Abstract
Objectives
To provide safety and efficacy data on infants implanted below 12 months of age.
Methods
With the wide application of newborn hearing screening programs, infants with deafness are being identified at birth. When a hearing aid trial fails, cochlear implantation is the only option to restore hearing. Mounting evidence suggests that age at implantation is a strong predictor of language outcomes. Using the minimally invasive surgical technique we have employed for nearly two decades, a limited clinical trial was initiated in the year 2000 because this age limitation fell outside of FDA guidelines. The infants were initially assessed using the preferential listening paradigm to confirm that they could learn associations between speech sounds and objects. Sufficient time was allowed to pass to administer more traditional language measures.
Results
No surgical or anesthetic complications occurred in this group of infants. The pattern of listening skill development mirrored that seen in normal hearing infants. Long-term language assessments using the Peabody Picture Vocabulary Test (PPVT) and other measures have demonstrated that many of infants achieved age appropriate language skills.
Conclusion
Cochlear implantation in children less than 12 months of age is safe and efficacious as demonstrated by long-term PPVT language data.
Keywords: Infants, Cochlear implantation, Treatment outcome
Introduction
In the early 1990's the first universal hearing screening programs were initiated. Significant progress has been made in the protocols for hearing screening, audiologic evaluation, fitting of amplification and the medical management of deaf and hard of hearing infants.1 It has been recommended that all babies be screened for hearing loss by 1 month of age, appropriate audiologic evaluation be completed by 3 months of age and appropriate intervention initiated by 6 months of age. The Centers for Disease Control and Prevention (CDC) reported that over 96.9% of all newborns were screened in 2008.2 However, early identification of hearing loss is only effective if appropriate intervention is available. When a hearing aid trial fails, cochlear implantation is the only remaining option.
This study describes the surgical technique used throughout our study and reports long-term longitudinal language scores using the Peabody Picture Vocabulary Test (PPVT).
Methods
Participants
Seventeen deaf infants (4 females and 13 males) with congenital profound hearing loss participated in this study. All received a cochlear implant before the age of 12 months. The subjects were developmentally normal with no additional disabilities (visual, motor, or cognitive) although one was later diagnosed with Usher's syndrome and one was found to have mutations in the Connexin 26 gene. All were native speakers of English. Four were bilaterally implanted (one sequential and three simultaneous). This prospective study began in the year 2000 and includes all patients below the age of 12 months of age who were implanted by the senior author (RTM). Subject accrual was very slow because few patients were successful in obtaining insurance coverage for the implant and postoperative rehabilitation because the FDA age of approval was 1 year.
Preimplant measures
All subjects failed their neonatal hearing screen and audiological assessment. Preimplant assessments included otomicroscopy, tympanometry, acoustic reflex and auditory brainstem (ABR) testing. All subjects were given a hearing aid trial.
All subjects were evaluated radiographically with computed tomography (CT). The degree of mastoid pneumatization, the presence or absence of middle ear or mastoid disease, facial nerve course, and cochlear patency were noted. If the imaging study excluded a Michel deformity or severe malformation of the cochlea and the infant failed the hearing aid trial, the profoundly deaf infant was deemed a suitable candidate for a cochlear implant.
Surgical technique
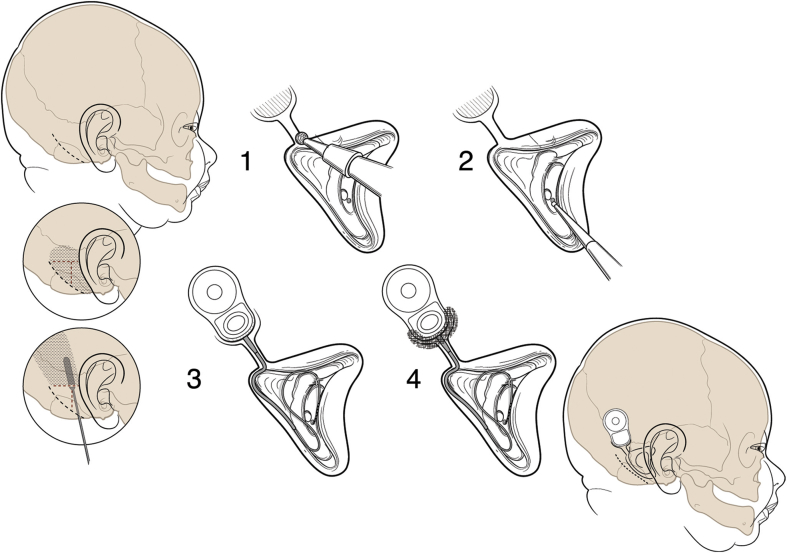
The same minimally invasive cochlear implant surgical technique which we have used for nearly two decades was used in all subjects.3, 4 The technique is illustrated in Fig. 1. Meticulous attention to the delicate tissues and small dimensions must be taken. In infants, the facial nerve is more superficial in the region of the mastoid tip and may lie just deep to the skin. A small 4–5 cm retro-auricular incision which does not cross either the implant package or the electrode is made. Careful attention to hemostasis is taken. Skin vessels and emissary veins are coagulated. A pericranial pocket is developed between the bone and periosteum leaving the pericranium lateral to the device intact. The pocket is designed to be slightly larger that the device to be inserted. Only adequate exposure to perform the mastoidectomy is required. During the mastoidectomy, oozing from exposed bone marrow is controlled using a diamond burr and bone wax. The device is recessed but only partially at its lower margin because of the thin cranial bone. The approach through the facial recess and the cochleostomy are performed in the usual manner. A redundant loop of the electrode is left coiled in the mastoid defect to allow for later skull growth. At the completion of the procedure, the cortical bone at the lower margin of the implant device is built up with the bone pate which had been previously collected during the mastoidectomy and the creation of the shallow well. A deeper well is thus created. Intraoperative facial nerve electromyographic monitoring and intraoperative plain film radiographics are used to confirm electrode placement.
Figure 1.
Cochlear implant procedure used in the infant study (Figure included in reference 22, copyright to Indiana University).
The subjects in this series were fitted with either a Nucleus or MedEl cochlear implant (Cochlear Nucleus Series, Cochlear Ltd, Sydney Australia; or Med-El, Corp., Innsbruch, Austria).
Language assessments
All language assessments were administered and scored by certified speech-language pathologists (B.C. and S.H.).
Peabody Picture Vocabulary Test (PPVT)
The Peabody Picture Vocabulary Test (PPVT) is an untimed measure of single word receptive vocabulary for Standard American English. It provides a quick estimate of verbal ability and scholastic aptitude. The test is appropriate for individuals age 2.6–90+ years. The test is given verbally and no reading is required by the individual. A series of four pictures to a page are presented by the examiner. The examiner states a word describing one of the pictures and the examiner asks the individual to point to the picture the word describes. The total score can be converted to a percentile rank, mental age, or a standard score. An age appropriate version of the (PPVT) was sequentially given through the longitudinal follow-up period.
Since the PPVT was first released, a number of versions have been developed. Scores obtained on the PPVT-III,5 and PPVT-IV6 are highly correlated with one another. Thus, data from both versions were used. The children's most recent post-implant PPVT scores were analyzed. Standard scores were used because they are corrected for chronological age and provide information about performance relative to the average score of normal hearing children (M = 100, SD = 15).
Results
Safety
Using this surgical technique in 21 surgeries in 17 deaf infants implanted between 6 months and 12 months of age, no anesthetic or surgical complications were encountered. There were no pulmonary complications and no instances of flap breakdown overlying the implant package. There were no facial nerve injuries.
A redundant loop of electrode was left in the mastoid at the time of surgery to allow for later skull growth. To date no growth related problems have been encountered.
Receptive vocabulary (PPVT)
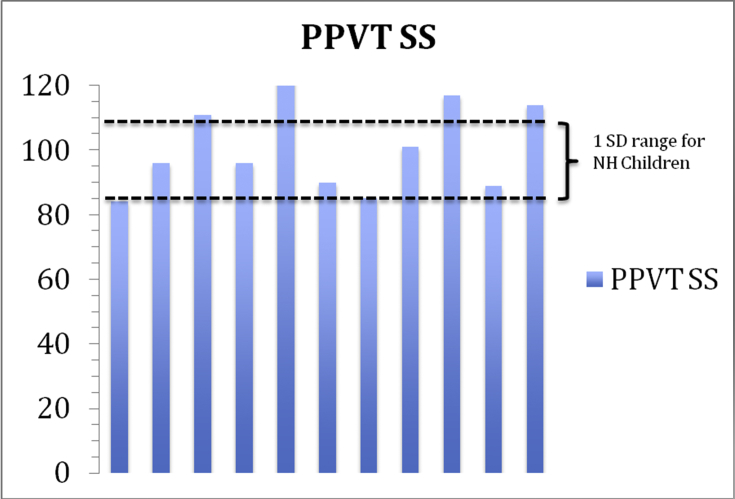
After the infants reached an age where the Peabody Picture Vocabulary Test (PPVT) could be reliably administered (usually greater than 2 years of age), the test was given longitudinally. Raw scores and standard scores were sequentially recorded over these multiple test intervals. Data points were added biennially in many and annually in others. A gradual growth pattern was recorded in many but nearly all subjects reached the lower limits of the normal range and some attained the upper limits of normal at their most recent test intervals. Fig. 2 illustrates the standard PPVT scores of eleven subjects all of whom were implanted before the age of 1 year and who attained a minimal chronological of at least six years of age at the time of the most recent test (range 6–12 years). The box outlines the range of normal standard scores on the Peabody Picture Vocabulary Test for normally hearing children (1 standard deviation from a mean of 100 with a range of 85–115 on the standard scores).
Figure 2.
Peabody Picture Vocabulary Test – Standard scores (PPVT SS).
Six subjects did not participate in this long-term longitudinal receptive language analysis either because they were not available (N = 1) or the post-surgical interval was short (N = 5). However, two of the subjects had already achieved scores in the normal range as early as age 3 (standard scores of 100 and 108). Three subjects fell outside of the normal standard score range (83, 79 and 68) but their follow-up interval was short (less than 4 years).
Discussion
Development of the auditory system begins well before birth. Auditory sensory abilities are observed in the fetus from about 26 to 28 weeks gestational age.7 At birth, the auditory system of the human neonate is fully functional and capable of establishing neural connections based upon auditory input. The ability to hear sounds of the ambient language environment plays an important role in shaping an infant's speech perception throughout the first year of life. It is postulated that the development of language concepts throughout the remainder of the infant's development will be influenced by enhanced auditory input.8
With this as a background, age at implantation would be a strong predictor of language outcomes in profoundly deaf infants who use a cochlear implant.
Confounding variables include the question whether the signal provided by current cochlear implants is rich enough for a congenitally deaf infant to develop a functional oral language system. Second, a surgical procedure is required to safely place the device.
This study was designed to address these two questions. Surgery in an infant carries more risk than in older children or adults. An anesthetic is required and a key member of the surgical team is the pediatric anesthesiologist. Pulmonary concerns are particularly prominent and must be carefully managed. Using the technique we describe in 21 surgeries in 17 infants between 6 months and 12 months of age, no complications were encountered. There were no instances of flap breakdown, facial paralysis, anesthetic problems, or pulmonary complications. There were no instances of growth related electrode problems.
Several lines of research have progressively highlighted the importance of early intervention with a cochlear implant on language outcomes. For example, Kirk et al9 found that children implanted before 2 years of age showed faster gains in vocabulary and receptive language than children implanted between 2 and 4 years of age and children implanted after 5 years of age. Nicholas and Geers10 took language samples from 3.5 and 4.5 year-old children implanted between 12 and 38 months of age and found that earlier implanted children showed a greater number of morphemes, a greater number of utterances, and a greater number of root words than later implanted children. Dettman et al11 found greater rates of expressive and receptive language growth in children implanted under 12 months of age than in children implanted between 12 and 24 months of age.
Children implanted before 3 years of age show better speech perception than children implanted at later ages.12 Similar findings were not found when comparisons were made among children implanted under 3 years of age.13, 14, 15
The assessment of speech and language skills in infants required the development of new behavioral methodologies. The clinical tests available in the year 2000 required the children to follow verbal instructions. The infants had not yet acquired those skills. To address this need, modified versions of the visual habituation (VH) procedure and the preferential looking paradigm (PLP) were developed and applied.16, 17 The infant's looking times were paired with an audible signal heard through a cochlear implant. These tests demonstrated that soon after cochlear implantation, young deaf infants were able to discriminate continuous versus discontinuous speech sounds. The infants were also able learn associations between speech sounds and objects. Given this information, confidence was gained that the infants would eventually demonstrate progress in more traditional measures such as the PPVT. This study has demonstrated that nearly all infants implanted before the age of 12 months achieved language scores in the normal range. The high degree of variability usually seen in cochlear implanted children is not seen.
The longitudinal observations made in this group of implanted infants lends credence to the notion that much of what in accomplished by deaf infants is attained vicariously. As noted by Yoshinaga-Itano,18 the developing nervous system is significantly shaped by early language exposure through social interactions. Only early access to language can provide a profoundly deaf infant the opportunity to develop within the normal range.
The Food and Drug Administration (FDA) approves cochlear implantation in children 12 months old and older. However, significant auditory and language acquisition typically occurs prior to this time in normal hearing infants. If the presence of profound deafness can be demonstrated in infants using available objective tests and the infant is not progressing in his or her speech and hearing development with appropriate amplification, there seems to be no reason to delay intervention if cochlear implantation can be safely performed. Allowing normal brain plasticity to augment the input through a cochlear implant at a younger age will obviate some the need to close a language gap. Several centers including ours provide cochlear implants at earlier ages “off label” when this is the case. Evidence supporting the safety of implanting infants younger than 12 months is mounting.19, 20, 21, 22, 23 With the development of smaller, thinner devices and improved technology, this argument becomes even more compelling.
Conclusions
Very early access to sound through a cochlear implant maximizes the listening and language acquisition skills of deaf infants below the age of 12 months.
The surgical procedure we have used is presented and no surgical complications were encountered. Long-term results (>6 years) on the PPVT have demonstrated a clear advantage to early implantation.
Disclosures
This entire study was performed off-label.
Supported by NIH-NIDCD.
Acknowledgements
This research was supported by research grants from the National Institute on Deafness and Other Communication Disorders (NIDCD) to Richard Miyamoto (NIDCD RO1 DC00064, RO1 DC00423), and NIDCD Training Grant (T32 DC00012) to David Pisoni.
Edited by Yu-Xin Fang
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Joint Committee on Infant Hearing of the American Academy of Pediatrics, Muse C., Harrison J. Supplement to the JCIH 2007 position statement: principles and guidelines for early intervention after confirmation that a child is deaf or hard of hearing. Pediatrics. 2013;131:e1324–e1349. doi: 10.1542/peds.2013-0008. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Summary of 2008 national CDC EHDA data. https://www.cdc.gov/ncbddd/hearingloss/2008-data/2008_ehdi_hsfs_summary.pdf.
- 3.Miyamoto R.T. In: Essential Otolaryngology. 10th ed. Lee K.J., editor. 2012. pp. 154–161. New York. [Google Scholar]
- 4.Miyamoto R.T., Miyamoto R.C., Iler-Kirk K. Cochlear implants in children. In: Bluestone C., Stoole S., editors. Pediatric Otolaryngology. 5th ed. 2014. pp. 547–560. [Google Scholar]
- 5.Dunn, L.M.; Dunn D.M., Peabody Picture Vocabulary Test-third Edition (PPVT-III). American Guidance Service: Circle Pines, MN.
- 6.Dunn L.M., Dunn D.M. Pearson Education; Minneapolis, MN: 2007. Peabody Picture Vocabulary Test-4th Edition (PPVT-IV) [Google Scholar]
- 7.Lecanuet J.P., Graniere-Deferre C., Jacquet A.Y., DeCasper A.J. Fetal discrimination of low-pitched musical notes. Dev Psychobiol. 2000;36:29–39. [PubMed] [Google Scholar]
- 8.Houston D.M., Stewart J., Moberly A., Hollich G., Miyamoto R.T. Word learning in deaf children with cochlear implants: effects of early auditory experience. Dev Sci. 2012;15:448–461. doi: 10.1111/j.1467-7687.2012.01140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirk K.I., Miyamoto R.T., Ying E.A., Perdew A.E., Zuganelis H. Cochlear implantation in young children: effects of age at implantation and communication mode. Volta Rev. 2002;102:127–144. [Google Scholar]
- 10.Nicholas J.G., Geers A.E. Will they catch up? The role of age at cochlear implantation in the spoken language development of children with severe to profound hearing loss. J Speech Lang Hear Res. 2007;50:1048–1062. doi: 10.1044/1092-4388(2007/073). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dettman S.J., Pinder D., Briggs R.J., Dowell R.C., Leigh J.R. Communication development in children who receive the cochlear implant younger than 12 months: risks versus benefits. Ear Hear. 2007;28:11S–18S. doi: 10.1097/AUD.0b013e31803153f8. [DOI] [PubMed] [Google Scholar]
- 12.Zwolan T.A., Ashbaugh C.M., Alarfaj A. Pediatric cochlear implant patient performance as a function of age at implantation. Otol Neurotol. 2004;25:112–120. doi: 10.1097/00129492-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Horn D.L., Houston D.M., Miyamoto R.T. Speech discrimination skills in deaf infants before and after cochlear implantation. Audiol Med. 2007;5:232–241. [Google Scholar]
- 14.McConkey Robbins A., Koch D.B., Osberger M.J., Zimmerman-Phillips S., Kishon-Rabin L. Effect of age at cochlear implantation on auditory skill development in infants and toddlers. Arch Otolaryngol Head Neck Surg. 2004;130:570–574. doi: 10.1001/archotol.130.5.570. [DOI] [PubMed] [Google Scholar]
- 15.Tajudeen B.A., Waltzman S.B., Jethanamest D., Svirsky M.A. Speech perception in congenitally deaf children receiving cochlear implants in the first year of life. Otol Neurotol. 2010;31:1254–1260. doi: 10.1097/MAO.0b013e3181f2f475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyamoto R.T., Houston D.M., Bergeson T. Cochlear implantation in deaf infants. Laryngoscope. 2005;115:1376–1380. doi: 10.1097/01.mlg.0000172039.26650.9b. [DOI] [PubMed] [Google Scholar]
- 17.Houston D.M., Ying E.A., Pisoni D.B., Kirk K.I. Development of pre-word-learning skills in infants with cochlear implants. Volta Rev. 2001;103:303–326. [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshinaga-Itano C. From screening to early identification and intervention: discovering predictors to successful outcomes for children with significant hearing loss. J Deaf Stud Deaf Educ. 2003;8:11–30. doi: 10.1093/deafed/8.1.11. [DOI] [PubMed] [Google Scholar]
- 19.Lesinski-Schiedat A., Illg A., Heermann R., Bertram B., Lenarz T. Paediatric cochlear implantation in the first and in the second year of life: a comparative study. Cochlear Implants Int. 2004;5:146–159. doi: 10.1179/cim.2004.5.4.146. [DOI] [PubMed] [Google Scholar]
- 20.James A.L., Papsin B.C. Cochlear implant surgery at 12 months of age or younger. Laryngoscope. 2004;114:2191–2195. doi: 10.1097/01.mlg.0000149456.75758.4c. [DOI] [PubMed] [Google Scholar]
- 21.Colletti V., Carner M., Miorelli V., Guida M., Colletti L., Fiorino F.G. Cochlear implantation at under 12 months: report on 10 patients. Laryngoscope. 2005;115:445–449. doi: 10.1097/01.mlg.0000157838.61497.e7. [DOI] [PubMed] [Google Scholar]
- 22.Valencia D.M., Rimell F.L., Friedman B.J., Oblander M.R., Helmbrecht J. Cochlear implantation in infants less than 12 months of age. Int J Pediatr Otorhinolaryngol. 2008;72:767–773. doi: 10.1016/j.ijporl.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Roland J.T., Jr., Cosetti M., Wang K.H., Immerman S., Waltzman S.B. Cochlear implantation in the very young child: long-term safety and efficacy. Laryngoscope. 2009;119:2205–2210. doi: 10.1002/lary.20489. [DOI] [PubMed] [Google Scholar]