage of detectable samples.
Serum levels of IL-31 were higher for the ASAS+ than control group (mean 12.6 ± 15.4 pg/ml vs mean 1.8 ± 4.0 pg/ml; p < 0.0001) (Fig. 1). Increased IL-31 serum level was confirmed when using other classification criteria commonly used in SpA. In fact, IL-31 was significantly increased in the AMOR-positive group (n = 556) (mean 12.8 ± 16.2) versus the controls (p < 0.0001). The results were similar for the ESSG-positive group (mean 11.8 ± 15.2) versus the controls (p < 0.0001) (Fig. 1B).
Level of sCD40L, a T-lymphocyte activation biomarker25, was greater for ASAS-positive than control participants (mean 516.51 ± 349.8 pg/ml vs mean 412.43±318.45 pg/ml, p = 0.012). The 2 groups did not differ in serum levels of the cytokines IL-1β, IL-6, IL-10, IL-17A, IL-17F, IL-21, IL-22, IL-23, IFNγ, or TNFα (Table 2), nor in levels of the other Th2 investigated cytokines (IL-33, IL-4 and IL-25).
Correlation of IL-31 with pro-inflammatory cytokines
We found a strong correlation between IL-31 and sCD40L levels (r = 0.6; p < 0.0001). The correlation with IL-6 and TNFα levels was weak but significant (r = 0.20; p < 0.001, and r = 0.23; p < 0.001, respectively). IL-31 level was correlated with DKK-1 level (r = 0.27; p = 0.0001) but not with the other studied cytokines (IL-1β, IL-10, IL-17A, IL-17F, IL-21, IL-22, IL-23, IL-25). These results were confirmed on multivariable analysis, finding an independent and significant association between IL-31 and sCD40L levels (p < 0.0001). We found no relevant correlation between IL-31 level and that of other Th2 cytokines known to be involved in IL-31 secretion: IL-4 (r = 0.13; p = 0.005) and IL-33 (r = −0.025; p = 0.59) (Suppl. Figure 1).
Serum IL-31 level and clinical characteristics
IL-31 level was not associated with any of the patient characteristics of articular peripheral involvement (defined by peripheral painful joint), dactylitis, synovitis, enthesitis, uveitis, psoriasis, inflammatory bowel disease or HLA-B27 status. Also, NSAID or DMARD use was not associated with increased IL-31 level.
Association of serum IL-31 level with disease activity in ASAS-positive patients
We found no relevant correlation between IL-31 level and the main acute-phase reactants among ASAS-positive patients (Suppl. Table 1). Similarly, we did not find any association of IL-31 serum level with disease activity or handicap scores (Suppl. Table 1). These results were confirmed by both univariate and multivariable analyses (data not shown).
Association of increased IL-31 level with fewer structural lesions of the spine
Higher serum level of IL-31 were observed in axSpA patients with mean mSASSS score <1: [mSASSS ≥ 1 (n = 65): 7.7 ± 10.7 vs mSASSS score <1 (n = 358) 13.02 ± 15.07, p = 0.0054] (Fig. 2).
Figure 2.
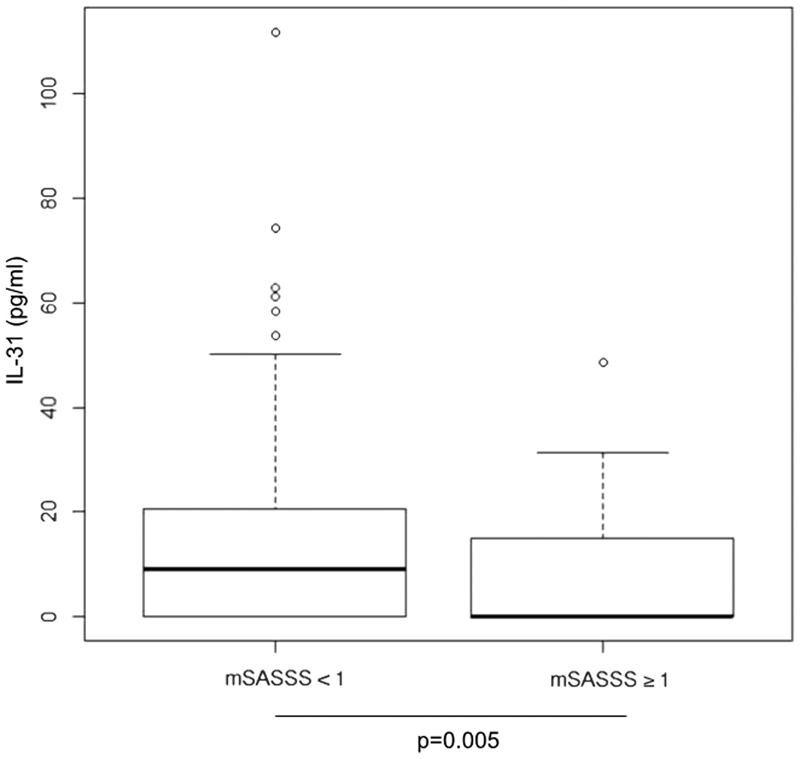
Levels of IL31 in mSASSS <1, and mSASSS ≥1 groups.
No differences in serum IL-31 level were observed depending on the presence of radiographic sacroiliitis (mNY-positive (n = 121)14.0 ± 14.7 vs mNY-negative (n = 317) 11.6 ± 14.4; p = 0.09) nor inflammatory sacroiliitis seen on MRI (MRI-SI–positive (n = 195) 12.9 ± 15.6 vs MRI-SI–negative (n = 224) 12.3 ± 15.5; p = 0.58). Univariate analysis showed increased IL-31 level associated with mean mSASSS score <1 (p = 0.02) (Suppl. Table 2), which was confirmed by multivariable analysis (p = 0.03) (Suppl. Table 3).
We then assessed factors associated with structural damage (defined by mSASSS score ≥1) among ASAS+ patients. On univariate analysis, mSASSS score ≥1 was significantly associated with IL-31 level (p = 0.009) but not sCD40L level (p = 0.84) (Suppl. Table 4). Multivariable analysis further confirmed that each 10-pg/ml increase in IL-31 level protected against structural damage (odds ratio [OR] 0.948 [95% confidence interval [95% CI] 0.917–0.979], p = 0.001), while age, inflammation seen on spine MRI, and active disease assessed by ASDAS-ESR was associated with spinal structural lesions (Table 3).
Table 3.
Multivariable analysis of factors associated with spinal structural lesions defined by mSASSS ≥1.
| Variables | Odds ratio (95% CI) | P-value |
|---|---|---|
| Age | 1.053 [1.007–1.101] | 0.0227 |
| hsCRP | 0.992 [0.968–1.016] | 0.5071 |
| MRI Spine | 4.522 [2.223–9.198] | <0.0001 |
| ASDAS-ESR | 1.654 [1.135–2.412] | 0.0089 |
| DKK1 | 1.010 [0.986–1.033] | 0.4188 |
| IL-31* | 0.587 [0.917–0.979] | 0.0014 |
*Odds ratio (95% CI) for each serum 10-pg/mL increase in IL-31 level.
We found the same statistically significant association for patients fulfilling the AMOR criteria (OR 0.674 [0.529–0.858], p = 0.0014) and the ESSG criteria (OR 0.738 [0.583–0.933], p = 0.011).
Association of increased IL-31 level and low BMD
Both univariate and multivariable analyses showed IL-31 associated but not significantly with low BMD among the ASAS positive patients (ptrend = 0.058 and ptrend = 0.06, respectively) (Suppl. Tables 1 and 3).
Briot et al.26 has previously showed in the same cohort that 13.0% of patients had a low BMD and that the main risk factor associated with low BMD was inflammation on MRI. We then decided to assess factors associated with low BMD (defined by Z-score ≤ −2 SD) including IL-31 serum level in the model. On univariate analysis, among ASAS-positive AxSpA patients, gender (p = 0.001), enthesitis (p = 0.02), hsCRP level (p = 0.0001), Sacroilitis X-ray (p = 0.0002), MRI spine (p = 0.003) and ASDAS-ESR (p = 0.002) were significantly associated. Both IL-31 and sCD40L levels were associated but not significantly with low BMD (p = 0.11 and p = 0.06, respectively) (Suppl. Table 5).
On multivariable analysis, risk of low BMD was increased with each 10-pg/ml increase in IL-31 (OR 1.385 [95% CI 1.051–1.823], p = 0.01), together with male gender, enthesitis and MRI-SI-positivity (Table 4). Considering the high correlation between IL-31 and sCD40L levels, we further confirmed that sCD40L was not a confounding factor (Suppl. Table 6).
Table 4.
Multivariable analysis of low BMD in ASAS-positive group.
| Variables | Odds ratio (95% CI) | P-value |
|---|---|---|
| Male | 6.738 [1.229–36.940] | 0.0280 |
| Enthesitis | 3.847 [1.227–12.058] | 0.0208 |
| Hs CRP | 1.002 [0.967–1.039] | 0.9041 |
| MRI SI-positive | 2.375 [0.688–8.199] | 0.1711 |
| Sacroilitis X-ray | 3.229 [0.846–12.231] | 0.0863 |
| MRI Spine | 1.248 [0.318–4.896] | 0.7504 |
| ASDAS ESR | 2.100 [0.958–4.603] | 0.0639 |
| BASFI | 0.964 [0.711–1.308] | 0.8149 |
| IL-31* | 1.367 [1.007–1.855] | 0.0449 |
*Odds ratio for each 10-pg/mL increase in IL-31 level.
In the AMOR-positive group, risk of low BMD was increased with each 10-pg/ml increase in IL-31 (OR 1.335 [95% CI 1.050–1.697], p = 0.018). In the ESSG-positive group, the results for low BMD were not significant (OR 1.189 [0.834–1.695], p = 0.34).
Discussion
This study, involving a large cohort of young adults with early IBP suggestive of SpA found 61% of patients from the ASAS-positive of the DESIR cohort with increased level of IL-31 as compared with only 18% in the control group. Increased serum level of IL-31 was significantly associated with less structural damage but also low BMD, which suggests that IL-31 might be involved in the bone formation/resorption balance in AxSpA. The lack of a statistically significant association of IL-31 with acute-phase reactants despite a statistically significant correlation with sCD40L level suggests that this effect could be independent of inflammation but could rely on T-cell activation.
IL-31 and sCD40L were the only 2 cytokines from the 17-plex ELISA showing increased levels in the DESIR cohort. Levels of the other studied cytokines, especially Th17 cytokines, were not significantly increased, despite the use of a Luminex technology that is usually considered quite sensitive. These results suggest that 1) serum Th17 cytokines may poorly reflect the level of activation of this pathway in AxSpA, at least at an early stage of the disease; 2) Th17 cytokines producing cells in peripheral blood mononuclear cells may be in a steady state, expressing low serum levels of cytokines that should be quantified in cell-stimulation conditions; and 3) Luminex assays, even if considered sensitive to assess cytokine serum levels may not be sensitive enough to quantify Th17 cytokines in early AxSpA.
IL-31 is a four-bundle helix cytokine belonging to the gp130/IL-6 cytokine family. It binds an heterodimeric receptor consisting of the IL-31 receptor A (IL-31RA) and the Oncostatin M receptor (OSMR). This receptor can recruit 3 signaling pathways: the Jak/STAT pathway, the PI3K/AKT pathway and the MAPK pathway27–29. The 2 subunits of the receptor are preferentially expressed in skin, testis, bone marrow, brain and thymus for IL-31RA28,30,31 and is more ubiquitous in humans organs for OSMR28,31. Upon stimulation, monocytes, macrophages, dendritic cells and mast cells are also able to produce IL-31.
IL-31 level was strongly correlated with level of sCD40L, a T-cell activation marker. The biological effect of IL-31 is currently unclear, and its involvement in some inflammatory processes is controversial. In 2004, Dillon et al. reported for the first time the expression of IL-31RA in bone marrow and the ability of IL-31 to induce Th1 polarization under specific conditions30. Subsequently, various conflicting data emerged: IL-31 could limit Th2-inflammation in the lung32 and the intestine33 but could have a pro-inflammatory effect in the skin34–36. Yagi et al. reported that in human colonic sub-epithelial myofibroblasts, the effect of IL-31 on the secretion of cytokines, chemokines and matrix metalloproteinases was similar to IL-17A37, so IL-31 might be a Th17-related cytokine. Several groups have reported an elevation of IL-31 in skin from patients with atopic dermatitis, in bronchoalveolar lavage fluid from patients with allergic asthma, and rhinitis; in intestinal mucosa from patients with inflammatory bowel disease; and recently in sera and aqueous humour from patients with acute anterior uveitis associated with HLA-B27 and osteoporosis27,34,35,38–44.
We observed a significant correlation between IL-31 and DKK-1 levels. A recent publication45 reported that DKK-1 polarizes CD4+ T cells to the Th2 cell lineage. DKK-1 may favour a Th2 response in SpA patients, consequently associated with increased IL-31. Nevertheless, levels of other cytokines (IL-4 and IL-10), usually considered surrogate markers for Th2 polarization, were not increased in AxSpA patients from the DESIR cohort. However, Notani et al. proposed that high concentrations of DKK-1 or Wnt3a could inhibit Th2 differentiation46. Considering that one third of AxSpA patients have high serum levels of DKK-124, the Th1/Th2 polarization induced by DKK-1 might be equivocal in AxSpA. In fact, in our study, IL-31 level was rather consistently correlated with levels of pro-inflammatory cytokines such as IL-6 or TNFα. These data support that IL-31 may have a role in SpA and more generally in the Th1/Th2 balance of the disease in a context of high levels of DKK-1 expression. IL-31 appears to be a cytokine at the border of the Th1 and Th2 polarization. Our data suggest that activated T cells could be the source of IL-31 production, but mast cells are also another source of IL-31. This latter cell type seems to play an important role in SpA pathogenesis via secretion of IL-1747, but its role as an IL-31–secreting cell could also be further assessed.
To the best of our knowledge, this is the first report suggesting a potential role for IL-31 in low BMD and absence of structural damage in SpA. Indeed, patients with low BMD had high levels of IL-31. An increase in IL-31 serum levels was previously observed in post-menopausal osteoporosis44. Nevertheless, the accurate role of IL-31 in bone remodelling is currently an unexplored field. IL-31 may be involved in the activation of the RANK/RANKL pathway or alternatively act by inhibiting Wnt signaling. This suggestion could be consistent with our analysis, because IL-31 level was correlated with serum DKK-1 level. In patients with ankylosing spondylitis, syndesmophyte formation was reduced with high functional DKK-1 level, which suggests that blunted Wnt signaling suppresses new bone formation and thereafter syndesmophyte growth in ankylosing spondylitis48.
Our study had some limitations. Although the Luminex technology does not have the highest sensitivity to quantify cytokines, our results show a significant difference in IL-31 and sCD40L levels between healthy controls and SpA patients in a large sample. Moreover, unlike Th17 cytokines, IL-31 level was increased at a steady state without any stimulation. Such lack of cell stimulation probably explains why we did not find an increase in serum levels of Th1 and Th17 cytokines. Although the initial cohort was large, the proportion of patients with low BMD was low; thus, the relationship between serum level of IL-31 and the measurement of BMD should be interpreted with caution. As for all cross-sectional studies, causality cannot be ascertained with our observations. These results must be confirmed with prospective data. Lack of centralized quality control of BMD measurements (i.e., use of different devices, absence of cross-calibration) and the low sample size of patients with BMD measurements is a limitation of our cross-sectional study; however, participating centers had expertise in BMD measurements and followed the recommendations for quality control of the device.
To conclude, our study, performed in a large cohort of patients, highlights the potential participation of IL-31 in the pathogenesis of structural damage in AxSpA. Our data support that IL-31 may participate in the bone remodelling processes responsible for the disease. We demonstrated an association of high IL-31 level with less structural damage in the spine and with the osteoporotic/osteopenic phenotype of patients. IL-31 may be involved in the bone loss found in SpA patients. The reason for the increased IL-31 level in AxSpA has yet to be determined. These data need to be confirmed in the most severe forms of the disease and assessed among AxSpA patients with longer disease duration. Nevertheless, these findings highlight new lines of thought about the pathophysiology of the structural changes in this disease, especially regarding the role of IL-31 in bone formation and could open new therapeutic perspectives.
Electronic supplementary material
Acknowledgements
An unrestricted grant from Wyeth Pharmaceuticals was allocated for the first 5 years of the follow-up of the recruited patients. The DESIR-cohort is financially supported by unrestricted grants from both the French Society of Rheumatology, and Pfizer Ltd, France. A research grant from AVIESAN INSERM (2013) “Cohortes cliniques et collections - Soutien à la recherche translationnelle” was obtained for cytokines quantifications by ELISA. The Variété cohort was supported by a grant from the Programme Hospitalier de Recherche Clinique, French Ministry of Health (no. P081216 / IDRCB 2009-A00892-55). No funding bodies had any roles in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The DESIR cohort was sponsored by the Département de la Recherche Clinique et du Développement de l’Assistance Publique–Hôpitaux de Paris. This study is conducted under the umbrella of the French Society of Rheumatology and INSERM (Institut National de la Santé et de la Recherche Médicale). The database management is performed within the department of epidemiology and biostatistics (Professor Paul Landais, D.I.M., Nîmes, France). An unrestricted grant from Pfizer was allocated for the first 10 years of the follow-up of the recruited patients. The authors thank the different regional participating centers: Pr M. Dougados (Paris–Cochin B), Pr A. Kahan (Paris - Cochin A), Pr P. Dieudé (Paris - Bichat), Pr L. Gossec (Paris–Pitié-Salpetrière), Pr F. Berenbaum (Paris - Saint Antoine), Pr P. Claudepierre (Créteil), Pr M. Breban (Boulogne Billancourt), Dr B. Saint-Marcoux (Aulnay-sous-Bois), Pr P. Goupille (Tours), Pr J-F. Maillefert (Dijon), Dr E. Dernis (Le Mans), Pr D. Wendling (Besançon), Pr B. Combe (Montpellier), Pr L. Euller-Ziegler (Nice), Pr P. Orcel, Pr P. Richette (Paris - Lariboisière), Pr P. Lafforgue (Marseille), Dr P. Boumier (Amiens), Pr M. Soubrier (Clermont-Ferrand), Dr N. Mehsen (Bordeaux), Pr D. Loeuille (Nancy), Pr R-M. Flipo (Lille), Pr A. Saraux (Brest), Dr S. Pavy (Kremlin Bicêtre), Pr A. Cantagrel (Toulouse), Pr O. Vittecoq (Rouen). The authors also thank URC-CIC Paris Centre for the coordination and monitoring of the study.
Author Contributions
Conceived and designed the experiments: C.M.R. Performed the experiments: H.C. Analyzed the data: N.R., A.E., H.C., R.S., K.B., A.M., P.C., Y.T., D.W., R.L., F.B., R.d.v.B., P.C., A.F., M.D., C.R., C.M.R. Central reading of DESIR imaging: P.C., A.F., R.v.d.B. Sample collection: all investigators from DESIR cohort for serum samples from DESIR cohort, P.C. for healthy controls samples. Wrote the manuscript: N.R., A.E., H.C., R.S.,K.B., A.M., P.C., Y.T., D.W., R.L., F.B., R.d.v.B., P.C., A.F., M.D., C.R., C.M.R. C.M.R. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-25722-z.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Haroon N, et al. The Impact of TNF-inhibitors on radiographic progression in Ankylosing Spondylitis. Arthritis Rheum. 2013;65:2645–2654. doi: 10.1002/art.38070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baraliakos X, et al. Radiographic progression in patients with ankylosing spondylitis after 4 yrs of treatment with the anti-TNF-alpha antibody infliximab. Rheumatol. Oxf. Engl. 2007;46:1450–1453. doi: 10.1093/rheumatology/kem166. [DOI] [PubMed] [Google Scholar]
- 3.Wanders A, et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum. 2005;52:1756–1765. doi: 10.1002/art.21054. [DOI] [PubMed] [Google Scholar]
- 4.Cañete JD, et al. Differential Th1/Th2 cytokine patterns in chronic arthritis: interferon gamma is highly expressed in synovium of rheumatoid arthritis compared with seronegative spondyloarthropathies. Ann. Rheum. Dis. 2000;59:263–268. doi: 10.1136/ard.59.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jandus C, et al. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum. 2008;58:2307–2317. doi: 10.1002/art.23655. [DOI] [PubMed] [Google Scholar]
- 6.Nistala K, et al. Interleukin-17-producing T cells are enriched in the joints of children with arthritis, but have a reciprocal relationship to regulatory T cell numbers. Arthritis Rheum. 2008;58:875–887. doi: 10.1002/art.23291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowness P, et al. Th17 cells expressing KIR3DL2 + and responsive to HLA-B27 homodimers are increased in ankylosing spondylitis. J. Immunol. Baltim. Md 1950. 2011;186:2672–2680. doi: 10.4049/jimmunol.1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen H, Goodall JC, Hill Gaston JS. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 2009;60:1647–1656. doi: 10.1002/art.24568. [DOI] [PubMed] [Google Scholar]
- 9.Kenna TJ, et al. Enrichment of circulating interleukin-17-secreting interleukin-23 receptor-positive γ/δ T cells in patients with active ankylosing spondylitis. Arthritis Rheum. 2012;64:1420–1429. doi: 10.1002/art.33507. [DOI] [PubMed] [Google Scholar]
- 10.Claudepierre P, Rymer JC, Chevalier X. IL-10 plasma levels correlate with disease activity in spondyloarthropathy. J. Rheumatol. 1997;24:1659–1661. [PubMed] [Google Scholar]
- 11.Rudwaleit M, et al. Atopic disorders in ankylosing spondylitis and rheumatoid arthritis. Ann. Rheum. Dis. 2002;61:968–974. doi: 10.1136/ard.61.11.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dougados M, et al. The DESIR cohort: a 10-year follow-up of early inflammatory back pain in France: study design and baseline characteristics of the 708 recruited patients. Jt. Bone Spine Rev. Rhum. 2011;78:598–603. doi: 10.1016/j.jbspin.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Calin A, Porta J, Fries JF, Schurman DJ. Clinical history as a screening test for ankylosing spondylitis. JAMA. 1977;237:2613–2614. doi: 10.1001/jama.1977.03270510035017. [DOI] [PubMed] [Google Scholar]
- 14.Rudwaleit M, Metter A, Listing J, Sieper J, Braun J. Inflammatory back pain in ankylosing spondylitis: a reassessment of the clinical history for application as classification and diagnostic criteria. Arthritis Rheum. 2006;54:569–578. doi: 10.1002/art.21619. [DOI] [PubMed] [Google Scholar]
- 15.van der Heijde D, et al. ASDAS, a highly discriminatory ASAS-endorsed disease activity score in patients with ankylosing spondylitis. Ann. Rheum. Dis. 2009;68:1811–1818. doi: 10.1136/ard.2008.100826. [DOI] [PubMed] [Google Scholar]
- 16.Berg Rvanden, et al. Classification of axial SpA based on positive imaging (radiographs and/or MRI of the sacroiliac joints) by local rheumatologists or radiologists versus central trained readers in the DESIR cohort. Ann. Rheum. Dis. 2015;74:2016–2021. doi: 10.1136/annrheumdis-2014-205432. [DOI] [PubMed] [Google Scholar]
- 17.Claudepierre P, et al. Reliability of mSASSS scoring in everyday practice in DESIR-cohort study centres: cross-sectional study of agreement with trained readers. Ann. Rheum. Dis. 2016;75:2213–2214. doi: 10.1136/annrheumdis-2016-209906. [DOI] [PubMed] [Google Scholar]
- 18.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361–368. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 19.Creemers MCW, et al. Assessment of outcome in ankylosing spondylitis: an extended radiographic scoring system. Ann. Rheum. Dis. 2005;64:127–129. doi: 10.1136/ard.2004.020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudwaleit M, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann. Rheum. Dis. 2009;68:777–783. doi: 10.1136/ard.2009.108233. [DOI] [PubMed] [Google Scholar]
- 21.Hermann K-GA, et al. Descriptions of spinal MRI lesions and definition of a positive MRI of the spine in axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI study group. Ann. Rheum. Dis. 2012;71:1278–1288. doi: 10.1136/ard.2011.150680. [DOI] [PubMed] [Google Scholar]
- 22.Lewiecki EM, et al. Special report on the 2007 adult and pediatric Position Development Conferences of the International Society for Clinical Densitometry. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA. 2008;19:1369–1378. doi: 10.1007/s00198-008-0689-9. [DOI] [PubMed] [Google Scholar]
- 23.Chanson P, et al. Reference Values for IGF-I Serum Concentrations: Comparison of Six Immunoassays. J. Clin. Endocrinol. Metab. 2016;101:3450–3458. doi: 10.1210/jc.2016-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nocturne G, et al. Increase in Dickkopf-1 Serum Level in Recent Spondyloarthritis. Data from the DESIR Cohort. PloS One. 2015;10:e0134974. doi: 10.1371/journal.pone.0134974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matthies KMG, Newman JL, Hodzic A, Wingett DG. Differential regulation of soluble and membrane CD40L proteins in T cells. Cell. Immunol. 2006;241:47–58. doi: 10.1016/j.cellimm.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Briot K, et al. Bone oedema on MRI is highly associated with low bone mineral density in patients with early inflammatory back pain: results from the DESIR cohort. Ann. Rheum. Dis. 2013;72:1914–1919. doi: 10.1136/annrheumdis-2012-201845. [DOI] [PubMed] [Google Scholar]
- 27.Dambacher J, et al. Interleukin 31 mediates MAP kinase and STAT1/3 activation in intestinal epithelial cells and its expression is upregulated in inflammatory bowel disease. Gut. 2007;56:1257–1265. doi: 10.1136/gut.2006.118679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Q, Putheti P, Zhou Q, Liu Q, Gao W. Structures and biological functions of IL-31 and IL-31 receptors. Cytokine Growth Factor Rev. 2008;19:347–356. doi: 10.1016/j.cytogfr.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kasraie S, Niebuhr M, Werfel T. Interleukin (IL)-31 activates signal transducer and activator of transcription (STAT)-1, STAT-5 and extracellular signal-regulated kinase 1/2 and down-regulates IL-12p40 production in activated human macrophages. Allergy. 2013;68:739–747. doi: 10.1111/all.12152. [DOI] [PubMed] [Google Scholar]
- 30.Dillon SR, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat. Immunol. 2004;5:752–760. doi: 10.1038/ni1084. [DOI] [PubMed] [Google Scholar]
- 31.Diveu C, et al. GPL, a novel cytokine receptor related to GP130 and leukemia inhibitory factor receptor. J. Biol. Chem. 2003;278:49850–49859. doi: 10.1074/jbc.M307286200. [DOI] [PubMed] [Google Scholar]
- 32.Perrigoue JG, et al. IL-31-IL-31R interactions negatively regulate type 2 inflammation in the lung. J. Exp. Med. 2007;204:481–487. doi: 10.1084/jem.20061791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perrigoue JG, Zaph C, Guild K, Du Y, Artis D. IL-31-IL-31R interactions limit the magnitude of Th2 cytokine-dependent immunity and inflammation following intestinal helminth infection. J. Immunol. Baltim. Md 1950. 2009;182:6088–6094. doi: 10.4049/jimmunol.0802459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bilsborough J, et al. IL-31 is associated with cutaneous lymphocyte antigen-positive skin homing T cells in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2006;117:418–425. doi: 10.1016/j.jaci.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 35.Raap U, et al. Correlation of IL-31 serum levels with severity of atopic dermatitis. J. Allergy Clin. Immunol. 2008;122:421–423. doi: 10.1016/j.jaci.2008.05.047. [DOI] [PubMed] [Google Scholar]
- 36.Nobbe S, et al. IL-31 expression by inflammatory cells is preferentially elevated in atopic dermatitis. Acta Derm. Venereol. 2012;92:24–28. doi: 10.2340/00015555-1191. [DOI] [PubMed] [Google Scholar]
- 37.Yagi Y, et al. Interleukin-31 stimulates production of inflammatory mediators from human colonic subepithelial myofibroblasts. Int. J. Mol. Med. 2007;19:941–946. [PubMed] [Google Scholar]
- 38.Sonkoly E, et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J. Allergy Clin. Immunol. 2006;117:411–417. doi: 10.1016/j.jaci.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 39.Lai T, et al. Interleukin-31 expression and relation to disease severity in human asthma. Sci. Rep. 2016;6:22835. doi: 10.1038/srep22835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lei Z, et al. SCF and IL-31 rather than IL-17 and BAFF are potential indicators in patients with allergic asthma. Allergy. 2008;63:327–332. doi: 10.1111/j.1398-9995.2007.01566.x. [DOI] [PubMed] [Google Scholar]
- 41.Okano M, et al. Characterization of pollen antigen-induced IL-31 production by PBMCs in patients with allergic rhinitis. J. Allergy Clin. Immunol. 2011;127(277–279):279.e1–11. doi: 10.1016/j.jaci.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 42.Andoh A, et al. Mucosal cytokine network in inflammatory bowel disease. World J. Gastroenterol. WJG. 2008;14:5154–5161. doi: 10.3748/wjg.14.5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen, W. et al. Cytokine expression profile in aqueous humor and sera of patients with acute anterior uveitis. Curr. Mol. Med. (2015). [DOI] [PubMed]
- 44.Ginaldi L, et al. Increased levels of interleukin 31 (IL-31) in osteoporosis. BMC Immunol. 2015;16:60. doi: 10.1186/s12865-015-0125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chae W-J, et al. The Wnt Antagonist Dickkopf-1 Promotes Pathological Type 2 Cell-Mediated Inflammation. Immunity. 2016;44:246–258. doi: 10.1016/j.immuni.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Notani D, et al. Global regulator SATB1 recruits beta-catenin and regulates T(H)2 differentiation in Wnt-dependent manner. PLoS Biol. 2010;8:e1000296. doi: 10.1371/journal.pbio.1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noordenbos T, et al. Interleukin-17-positive mast cells contribute to synovial inflammation in spondylarthritis. Arthritis Rheum. 2012;64:99–109. doi: 10.1002/art.33396. [DOI] [PubMed] [Google Scholar]
- 48.Heiland GR, et al. Neutralisation of Dkk-1 protects from systemic bone loss during inflammation and reduces sclerostin expression. Ann. Rheum. Dis. 2010;69:2152–2159. doi: 10.1136/ard.2010.132852. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.