Abstract
Objective
A design comparison of current perimodiolar and lateral wall electrode arrays of the cochlear implant (CI) is provided. The focus is on functional features such as acoustic frequency coverage and tonotopic mapping, battery consumption and dynamic range. A traumacity of their insertion is also evaluated.
Methods
Review of up-to-date literature.
Results
Perimodiolar electrode arrays are positioned in the basal turn of the cochlea near the modiolus. They are designed to initiate the action potential in the proximity to the neural soma located in spiral ganglion. On the other hand, lateral wall electrode arrays can be inserted deeper inside the cochlea, as they are located along the lateral wall and such insertion trajectory is less traumatic. This class of arrays targets primarily surviving neural peripheral processes. Due to their larger insertion depth, lateral wall arrays can deliver lower acoustic frequencies in manner better corresponding to cochlear tonotopicity. In fact, spiral ganglion sections containing auditory nerve fibres tuned to low acoustic frequencies are located deeper than 1 and half turn inside the cochlea. For this reason, a significant frequency mismatch might be occurring for apical electrodes in perimodiolar arrays, detrimental to speech perception. Tonal languages such as Mandarin might be therefore better treated with lateral wall arrays. On the other hand, closer proximity to target tissue results in lower psychophysical threshold levels for perimodiolar arrays. However, the maximal comfort level is also lower, paradoxically resulting in narrower dynamic range than that of lateral wall arrays. Battery consumption is comparable for both types of arrays.
Conclusions
Lateral wall arrays are less likely to cause trauma to cochlear structures. As the current trend in cochlear implantation is the maximal protection of residual acoustic hearing, the lateral wall arrays seem more suitable for hearing preservation CI surgeries. Future development could focus on combining the advantages of both types: perimodiolar location in the basal turn extended to lateral wall location for higher turn locations.
Keyword: Cochlear implant
Introduction
An electrode array is the essential part of a cochlear implant (CI).It is inserted into the cochlea of the inner ear in the near proximity of auditory nerve fibres and allows their electrical stimulation, Fig. 1. The design of a CI electrode array as well as its exact intracochlear position determines to great extend sound audibility with the CI technology. While the external part of CI, containing an audio processor, can be upgraded in line with technological development, the implanted electrode array remains inside the cochlea typically for the whole duration of implantation. Such intended long-term implantability puts high requirements on array's biocompatibility, durability and its functional design as it cannot be changed easily. At present, more than 20 years since the introduction of such hearing restorative treatment to clinical practice, the surgical implantation has reached very a traumatic levels. On the one hand, such a traumacity was achieved by improving the design of the array making it maximally compatible with the anatomical shape of the cochlea. On the other hand, the surgical advancement has minimised trauma during the insertion process, maximally protecting the intracochlear cellular structures. The cochlear implantation has therefore become a gold standard for the treatment of all sorts of deafness forms, which cannot be helped by conventional hearing aids. The CI candidacy nowadays extends extensively the original group of profoundly deaf persons and includes also patients with significant levels of residual hearing.
Fig. 1.
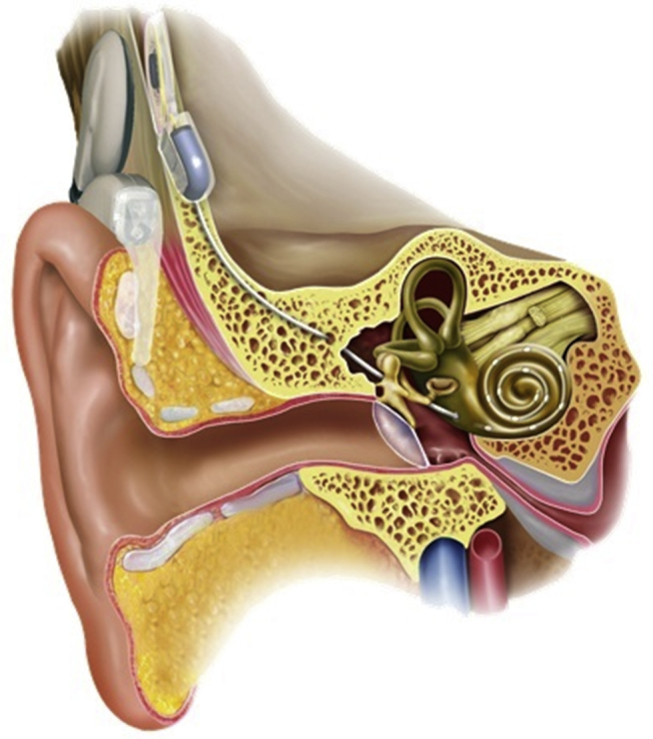
Schematic representation of the cochlear implant. The behind-the-ear external processor with ear hook and a battery case uses a microphone to pick up sound, converts the sound into a digital signal, processes and encodes it into a radio frequency (RF) signal and sends it to an internal receiver placed under the skin behind the ear. A hermetically sealed stimulator decodes the signal using power derived from the RF signal, converts it into electric currents and sends them along wires into the cochlea. The electrodes at the end of the wire stimulate the auditory nerve fibres. Such electrical impulses are interpreted in the central nervous system as sound.
All current CI electrode arrays have evolved along two distinctive design concepts. More common are so called lateral wall electrode arrays. These are free-fitting arrays occupying space in scala tympani in their final location just under the organ of Corti next to the lateral wall. On the contrary, the second class, perimodiolar electrode arrays, are located along the cochlear modiolar wall to minimise the distance to the modiolus to increase stimulation specificity and reduce battery consumption. To reach such a specific location, perimodiolar arrays are typically pre-shaped and implanted with the help of a stiffening element to keep the array relatively straight as needed for insertion. The array resumes its curved form after a removal of the stiffening element when insertion is advanced far enough. Examples of both types of arrays are given in Table 1.
Table 1.
Dimension specifications of different CI electrode arrays. “Insertion length” stands for the distance from the tip to the stopper (marker), while “Active length” for the distance from the first to the last electrode. “Spacing” indicates the distance between electrodes.
| Electrode array | Type | Cochlear position | Manufacturer | Insertion length (mm) | Active length (mm) | Diameter at apical end (mm) | Diameter at basal end (mm) | Spacing (mm) | Number of electrodes |
|---|---|---|---|---|---|---|---|---|---|
| Flexsoft | Straight | Lateral wall | MED-EL | 31.3 | 26.4 | 0.5 × 0.4 | 1.3 | 2.4 | 12 |
| Flex28 | Straight | Lateral wall | MED-EL | 28.0 | 23.1 | 0.5 × 0.4 | 0.8 | 2.1 | 12 |
| Flex24 | Straight | Lateral wall | MED-EL | 24.0 | 20.9 | 0.5 × 0.3 | 0.8 | 1.9 | 12 |
| Flex20 | Straight | Lateral wall | MED-EL | 20.0 | 15.4 | 0.5 × 0.3 | 0.8 | 1.3 | 12 |
| Contour advance™ | Spiral | Perimodiolar | Cochlear Limited | 17.8 | 15.0 | 0.5 | 0.8 | 0.7 | 22 |
| Slim straight | Straight | Lateral wall | Cochlear Limited | 25.0 | 20.0 | 0.3 | 0.6 | 0.9 | 22 |
| Hybrid™ l24 | Straight | Lateral wall | Cochlear Limited | 16.0 | 14.5 | 0.25 | 0.4 | 0.7 | 22 |
| Full-band straight | Straight | Lateral wall | Cochlear Limited | 23.9 | 16.4 | 0.4 | 0.6 | 0.7 | 22 |
| HiFocus™ V | Spiral with Stylet | Mid scala | Advanced Bionics | 18.5 | 15.0 | 0.5 | 0.7 | 0.9 | 16 |
| HiFocus™ Helix |
Spiral | Perimodiolar | Advanced Bionics | 18.5–21.5 | 13.0 | 0.6 | 1.1 | 0.85 | 16 |
| HiFocus™ 1J | Straight | Lateral wall | Advanced Bionics | 22.0–24.0 | 17.0 | 0.4 | 0.8 | 1.1 | 16 |
Perimodiolar electrode arrays can hug the modiolus only in the basal turn where it has a diameter large enough to resist its wrapping with an electrode array. Therefore, they are relatively short and cover mainly the basal turn. Furthermore, as these arrays are mostly inserted through the cochleostomy, typically drilled some distance from the round window, they don't wrap the most basal part of the modiolus and the distance to it might be larger than for the lateral wall arrays in this cochlear region.1 In the future developments, it would be desirable to design a perimodiolar electrode array which continues in higher turns along the lateral wall, where modiolus is too fragile, to provide the complete cochlear coverage of required acoustic frequencies.
Discussion
-
1.
Complete cochlear coverage not only for Mandarin-speakers
Tonal languages such as Mandarin require that CI transmits highly effectively also temporal information about low frequency components in speech, encoded by the most apical auditory nerve fibres.2, 3, 4 Therefore, the CI electrode array must be designed and positioned in such a way in the cochlea to cover even relatively low acoustic frequency (∼200 Hz). This is not a trivial task due to the anatomic/tonotopic compression and mismatch of the auditory nerve somas in spiral ganglion (SG) with respects to their peripheral processes projecting towards sensory cells in the organ of Corti (OC).5 Surviving processes run from SG in strictly radial direction only in the basal turn, Fig. 2. The frequency tuning of both somas (SG) and dendrites (OC) is in this region governed by the same Greenwood's frequency-position function.6 However, towards the apical end, surviving peripheral processes increasingly deviate from the radial direction since SG somas reach only one and three-fourth of a turnover a rather short distance, while the sensory cell region extends to 2 and three-fourth turns in total. These neurons, even though tonotopically arranged, have their soma and peripheral processes in different radial cochlear sections and a modified version of Greenwood's frequency-position function was proposed to describe the tuning at the level of neural somas in SG.7 Such anatomical hindrances are further complicated by the uncertainty about the site of spike initiation, most likely located in initial Ranviers nodes as the unmyelinated soma has too high capacitance.8 Despite such anatomical challenges, the selective electrical stimulation of neurons towards apexis possible with lateral wall electrode arrays with insertion depth at least 28 mm such as FLEX28.9, 10
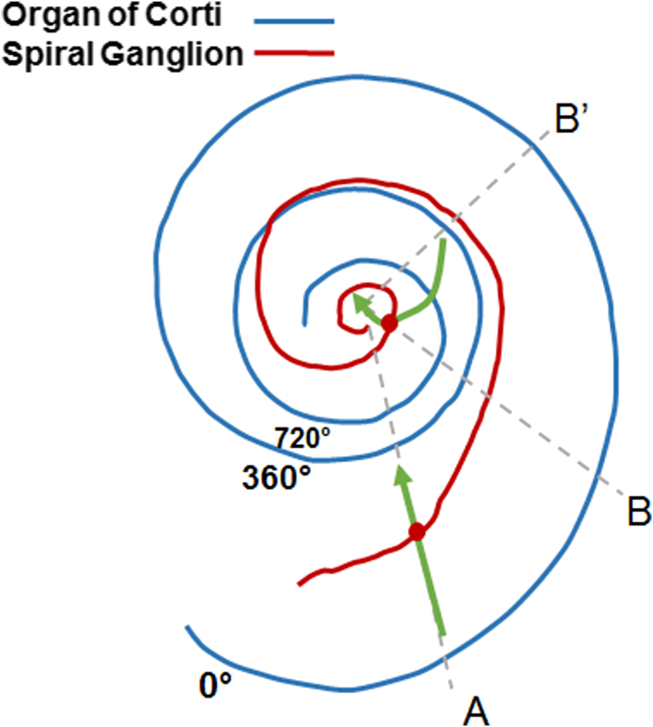
Fig. 2.
Schematic drawing of the organ of Corti (in blue) and spiral ganglion (in red) in the cochlea. Two exemplary neural fibres at different positions are also shown (in green). Each of them runs from the organ of Corti across spiral ganglion, where the soma is located, and then away from the cochlea to the cochlear nucleus in the brainstem (not shown). The course of the first one in the basal turn (position A) is radial with respect to the organ of Corti, and both the soma and the peripheral process are in the same cochlear cross-section with the same best frequency. On the other hand, the course of the second nerve fibre in the second turn is not radial with respect to the organ of Corti anymore and the soma and the peripheral process are at different cross-sections with different best frequencies (position B and B′, respectively).
An alternative way to achieve a selective electric stimulation, but with a relatively shallow electrode insertion, is to use perimodiolar electrode arrays designed to stimulate initial axonal Ranviers nodes near neural soma. Spiral ganglion is relatively short in comparison to the organ of Corti, under which the lateral wall electrode arrays are located, (15.49 ± 0.69) mm and (33.13 ± 2.11) mm, respectively.7 Therefore, it can be argued that relatively short perimodiolar electrode array is sufficient to provide the complete coverage of acoustic frequencies by stimulating the neural soma (SG) instead of peripheral processes of auditory nerve fibres (OC). Intuitively, the advantage of shorter electrode is smaller electrode insertion-associated trauma, higher residual hearing and better speech perception with CI. For example, the Contour Advance™ electrode array has the length of 15 mm only and HiFocus Helix™ of 13.25 mm, while the length of a lateral wall electrode such as FLEX28 is 28 mm. However, it must be noted that shorter SG length doesn't translate directly into the required smaller insertion angle of the electrode array tip for the complete coverage due to different trajectories of SG and OC, Fig. 2. According to Stakhovskaya et al,7 the insertion angle of 630° is needed to reach the somas of low sound frequency-specific neurons at the cochlear apex (170–262 Hz). The same insertion angle corresponds for the lateral wall electrodes to very similar frequencies 208–302 Hz. However, the insertion angle for the Contour Advance™ Electrode was established experimentally in the range of 240°–430° only,11, 12, 13, 14 which is far less than the desired 630° needed for a good anatomical pitch mapping for low frequencies. Similarly, averaged insertion angle for the HiFocusV Mid-Scala electrode is 422° ± 20.7° (the Advanced Bionics website), again much smaller than that required for the full coverage even if at the SG anatomical level. On the other hand, the lateral wall electrodes such as FlexSOFT or Flex28 with the length of 28 mm and 31 mm, respectively, have typical insertion angle of at least 640° and 530°, respectively,14, 15 sufficient to stimulate also the low frequency neurons. Based on this comparison, it is unlikely that the perimodiolar electrode arrays could stimulate the low-frequency neural fibres as effectively as the lateral wall electrodes in a simple pitch corresponding matter. A significant amount of pitch compression at low frequencies might be occurring, which could be detrimental to the speech audibility if the brain doesn't possess sufficient plasticity, which cannot be always guaranteed.16, 17
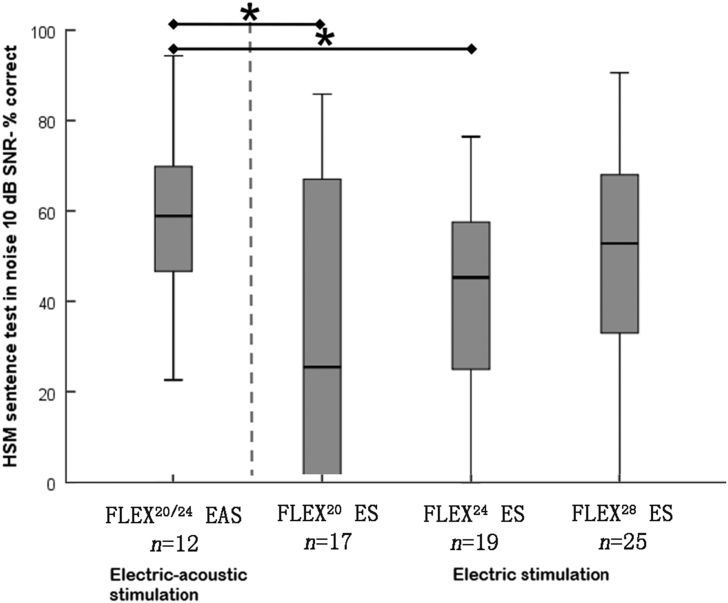
In fact, a direct comparison of CI electrode arrays (lateral wall type) with the same overall design and differing only in the length (20, 24 and 28 mm) showed that longer electrode arrays provide better hearing performance under normal (noisy) hearing conditions, Fig. 3. Only the longest array, Flex28, came closer in performance to the Electro-Acoustic Stimulation (EAS) situation, where the apical region of the cochlea is not stimulated electrically, but via the surviving sensory cells (with sound amplification). Such result indicates the importance of reaching the cochlear apex also with the conventional CI and purely electric stimulation.
-
2.
Stimulation thresholds
Fig. 3.
HSM sentence test in noise 10 dB SNR after 6 months of purely electrical stimulation (ES) or Electro-Acoustic Stimulation (EAS). All subjects were postlingually hearing-impaired adult CI users who were implanted with a FLEX20, FLEX24, or FLEX28 electrode array. Median, interquartile, and minimum and maximum scores are shown, statistical significance is marked with * for P < 0.05 and ** for P < 0.01. From Büchner et al.18
Perimodiolar electrode arrays were developed to improve stimulation of specific neuronal populations and to decrease power consumption. Surprisingly, in a study with patients who received a different type of bilateral cochlear implant in each ear (either Contour Advance or Slim Straight) there was little difference in battery life for most patients.1 Such unexpected finding could be explained by the fact that significant amounts of battery consumption are used by radiofrequency transmission and signal processing. On the other hand, psychophysical threshold level was lower for the perimodiolar electrode array than for the lateral wall array, but so was also the comfort level and the resulting dynamic range for the perimodiolar array was narrower than for the lateral array. Furthermore, in 6 out of 7 bilateral sequentially implanted patients, a perimodiolar array was implanted first, while a lateral wall electrode array later in the second ear to preserve the anatomic structure of the cochlea, despite higher thresholds. Only in one case it was done the other way round, a lateral wall array in the first ear and a perimodiolar array in the second ear. This example emphasises the current preference of a traumacity over lower stimulation thresholds, as clinical indications for CIs have been expanded to patients with significant residual hearing and only partial deafness.
Other studies comparing perimodiolar and lateral wall electrode arrays in terms of eCAP measurements often failed to confirm lower eCAP thresholds for the perimodiolar electrode arrays.19, 20, 21, 22 The differences in the current amplitude or the distance between the electrode and the cochlear middle wall were often not statistically significant. One reason is a small patient size in such studies. Another, more fundamental, could be the relatively slow retrograde degeneration of peripheral process of the auditory nerve fibres, running from the neural soma towards the organ of Corti, especially at the mid- and low-frequency region.23, 24 Consequently, as much as 30% of the neural fibres might still be bipolar even after many years of deafness.25 Therefore, the Ranviers nodes at peripheral processes might be as valuable target for the electrical stimulation with CI as those on central axon and while located closely to the organ of Corti, well targeted by the lateral wall electrode arrays.26 Such functional and anatomical considerations were confirmed recently by the clinical finding that speech perception outcomes for patients who received a lateral wall or perimodiolar electrode array (Slim Straight and Contour, respectively) were found similar at 3 or 9 months after implant activation.27 Bamford-Kowal-Bench (BKB) speech perception scores were compared in this study and no statistical difference between the two types of arrays was found.
-
3.
Electrode impedance and fibrotic tissue encapsulation
Perimodiolar electrode arrays are primarily designed for cochleostomy and extended round window insertions,28 while lateral wall arrays can be inserted also through the round window (RW). However, cochleostomy and related surgical approaches were found to be more associated with new bone formation and fibrotic tissue encapsulation of the electrode array due to larger initial intracochlear damage than RW, partly because they can lead to unintended involvement of scala vestibuli.29, 30 In fact, a direct comparison of perimodiolar arrays (ClarionTM or HiRes90K™) with a lateral wall array (Full-Band Straight) confirmed thickened fibrous capsule at the medial aspect of the electrode array in individuals implanted with a perimodiolar device.31 This finding was attributed to closer proximity to the modiolus and differences in the shape of electrical field. Increased electrode impedance, which might result from such isolating encapsulation,32, 33 although the association between the longitudinal impedance data and the development of fibrous tissue is difficult,31 negates any hypothetical reduction in threshold currents linked with shorter distance to the target neural tissue at perimodiolar location.
-
4.
Insertion trauma
Finally, and most importantly, when comparing the two types of arrays, perimodiolar electrodes are much more traumatic. Due to their shape and higher stiffness, affected by the presence of stylet, and close position to the modiolar wall, they may cause trauma to the cochlear structures. Such trauma could consist of fracture of the osseous spiral lamina, disruption of the basilar membrane, dissection into the spiral ligament and stria vascularis, or injury to the modiolus.34 Depending on its severity, immediate irreversible necrosis and apoptosis of sensorineural cells in the organ of Corti as well as of spiral ganglion neurons, positioned just behind the thin bony wall of the modiolus, Fig. 4, can occur. Reduced numbers of such cells might have direct impact on the CI performance, which relies on healthy excitable neural substrate.
Fig. 4.
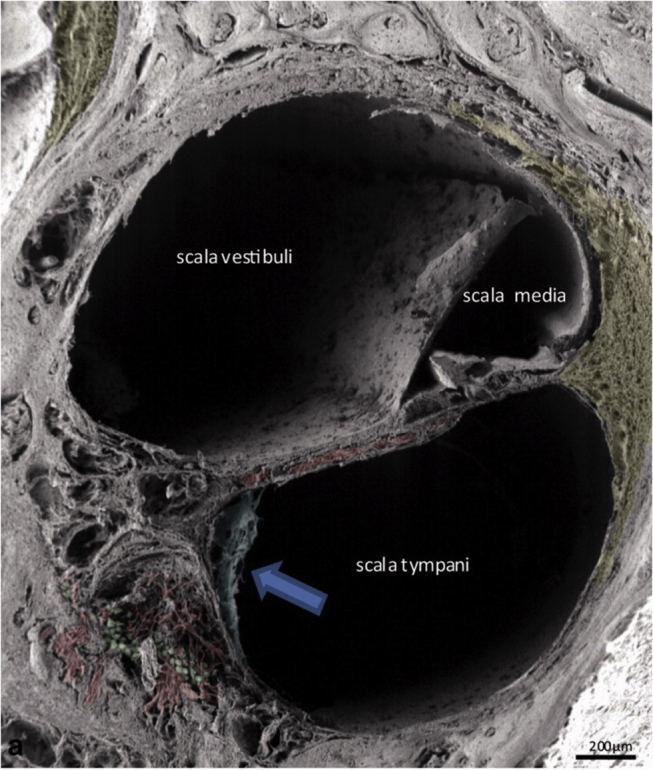
Possible trauma sites for cochlear implantation and position of neural elements in the human cochlea lower basal turn. Between scala tympani and the somas of spiral ganglion neurons (coloured green), a thin mesothelial sheet (arrow) spans between bony columns that guide nerve fibres (coloured red) to the osseous spiral lamina. Taken from Rask-Andersen et al.5 Copyright© 2012, John Wiley and Sons.
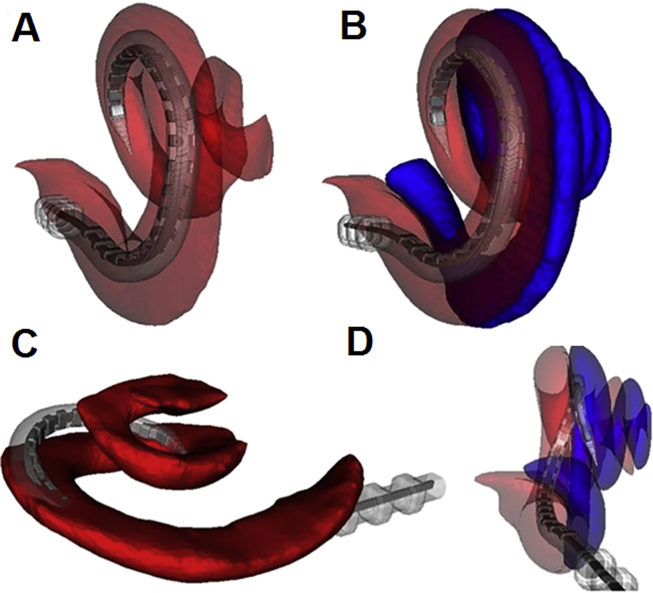
Furthermore, due to their larger rigidity, especially around the tip, perimodiolar electrode arrays tend to undergo translocation from scala tympani to scala vestibuli, Fig. 5. This phenomenon was observed in up to 30% of patients.21, 35, 36, 37, 38 Such scala change involves rapture of basilar membrane resulting in mixing of perilymph and endolymph from scala tympani and scala media, respectively. Intracochlear fluids have very different ionic composition, potassium being dominant in the endolymph (157 mmol/L) and sodium in the perilymph (138 mmol/L). Undisturbed cochlear ionic homoeostasis is essential for residual acoustic hearing as the difference in ionic composition forms the electrochemical base (+80 mV) for the active sound amplification driven by a molecular motor prestin, localized in the outer hair cells (OHC) in the organ of Corti.39 This so called OHC motility can provide up to 40 dB sound amplification in a healthy cochlea.40 The rapture of basilar membrane therefore typically results in drastic damage to the residual hearing and for this reason the perimodiolar electrode arrays should be avoided in the CI hearing preservation surgeries. On the other hand, strain to the lateral wall and basilar membrane by a laterally positioned electrode array has not been reported to cause any noticeable damage to cochlear structures.
Fig. 5.
Reconstructed CT images showing a CI electrode array either completely within the scala tympani (A–B) or partially translocated to scala vestibuli (C–D). ST is shown in red and SV in blue. From O'Connell et al.38
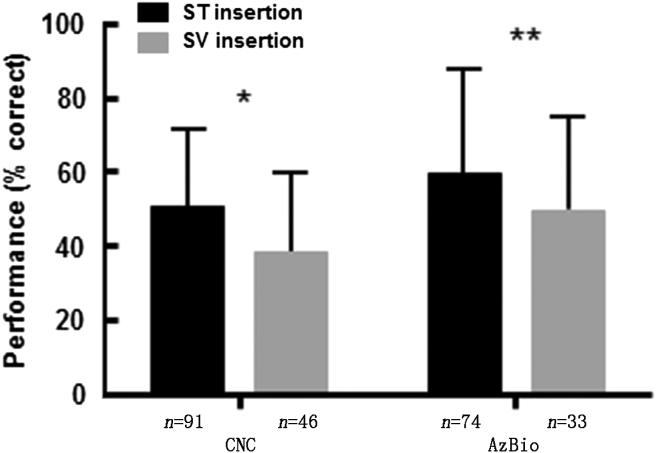
Also importantly, a traumatic cochlear surgery has a direct impact on CI performance, not only on the residual acoustic hearing. As shown in Fig. 6, better performance on CNC and AzBio testing was found for straightforward scala tympani (ST) insertions when compared to cases where the array deviated from ST to scala vestibuli (SV) as determined at 12–16 months postoperatively.41 Such comparison indicates that protection of cochlear cellular structures during the electrode array insertion reduces the associated trauma maintaining the auditory nerve fibres in better healthy state and more excitable by electrical stimulation with CI.
Fig. 6.
Impact of electrode location on speech perception. Better performance on CNC and AzBio audiologic testing for scala tympani (ST) insertions when compared with transscalar deviations to scala vestibuli (SV) as measured 12–16 months postoperatively. *P = 0.005; **P = 0.04. From O'Connell et al.41 Promotional and commercial use of the material in print, digital or mobile device format is prohibited without the permission from the publisher Wolters Kluwer. Please contact healthpermissions@wolterskluwer.com for further information.
Furthermore, perimodiolar electrode arrays have been also found to undergo more frequently tip fold-over. It was reported in 1.4%–6.5% of cases implanted with perimodiolar arrays in contrast with only 0.005% cases for lateral wall electrode arrays. Such partial array misposition again contributes to higher insertion trauma, smaller cochlear coverage and fewer channels available for electrical stimulation, making the stimulation less frequency specific.
Perimodiolar electrode arrays are not optimal neither for revision surgeries, when more force may be required to extract a CI electrode array hugged around the modiolus. This could be again accompanied by significant trauma to delicate cochlear cellular structures, which must be maximally protected especially in case of young children, who can be expected to undergo revision surgery several times during their life time and might in future be candidates for novel hearing therapies based on regeneration of such cellular structures.
Conclusions
Both perimodiolar and lateral wall electrode arrays seem to have similar battery consumption despite lower psychophysical thresholds for the perimodiolar type. On the other hand, dynamic range is wider for the laterally placed array type, which is also less traumatic and more suitable for patients with significant levels of residual hearing. Regarding patients using tonal languages such as Mandarin, longer lateral wall electrode arrays allowing effective stimulation towards the cochlear apex seem preferred for optimal speech recognition.
Conflict of interest
Authors are employees of MED-EL company.
Acknowledgement
We would like to thank Konrad Schwarz for a very useful discussion during the preparation of manuscript.
Edited by Jing Li
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Jeong J., Kim M., Heo J.H. Intraindividual comparison of psychophysical parameters between perimodiolar and lateral-type electrode arrays in patients with bilateral cochlear implants. Otol Neurotol. 2015;36:228–234. doi: 10.1097/MAO.0000000000000672. [DOI] [PubMed] [Google Scholar]
- 2.Qi B., Liu B., Krenmayr A. The contribution of apical stimulation to Mandarin speech perception in users of the MED-EL COMBI 40+ cochlear implant. Acta Otolaryngol. 2011;131:52–58. doi: 10.3109/00016489.2010.506652. [DOI] [PubMed] [Google Scholar]
- 3.Lin Y.S., Peng S.C. Effects of frequency allocation on lexical tone identification by Mandarin-speaking children with a cochlear implant. Acta Otolaryngol. 2009;129:289–296. doi: 10.1080/00016480701596047. [DOI] [PubMed] [Google Scholar]
- 4.Lee F.P., Hsu H.T., Lin Y.S., Hung S.C. Effects of the electrode location on tonal discrimination and speech perception of Mandarin-speaking patients with a cochlear implant. Laryngoscope. 2012;122:1366–1378. doi: 10.1002/lary.23313. [DOI] [PubMed] [Google Scholar]
- 5.Rask-Andersen H., Liu W., Erixon E. Human cochlea: anatomical characteristics and their relevance for cochlear implantation. Anat Rec (Hoboken) 2012;295:1791–1811. doi: 10.1002/ar.22599. [DOI] [PubMed] [Google Scholar]
- 6.Greenwood D.D. A cochlear frequency-position function for several species–29 years later. J Acoust Soc Am. 1990;87:2592–2605. doi: 10.1121/1.399052. [DOI] [PubMed] [Google Scholar]
- 7.Stakhovskaya O., Sridhar D., Bonham B.H., Leake P.A. Frequency map for the human cochlear spiral ganglion: implications for cochlear implants. J Assoc Res Otolaryngol. 2007;8:220–233. doi: 10.1007/s10162-007-0076-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rattay F., Potrusil T., Wenger C., Wise A.K., Glueckert R., Schrott-Fischer A. Impact of morphometry, myelinization and synaptic current strength on spike conduction in human and cat spiral ganglion neurons. PLoS One. 2013;8 doi: 10.1371/journal.pone.0079256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brill S., Müller J., Hagen R. Site of cochlear stimulation and its effect on electrically evoked compound action potentials using the MED-EL standard electrode array. Biomed Eng Online. 2009;8:40. doi: 10.1186/1475-925X-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schatzer R., Vermeire K., Visser D. Electric-acoustic pitch comparisons in single-sided-deaf cochlear implant users: frequency-place functions and rate pitch. Hear Res. 2014;309:26–35. doi: 10.1016/j.heares.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Tykocinski M., Saunders E., Cohen L.T. The contour electrode array: safety study and initial patient trials of a new perimodiolar design. Otol Neurotol. 2001;22:33–41. doi: 10.1097/00129492-200101000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Mittmann P., Todt I., Wesarg T., Arndt S., Ernst A., Hassepass F. Electrophysiological detection of intracochlear scalar changing perimodiolar cochlear implant electrodes: a blinded study. Otol Neurotol. 2015;36:1166–1171. doi: 10.1097/MAO.0000000000000766. [DOI] [PubMed] [Google Scholar]
- 13.Mittmann P., Rademacher G., Mutze S., Hassepass F., Ernst A., Todt I. Evaluation of the relationship between the NRT-ratio, cochlear anatomy, and insertions depth of perimodiolar cochlear implant electrodes. Biomed Res Int. 2015;2015:706253. doi: 10.1155/2015/706253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyer E., Karkas A., Attye A., Lefournier V., Escude B., Schmerber S. Scalar localization by cone-beam computed tomography of cochlear implant carriers: a comparative study between straight and periomodiolar precurved electrode arrays. Otol Neurotol. 2015;36:422–429. doi: 10.1097/MAO.0000000000000705. [DOI] [PubMed] [Google Scholar]
- 15.Venail F., Mathiolon C., de Champfleur S.M. Effects of electrode array length on frequency-place mismatch and speech perception with cochlear implants. Audiol Neurootol. 2015;20:102–111. doi: 10.1159/000369333. [DOI] [PubMed] [Google Scholar]
- 16.Baskent D., Shannon R.V. Speech recognition under conditions of frequency-place compression and expansion. J Acoust Soc Am. 2003;113:2064–2076. doi: 10.1121/1.1558357. [DOI] [PubMed] [Google Scholar]
- 17.Fu Q.J., Shannon R.V. Recognition of spectrally degraded and frequency-shifted vowels in acoustic and electric hearing. J Acoust Soc Am. 1999;105:1889–1900. doi: 10.1121/1.426725. [DOI] [PubMed] [Google Scholar]
- 18.Büchner A., Illg A., Majdani O., Lenarz T. Investigation of the effect of cochlear implant electrode length on speech comprehension in quiet and noise compared with the results with users of electro-acoustic-stimulation, a retrospective analysis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0174900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Weert S., Stokroos R.J., Rikers M.M., van Dijk P. Effect of peri-modiolar cochlear implant positioning on auditory nerve responses: a neural response telemetry study. Acta Otolaryngol. 2005;125:725–731. doi: 10.1080/00016480510028492. [DOI] [PubMed] [Google Scholar]
- 20.Xi X., Ji F., Han D., Hong M., Chen A. Electrode interaction in cochlear implant recipients: comparison of straight and contour electrode arrays. ORL J Otorhinolaryngol Relat Spec. 2009;71:228–237. doi: 10.1159/000229303. [DOI] [PubMed] [Google Scholar]
- 21.Venail F., Mura T., Akkari M. Modeling of auditory neuron response thresholds with cochlear implants. Biomed Res Int. 2015;2015:394687. doi: 10.1155/2015/394687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis T.J., Zhang D., Gifford R.H. For adults with cochlear implants. Otol Neurotol. 2016;37:31–37. doi: 10.1097/MAO.0000000000000896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nadol J.B., Young Y.S., Glynn R.J. Survival of spiral ganglion cells in profound sensorineural hearing loss: implications for cochlear implantation. Ann Otol Rhinol Laryngol. 1989;98:411–416. doi: 10.1177/000348948909800602. [DOI] [PubMed] [Google Scholar]
- 24.Linthicum F.H., Fayad J.N. Spiral ganglion cell loss is unrelated to segmental cochlear sensory system degeneration in humans. Otol Neurotol. 2009;30:418–422. doi: 10.1097/mao.0b013e31819a8827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu W., Edin F., Atturo F. The pre- and post-somatic segments of the human type I spiral ganglion neurons–structural and functional considerations related to cochlear implantation. Neuroscience. 2015;284:470–482. doi: 10.1016/j.neuroscience.2014.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frijns J.H., Kalkman R.K., Vanpoucke F.J., Bongers J.S., Briaire J.J. Simultaneous and non-simultaneous dual electrode stimulation in cochlear implants: evidence for two neural response modalities. Acta Otolaryngol. 2009;129:433–439. doi: 10.1080/00016480802610218. [DOI] [PubMed] [Google Scholar]
- 27.Doshi J., Johnson P., Mawman D. Straight versus modiolar hugging electrodes: does one perform better than the other? Otol Neurotol. 2015;36:223–227. doi: 10.1097/MAO.0000000000000603. [DOI] [PubMed] [Google Scholar]
- 28.Jayawardena J., Kuthubutheen J., Rajan G. Hearing preservation and hearing improvement after reimplantation of pediatric and adult patients with partial deafness: a retrospective case series review. Otol Neurotol. 2012;33:740–744. doi: 10.1097/MAO.0b013e318255dd91. [DOI] [PubMed] [Google Scholar]
- 29.Richard C., Fayad J.N., Doherty J., Linthicum F.H. Round window versus cochleostomy technique in cochlear implantation: histologic findings. Otol Neurotol. 2012;33:1181–1187. doi: 10.1097/MAO.0b013e318263d56d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishiyama A., Doherty J., Ishiyama G., Quesnel A.M., Lopez I., Linthicum F.H. Post hybrid cochlear implant hearing loss and endolymphatic hydrops. Otol Neurotol. 2016;37:1516–1521. doi: 10.1097/MAO.0000000000001199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishai R., Herrmann B.S., Nadol J.B., Quesnel A.M. The pattern and degree of capsular fibrous sheaths surrounding cochlear electrode arrays. Hear Res. 2017;348:44–53. doi: 10.1016/j.heares.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shepherd R.K., Matsushima J., Martin R.L., Clark G.M. Cochlear pathology following chronic electrical stimulation of the auditory nerve: II. Deafened kittens. Hear Res. 1994;81:150–166. doi: 10.1016/0378-5955(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 33.Clark G.M., Shute S.A., Shepherd R.K., Carter T.D. Cochlear implantation: osteoneogenesis, electrode-tissue impedance, and residual hearing. Ann Otol Rhinol Laryngol Suppl. 1995;166:40–42. [PubMed] [Google Scholar]
- 34.Rask-Andersen H., Schrott-Fischer A., Pfaller K., Glueckert R. Perilymph/modiolar communication routes in the human cochlea. Ear Hear. 2006;27:457–465. doi: 10.1097/01.aud.0000233864.32183.81. [DOI] [PubMed] [Google Scholar]
- 35.Briggs R.J., Tykocinski M., Saunders E. Surgical implications of perimodiolar cochlear implant electrode design: avoiding intracochlear damage and scala vestibuli insertion. Cochlear Implants Int. 2001;2:135–149. doi: 10.1179/cim.2001.2.2.135. [DOI] [PubMed] [Google Scholar]
- 36.Coordes A., Ernst A., Brademann G., Todt I. Round window membrane insertion with perimodiolar cochlear implant electrodes. Otol Neurotol. 2013;34:1027–1032. doi: 10.1097/MAO.0b013e318280da2a. [DOI] [PubMed] [Google Scholar]
- 37.Wanna G.B., Noble J.H., Carlson M.L. Impact of electrode design and surgical approach on scalar location and cochlear implant outcomes. Laryngoscope. 2014;124(Suppl 6):S1–S7. doi: 10.1002/lary.24728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Connell B.P., Hunter J.B., Wanna G.B. The importance of electrode location in cochlear implantation. Laryngoscope Investig Otolaryngol. 2016;1:169–174. doi: 10.1002/lio2.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rask-Andersen H., Liu W., Boström M.A. Immunolocalization of prestin in the human cochlea. Audiol Med. 2010;8:56–62. [Google Scholar]
- 40.Liberman M.C., Zuo J., Guinan J.J. Otoacoustic emissions without somatic motility: can stereocilia mechanics drive the mammalian cochlea. J Acoust Soc Am. 2004;116:1649–1655. doi: 10.1121/1.1775275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Connell B.P., Cakir A., Hunter J.B. Electrode location and angular insertion depth are predictors of audiologic outcomes in cochlear implantation. Otol Neurotol. 2016;37:1016–1023. doi: 10.1097/MAO.0000000000001125. [DOI] [PMC free article] [PubMed] [Google Scholar]