Abstract
Patients with cystic fibrosis (CF) experience chronic or recurrent bacterial and fungal lung infections. Many patients with CF cannot effectively clear Aspergillus from their lungs. This may result in IgE sensitization and the development of allergic bronchopulmonary aspergillosis, or invasive infections, such as Aspergillus bronchitis. Lung disease in patients with CF is associated with neutrophil-dominated inflammation and elevated levels of the serine protease, neutrophil elastase (NE). Various C-type lectin-like receptors (CLRs), including Dectin-1 and Dectin-2, are involved in the immune response to Aspergillus. Here, we show that purified NE cleaves Dectin-1 in an isoform-specific manner. Bronchoalveolar lavage fluid from patients with CF, which contains high NE activity, induces Dectin-1 cleavage. Similarly, filtrate from a protease-producing strain of Aspergillus fumigatus induces isoform-specific cleavage of Dectin-1. Dectin-1 knockout (KO) cells and NE-treated cells demonstrated reduced phagocytosis of zymosan, a fungal cell wall preparation. In addition, NE cleaves 2 other CLRs, Dectin-2 and Mincle, and fungal-induced cytokine production was reduced in Dectin-1 KO cells, Dectin-2 KO cells, and NE-treated cells. Thus, Dectin-1 and Dectin-2 cleavage by NE and/or A. fumigatus–derived proteases results in an aberrant antifungal immune response that likely contributes to disease pathology in patients with CF.—Griffiths, J. S., Thompson, A., Stott, M., Benny, A., Lewis, N. A., Taylor, P. R., Forton, J., Herrick, S., Orr, S. J., McGreal, E. P. Differential susceptibility of Dectin-1 isoforms to functional inactivation by neutrophil and fungal proteases.
Keywords: elastase, C-type lectin-like receptor, cystic fibrosis, Aspergillus
Aspergillus fumigatus has long been recognized as a serious invasive pathogen in immunocompromised patients and carries a high mortality rate. More recently, it has increasingly been acknowledged as an important pathogen in patients with preexisting airway disease who are otherwise immunocompetent. Common diseases, including asthma, chronic obstructive pulmonary disease, and cystic fibrosis (CF), are frequently complicated by this fungus (1). The spectrum of disease caused by A. fumigatus in these patients includes invasive infection and allergic hypersensitivity, both of which cause progressive lung disease. Worldwide, the number of patients with underlying airway disease who are affected by this fungus exceeds 10 million (1). To elicit disease, the fungus must first access and persist in the airway. The normally functioning host is well adapted to prevent such persistence; however, A. fumigatus is isolated in 10–57% of airway secretions from patients with CF (2). A. fumigatus–specific IgG has also been detected in 41% of patients with CF by age 4 yr, increasing to 98% by age 10 yr. These seropositivity rates were significantly higher than either control or asthmatic patients (3). Allergic bronchopulmonary aspergillosis (ABPA), a serious allergic entity that is characterized by airway obstruction and irreversible bronchiectasis, affects 17.7% of the adult CF population, whereas Aspergillus bronchitis is present in 30% of patients (4). A central question, therefore, is why patients with underlying airway disease are susceptible to A. fumigatus.
In addition to the critical action of the mucocilliary escalator in clearing pathogens and other foreign particles from the airway, the immune system takes a multifaceted approach to the recognition and elimination of pathogenic fungi. TLRs and C-type lectin-like receptors (CLRs) expressed by myeloid cells are essential components of these responses (5). Macrophages from mice that lack functional TLR2, TLR4, or both receptors displayed defective inflammatory responses to A. fumigatus (6). In addition, a study that examined single nucleotide polymorphisms (SNPs) in TLR2, TLR3, TLR4, and TLR9 identified an association between a TLR4 haplotype and an increased risk of developing invasive aspergillosis in a cohort of patients who underwent hematopoietic stem cell transplantation (7). Immunocompetent mice that are deficient in the CLR, Dectin-1, are exquisitely sensitive to A. fumigatus airway infection, with an 80% mortality rate (8). The β-glucan receptor, Dectin-1, binds specific morphologies of A. fumigatus, including swollen conidia, early germlings, and hyphae, but not resting conidia (9, 10). Dectin-1 is important for A. fumigatus–associated phagocytosis and cytokine production (8, 9), and a premature stop codon polymorphism in Dectin-1 doubles the risk of invasive fungal disease in immunocompromised patients (11). Another CLR, Dectin-2, recognizes mannose-like structures in fungal cell walls, including that of A. fumigatus, and mediates important inflammatory responses upon fungal challenge (12–15). Mincle also recognizes mannans in fungal cell walls, and whereas Mincle expression is up-regulated in response to A. fumigatus, A. fumigatus did not activate Mincle-expressing cells (16, 17). SNPs in Dectin-1 and an additional CLR, dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN), are associated with increased susceptibility to invasive aspergillosis. In addition, SNP–SNP interaction analysis demonstrated increased susceptibility in patients with SNPs in the genes that encode C-C motif chemokine ligand 2 (CCL2), Dectin-1, C-C motif chemokine receptor 2 (CCR2), and Dectin-2 (18).
Whereas defects in these receptors markedly increase susceptibility to fungal disease, little is known of their functional status in patients with underlying airway disease. Given the important complicating influence of fungal pathogens in CF, understanding the behavior of these receptors in the CF airway is of critical importance. Declining lung function in patients with CF is associated with a profound neutrophilia and the presence of high levels of free neutrophil-derived serine protease activity (19). Such proteases as neutrophil elastase (NE), cathepsin-G, and proteinase 3 have important antimicrobial functions, but are also capable of damaging host tissues and molecules of the immune system if not tightly regulated by the serpin family of antiproteases. A protease:antiprotease imbalance is widely recognized as an important pathologic mechanism in the CF airway (20). We and others have previously demonstrated that several arms of the immune system, including C5aR, IL-6, soluble IL-6R, surfactant protein-D, and TLRs (21–24), are subject to profound functional inactivation in the CF airway. Of note, Dectin-1 recognition of fungal ligands is trypsin sensitive (25), which raises the possibility that this critical fungal CLR may also be susceptible to inactivation by neutrophil-derived proteases in diseases such as CF. Here, we address this possibility and demonstrate that NE cleaves Dectin-1 and other CLRs, Dectin-2, and Mincle, which results in reduced antifungal responses.
MATERIALS AND METHODS
Mice
C57BL6/J, Dectin-1 knockout (KO) (26), Dectin-2 KO (27), Mincle KO (28), and BALB/c mice were maintained and handled according to institutional and UK Home Office guidelines. This study was performed in accordance with a project license approved by the Cardiff University Animal Welfare and Ethical Review Body and the UK Home Office. The animal care and use protocol adhered to the Animals (Scientific Procedures) Act 1986.
Ethics and sample collection
Bronchoalveolar lavage (BAL) was collected from patients with CF who attended the Children’s Hospital for Wales. BAL samples from 35 patients are included in this study, with a median age at the time of sampling of 10.5 yr (interquartile range, 7–13.5 yr) and a gender distribution of 20:15 (female:male). Ethical approval for the collection and use of BAL samples in this study was obtained from the Wales Research Ethics Committee as part of the CF-SpIT study (11/WA/0334). BAL samples were obtained by the instillation and retrieval of normal saline at the right middle lobe by flexible bronchoscopy under general anesthetic as part of routine clinical care. BAL sampling was performed in accordance with the European Respiratory Society Task Force on BAL in children (29). In brief, a flexible bronchoscope was used to instill 0.9% sterile saline at of volume of 1 ml/kg to a maximum volume of 20 ml and immediately suctioned. Samples were stored at 4°C after collection and were processed in the laboratory within 1 h of collection. Samples were centrifuged at 500 g for 5 min at 4°C, and cell-free BAL fluid (BALF) was portioned into aliquots and stored at −80°C. For cytospins, cell pellets were resuspended in 10 mM EDTA/PBS and counted by using a hemocytometer. After cellular resuspension, 5 × 104 cells were fixed to each polysine slide by using a cytofuge at 500 g for 5 min. For differential cell counts, cytospins were stained with hemacolor (EMD Millipore, Billerica, MA, USA) according to manufacturer’s instructions and fixed in DPX mounting medium. Cell differentiation was performed on at least 4 separate fields per cytospin at ×20 magnification, counting at least 300 events. Differential cell counts in recovered BAL from the 35 patients were as follows [median (interquartile range)]: total cell counts: 1.1 × 106 cells/ml (0.76–2.56); polymorphonuclear cells: 0.83 × 106 cells/ml (0.31–1.8); and mononuclear cells: 0.21 × 106 cells/ml (0.11–0.31).
Reagents
Elastase that was purified from primary human neutrophils was purchased from Athens Research and Technology (Athens, GA, USA). α-1 Antitrypsin (AAT) and PMSF were purchased from Sigma-Aldrich (St. Louis, MO, USA). Anti-hemagglutinin (HA) was purchased from Miltenyi Biotec (Bergisch Gladbach, Germany). Soluble recombinant human Dectin-1 was purchased from R&D Systems (Minneapolis, MN, USA). Anti–Dectin-1 (human and mouse) was purchased from Bio-Rad (Hercules, CA, USA). Anti-Ly6G, anti-CD11b, anti-F4/80, anti-CD19, and anti-TNF were purchased from BioLegend (San Diego, CA, USA). Anti-Mincle was purchased from Caltag MedBiosystems (Buckingham, United Kingdom). Anti–Dectin-2 mAb D2.11E4 has been previously described (30). Geneticin was purchased from Thermo Fisher Scientific (Waltham, MA, USA).
Cell isolation and culture
NIH3T3 cell lines that expressed human or mouse Dectin-1 HA were a gift from Prof. Gordon Brown (University of Aberdeen, Aberdeen, United Kingdom). Cells were maintained in DMEM that was supplemented with 10% fetal bovine serum, 2 mM l-glutamine, and penicillin/streptomycin. Geneticin (G418) was added at a concentration of 0.4–0.6 mg/ml for selection. For passaging, cells were incubated with trypsin for 4–5 min at 37°C and detached by tapping the flask; however, for experiments, cells were removed from flasks by incubating with PBS that was supplemented with 10 mM EDTA and 4 mg/ml lidocaine (Sigma-Aldrich) to avoid receptor cleavage by trypsin. Medium was added to cells in trypsin or lidocaine and cells were centrifuged. Cells were washed twice in PBS before use.
To culture bone marrow–derived macrophages (BMDMs) (31), bone marrow cells were flushed from femurs and tibiae of mice. Bone marrow cells were resuspended at 2 × 105 cells/ml in DMEM that was supplemented with 10% fetal bovine serum, 5% horse serum, 2 mM l-glutamine, penicillin/streptomycin, HEPES, and 10 ng/ml M-CSF. Cells were plated at a density of 7 × 106 cells per 145-mm2 plate. Cells were cultured for 6–7 d, and 15 ml of fresh medium and M-CSF was added on d 3. Medium was removed from BMDM on d 6–7 and PBS that was supplemented with 8 mg/ml lidocaine was incubated with BMDMs for 4–5 min at 37°C. Cells were detached upon tapping and medium was added to cells. BMDMs were centrifuged and washed twice before use.
To recruit inflammatory cells, mice were injected intraperitoneally with 0.5 ml 2% (w/v) Biogel P-100 polyacrylamide beads (Bio-Rad) (32). Mice were sacrificed 16–18 h later, and inflammatory cells were collected by peritoneal lavage with 5 ml ice-cold RPMI 1640 that was supplemented with 10% fetal bovine serum and penicillin/streptomycin. Cells were passed through 40-µm cell strainers (BD Biosciences, San Jose, CA, USA) to remove Biogel beads. Cells were washed twice with assay buffer, PBS that was supplemented with 0.1% bovine serum albumin (BSA) and 5 mM EDTA, before use. Using Biogel injection, 9.37 × 106 ± 0.80 × 106 cells/mouse were elicited, and the cell population consisted of 71.68 ± 2.59% Ly6G+CD11b+ neutrophils and 15.48 ± 3.57% Ly6G−CD11b+ inflammatory monocytes.
Aspergillus culture
A. fumigatus (CEA10 and Af293) strains were cultured on Sabouraud dextrose agar and incubated at 37°C for 48–72 h. Conidia were harvested with a PBS–Tween (0.05% Tween 20) wash. A flask that contained 500 ml Vogel’s minimal medium was inoculated with 1 × 106 conidia/ml and cultured for 48 h at 37°C and 320 rpm. A. fumigatus cultures were then prefiltered through J cloth and sterile filtered through a 0.2-µm filter. Resultant culture filtrate was dialyzed overnight against repeated changes of distilled water and freeze dried before being stored at −80°C. Freeze-dried aliquots were reconstituted with sterile PBS, and total protein content was determined by using a BCA protein assay (Thermo Fisher Scientific). Total protease activity of culture filtrate was determined by using Universal Protease Substrate (Casein, resorufin labeled; Roche, Basel, Switzerland) according to manufacturer’s instructions. Filtrates were resuspended at 2 mg/ml in PBS. For CEA10, this was equivalent to 19 U/ml protease activity as measured by using the casein protease assay.
Cleavage assays
For receptor cleavage experiments, cells were resuspended at 1–2 × 106 cells/ml in assay buffer (PBS, 5 mM EDTA, 0.1% BSA, 10 mM NaN3). Cells (50 μl; 5–10 × 104) were added to a 96-well plate and incubated with 50 µl of purified proteases in assay buffer or A. fumigatus filtrates at indicated concentrations for the indicated times at 37°C. For experiments that used CF BALF, cells were exposed to 50 µl of cell-free BALF for 30 min at 37°C. NE activity was neutralized either by using the indicated concentration of AAT or by heat inactivation (HI NE) at 95°C for 10 min. Cells were centrifuged, washed, and stained with anti-HA, anti–Dectin-1, anti–Dectin-2, or anti-Mincle. Biogel elicited cells were also stained with anti-Ly6G and anti-CD11b. Cells were then analyzed by flow cytometry on a Beckman Coulter CyAn, BD Canto, or FACScalibur flow cytometer (Beckman Coulter, Brea, CA, USA). Data were analyzed by using Flowing or FlowJo software (FlowJo, Ashland, OR, USA).
For experiments that examined the cleavage of soluble recombinant Dectin-1, 1 µg/ml of the recombinant protein in Tris-buffered saline (pH 7.4) was treated with the indicated concentration of NE or A. fumigatus filtrate for 60 min at 37°C. Reactions were stopped with the addition of 10 mM PMSF. Samples were subsequently prepared for SDS-PAGE under nonreducing conditions on a 15% Tris-glycine gel. Proteins were transferred to a nitrocellulose membrane, blocked with PBS-5% (w/v) milk, and probed with goat anti-human Dectin-1 (R&D Systems), followed by horseradish peroxidase conjugated donkey anti-goat IgG. Blots were visualized by using ECL radiography.
NE activity assay
BALF was removed from −80°C and thawed on ice. BALF was diluted to <1:2 using activity buffer (0.1 M Tris, 0.5 M NaCl, 0.05% v/v Triton X-100, pH 7.5), and additional dilutions were made for samples with high activity. For standards, purified human NE was diluted 2-fold serially from 80 to 1.25 nM. Activity buffer was used as a negative control. BALF or standard (50 μl) was added to a flat-bottomed 96-well plate in duplicate. NE-specific chromogenic substrate (50 μl/well, 2mM), Suc-Ala-Ala-Pro-Val-pNA in activity buffer, was added. Optical density at 405 nm was read every minute for 20 min on a Dynex Revelation MRX TC spectrophotometer (Dynex Technologies, Chantilly, VA, USA). For each sample, the average change in optical density over time was calculated. NE activity in BALF was interpolated from a 7-point standard curve.
Zymosan recognition assay
Zymosan (Thermo Fisher Scientific) was labeled with FITC (Sigma-Aldrich) as previously described (32). Labeled zymosan was washed 4 times with PBS and resuspended at 50 µg/ml in assay buffer ready for use. BMDMs or NIH3T3 cells that expressed human Dectin-1 isoform A or B were resuspended at 1 × 106 cells/ml in assay buffer. Cells (50 μl; 5 × 104) were added to a 96-well plate. Cells were centrifuged and supernatants were removed. Assay buffer, NE, or HI NE (50 μl) at the indicated concentrations were added for 1 h before the addition of 50 µl zymosan at 50 µg/ml for 15 min. Cells were centrifuged and washed. BMDMs were stained with anti-F4/80, anti-CD11b, and anti–Dectin-1. NIH3T3 cells that expressed human Dectin-1 isoform A or B were stained with anti–Dectin-1. Cells were analyzed by flow cytometry.
Intracellular cytokine flow cytometry assay
A. fumigatus isolate 13073 (American Type Culture Collection, Manassas, VA, USA) swollen conidia (SC) were obtained by culturing 1 × 108 A. fumigatus resting conidia in 1 ml RPMI 1640 and polymixin B (2 mg/ml) for 6 h at 37°C. SC were washed 3 times with PBS and labeled with 4.5 µM cell trace far red (Thermo Fisher Scientific) for 30 min at room temperature. SC were washed 3 times with PBS. Biogel elicited cells (100 μl; 4 × 105) in RPMI 1640 that was supplemented with 10% fetal bovine serum and penicillin/streptomycin were added to a 96-well ultra-low adherence plate and left to rest for 1 h at 37°C. Medium or A. fumigatus SC (100 μl; 4 × 105) was added for 3 h at 37°C in the presence of Brefeldin (BioLegend). Cells were stained with anti-Ly6G, anti-CD11b, anti-CD19, anti-TNF, and a live-dead stain (Thermo Fisher Scientific) and analyzed on a BD Canto flow cytometer. The following gating strategy was used to determine percent A. fumigatus+ inflammatory monocytes producing TNF: live cells, CD19−, Ly6G−CD11b+, A. fumigatus+.
Zymosan-induced ELISA cytokine assay
Biogel-elicited cells were resuspended at 8 × 106 cells/ml in assay buffer. Cells (25 μl; 2 × 105) were plated in a 96-well ultra-low adherence plate. Assay buffer, 4 µM NE, or HI NE (25 μl) was added for 1 h. Zymosan (50 μl; 50 µg/ml) was added for 4 h. NE and HI NE remained in the cultures during stimulation with zymosan. Cell culture supernatants were recovered and assayed for cytokine by ELISA (Thermo Fisher Scientific) according to the manufacturer’s protocol.
A. fumigatus–induced ELISA cytokine assay
Biogel-elicited cells were washed with MACS buffer, PBS that was supplemented with 0.5% BSA and 2 mM EDTA, and stained with anti-Ly6G biotin (Miltenyi Biotec). Cells were incubated with anti-biotin microbeads (Miltenyi Biotec), and inflammatory monocytes (Ly6G− cells) were enriched by MACS separation according to manufacturer’s instructions. Cells were resuspended in assay buffer, and 2–4 × 105 cells were plated in a 96-well ultra-low adherence plate in 25 µl assay buffer. Assay buffer, 4 µM NE, or HI NE (25 μl) was added for 1 h. A. fumigatus SC (0.6–1.2 × 106) in 50 µl Aim V medium was added for 4 h to stimulate cells at a 3:1 A. fumigatus:cell ratio. NE and HI NE remained in the cultures during stimulation with A. fumigatus. Cell culture supernatants were recovered and assayed for cytokine by ELISA according to manufacturer’s protocol.
Statistical methods
Data were analyzed on GraphPad Prism (GraphPad Software, La Jolla, CA, USA). Data are presented as means ± sd or sem and are representative of 3–4 independent experiments. One-way ANOVA followed by Bonferroni’s posttest was used for statistical analysis when multiple groups were analyzed. A Student’s t test was used for statistical analysis when 2 groups were analyzed. A Spearman’s rank test was used to test correlations. Statistical significance was set at P < 0.05, P < 0.01, or P < 0.001.
RESULTS
Dectin-1A is cleaved by NE
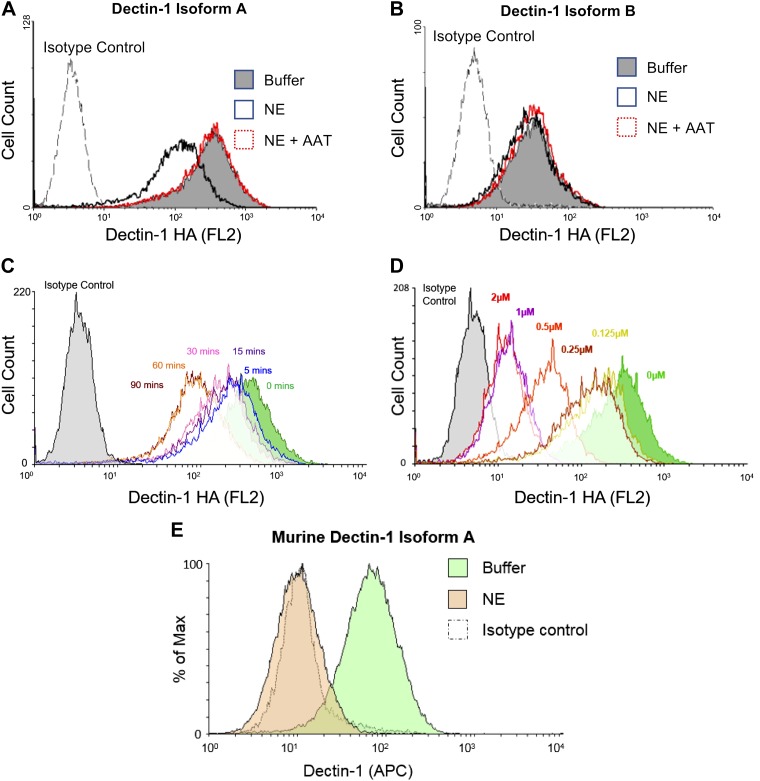
As NE cleaves various cell surface receptors, such as TLR4 and C5aR (23, 24), and Dectin-1 activity was trypsin sensitive (25), we postulated that NE may also cleave Dectin-1. To investigate this possibility, we incubated NIH3T3 cell lines that expressed 2 different isoforms of human Dectin-1 (hDectin-1) with NE. Dectin-1 isoform A is the full-length isoform, whereas isoform B lacks the stalk region (33). Of interest, NE only cleaved full-length Dectin-1 (isoform A; Fig. 1A), but not the short isoform (isoform B; Fig. 1B), which suggests that the NE cleavage site is within the stalk region. NE-mediated cleavage of Dectin-1 was inhibited by the serine protease inhibitor, AAT (Fig. 1A). Similar to other receptors, NE-mediated cleavage of hDectin-1 isoform A occurred in a time- and dose-dependent manner (Fig. 1C, D). In addition to hDectin-1, NE also cleaved murine Dectin-1 isoform A (Fig. 1E). Thus, NE cleaves Dectin-1 isoform A, but not isoform B, which will likely have functional consequences in the presence of fungal pathogens.
Figure 1.
Dectin-1A is cleaved by NE. A–C) NIH-3T3 cells that express human HA-tagged Dectin-1 isoform A (A, C) or Dectin-1 isoform B (B) were exposed to 0.5 µM NE, in the presence or absence of AAT, for 30 min (A, B) or for the indicated times (C) at 37°C. Cells were stained with an anti-HA Ab and analyzed by flow cytometry. D) NIH-3T3 cells that express human HA-tagged Dectin-1 isoform A were exposed to the indicated concentrations of NE for 30 min at 37°C. Cells were stained with an anti-HA Ab and analyzed by flow cytometry. E) NIH-3T3 cells that express murine Dectin-1 isoform A were exposed to NE for 1 h at 37°C. Cells were stained with an anti–Dectin-1 Ab and analyzed by flow cytometry. Data are representative of 3–4 independent experiments. APC, allophycocyanin.
CF BALF induces cleavage of Dectin-1
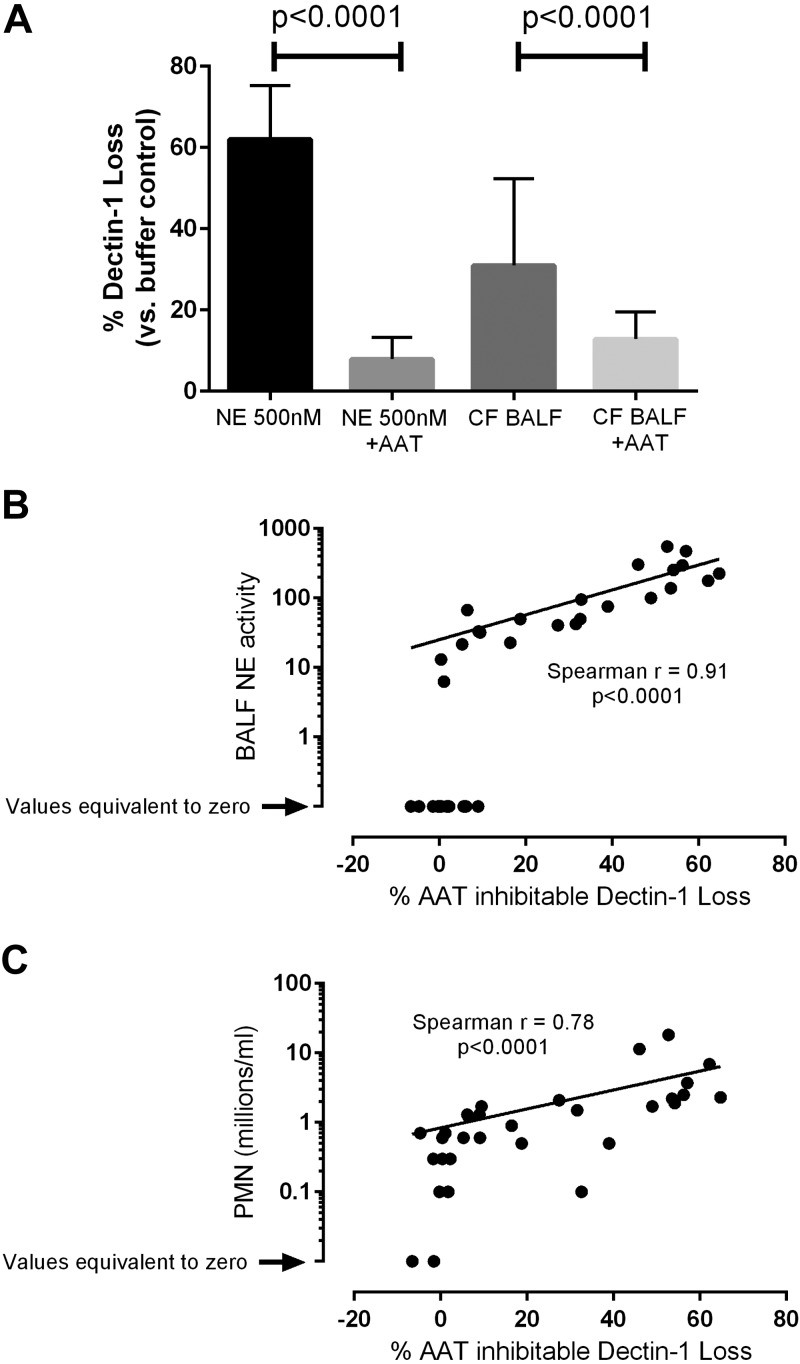
Increased neutrophil influx occurs in the lungs of patients with CF. This results in cellular activation, apoptosis, and necrosis, with a subsequent release of excess levels of NE (23). To determine whether BALF from the lungs of patients with CF has the ability to cleave Dectin-1, we incubated NIH3T3 cells that expressed hDectin-1 isoform A with either NE or BALF from patients with CF. Both NE and CF BALF substantially reduced cell surface Dectin-1 levels, an effect that was inhibited by the serine protease inhibitor, AAT (Fig. 2A), thus demonstrating that CF BALF-induced cleavage of Dectin-1 was dependent on a serine protease. In addition to measuring the BALF-induced loss of Dectin-1, NE activity and neutrophil numbers in CF BALF samples were determined and examined for any relationship with the BALF-induced loss of Dectin-1 in the in vitro NIH-3T3 assay. A strong correlation was observed between NE activity and the loss of Dectin-1 (Fig. 2B), and between the number of neutrophils that are present in CF BALF and the loss of Dectin-1 (Fig. 2C). Although this does not rule out the possibility that other neutrophil serine proteases are also involved, these data indicate that Dectin-1 is cleaved by NE, and there is a strong association between Dectin-1 cleavage and neutrophil-mediated secretion of NE in the lungs of patients with CF.
Figure 2.
CF BALF induces cleavage of Dectin-1. A) NIH-3T3 cells that express human HA-tagged Dectin-1 isoform A were exposed to 0.5 µM NE or 50 µl CF BALF, in the presence or absence of AAT, for 30 min at 37°C. Cells were stained with anti-HA Ab and analyzed by flow cytometry. Graph displays means ± sd. Data are the cumulative result of 7 independent experiments. B, C) NIH3T3 cells that express human HA-tagged Dectin-1 isoform A were exposed to CF BALF for 30 min at 37°C. Cells were stained with an anti-HA Ab and analyzed by flow cytometry. NE activity in CF BALF samples was measured (B). Correlation analysis between NE activity and percentage loss of Dectin-1 was performed. Neutrophil numbers in the CF BALF were counted and correlation analysis between neutrophil numbers in CF BALF samples and percentage loss of Dectin-1 was performed (C). Graphs are the cumulative result of at least 7 independent experiments (B, C). Each symbol represents data for CF BALF from a single patient.
A. fumigatus–derived proteases cleave Dectin-1
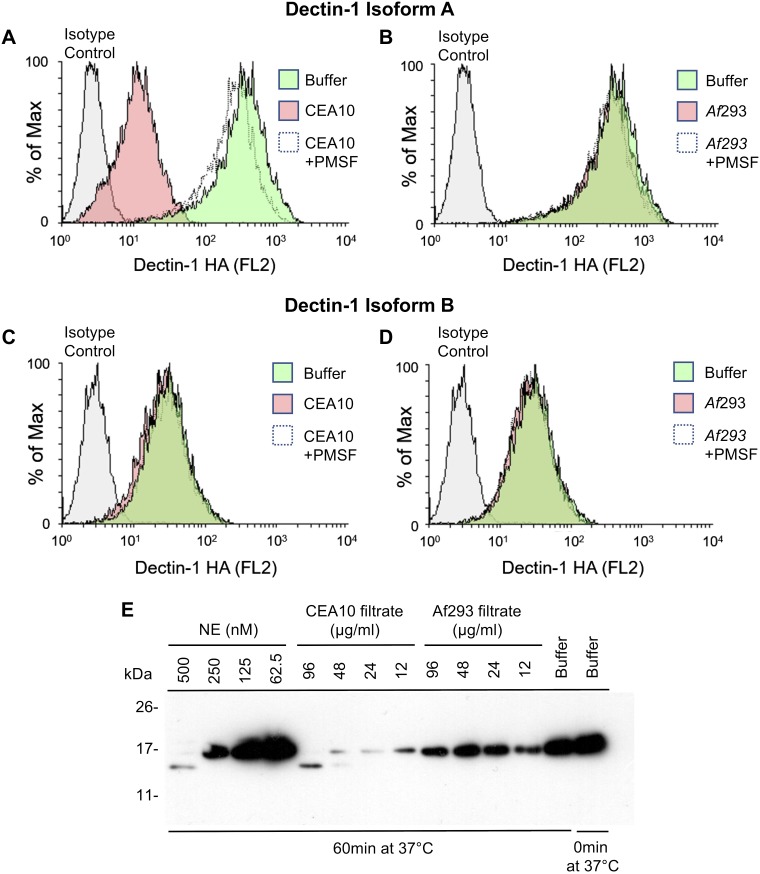
A. fumigatus has been isolated from the lungs of 10–57% of patients with CF, with increasing isolation from older patients and patients with worsening respiratory function (2). A. fumigatus secretes proteases, some of which display serine protease activity that is similar to NE (34). To investigate whether A. fumigatus–secreted proteases have the ability to cleave Dectin-1 in a manner similar to NE, we investigated 2 strains of A. fumigatus: CEA10, a high protease producer, and Af293, a low protease producer. Fungi were cultured in Vogel’s medium and the resulting culture filtrates were incubated with NIH3T3 cells that expressed hDectin-1. Culture filtrate (100 µg/ml total protein) from the high protease producer (A. fumigatus CEA10) induced the cleavage of hDectin-1 isoform A (Fig. 3A), and this was inhibited by the serine protease inhibitor, PMSF; however, as expected, an equivalent quantity of culture filtrate from the low protease producer, A. fumigatus 293, did not induce the cleavage of hDectin-1 isoform A (Fig. 3B). Neither filtrate induced the cleavage of hDectin-1 isoform B (Fig. 3C, D). In addition to cleaving surface-bound Dectin-1, both NE and A. fumigatus CEA10 filtrate cleaved soluble Dectin-1, which resulted in a similar-sized fragment (Fig. 3E), whereas A. fumigatus 293 filtrate did not cleave soluble Dectin-1. These data indicate that, in addition to the NE-mediated cleavage of Dectin-1 in the lungs of patients with CF, A. fumigatus–secreted protease(s) may also cleave Dectin-1 in the lungs of patients with CF who are colonized with A. fumigatus.
Figure 3.
A. fumigatus–derived proteases cleave Dectin-1. A–D) NIH3T3 cells that express human HA-tagged Dectin-1 isoform A (A, B) or isoform B (C, D) were exposed to A. fumigatus CEA10 supernatant (A, C) or Af293 supernatant (B, D)—in the presence or absence of PMSF—for 30 min at 37°C. Cells were stained with anti-HA Ab and analyzed by flow cytometry. Plots are representative of 3 independent experiments. E) Soluble Dectin-1 was exposed to NE or A. fumigatus filtrates from protease-producing (CEA10) or non–protease-producing (Af293) fungal strains at the indicated concentrations for 60 min at 37°C. Proteases were inhibited with PMSF, and Dectin-1 was separated by SDS-PAGE, blotted to nitrocellulose membrane, and probed with anti–Dectin-1. Data are representative of 3 independent experiments.
NE cleaves Dectin-1 on myeloid cells
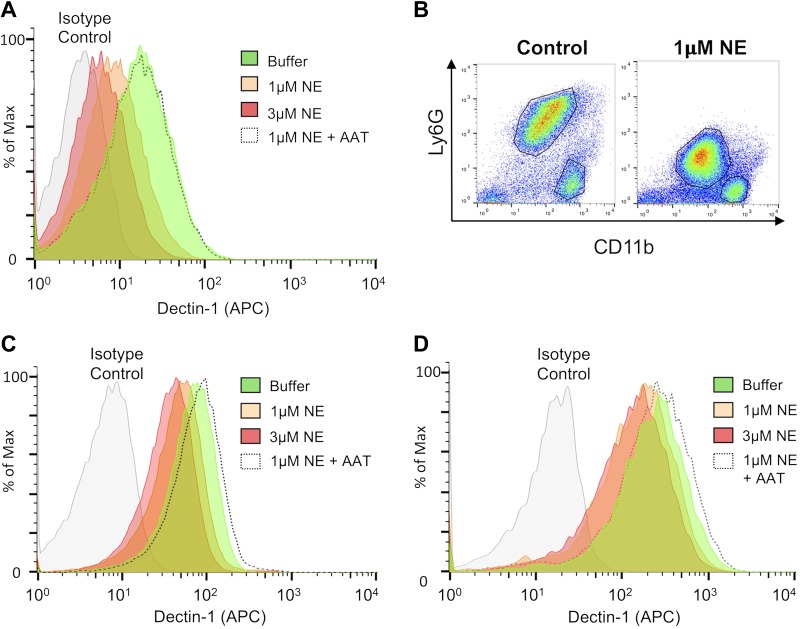
As we have shown that NE cleaves Dectin-1 on NIH3T3 cells that overexpress Dectin-1, we undertook experiments to determine whether this occurs on myeloid cells that naturally express Dectin-1. Heinsbroek et al. (33) demonstrated that macrophages from BALB/c mice expressed similar levels of Dectin-1 isoform A and isoform B, whereas macrophages from C57BL/6 mice predominantly expressed isoform B; therefore, as only the Dectin-1A isoform is cleaved by NE, we examined NE-mediated Dectin-1 cleavage on myeloid cells from BALB/c mice. Consistent with our findings with NIH3T3 cells that overexpressed Dectin-1, NE cleaved Dectin-1 on BMDMs from BALB/c mice in a dose-dependent manner, and this was inhibited by the serine protease inhibitor, AAT (Fig. 4A). As neutrophils and inflammatory cells are recruited to the lungs of patients with CF, we elicited neutrophils and inflammatory monocytes/macrophages by injecting Biogel into the peritoneal cavity. These 2 populations were clearly identifiable as Ly6G+CD11b+ neutrophils and Ly6G−CD11b+ inflammatory monocytes/macrophages in the absence of NE treatment (Fig. 4B). After treatment with NE for 1 h, the 2 populations could still be identified by using these markers; however, Ly6G expression was substantially reduced (Fig. 4B), which indicated NE-mediated cleavage of Ly6G. NE also induced Dectin-1 cleavage in Biogel-elicited neutrophils (Fig. 4C) and inflammatory monocytes/macrophages (Fig. 4D) in a dose-dependent manner, and this was inhibited by AAT; therefore, NE cleaves Dectin-1 from the surface of various myeloid cell populations.
Figure 4.
NE cleaves Dectin-1 on myeloid cells. A) BMDMs from BALB/c mice were exposed to NE, in the presence or absence of AAT, for 1 h at 37°C. Cells were stained with anti–Dectin-1 and analyzed by flow cytometry. B–D) BALB/c mice were injected i.p. with 0.5 ml Biogel, and inflammatory infiltrates were recovered 16 h later by peritoneal lavage. Cells were left unexposed or exposed to NE in the presence or absence of AAT for 1 h at 37°C. Cells were stained with anti-Ly6G, anti-CD11b, and anti–Dectin-1, and analyzed by flow cytometry. Dectin-1 levels were measured in the LyG+CD11b+ (neutrophil) population (C) and LyG−CD11b+ (inflammatory monocyte/macrophage) population (D). Data are representative of 3 independent experiments.
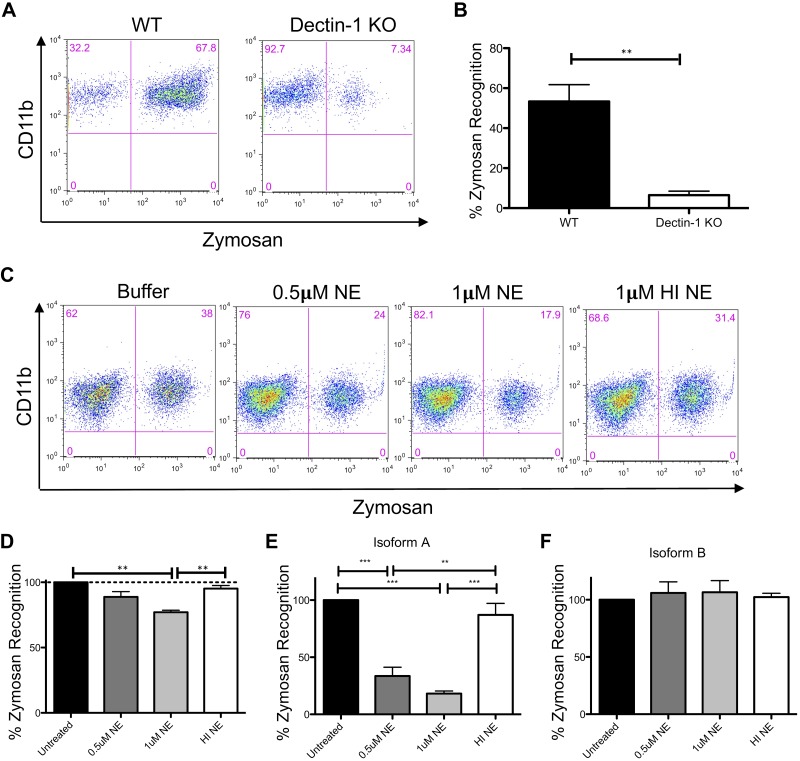
NE cleavage of Dectin-1 impairs zymosan recognition
Dectin-1 is a phagocytic receptor for various fungal pathogens (9). To determine whether NE-induced cleavage of Dectin-1 resulted in the reduced recognition of β-glucan–containing substrates, we used the fungal cell wall preparation, zymosan, which is rich in β-glucans. In agreement with previous findings, we showed that Dectin-1 KO BMDMs displayed significantly reduced zymosan recognition (Fig. 5A, B). In addition, BMDMs from BALB/c mice that were treated with NE demonstrated reduced zymosan recognition in a dose-dependent manner (Fig. 5C, D). HI NE did not substantially reduce zymosan recognition (Fig. 5C, D). Similarly, NE treatment of NIH3T3 cells that expressed hDectin-1 isoform A resulted in reduced zymosan recognition in a dose-dependent manner (Fig. 5E); however, NE treatment of NIH3T3 cells that expressed hDectin-1 isoform B did not affect zymosan recognition (Fig. 5F). This is consistent with the inability of NE to cleave hDectin-1 isoform B (Fig. 1B). Therefore, reduced Dectin-1 expression as a result of NE-induced cleavage in the lungs of patients with CF would likely result in the reduced recognition of fungal pathogens that are rich in β-glucans.
Figure 5.
NE cleavage of Dectin-1 impairs zymosan recognition. A–D) Wild-type (WT) and Dectin-1 KO BMDMs (A, B) and NE-treated BMDMs from BALB/c mice (C, D) were incubated with 2.5 µg FITC-labeled zymosan for 15 min. Cells were stained with anti-F4/80, anti-CD11b, and anti–Dectin-1, and analyzed by flow cytometry. Data are representative of 3 independent experiments. Zymosan recognition in Dectin-1 KO cells (B) and NE/HI NE-treated cells (D) is relative to WT cells (set at 100%; B) or buffer control cells (set at 100%; D). E, F) NE-treated NIH3T3 cells that express hDectin-1 isoform A (E) or isoform B (F) were incubated with 2.5 µg FITC-labeled zymosan for 15 min. Cells were stained with anti–Dectin-1 and analyzed by flow cytometry. Data are representative of 3 independent experiments. Zymosan recognition in NE/HI NE-treated cells is relative to buffer control cells (set at 100%). Graphs display means ± sem. Representative data from 1 of the 3 independent experiments (A, C). **P < 0.01, Student’s t test (B); **P < 0.01, ***P < 0.001, 1-way ANOVA with Bonferroni’s posttest (D, E).
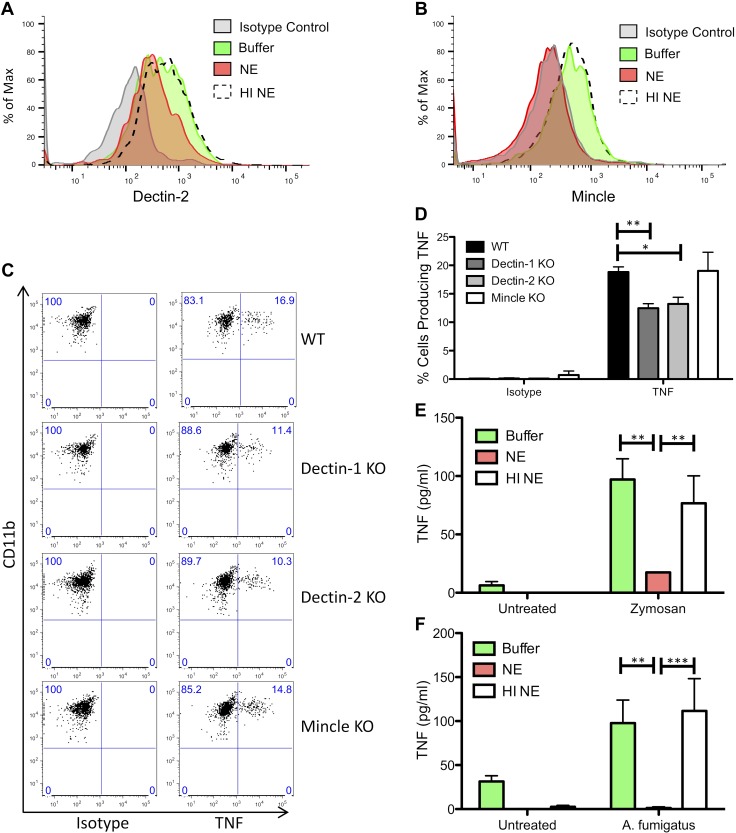
NE cleaves CLRs and reduces TNF production
As we have shown that Dectin-1 is cleaved by NE, we conducted experiments to determine whether additional CLRs, such as Dectin-2 or Mincle, were also cleaved by NE. To this end, Biogel-elicited cells were treated with NE, and Dectin-2 and Mincle expression levels were determined by flow cytometry. Similar to our findings with Dectin-1, NE cleaved both Dectin-2 and Mincle on inflammatory monocytes/macrophages (Fig. 6A, B) and neutrophils (data not shown). A. fumigatus–induced TNF production was reduced in inflammatory monocytes/macrophages from Dectin-1 KO and Dectin-2 KO mice, but not Mincle KO mice (Fig. 6C, D). In addition, zymosan-induced TNF production from Biogel-elicited cells was reduced after incubation with NE, whereas HI NE did not substantially reduce TNF production (Fig. 6E). Similarly, A. fumigatus–induced TNF production from an enriched population of Biogel-elicited inflammatory monocytes was reduced after treatment with NE, whereas HI NE did not reduce TNF production (Fig. 6F). As both Dectin-1 and Dectin-2 contribute to fungal-induced TNF production, and as both CLRs are cleaved by NE, NE-mediated blockade of TNF production is likely a result, in part, of the loss of Dectin-1 and Dectin-2, although contributing roles for other receptors, such as TLRs, cannot be ruled out.
Figure 6.
NE cleaves CLRs and reduces TNF production. A, B) BALB/c mice were injected with Biogel (0.5 ml, i.p.), and inflammatory infiltrates were recovered 16 h later by peritoneal lavage. Cells were left unexposed or exposed to 1 µM NE or HI NE for 1 h at 37°C. Cells were stained with anti-Ly6G, anti-CD11b, anti–Dectin-2, and anti-Mincle, and analyzed by flow cytometry. Dectin-2 (A) and Mincle (B) levels were measured in the LyG−CD11b+ inflammatory monocyte/macrophage population. C, D) Wild-type (WT), Dectin-1 KO, Dectin-2 KO, and Mincle KO mice were injected intraperitoneally with 0.5 ml Biogel, and inflammatory infiltrates were recovered 16 h later by peritoneal lavage. Cells were stimulated with cell trace far red-labeled A. fumigatus for 3 h in the presence of Brefeldin, and cells were stained with anti-Ly6G, anti-CD11b, anti-CD19, and anti-TNF, and analyzed by flow cytometry. Percentages of TNF-producing inflammatory monocytes with bound/phagocytosed A. fumigatus were measured. Representative data from 1 of 3 independent experiments is shown in panel C. Graph displays means ± sem (D). Graph displays cumulative data from 3 independent experiments. *P < 0.05, **P < 0.01, 2-way ANOVA with Bonferroni’s posttest. E) BALB/c mice were injected with Biogel (0.5 ml, i.p.), and inflammatory infiltrates were recovered 16 h later by peritoneal lavage. Cells were left unexposed or exposed to 2 µM NE or HI NE for 1 h at 37°C, followed by stimulation with 25 µg/ml zymosan for 4 h. TNF levels in the supernatants were measured by ELISA. Graph displays means ± sem. Graph displays cumulative data from 3 independent experiments. **P < 0.01, 2-way ANOVA with Bonferroni’s posttest. F) BALB/c mice were injected with Biogel (0.5 ml, i.p.), and inflammatory infiltrates were recovered 16 h later by peritoneal lavage. The inflammatory monocyte population was enriched by Ly6G+ cell depletion. Cells were left unexposed or exposed to 2 µM NE or HI NE for 1 h at 37°C, followed by stimulation with A. fumigatus for 4 h. TNF levels in the supernatants were measured by ELISA. Graph displays means ± sem. Graph displays cumulative data from 4 independent experiments. **P < 0.01, ***P < 0.001, paired 2-way ANOVA with Bonferroni’s posttest.
DISCUSSION
Here, for the first time, we have demonstrated that Dectin-1 is cleaved by NE in an isoform-specific manner, and we have shown that BALF from patients with CF also induces Dectin-1 cleavage. The percentage loss of Dectin-1 at the surface of cells in vitro correlates with NE activity and neutrophil numbers in BALF. We also determined that culture filtrate from a high protease-producing strain of A. fumigatus induces the cleavage of Dectin-1 in an isoform-specific manner, similar to that observed with NE treatment. In addition, Dectin-1 KO BMDMs and NE-treated BMDMs demonstrated reduced recognition/phagocytosis of zymosan, a fungal cell wall preparation. Finally, NE cleaves two other CLR family members, Dectin-2 and Mincle, and fungal-induced TNF production was reduced in Dectin-1 KO cells, Dectin-2 KO cells, and NE-treated cells; therefore, cleavage of Dectin-1 and Dectin-2 by NE and/or A. fumigatus–derived proteases results in an aberrant antifungal immune response. Taken together, these data indicate that both host-derived NE and Aspergillus-derived proteases have the capacity to cleave Dectin-1 and other CLRs in the lungs of patients with CF, potentially inhibiting immune responses to Aspergillus, thereby reducing the ability to clear Aspergillus from the airway.
CF affects ∼1 in 2500 live births in the United Kingdom. Although it is a multiorgan disease, the majority of morbidity and mortality is associated with progressive airway disease as a result of recurrent and chronic infection. The neutrophils that dominate the airway during disease exacerbation, and the proteases they secrete, are primary contributors to declining airway function in CF (20). In addition to their damaging effects on the mucocilliary system and other local tissue structures, neutrophil serine proteases (NSPs) cleave and inactivate several important components of the immune system, which compromises effective immunity. For example, we demonstrated that NE and other NSPs in BALF from patients with CF cleave and inactivate C5aR (23). Of interest, another study demonstrated that TLR4 is cleaved by NE; however, that study showed that NE promotes cytokine production mediated, in part, by TLR4 (24). This is in contrast to our results with Dectin-1; however, this may be a result of different experimental conditions. That previous study incubated cells with NE for 1 h, then removed NE before additional culture for 4 h (24). In contrast, we incubated cells with NE for 1 h and added zymosan for an additional 4 h in the presence of NE. This resulted in diminished cytokine production in response to zymosan in the presence of NE, which indicated an aberrant response to A. fumigatus in settings in which high levels of NE are present for a prolonged time. Whereas CF lungs are frequently exposed to high levels of NE and other NSPs, and they express high levels of proinflammatory cytokines, there are likely multiple factors that are involved in the induction of these proinflammatory cytokines. On the basis of our data, we hypothesize that exposure to A. fumigatus in patients with CF who undergo an airway exacerbation may lead to aberrant antifungal immune responses as a result of protease-mediated deficiency of Dectin-1 and other antifungal receptors. This may result in an inability to effectively clear A. fumigatus from the lungs, and may also lead to sensitization to A. fumigatus and the development of ABPA.
Whereas much of our data focus on the potential for neutrophil-derived proteases to functionally inactivate Dectin-1, we also present data that show that proteases expressed by A. fumigatus itself have the potential to inactivate this important fungal receptor. A wide range of fungal species are known to express proteases, and these are thought to play important roles in normal fungal growth and propagation as well as facilitating tissue invasion and catabolizing extracellular macromolecules as a food source (35). Proteases that are secreted by A. fumigatus are also recognized as important antigens that mediate allergic immune responses. Whereas there is little evidence to suggest that they act in a directly immune-evasive manner, a previous report has demonstrated their capacity to cleave the innate immune pattern recognition receptor, Pentraxin-3 (34). Data presented in this study indicate that these proteases may further benefit the pathogen via inactivation of fundamental antifungal innate immune receptors. As A. fumigatus secretion of proteases is strain dependent, A. fumigatus protease-dependent Dectin-1 cleavage may vary between patients, depending on which strain(s) of A. fumigatus are present. Additional studies that focus on in vivo models with protease-sufficient and -deficient fungal strains will be required to fully understand the importance of this observation to fungal pathogenesis.
CLRs, Dectin-1, Dectin-2, and Mincle are important for antifungal responses to A. fumigatus and/or other fungal spp. The immunologically inert surface RodA layer on resting A. fumigatus conidia masks other immunologically active cell wall components, such as β-glucans and mannans. Upon removal of this layer by swelling/germination, host immune pathogen recognition receptors recognize cell wall components and induce an immune response (36). In agreement with this, Dectin-1 does not bind A. fumigatus resting conidia, but binds to SC, early germlings, and hyphae (9, 10). Dectin-1 is important for phagocytosis of fungal pathogens, and we observed reduced zymosan recognition/binding by Dectin-1 KO BMDMs and NE-treated BMDMs, with reduced Dectin-1 expression. A. fumigatus SC and early germlings induce a robust cytokine response that is mediated, in part, by Dectin-1 and TLR2 (8–10). In addition, Dectin-2 is partially responsible for A. fumigatus SC and hyphae-induced reactive oxygen species and/or cytokine production (14, 37). Similar to Dectin-1–induced responses, Dectin-2–mediated responses are blocked by the presence of the RodA layer on resting conidia (12). Whereas Mincle expression is up-regulated in response to A. fumigatus stimulation (16), a role for Mincle during the response to A. fumigatus has not been identified to date (17). In agreement with these data, we observed that A. fumigatus SC-induced TNF from Biogel-elicited inflammatory monocytes/macrophages was partially dependent on Dectin-1 and Dectin-2, but independent of Mincle. This does not rule out the involvement of other receptors, such as TLR2, which has previously been shown to play a role in A. fumigatus–induced cytokine production from macrophages (9, 10). NE-treated cells, with reduced Dectin-1, Dectin-2, and Mincle expression, also displayed reduced fungal-induced cytokine responses, which is in agreement with the reduced cytokine responses that we observed in Dectin-1 and Dectin-2 KO cells. Therefore, CLRs, Dectin-1, and Dectin-2 are involved in the antifungal response to A. fumigatus, and loss of these receptors as a result of protease-induced cleavage results in an aberrant response.
In addition to pathogen recognition and cytokine production by innate immune cells, TLRs and/or CLRs are involved in inflammatory cell recruitment, fungal killing, and the induction of T-cell responses. Werner et al. (8) demonstrated that Dectin-1 KO mice displayed reduced neutrophil recruitment compared with wild-type mice in a pulmonary model of Aspergillus infection. Another study demonstrated reduced inflammatory cell recruitment in Dectin-1 KO mice during a corneal infection model with Aspergillus, whereas cell recruitment was unimpaired in TLR2 KO or TLR4 KO mice; however, fungal killing was impaired in TLR4 KO mice (38). A. fumigatus induces both T helper (Th)1 and Th17 responses, which depend on TLR/MyD88 and Dectin-1 signaling, respectively. Dectin-1 signals reduce IL-12 and IFN-γ production in innate cells, which results in decreased T-bet expression in A. fumigatus–specific CD4 T cells, thereby facilitating Th17 differentiation (39). Dectin-2 is important for the induction of Th17 responses to Candida albicans (27, 40), and A. fumigatus–induced Dectin-2 signaling promotes IL-1β and IL-23 production (37), which suggests that it will also be involved in the induction of A. fumigatus–associated Th17 responses. We could speculate that NE-induced cleavage of these CLRs, and potentially TLR4, could reduce Th17 and Th1 responses, leading to increased Th2 responses like that observed in ABPA; however, additional study is required to determine if this is the case. The isoform-specific susceptibility of Dectin-1 to such proteolysis is intriguing. The functional relevance of the 2 main human isoforms of Dectin-1 (A and B) remains unclear, although it is known that they are differentially expressed by myeloid subsets and at different sites, and recent evidence demonstrates distinct inflammatory responses to the ligation of either isoform (41). As isoform B seems to be resistant to proteolysis, we speculate that this isoform may play a particularly important role in antifungal immunity during periods of intense neutrophilic inflammation.
It may be difficult to establish whether the protease-mediated reduction of Dectin-1 expression on airway cells correlates with the incidence of APBA and/or A. fumigatus colonization in the human population. This is a result of the fact that patient BAL samples are collected intermittently and may not coincide with the original sensitization or colonization event. In addition, the patient cohort under investigation in this study is relatively small and is derived from a pediatric population in whom fungal pathology is less prevalent than in older patients with CF (3).
Overall, our data suggest a novel role for neutrophil and fungal-derived proteases in the modulation of host immune responses to fungal pathogens. We have demonstrated the functionally relevant and isoform-specific inactivation of Dectin-1 and other fungal receptors by these proteases in both man and mouse. Additional studies are required to shed light on the pathologic importance of these observations in various diseases in which excess protease activity is observed.
ACKNOWLEDGMENTS
The authors thank Gordon Brown and Janet Willment [Medical Research Council (MRC) Centre for Medical Mycology, University of Aberdeen, United Kingdom] for providing the NIH3T3 cell lines that expressed human and mouse wild-type Dectin-1. A. fumigatus (CEA10 and Af293) strains were a kind gift from Dr. Paul Bowyer (University of Manchester). The authors wish to acknowledge the U.S. National Institutes of Health (NIH)–sponsored Mutant Mouse Regional Resource Center (MMRRC) National System as the source of genetically altered mice (C57BL/6-Clec4etm1.1Cfg/Mmucd 031936-UCD) for use in this study. The mice were produced and deposited to the MMRRC by the Consortium for Functional Glycomics, supported by the National Institute of General Medical Sciences (Grant GM62116). S.J.O. is funded by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (Grant 099953/Z/12/Z). J.F. is funded by a National Institute for Social Care and Health Research Academic Health Science collaboration (Wales, United Kingdom). P.R.T. is funded by a Wellcome Trust Investigator Award (WT107964MA). M.S. is funded by a Ph.D. Studentship awarded to E.P.M. by Complement UK. Funding for this Studentship was part of an unencumbered educational funding agreement between Complement UK and Alexion Pharmaceuticals. Alexion had no involvement at any stage of the project, including its design, implementation, and execution, nor were they involved in drafting or approving the manuscript.
Glossary
- AAT
α-1 antitrypsin
- ABPA
allergic bronchopulmonary aspergillosis
- BAL
bronchoalveolar lavage
- BALF
bronchoalveolar lavage fluid
- BMDM
bone marrow–derived macrophages
- BSA
bovine serum albumin
- CF
cystic fibrosis
- CLR
C-type lectin-like receptor
- hDectin-1
human Dectin-1
- HA
hemagglutinin
- HI NE
heat-inactivated neutrophil elastase
- KO
knockout
- NE
neutrophil elastase
- NSP
neutrophil serine protease
- SC
swollen conidia
- SNP
single nucleotide polymorphism
- Th
T helper
AUTHOR CONTRIBUTIONS
J. S. Griffiths, A. Thompson, M. Stott, A. Benny, and N. A. Lewis performed experiments and edited the manuscript; P. R. Taylor designed experiments and edited the manuscript; J. Forton collected patient samples and edited the manuscript; S. Herrick performed experiments and edited the manuscript; and S. J. Orr and E. P. McGreal conceived the project, designed experiments, performed experiments, and wrote the manuscript.
REFERENCES
- 1.Brown G. D., Denning D. W., Gow N. A., Levitz S. M., Netea M. G., White T. C. (2012) Hidden killers: human fungal infections. Sci. Transl. Med. 4, 165rv13 [DOI] [PubMed] [Google Scholar]
- 2.King J., Brunel S. F., Warris A. (2016) Aspergillus infections in cystic fibrosis. J. Infect. 72(Suppl), S50–S55 [DOI] [PubMed] [Google Scholar]
- 3.El-Dahr J. M., Fink R., Selden R., Arruda L. K., Platts-Mills T. A., Heymann P. W. (1994) Development of immune responses to Aspergillus at an early age in children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 150, 1513–1518 [DOI] [PubMed] [Google Scholar]
- 4.Baxter C. G., Dunn G., Jones A. M., Webb K., Gore R., Richardson M. D., Denning D. W. (2013) Novel immunologic classification of aspergillosis in adult cystic fibrosis. J. Allergy Clin. Immunol. 132, 560–566.e510 [DOI] [PubMed] [Google Scholar]
- 5.Plato A., Hardison S. E., Brown G. D. (2015) Pattern recognition receptors in antifungal immunity. Semin. Immunopathol. 37, 97–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meier A., Kirschning C. J., Nikolaus T., Wagner H., Heesemann J., Ebel F. (2003) Toll-like receptor (TLR) 2 and TLR4 are essential for Aspergillus-induced activation of murine macrophages. Cell. Microbiol. 5, 561–570 [DOI] [PubMed] [Google Scholar]
- 7.Bochud P. Y., Chien J. W., Marr K. A., Leisenring W. M., Upton A., Janer M., Rodrigues S. D., Li S., Hansen J. A., Zhao L. P., Aderem A., Boeckh M. (2008) Toll-like receptor 4 polymorphisms and aspergillosis in stem-cell transplantation. N. Engl. J. Med. 359, 1766–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Werner J. L., Metz A. E., Horn D., Schoeb T. R., Hewitt M. M., Schwiebert L. M., Faro-Trindade I., Brown G. D., Steele C. (2009) Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J. Immunol. 182, 4938–4946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gersuk G. M., Underhill D. M., Zhu L., Marr K. A. (2006) Dectin-1 and TLRs permit macrophages to distinguish between different Aspergillus fumigatus cellular states. J. Immunol. 176, 3717–3724 [DOI] [PubMed] [Google Scholar]
- 10.Steele C., Rapaka R. R., Metz A., Pop S. M., Williams D. L., Gordon S., Kolls J. K., Brown G. D. (2005) The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog. 1, e42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cunha C., Di Ianni M., Bozza S., Giovannini G., Zagarella S., Zelante T., D’Angelo C., Pierini A., Pitzurra L., Falzetti F., Carotti A., Perruccio K., Latgé J. P., Rodrigues F., Velardi A., Aversa F., Romani L., Carvalho A. (2010) Dectin-1 Y238X polymorphism associates with susceptibility to invasive aspergillosis in hematopoietic transplantation through impairment of both recipient- and donor-dependent mechanisms of antifungal immunity. Blood 116, 5394–5402 [DOI] [PubMed] [Google Scholar]
- 12.Carrion S. J., Leal S. M., Jr., Ghannoum M. A., Aimanianda V., Latgé J. P., Pearlman E. (2013) The RodA hydrophobin on Aspergillus fumigatus spores masks dectin-1- and dectin-2-dependent responses and enhances fungal survival in vivo. J. Immunol. 191, 2581–2588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGreal E. P., Rosas M., Brown G. D., Zamze S., Wong S. Y., Gordon S., Martinez-Pomares L., Taylor P. R. (2006) The carbohydrate-recognition domain of Dectin-2 is a C-type lectin with specificity for high mannose. Glycobiology 16, 422–430 [DOI] [PubMed] [Google Scholar]
- 14.Loures F. V., Röhm M., Lee C. K., Santos E., Wang J. P., Specht C. A., Calich V. L., Urban C. F., Levitz S. M. (2015) Recognition of Aspergillus fumigatus hyphae by human plasmacytoid dendritic cells is mediated by dectin-2 and results in formation of extracellular traps. PLoS Pathog. 11, e1004643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor P. R., Roy S., Leal S. M., Jr., Sun Y., Howell S. J., Cobb B. A., Li X., Pearlman E. (2014) Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORγt and dectin-2. Nat. Immunol. 15, 143–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao G., Xu Q., Lin J., Chen W., Cui T., Hu L., Jiang N. (2017) The role of Mincle in innate immune to fungal keratitis. J. Infect. Dev. Ctries. 11, 89–97 [DOI] [PubMed] [Google Scholar]
- 17.Yamasaki S., Matsumoto M., Takeuchi O., Matsuzawa T., Ishikawa E., Sakuma M., Tateno H., Uno J., Hirabayashi J., Mikami Y., Takeda K., Akira S., Saito T. (2009) C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia. Proc. Natl. Acad. Sci. USA 106, 1897–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sainz J., Lupiáñez C. B., Segura-Catena J., Vazquez L., Ríos R., Oyonarte S., Hemminki K., Försti A., Jurado M. (2012) Dectin-1 and DC-SIGN polymorphisms associated with invasive pulmonary aspergillosis infection. PLoS One 7, e32273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayer-Hamblett N., Aitken M. L., Accurso F. J., Kronmal R. A., Konstan M. W., Burns J. L., Sagel S. D., Ramsey B. W. (2007) Association between pulmonary function and sputum biomarkers in cystic fibrosis. Am. J. Respir. Crit. Care Med. 175, 822–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Twigg M. S., Brockbank S., Lowry P., FitzGerald S. P., Taggart C., Weldon S. (2015) The role of serine proteases and antiproteases in the cystic fibrosis lung. Mediators Inflamm. 2015, 293053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotecha S., Doull I., Davies P., McKenzie Z., Madsen J., Clark H. W., McGreal E. P. (2013) Functional heterogeneity of pulmonary surfactant protein-D in cystic fibrosis. Biochim. Biophys. Acta 1832, 2391–2400 [DOI] [PubMed] [Google Scholar]
- 22.McGreal E. P., Davies P. L., Powell W., Rose-John S., Spiller O. B., Doull I., Jones S. A., Kotecha S. (2010) Inactivation of IL-6 and soluble IL-6 receptor by neutrophil derived serine proteases in cystic fibrosis. Biochim. Biophys. Acta 1802, 649–658 [DOI] [PubMed] [Google Scholar]
- 23.Van den Berg C. W., Tambourgi D. V., Clark H. W., Hoong S. J., Spiller O. B., McGreal E. P. (2014) Mechanism of neutrophil dysfunction: neutrophil serine proteases cleave and inactivate the C5a receptor. J. Immunol. 192, 1787–1795 [DOI] [PubMed] [Google Scholar]
- 24.Benabid R., Wartelle J., Malleret L., Guyot N., Gangloff S., Lebargy F., Belaaouaj A. (2012) Neutrophil elastase modulates cytokine expression: contribution to host defense against Pseudomonas aeruginosa-induced pneumonia. J. Biol. Chem. 287, 34883–34894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown G. D., Gordon S. (2001) Immune recognition. A new receptor for beta-glucans. Nature 413, 36–37 [DOI] [PubMed] [Google Scholar]
- 26.Taylor P. R., Tsoni S. V., Willment J. A., Dennehy K. M., Rosas M., Findon H., Haynes K., Steele C., Botto M., Gordon S., Brown G. D. (2007) Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat. Immunol. 8, 31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saijo S., Ikeda S., Yamabe K., Kakuta S., Ishigame H., Akitsu A., Fujikado N., Kusaka T., Kubo S., Chung S. H., Komatsu R., Miura N., Adachi Y., Ohno N., Shibuya K., Yamamoto N., Kawakami K., Yamasaki S., Saito T., Akira S., Iwakura Y. (2010) Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 32, 681–691 [DOI] [PubMed] [Google Scholar]
- 28.Wells C. A., Salvage-Jones J. A., Li X., Hitchens K., Butcher S., Murray R. Z., Beckhouse A. G., Lo Y. L., Manzanero S., Cobbold C., Schroder K., Ma B., Orr S., Stewart L., Lebus D., Sobieszczuk P., Hume D. A., Stow J., Blanchard H., Ashman R. B. (2008) The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J. Immunol. 180, 7404–7413 [DOI] [PubMed] [Google Scholar]
- 29.De Blic J., Midulla F., Barbato A., Clement A., Dab I., Eber E., Green C., Grigg J., Kotecha S., Kurland G., Pohunek P., Ratjen F., Rossi G.; European Respiratory Society . (2000) Bronchoalveolar lavage in children. ERS Task Force on bronchoalveolar lavage in children. Eur. Respir. J. 15, 217–231 [DOI] [PubMed] [Google Scholar]
- 30.Taylor P. R., Reid D. M., Heinsbroek S. E., Brown G. D., Gordon S., Wong S. Y. (2005) Dectin-2 is predominantly myeloid restricted and exhibits unique activation-dependent expression on maturing inflammatory monocytes elicited in vivo. Eur. J. Immunol. 35, 2163–2174 [DOI] [PubMed] [Google Scholar]
- 31.Patin E. C., Jones A. V., Thompson A., Clement M., Liao C. T., Griffiths J. S., Wallace L. E., Bryant C. E., Lang R., Rosenstiel P., Humphreys I. R., Taylor P. R., Jones G. W., Orr S. J. (2016) IL-27 induced by select Candida spp. via TLR7/NOD2 signaling and IFN-β production inhibits fungal clearance. J. Immunol. 197, 208–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDonald J. U., Rosas M., Brown G. D., Jones S. A., Taylor P. R. (2012) Differential dependencies of monocytes and neutrophils on dectin-1, dectin-2 and complement for the recognition of fungal particles in inflammation. PLoS One 7, e45781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heinsbroek S. E., Taylor P. R., Rosas M., Willment J. A., Williams D. L., Gordon S., Brown G. D. (2006) Expression of functionally different dectin-1 isoforms by murine macrophages. J. Immunol. 176, 5513–5518 [DOI] [PubMed] [Google Scholar]
- 34.Hamon Y., Jaillon S., Person C., Giniès J. L., Garo E., Bottazzi B., Ghamrawi S., Urban T., Subra J. F., Bouchara J. P., Mantovani A., Jeannin P., Delneste Y. (2013) Proteolytic cleavage of the long pentraxin PTX3 in the airways of cystic fibrosis patients. Innate Immun. 19, 611–622 [DOI] [PubMed] [Google Scholar]
- 35.Yike I. (2011) Fungal proteases and their pathophysiological effects. Mycopathologia 171, 299–323 [DOI] [PubMed] [Google Scholar]
- 36.Aimanianda V., Bayry J., Bozza S., Kniemeyer O., Perruccio K., Elluru S. R., Clavaud C., Paris S., Brakhage A. A., Kaveri S. V., Romani L., Latgé J. P. (2009) Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 460, 1117–1121 [DOI] [PubMed] [Google Scholar]
- 37.Sun H., Xu X. Y., Tian X. L., Shao H. T., Wu X. D., Wang Q., Su X., Shi Y. (2013) Activation of NF-kappaB and respiratory burst following Aspergillus fumigatus stimulation of macrophages. Immunobiology 219, 25–36 [DOI] [PubMed] [Google Scholar]
- 38.Leal S. M., Jr., Cowden S., Hsia Y. C., Ghannoum M. A., Momany M., Pearlman E. (2010) Distinct roles for Dectin-1 and TLR4 in the pathogenesis of Aspergillus fumigatus keratitis. PLoS Pathog. 6, e1000976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rivera A., Hohl T. M., Collins N., Leiner I., Gallegos A., Saijo S., Coward J. W., Iwakura Y., Pamer E. G. (2011) Dectin-1 diversifies Aspergillus fumigatus-specific T cell responses by inhibiting T helper type 1 CD4 T cell differentiation. J. Exp. Med. 208, 369–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robinson M. J., Osorio F., Rosas M., Freitas R. P., Schweighoffer E., Gross O., Verbeek J. S., Ruland J., Tybulewicz V., Brown G. D., Moita L. F., Taylor P. R., Reis e Sousa C. (2009) Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J. Exp. Med. 206, 2037–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fischer M., Müller J. P., Spies-Weisshart B., Gräfe C., Kurzai O., Hünniger K., Hochhaus A., Scholl S., Schnetzke U. (2017) Isoform localization of Dectin-1 regulates the signaling quality of anti-fungal immunity. Eur. J. Immunol. 47, 848–859 [DOI] [PubMed] [Google Scholar]