Abstract
Eradication of cervical cancer involves the expansion of human papillomavirus (HPV) vaccine coverage and the development of efficient screening guidelines that take vaccination into account. In Korea, the HPV National Immunization Program was launched in 2016 and is expected to shift the prevalence of HPV genotypes in the country, among other effects. The experiences of another countries that implement national immunization programs should be applied to Korea. If HPV vaccines spread nationwide with broader coverage, after a few decades, cervical intraepithelial lesions or invasive cancer should become a rare disease, leading to a predictable decrease in the positive predictive value of cervical screening cytology. HPV testing is the primary screening tool for cervical cancer and has replaced traditional cytology-based guidelines. The current screening strategy in Korea does not differentiate women who have received complete vaccination from those who are unvaccinated. However, in the post-vaccination era, newly revised policies will be needed. We also discuss on how to increase the vaccination rate in adolescence.
Keywords: HPV vaccines, Human papillomavirus, Cancer screening, Immunization program
Introduction
Cervical cancer is the fourth most common female cancer worldwide, with an estimated 265,700 deaths per year, and remains the most common female cancer in 42 countries (primarily developing countries) [1]. In addition, cervical cancer has relatively early onset, occurring primarily during reproductive ages, and is one of the 3 most common cancers among women under age 45 in most countries [2]. There has been a lot of effort to prevent cervical cancer through primary screening and human papillomavirus (HPV) vaccination; as a result, the disease has been gradually reduced in several developed countries [3,4]. In Korea, for example, the incidence rate of cervical cancer has gradually decreased to a rate of 9.0 per 105 in 2014, compared to 16.3 per 105 in 1999 [5].
HPV has been clearly demonstrated as a cause of invasive cervical cancer [6]. It is the most common sexually transmitted virus, and the progression of it is unusual in that the greatest prevalence is within 5 years from the initiation of first coitus, then decreases with age [7]. Most women infected with high-risk HPV self-clear and acquire immunity against certain types. However, in about 15% of HPV infections, the virus persists and induces precancerous lesions or invasive cervical cancer [8]. HPV 16 and 18 have been the most causative types among high-risk HPV viruses, and up to now those 2 genotypes have accounted for 70% of all cervical cancer [9]. HPV 6 and 11, which are also covered by the quadrivalent vaccine, are responsible for most anogenital warts [10].
In a randomized controlled trial, HPV testing in combination with liquid-based cytology or alone was more effective than cytology for cervical cancer screening, although HPV screening might result in over-diagnosis in patients with regressive moderate dysplasia [11]. The primary HPV DNA test has a higher sensitivity and reproducibility than cervical cytology for detecting cervical intraepithelial lesions [12]. In the HPV vaccination era, the prevalence of cervical lesions as precancerous lesions or invasive cancer should decrease, and as a result, the HPV test will largely replace cytology for screening [13].
The introduction of a national HPV immunization program in Korea is expected to make various changes in Korea, including the eradication of specific HPV types and a shift in the distribution of HPV genotypes. As the vaccination rate increases, the prevalence of precancerous cervical lesions and cervical cancer will decrease, which will require revision of screening strategies in the post-vaccination era. Two major strategies for cancer prevention and eradication should be considered in future guidelines. First, the efficiency of screening should be improved through HPV DNA tests or new screening tools. Second, efforts should be made to improve the vaccination rate and coverage (Fig. 1).
Fig. 1.
Two different strategies against human papillomavirus (HPV) in the era of HPV vaccination.
Effects of HPV vaccination
1. Mechanism: antibodies against major L1 capsid proteins
The HPV vaccine plays both preventive and treatment roles for precancerous lesions or cervical cancer. HPV 16/18 E7 antigen-pulsed dendritic cell vaccination can be used as a treatment option for invasive cervical cancer [14]. In addition, recurrent laryngeal papillomatosis is treated successfully by HPV vaccination [15,16]. Previous studies have shown that the HPV vaccine produces HPV-specific antibodies against L1 capsid proteins into the cervical epithelium [17]. Furthermore, HPV vaccination induces T-cell responses and antigen-presenting cells for local cell-mediated immunity, enhancing adaptive immunity [18]. The major capsid antigen L1 synthesizes virus-like particles, which lead to the production of neutralizing antibodies and a humoral response [19].
In the United States, the National Center for Health Statistics reported the impact of vaccination on the prevalence of HPV in the population by comparing HPV DNA prevalence in the pre-vaccination era (2003–2006) and vaccination era (2009–2012). They showed a 64% decrease in the prevalence of quadrivalent HPV vaccine types [6,11,16,18] in women aged 14 to 19 years, and a 34% decrease in women aged 20 to 24 years [20].
2. HPV vaccine against high-grade cervical intraepithelial neoplasia
Clinical trials to evaluate the HPV vaccine against high-grade cervical intraepithelial lesions, including HPV-023, Patricia, Costa Rica, Future I, II, and NCT00543543, reached a consensus result of nearly 100% efficacy (Table 1) [21,22,23,24,25,26].
Table 1. Human papillomavirus (HPV) vaccine efficacy in various studies.
| Study | Number | Age (yr) | Study type | Type | Follow-up | Efficacy (%)a) |
|---|---|---|---|---|---|---|
| HPV-023 [21] | 437 | 15–25 | RCT | Bivalent | 9.4 yr | 100.0 |
| Patricia [23] | 18,644 | 15–25 | Phase III RCT | Bivalent | 34.9 mon | 98.2 |
| Cost Rica [22] | 7,466 | 18–25 | Phase III RCT | Bivalent | 8.4 yr | 89.8 |
| HPV-P-007 [26] | 1,158 | 16–23 | Phase II randomized double-blind, placebo-controlled study | Quadrivalent | 5 yr | 100.0 |
| Future I, II [24] | 17,622 | 16–26 | Randomized double-blind placebo-controlled | Quadrivalent | 3.7 yr | 100.0 |
| NCT00543543 [25] | 14,215 | 16–26 | Phase II/III RCT | Nonavalent | 4 yr | 96.7 |
RCT, randomized controlled trial; CIN, cervical intraepithelial neoplasia.
a)Efficacy against CIN 2 or more severe lesions.
The HPV vaccine impact monitoring project (HPV-IMPACT) in the United States was a sentinel system for monitoring the impact of HPV vaccination targeting cervical intraepithelial neoplasia (CIN) 2/3 in 18 to 39-year-old women from 2008 to 2012. The authors reported a decrease in screening rates, with the largest decreases among 18 and 20-year-olds, as well as a significant decrease in the incidence of CIN 2+. Nevertheless, an impact of vaccination on declining CIN 2+ was still demonstrated because the decrease in CIN 2+ was larger than the decrease in screening [27].
A phase 3 double-blind trial, Females United to Unilaterally Reduce Endo/Ectocervical Disease, was conducted to estimate the efficacy of the quadrivalent vaccine against high-grade cervical lesions. Vaccine efficacy for the prevention of CIN 2/3, adenocarcinoma in situ, or cervical cancer was 98.2% (95.89% confidence interval [CI], 86–100) [24].
3. Seroconversion rate after vaccination
HPV vaccination induces seroconversion in nearly all women who were vaccinated, and titer levels are higher than in women with seroconversion as a consequence of natural infection [28,29]. Although natural infection also induces cell-mediated immunity and protects against the identical HPV type, the seroconversion rate is much lower than HPV vaccination; 60% for HPV 16, 54% for HPV 18, and 69% for HPV 6. Natural infection results in lower titer levels and a delay of about 1 year for seroconversion compared to HPV vaccination [30]. Although HPV antibodies are sustained for at least 4.5 to 5 years, the sustainability of seropositivity after HPV vaccination has yet to be established since no long-term follow-up data are available [31,32].
4. Impact on HPV distribution
In the era of HPV vaccination, a shift in the prevalence of HPV genotypes is expected. In a German population-based cohort study, a significant decrease in HPV 16, 18, and 31 was found among women aged ≤22 years, compared with women aged 23 to 29 years [33]. Notably, HPV 31 was reduced via cross-protection. On the other hand, other types not included in the vaccine such as HPV 51, 53, and 56 occurred at a higher percentage in vaccinated women.
In Scotland, a national HPV immunization program was implemented for girls aged 12 and 13 years in 2008, with 90% of all subjects receiving the 3-dose uptake of the bivalent vaccine annually. They demonstrated a significant reduction in the prevalence of HPV 16 and 18, as well as HPV 31, 33, and 45 from a cross-protective effect. HPV 51 and 56 rose as most prevalent HPV genotypes among the HPV types not covered by the vaccine [34]. The Scottish HPV prevalence in Vaccinated women (SHEVa) study was designed to analyze the impact of vaccination on the performance of HPV testing [35]. Using clinically validated HPV assays which target both DNA and RNA, there was a 23% to 32% reduction of HPV prevalence in vaccinated women compared to unvaccinated women following the coverage rate was over 90% in the target population. The prevalence of high-risk types other than HPV 16 and 18 was not different between the vaccinated and unvaccinated groups. However, the prevalence of HPV 16 and 18 significantly decreased by 75%.
Korea
1. HPV prevalence and type distribution in Korea
In a meta-analysis of HPV type distribution between 1995 and 2007 in Korea, the overall HPV prevalence was 23.9% (95% CI, 23.8–24.1%) in women with normal cytology compared to 95.8% (95% CI, 95.4–96.2%) in women with cervical cancer. HPV 16 was the most common type regardless of cervical disease status. In cervical cancer, HPV 16 accounted for 65.1% of cases, followed by HPV 18 (11.9%), HPV 58 (8.6%), HPV 33 (3.7%), and HPV 52 (3.4%). In high-grade precancerous lesions (CIN 2, 3, and CIS), HPV 58 was the second most common type (14.1%), while HPV 16 accounted for 40.6% [36]. Likewise, Lee et al. [37] investigated liquid-based cytology, HPV DNA analysis, and cervical biopsies of 2,358 women, finding that HPV 16 was the most common in any cervical lesions, normal, CIN and squamous cell carcinoma (SCC) lesions. HPV 16 and 58 were the most common in CIN 2/3 patients and HPV 16, 18, 58, and 33 were common in patients with SCC.
Recent studies demonstrated that HPV type distribution has been changing and is different from previous studies, in that HPV 16 is no longer the most common genotype in Korea. A retrospective study in 7,014 women who received a health check-up indicated that the overall positivity for high-risk HPV was 8.4%; HPV 58 (23.8%) was most common, followed by HPV 16 (21.8%) and HPV 52 (16.6%). The type most strongly related to increasing severity of cervical cytology was HPV 56 [38]. In a single-center study of healthy women who received a health check-up in 2013, HPV 53 (6.5%) was the most common HPV genotype, followed by HPV 52 (6.1%) [39]. As expected, HPV 16 was the most common type in high-grade CIN lesions. In an analysis of 874 invasive cervical cancer cases over 47 years (1958–2004), HPV 16 accounted for 63.1% of cases, followed by HPV 18 (8.5%), HPV 33 (4.5%), HPV 58 (3.9%), and HPV 31 (3.0%) [40]. Continued monitoring of the shift in prevalence and distribution of HPV genotypes should continue as vaccination increases.
2. Korean guidelines for cervical cancer screening
The well-established national cancer screening program in Korea has led to 71% and 66% reduced risk of invasive cancer and carcinoma in situ compared to unscreened patients, respectively [41]. The distribution of age at cervical cancer diagnosis has been shifting, and revised guidelines regarding the timing for cervical cancer screening have been newly implemented in various organizations [42,43,44]. Moreover, cervical cancer is definitively influenced by HPV infection, and HPV tests have emerged as important screening tools for precancerous lesions and cervical cancer. Therefore, the practice guidelines for the early detection of cervical cancer by Korean Society of Gynecologic Oncology recommended the HPV DNA test in combination with a cervical cytology test is recommended for women aged ≥30 years old. The screening interval can be extended to 2 years if both tests are negative [45]. Because the mortality of cervical cancer in Korea and other countries increases with age, the recommendation was made to end cervical cancer screening after the age of 74 [46]. Within the guidelines, no special considerations were specified for HPV-vaccinated women.
3. HPV vaccination rate in Korea in the present and future
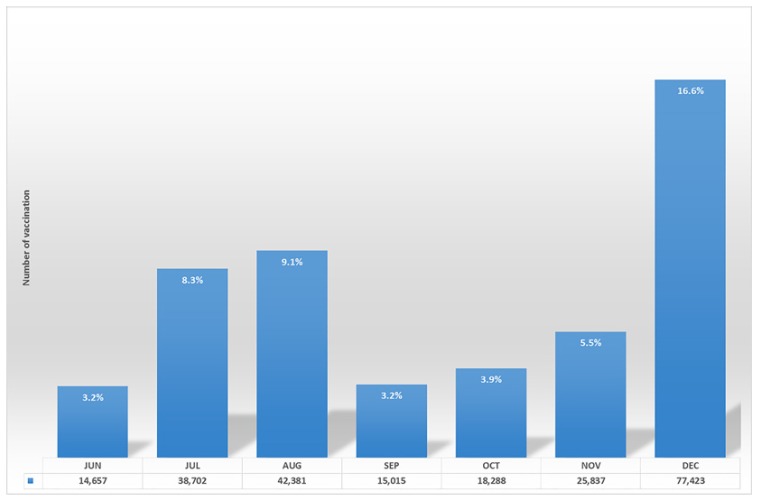
The Korean National Immunization Program (NIP) for HPV was first implemented in June 2016 for girls 11–12 years of age with a 2-dose schedule. Of the 464,932 total subjects aged 11–12 years, 232,303 (50.0%) girls initiated vaccination in the first year of the NIP, especially during the vacation period of July (8.3%), August (9.1%), and December (16.6%) (Fig. 2). Initiation of vaccination rates of girls born in 2004 were 86.3% in Gokseong, a county in South Jeolla, with a highly cooperative public health center and school-based vaccination program [47]. Regional disparities in HPV vaccination rate were reported as a maximum of 11% points up to June 2017 [48]. The greatest success was found when public health centers contacted the parents of girls, and they subsequently encouraged children to participate in the vaccination program. Educational newsletters handed out at school also helped enhance the vaccination rate in certain counties. In spite of these efforts, according to the latest analysis in June 2017, nationwide initiation rates were only 35.7% among girls born in 2004 and 2005 [49].
Fig. 2.
The monthly number of human papillomavirus vaccination rates in 2016. The data contained only from June to December because the Korean National Immunization Program for HPV was first implemented in June 2016 for girls 11–12 years of age with a 2-dose schedule. The vaccination rates were relatively higher during vacation period (July, August, and December).
4. Future strategies
Although various randomized controlled trials around the world have described the efficacy and impacts of HPV vaccination, the complete effect on future strategies for the prevention of cervical cancer remains undefined. Because the oncogenesis of HPV infection is slow progression from CIN to cervical cancer, it will take decades to thoroughly analyze the effects of vaccination on the prevalence of HPV and incidence of cervical cancer. In terms of immunology, the long-term effects of seropositivity and clinical protection following HPV vaccination should be studied with more vaccinated women, although antibody responses to HPV vaccination have been observed in previous studies [26].
1) The need for cervical cancer screening
As shown in a German population-based study, there has been a shift in the distribution of HPV types that are not included in the vaccine in the post-HPV vaccination era [33]. Vaccination for HPV 16/18 had a cross-protective effect against 4 non-vaccine HPV types (HPV 31, 33, 45, and 51) in the randomized, double-blind trial [50]. Induced cross-reactive T-cells and specific antibodies to other HPV genotypes not included in the quadrivalent HPV vaccine, such as HPV 31, 33, and 45, have been demonstrated in previous studies, and the prevalence of HPV 31, 33, and 45 is also declining [51]. Debates continue on whether the bivalent HPV vaccine is cross-protective against HPV 6 and HPV 11 [52,53,54]. For these reasons, screening for HPV DNA is still important for the time being, because none of the currently available vaccines has been proven to provide complete protection against all high-risk HPV genotypes.
As described above, there was a 75% reduction of HPV 16 and 18 in Scotland following a national vaccination program with a coverage rate of over 90% [35]. Nevertheless, other high-risk HPV types were prevalent in vaccinated women with low grade cervical lesions. The phenomenon of increasing non-HPV 16/18 genotypes highlights the importance of utilizing different HPV detection strategies in women who have been vaccinated and those who are unvaccinated. However, in a recent randomized trial evaluating type replacement after HPV vaccination, HPV type replacement did not occur in vaccinated population within 4 years, and the authors predicted that it was unlikely to occur in vaccinated populations [55].
2) Reassessment of HPV screening initiation age and intervals (distinguishing between vaccinated and unvaccinated women)
Since HPV 16 and 18 positivity is expected to decline rapidly over the decades following implementation of a national immunization program, specific screening protocols and intervals should be implemented for vaccinated groups. There have been some studies about the correlation between HPV vaccination and changes in cervical cancer screening rates, although none have focused on Korea. In spite of concerns that women who have been vaccinated would be less likely to seek out cervical cancer screening, women who received the HPV vaccine more often received cervical cancer screening than those who had not been vaccinated [56,57]. Research on awareness of cervical cancer screening for women who have been vaccinated is needed in Korea, as well as a serious discussion about strategies to induce unvaccinated women to seek screening.
In the era of vaccination, we should provide different strategies for cervical cancer screening. HPV 16 and 18 are expected to nearly disappear; as a consequence, the positivity of screening tests would be lower than 30% [58]. Furthermore, the prevalence of lesions more advanced than severe dysplasia would also be reduced more than half, making screening less predictive and decreasing the benefit-harm ratio [59]. Cervical cancer caused by HPV types other than HPV 16 and 18 appears at a median of 5 years later than that caused by HPV 16 and 18. In particular, short term persistence of HPV 16 infection more strongly predicts a subsequent moderate dysplasia or more advanced pathology compared to other HPV genotypes [60]. Although there are not enough data to suggest revised recommendations other than older initial screening age and extended screening intervals, one option would be routine screening with HPV testing at 30, 45, and 60 years of age for women who were fully vaccinated before first sexual contact [61]. It would be more efficient to provide separate screening guidelines for vaccinated and unvaccinated women. In Italy, primary HPV screening is recommended starting at 30 years and at 5-year intervals for vaccinated women who were vaccinated in 2007/2008 and became 25 years old in 2017 [62]. An optimal cervical cancer screening model for women who have been vaccinated with all 3 doses was proposed in 2017 from a US model based-analysis of benefits and costs. They suggested that screening could be modified to start later with decreased frequency, with either cervical cytology or HPV testing alone every 5 years starting at age 25 or 30, and only primary HPV testing recommended every 10 years starting at age 30 or 35 for women vaccinated with the nonavalent vaccine [63].
3) Shift in screening from cytology or cytology/HPV to HPV alone
In April 2014, an HPV DNA test was approved as a primary screening tool by the Food and Drug Administration. Nevertheless, further investigation is needed to evaluate the efficacy of using only an HPV DNA test and the adverse effects of increased false-positivity. The issues of the primary HPV screening test are to distinguish high-risk HPV positive with ≥CIN 2 from patients with transient positivity. There will be an increase in false positivity due to high-risk HPV infection without ≥CIN 2. As the number of patient with transient high-risk HPV infection increases, unnecessary follow-up and cost burdens will be a problem to be solved [64]. The positive predictive value of cervical cytology for cervical cancer screening is expected to decrease along with the incidence of precancerous or cancerous lesions in the cervix after implementation of the NIP of HPV. Normal cervical cytology will correspondingly increase, leading to an increase in false negative results and a decrease in the sensitivity of cytology, further reducing the value of cytology as screening tool [65,66]. However, endocervical adenocarcinoma with gastric type in which the HPV was rarely detected could be a potential pitfall of HPV vaccination and HPV DNA testing although the incidence was low [67].
A retrospective population-based cohort study documented the effect of HPV vaccination on abnormal cervical cytology in women born between 1988 and 1993, using data from the Scottish Cervical Screening Program [68]. The authors observed a significant reduction in positive predictive value and abnormal predictive values for detecting CIN 2+ in vaccinated women, as well as a significant reduction in abnormal cytology.
4) New screening tools
New screening tools are an alternative in the context of a lower prevalence of HPV-positive tests and related abnormal cytology of the cervix. Although various new tools have been proposed with more specific markers, additional verification and certification are needed before commercialization. First, HPV E6 protein detection is more specific than the HPV DNA test for high-grade cervical lesions, and so far at a lower cost. This test targets HPV 16, 18, and 45 and has the greatest positive values for detecting severe dysplasia or more severe lesions compared to high-risk HPV DNA testing [69,70]. Second, p16INK4a immunohistochemistry is useful for identifying moderate dysplasia or more severe lesions in high-risk HPV-positive women [71]. In a study of the endpoint of moderate dysplasia or more severe lesions in HPV-positive women, the sensitivity of p16INK4a immunohistochemistry was 88% (81 of 92; 95% CI, 80–94) and specificity was 61% (633 of 1,045; 95% CI, 57–64) without an increase in the implementation of colposcopy [72]. Finally, p16/Ki-67 dual-stained cytology is more sensitive than Pap cytology for detecting high-grade CIN. Even with normal cytology, p16/Ki-67 dual-stained cytology detected more than two-thirds of severe dysplasia lesions in women with high-risk HPV and helped select colposcopy referral patients [73,74,75].
5) How to increase vaccination coverage levels (i.e., school-based vaccination)
Because 2 doses of the HPV vaccine provided more compliance than 3 doses, 2 doses tended to increase the rate of vaccination completion. In a combined analysis of data from the Costa Rica Vaccine and PATRICIA trials, the efficacy of 2 dose-vaccination was evaluated. Both 3 doses and fewer than 3 doses of bivalent vaccine showed comparable efficacy 4 years following vaccination of women between 15 and 25 years old. Cross-protective activity against HPV 31, 33, and 45 was obtained only for cases in which the interval of the 2 doses was 6 months [76]. In a cluster-randomized trial, the vaccination coverage rate was increased by education delivered to mothers of adolescent daughters [77]. In addition, the vaccination and completion rate was improved by consistent recommendations from health care providers [78]. Social efforts such as educating providers and clinic-specific feedback to encourage patients will increase vaccination rates.
Conclusion
To eradicate cervical cancer in an era when HPV infection and related diseases rarely occur, screening methods must account for vaccine programs. Primary HPV DNA tests will be substituted for conventional Pap smears in screening tests, allowing Pap smears to be applied only to HPV-positive women. Education of patients and providers, an effective vaccination program to increase vaccine coverage rate, and school-based encouragement can help eliminate HPV-related disease and invasive cervical cancer.
In Korea, a national immunization program has been implemented since 2016, and strategies to further increase the vaccination rate should involve the government, schools, and parents. Because HPV vaccines do not cover all types of high-risk HPV, screening for precancerous lesions and cervical cancer will not be eliminated. In the decades following a national HPV vaccine program with a high coverage rate, existing screening strategies based on primary cytology such as Pap smear should be reviewed, because the low prevalence of abnormal cytology of the cervix will make screening less cost-effective and inefficient. Primary HPV testing will play an important role as a screening test and cytology should be reserved for women with an HPV positive test. In addition, reassessment of HPV screening initiation age and intervals that distinguish vaccinated women from unvaccinated women should be discussed in the near future.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012 v1.0 [Internet] Lyon: IARC; 2013. [cited 2017 Aug 1]. Available from: http://globocan.iarc.fr/Default.aspx. [Google Scholar]
- 3.Vaccarella S, Franceschi S, Engholm G, Lönnberg S, Khan S, Bray F. 50 years of screening in the Nordic countries: quantifying the effects on cervical cancer incidence. Br J Cancer. 2014;111:965–969. doi: 10.1038/bjc.2014.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaccarella S, Franceschi S, Zaridze D, Poljak M, Veerus P, Plummer M, et al. Preventable fractions of cervical cancer via effective screening in six Baltic, central, and eastern European countries 2017–40: a population-based study. Lancet Oncol. 2016;17:1445–1452. doi: 10.1016/S1470-2045(16)30275-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jung KW, Won YJ, Oh CM, Kong HJ, Lee DH, Lee KH. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2014. Cancer Res Treat. 2017;49:292–305. doi: 10.4143/crt.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 7.Sycuro LK, Xi LF, Hughes JP, Feng Q, Winer RL, Lee SK, et al. Persistence of genital human papillomavirus infection in a long-term follow-up study of female university students. J Infect Dis. 2008;198:971–978. doi: 10.1086/591625. [DOI] [PubMed] [Google Scholar]
- 8.Koshiol J, Lindsay L, Pimenta JM, Poole C, Jenkins D, Smith JS. Persistent human papillomavirus infection and cervical neoplasia: a systematic review and meta-analysis. Am J Epidemiol. 2008;168:123–137. doi: 10.1093/aje/kwn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(Suppl 5):F12–F23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 10.Bouscarat F, Pelletier F, Fouere S, Janier M, Bertolloti A, Aubin F, et al. External genital warts (condylomata) Ann Dermatol Venereol. 2016;143:741–745. doi: 10.1016/j.annder.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Ronco G, Giorgi-Rossi P, Carozzi F, Confortini M, Dalla Palma P, Del Mistro A, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249–257. doi: 10.1016/S1470-2045(09)70360-2. [DOI] [PubMed] [Google Scholar]
- 12.Leinonen M, Nieminen P, Kotaniemi-Talonen L, Malila N, Tarkkanen J, Laurila P, et al. Age-specific evaluation of primary human papillomavirus screening vs conventional cytology in a randomized setting. J Natl Cancer Inst. 2009;101:1612–1623. doi: 10.1093/jnci/djp367. [DOI] [PubMed] [Google Scholar]
- 13.Almonte M, Sasieni P, Cuzick J. Incorporating human papillomavirus testing into cytological screening in the era of prophylactic vaccines. Best Pract Res Clin Obstet Gynaecol. 2011;25:617–629. doi: 10.1016/j.bpobgyn.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Santin AD, Bellone S, Palmieri M, Zanolini A, Ravaggi A, Siegel ER, et al. Human papillomavirus type 16 and 18 E7-pulsed dendritic cell vaccination of stage IB or IIA cervical cancer patients: a phase I escalating-dose trial. J Virol. 2008;82:1968–1979. doi: 10.1128/JVI.02343-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mészner Z, Jankovics I, Nagy A, Gerlinger I, Katona G. Recurrent laryngeal papillomatosis with oesophageal involvement in a 2 year old boy: successful treatment with the quadrivalent human papillomatosis vaccine. Int J Pediatr Otorhinolaryngol. 2015;79:262–266. doi: 10.1016/j.ijporl.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 16.Yu Z. Recurrent laryngeal papillomatosis: the current treatment and future development. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2015;29:2107–2110. [PubMed] [Google Scholar]
- 17.Luxembourg A, Brown D, Bouchard C, Giuliano AR, Iversen OE, Joura EA, et al. Phase II studies to select the formulation of a multivalent HPV L1 virus-like particle (VLP) vaccine. Hum Vaccin Immunother. 2015;11:1313–1322. doi: 10.1080/21645515.2015.1012010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garçon N, Morel S, Didierlaurent A, Descamps D, Wettendorff M, Van Mechelen M. Development of an AS04-adjuvanted HPV vaccine with the adjuvant system approach. BioDrugs. 2011;25:217–226. doi: 10.2165/11591760-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Yokomine M, Matsueda S, Kawano K, Sasada T, Fukui A, Yamashita T, et al. Enhancement of humoral and cell mediated immune response to HPV16 L1-derived peptides subsequent to vaccination with prophylactic bivalent HPV L1 virus-like particle vaccine in healthy females. Exp Ther Med. 2017;13:1500–1505. doi: 10.3892/etm.2017.4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markowitz LE, Liu G, Hariri S, Steinau M, Dunne EF, Unger ER. Prevalence of HPV after introduction of the vaccination program in the United States. Pediatrics. 2016;137:e20151968. doi: 10.1542/peds.2015-1968. [DOI] [PubMed] [Google Scholar]
- 21.Naud PS, Roteli-Martins CM, De Carvalho NS, Teixeira JC, de Borba PC, Sanchez N, et al. Sustained efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine: final analysis of a long-term follow-up study up to 9.4 years post-vaccination. Hum Vaccin Immunother. 2014;10:2147–2162. doi: 10.4161/hv.29532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hildesheim A, Wacholder S, Catteau G, Struyf F, Dubin G, Herrero R, et al. Efficacy of the HPV-16/18 vaccine: final according to protocol results from the blinded phase of the randomized Costa Rica HPV-16/18 vaccine trial. Vaccine. 2014;32:5087–5097. doi: 10.1016/j.vaccine.2014.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–314. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 24.FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 25.Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372:711–723. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 26.Villa LL, Ault KA, Giuliano AR, Costa RL, Petta CA, Andrade RP, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus types 6, 11, 16, and 18. Vaccine. 2006;24:5571–5583. doi: 10.1016/j.vaccine.2006.04.068. [DOI] [PubMed] [Google Scholar]
- 27.Hariri S, Johnson ML, Bennett NM, Bauer HM, Park IU, Schafer S, et al. Population-based trends in high-grade cervical lesions in the early human papillomavirus vaccine era in the United States. Cancer. 2015;121:2775–2781. doi: 10.1002/cncr.29266. [DOI] [PubMed] [Google Scholar]
- 28.Petrosky EY, Hariri S, Markowitz LE, Panicker G, Unger ER, Dunne EF. Is vaccine type seropositivity a marker for human papillomavirus vaccination? National Health and Nutrition Examination Survey, 2003–2010. Int J Infect Dis. 2015;33:137–141. doi: 10.1016/j.ijid.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mesher D, Stanford E, White J, Findlow J, Warrington R, Das S, et al. HPV serology testing confirms high HPV immunisation coverage in England. PLoS One. 2016;11:e0150107. doi: 10.1371/journal.pone.0150107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carter JJ, Koutsky LA, Hughes JP, Lee SK, Kuypers J, Kiviat N, et al. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis. 2000;181:1911–1919. doi: 10.1086/315498. [DOI] [PubMed] [Google Scholar]
- 31.Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;367:1247–1255. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 32.Olsson SE, Villa LL, Costa RL, Petta CA, Andrade RP, Malm C, et al. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine. 2007;25:4931–4939. doi: 10.1016/j.vaccine.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 33.Fischer S, Bettstetter M, Becher A, Lessel M, Bank C, Krams M, et al. Shift in prevalence of HPV types in cervical cytology specimens in the era of HPV vaccination. Oncol Lett. 2016;12:601–610. doi: 10.3892/ol.2016.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kavanagh K, Pollock KG, Potts A, Love J, Cuschieri K, Cubie H, et al. Introduction and sustained high coverage of the HPV bivalent vaccine leads to a reduction in prevalence of HPV 16/18 and closely related HPV types. Br J Cancer. 2014;110:2804–2811. doi: 10.1038/bjc.2014.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhatia R, Kavanagh K, Cubie HA, Serrano I, Wennington H, Hopkins M, et al. Use of HPV testing for cervical screening in vaccinated women--Insights from the SHEVa (Scottish HPV Prevalence in Vaccinated Women) study. Int J Cancer. 2016;138:2922–2931. doi: 10.1002/ijc.30030. [DOI] [PubMed] [Google Scholar]
- 36.Bae JH, Lee SJ, Kim CJ, Hur SY, Park YG, Lee WC, et al. Human papillomavirus (HPV) type distribution in Korean women: a meta-analysis. J Microbiol Biotechnol. 2008;18:788–794. [PubMed] [Google Scholar]
- 37.Lee HS, Kim KM, Kim SM, Choi YD, Nam JH, Park CS, et al. Human papillomavirus genotyping using HPV DNA chip analysis in Korean women. Int J Gynecol Cancer. 2007;17:497–501. doi: 10.1111/j.1525-1438.2007.00871.x. [DOI] [PubMed] [Google Scholar]
- 38.Kim MJ, Kim JJ, Kim S. Type-specific prevalence of high-risk human papillomavirus by cervical cytology and age: data from the health check-ups of 7,014 Korean women. Obstet Gynecol Sci. 2013;56:110–120. doi: 10.5468/OGS.2013.56.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.So KA, Hong JH, Lee JK. Human papillomavirus prevalence and type distribution among 968 women in South Korea. J Cancer Prev. 2016;21:104–109. doi: 10.15430/JCP.2016.21.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oh JK, Alemany L, Suh JI, Rha SH, Munoz N, Bosch FX, et al. Type-specific human papillomavirus distribution in invasive cervical cancer in Korea, 1958–2004. Asian Pac J Cancer Prev. 2010;11:993–1000. [PubMed] [Google Scholar]
- 41.Jun JK, Choi KS, Jung KW, Lee HY, Gapstur SM, Park EC, et al. Effectiveness of an organized cervical cancer screening program in Korea: results from a cohort study. Int J Cancer. 2009;124:188–193. doi: 10.1002/ijc.23841. [DOI] [PubMed] [Google Scholar]
- 42.Dickinson J, Tsakonas E, Conner Gorber S, Lewin G, Shaw E, Singh H, et al. Recommendations on screening for cervical cancer. CMAJ. 2013;185:35–45. doi: 10.1503/cmaj.121505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moyer VA U.S. Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156:880–891.:W312. doi: 10.7326/0003-4819-156-12-201206190-00424. [DOI] [PubMed] [Google Scholar]
- 44.Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137:516–542. doi: 10.1309/AJCPTGD94EVRSJCG. [DOI] [PubMed] [Google Scholar]
- 45.Lee JK, Hong JH, Kang S, Kim DY, Kim BG, Kim SH, et al. Practice guidelines for the early detection of cervical cancer in Korea: Korean Society of Gynecologic Oncology and the Korean Society for Cytopathology 2012 edition. J Gynecol Oncol. 2013;24:186–203. doi: 10.3802/jgo.2013.24.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Min KJ, Lee YJ, Suh M, Yoo CW, Lim MC, Choi J, et al. The Korean guideline for cervical cancer screening. J Gynecol Oncol. 2015;26:232–239. doi: 10.3802/jgo.2015.26.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Korea Centers for Disease Control and Prevention. Cheif of KCDC, conference of public health and ministry of education was held in Gokseong where had the highest HPV vaccination rate (press release in 25th April, 2017) Cheongju: Korea Centers for Disease Control and Prevention; 2017. [Google Scholar]
- 48.Korea Centers for Disease Control and Prevention. National immunization program "Up to 3 times difference" regional disparity in HPV NIP vaccination rate (press release in June 2017) Cheongju: Korea Centers for Disease Control and Prevention; 2017. [Google Scholar]
- 49.Korea Centers for Disease Control and Prevention. National immunization program [Internet] Cheongju: Korea Centers for Disease Control and Prevention; 2017. [updated 2017 Jun 9]. [cited 2017 Jul 10]. Available from: https://nip.cdc.go.kr/irgd/index.html. [Google Scholar]
- 50.Wheeler CM, Castellsagué X, Garland SM, Szarewski A, Paavonen J, Naud P, et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13:100–110. doi: 10.1016/S1470-2045(11)70287-X. [DOI] [PubMed] [Google Scholar]
- 51.Tabrizi SN, Brotherton JM, Kaldor JM, Skinner SR, Liu B, Bateson D, et al. Assessment of herd immunity and cross-protection after a human papillomavirus vaccination programme in Australia: a repeat cross-sectional study. Lancet Infect Dis. 2014;14:958–966. doi: 10.1016/S1473-3099(14)70841-2. [DOI] [PubMed] [Google Scholar]
- 52.Woestenberg PJ, King AJ, van der Sande MA, Donken R, Leussink S, van der Klis FR, et al. No evidence for cross-protection of the HPV-16/18 vaccine against HPV-6/11 positivity in female STI clinic visitors. J Infect. 2017;74:393–400. doi: 10.1016/j.jinf.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 53.Westra TA, Stirbu-Wagner I, Dorsman S, Tutuhatunewa ED, de Vrij EL, Nijman HW, et al. Inclusion of the benefits of enhanced cross-protection against cervical cancer and prevention of genital warts in the cost-effectiveness analysis of human papillomavirus vaccination in the Netherlands. BMC Infect Dis. 2013;13:75. doi: 10.1186/1471-2334-13-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Howell-Jones R, Soldan K, Wetten S, Mesher D, Williams T, Gill ON, et al. Declining genital Warts in young women in england associated with HPV 16/18 vaccination: an ecological study. J Infect Dis. 2013;208:1397–1403. doi: 10.1093/infdis/jit361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tota JE, Struyf F, Merikukka M, Gonzalez P, Kreimer AR, Bi D, et al. Evaluation of type replacement following HPV16/18 vaccination: pooled analysis of two randomized trials. J Natl Cancer Inst. 2017;109:djw300. doi: 10.1093/jnci/djw300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chao C, Silverberg MJ, Becerra TA, Corley DA, Jensen CD, Chen Q, et al. Human papillomavirus vaccination and subsequent cervical cancer screening in a large integrated healthcare system. Am J Obstet Gynecol. 2017;216:151.e1–151.e9. doi: 10.1016/j.ajog.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hirth JM, Lin YL, Kuo YF, Berenson AB. Effect of number of human papillomavirus vaccine doses on guideline adherent cervical cytology screening among 19–26 year old females. Prev Med. 2016;88:134–139. doi: 10.1016/j.ypmed.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wright TC, Jr, Stoler MH, Sharma A, Zhang G, Behrens C, Wright TL, et al. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am J Clin Pathol. 2011;136:578–586. doi: 10.1309/AJCPTUS5EXAS6DKZ. [DOI] [PubMed] [Google Scholar]
- 59.Khan MJ, Castle PE, Lorincz AT, Wacholder S, Sherman M, Scott DR, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97:1072–1079. doi: 10.1093/jnci/dji187. [DOI] [PubMed] [Google Scholar]
- 60.Castle PE, Rodríguez AC, Burk RD, Herrero R, Wacholder S, Alfaro M, et al. Short term persistence of human papillomavirus and risk of cervical precancer and cancer: population based cohort study. BMJ. 2009;339:b2569. doi: 10.1136/bmj.b2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jeronimo J, Castle PE, Temin S, Shastri SS. Secondary prevention of cervical cancer: American Society of Clinical Oncology resource-stratified clinical practice guideline summary. J Oncol Pract. 2017;13:129–133. doi: 10.1200/JOP.2016.017889. [DOI] [PubMed] [Google Scholar]
- 62.Giorgi Rossi P, Carozzi F, Federici A, Ronco G, Zappa M, Franceschi S, et al. Cervical cancer screening in women vaccinated against human papillomavirus infection: Recommendations from a consensus conference. Prev Med. 2017;98:21–30. doi: 10.1016/j.ypmed.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 63.Kim JJ, Burger EA, Sy S, Campos NG. Optimal cervical cancer screening in women vaccinated against human papillomavirus. J Natl Cancer Inst. 2017;109:djw216. doi: 10.1093/jnci/djw216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meijer CJ, Berkhof J, Castle PE, Hesselink AT, Franco EL, Ronco G, et al. Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older. Int J Cancer. 2009;124:516–520. doi: 10.1002/ijc.24010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Franco EL, Cuzick J, Hildesheim A, de Sanjosé S. Chapter 20: Issues in planning cervical cancer screening in the era of HPV vaccination. Vaccine. 2006;24(Suppl 3):S3/171-7. doi: 10.1016/j.vaccine.2006.05.061. [DOI] [PubMed] [Google Scholar]
- 66.Cuzick J, Clavel C, Petry KU, Meijer CJ, Hoyer H, Ratnam S, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095–1101. doi: 10.1002/ijc.21955. [DOI] [PubMed] [Google Scholar]
- 67.Kusanagi Y, Kojima A, Mikami Y, Kiyokawa T, Sudo T, Yamaguchi S, et al. Absence of high-risk human papillomavirus (HPV) detection in endocervical adenocarcinoma with gastric morphology and phenotype. Am J Pathol. 2010;177:2169–2175. doi: 10.2353/ajpath.2010.100323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Palmer TJ, McFadden M, Pollock KG, Kavanagh K, Cuschieri K, Cruickshank M, et al. HPV immunisation and cervical screening--confirmation of changed performance of cytology as a screening test in immunised women: a retrospective population-based cohort study. Br J Cancer. 2016;114:582–589. doi: 10.1038/bjc.2015.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Valdez M, Jeronimo J, Bansil P, Qiao YL, Zhao FH, Chen W, et al. Effectiveness of novel, lower cost molecular human papillomavirus-based tests for cervical cancer screening in rural china. Int J Cancer. 2016;138:1453–1461. doi: 10.1002/ijc.29877. [DOI] [PubMed] [Google Scholar]
- 70.Zhao FH, Jeronimo J, Qiao YL, Schweizer J, Chen W, Valdez M, et al. An evaluation of novel, lower-cost molecular screening tests for human papillomavirus in rural China. Cancer Prev Res (Phila) 2013;6:938–948. doi: 10.1158/1940-6207.CAPR-13-0091. [DOI] [PubMed] [Google Scholar]
- 71.Carozzi F, Gillio-Tos A, Confortini M, Del Mistro A, Sani C, De Marco L, et al. Risk of high-grade cervical intraepithelial neoplasia during follow-up in HPV-positive women according to baseline p16-INK4A results: a prospective analysis of a nested substudy of the NTCC randomised controlled trial. Lancet Oncol. 2013;14:168–176. doi: 10.1016/S1470-2045(12)70529-6. [DOI] [PubMed] [Google Scholar]
- 72.Carozzi F, Confortini M, Dalla Palma P, Del Mistro A, Gillio-Tos A, De Marco L, et al. Use of p16-INK4A overexpression to increase the specificity of human papillomavirus testing: a nested substudy of the NTCC randomised controlled trial. Lancet Oncol. 2008;9:937–945. doi: 10.1016/S1470-2045(08)70208-0. [DOI] [PubMed] [Google Scholar]
- 73.Uijterwaal MH, Polman NJ, Witte BI, van Kemenade FJ, Rijkaart D, Berkhof J, et al. Triaging HPV-positive women with normal cytology by p16/Ki-67 dual-stained cytology testing: baseline and longitudinal data. Int J Cancer. 2015;136:2361–2368. doi: 10.1002/ijc.29290. [DOI] [PubMed] [Google Scholar]
- 74.Wright TC, Jr, Behrens CM, Ranger-Moore J, Rehm S, Sharma A, Stoler MH, et al. Triaging HPV-positive women with p16/Ki-67 dual-stained cytology: Results from a sub-study nested into the ATHENA trial. Gynecol Oncol. 2017;144:51–56. doi: 10.1016/j.ygyno.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 75.Wentzensen N, Schwartz L, Zuna RE, Smith K, Mathews C, Gold MA, et al. Performance of p16/Ki-67 immunostaining to detect cervical cancer precursors in a colposcopy referral population. Clin Cancer Res. 2012;18:4154–4162. doi: 10.1158/1078-0432.CCR-12-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kreimer AR, Struyf F, Del Rosario-Raymundo MR, Hildesheim A, Skinner SR, Wacholder S, et al. Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: combined analysis of data from the Costa Rica Vaccine and PATRICIA Trials. Lancet Oncol. 2015;16:775–786. doi: 10.1016/S1470-2045(15)00047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Winer RL, Gonzales AA, Noonan CJ, Buchwald DS. A Cluster-randomized trial to evaluate a mother-daughter dyadic educational intervention for increasing HPV vaccination coverage in American Indian girls. J Community Health. 2016;41:274–281. doi: 10.1007/s10900-015-0093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krantz L, Ollberding NJ, Beck AF, Carol Burkhardt M. Increasing HPV vaccination coverage through provider-based interventions. Clin Pediatr (Phila) 2018;57:319–326. doi: 10.1177/0009922817722014. [DOI] [PubMed] [Google Scholar]