Abstract
Thy1 (CD90), a glycosylated, glycophosphatidylinositol-anchored membrane protein highly expressed by subsets of mesenchymal stem cells and fibroblasts, inhibits adipogenesis. The role of Thy1 on bone structure and function has been poorly studied and represents a major knowledge gap. Therefore, we analyzed the long bones of wild-type (WT) and Thy1 knockout (KO) mice with micro-computed tomography (micro-CT) and histomorphometry to compare changes in bone architecture and overall bone structure. micro-CT analysis of long bones revealed Thy1 KO and WT mice fed a high-fat diet demonstrated bone structural parameters at 4 mo that differed significantly between WT and KO mice. A significant reduction in trabecular bone volume was noted in Thy1 KO mice. The most prominent differences were observed in trabecular bone volume ratio and trabecular bone connectivity density. Consistent with micro-CT measurements, histomorphometric analysis also showed decreased bone volume in the obese Thy1 KO mice compared to obese WT mice. In vitro assays revealed that osteogenic conditions increased Thy1 expression during OB differentiation and absence of Thy1 attenuated osteoblastogenesis. Together, these findings support the concept that Thy1 serves as a major mechanistic link to regulate bone formation and negatively regulate adipogenesis.—Paine, A., Woeller, C. F., Zhang, H., Garcia-Hernandez, M. L., Huertas, N., Xing, L., Phipps, R. P., Ritchlin, C. T. Thy1 is a positive regulator of osteoblast differentiation and modulates bone homeostasis in obese mice.
Keywords: adipocytes, CD90, mesenchymal stem cells, osteoblastogenesis, trabecular bone volume
The imbalance between bone formation and resorption is central to the pathogenesis of inflammatory arthritis and metabolic bone disease, which are prevalent disorders associated with chronic pain and disability. Bone is composed of a complex internal and external structure that is maintained by concerted activity of bone-forming osteoblasts (OBs) and bone-resorbing osteoclasts (OCs) (1). Bone formation by OBs and resorption by OCs are tightly regulated processes that continuously remodel bone to maintain structural and functional integrity (2). OCs originate from myeloid precursor cells of hematopoietic origin (1), whereas OBs are derived from bone marrow stem cells of mesenchymal origin (3). The key molecular and signaling events that promote osteoclastogenesis are well understood, and therapeutic agents to inhibit pathologic resorption are widely available but factors that promote differentiation of OBs and bone deposition are not well understood.
Formation of bone and cartilage in the embryo, as well as repair and turnover in the adult, involve the progeny of a small number of progenitor mesenchymal stem cells (MSCs) (4) that also give rise to adipocytes and other cells (5). As a common progenitor, the tightly controlled lineage commitment of MSCs is critically involved in the maintenance of bone homeostasis (5). Although MSCs can give rise to a variety of cell types, the commitment of MSCs to OBs and adipocytes has been strongly implicated in pathologic conditions of abnormal bone remodeling (6, 7). Therefore, improved understanding of the molecular events that control the lineage commitment of MSCs toward OBs or adipocytes are of central importance, and identification of the key molecular drivers may reveal new biomarkers and therapeutic targets.
Thy1 or cluster of differentiation 90 (CD90) is a glycophosphatidylinositol-anchored membrane protein highly expressed in the thymus of newborn and adult mice and in the lungs and other body spaces of adult mice (8). Thy1 is expressed on fibroblastic cells from numerous tissues and is a marker of lung cell diversity (9). Thy1 is also expressed on bone marrow-derived MSCs (10). The function of Thy1 is not well understood but diverse physiologic functions have been proposed. Based on experimental findings, it has been speculated that Thy1 is involved in cell-cell and cell–matrix interactions that promote nerve regeneration, neurite outgrowth, apoptosis, metastasis, inflammation, and fibrosis (11). Recent studies of Thy1 knockout (KO) mice and mesenchymal cell lines revealed that Thy1 regulates adipogenesis and absence of Thy1 results in increased adipogenesis and obesity (12). Studies further indicated that Thy1 is a key marker targeted by environmental chemicals, such as tetrabromobisphenol-A, that promote adipogenesis and obesity (13). The role of Thy1 in regulating adipogenesis is established, but its roles in OB differentiation and bone homeostasis remain unknown.
Thy1 is expressed on OBs and may act as a differentiation marker (14), but the mechanisms underlying its potential contribution to osteoblastogenesis have not been examined. To address this gap, we systematically studied changes in bone structural parameters in the Thy1 global KO mice and their wild-type (WT) counterparts with micro–computed tomography (micro-CT). We also monitored the changes in the expression patterns of Thy1 in mesenchymal cells cultured in osteogenic conditions. Finally, we examined the effect of Thy1 knockdown in the preosteoblastic cell line MC3T3 and on calcium mineralization capacity of mouse embryonic fibroblasts derived from Thy1 KO and WT mice.
MATERIALS AND METHODS
Animals
Thy1-KO mice on the C57BL/6J background and WT C57BL/6J mice were bred and maintained in a specific-pathogen–free laboratory animal center in the University of Rochester School of Medicine and Dentistry. We generated WT and Thy1 KO mice from a backcross of Thy1 heterozygous mice (on the C57BL/6 background) and have maintained these previously backcrossed strains of WT and Thy1 KO mice to perform our current studies. Thy1 KO and control WT mice were housed in colony cages in a room with the appropriate temperature and humidity with a 12-h light–dark cycle and fed ad libitum. For this study, 8-wk-old male mice were fed a high-fat diet (HFD) consisting of 60% kcal/fat (D12492; Research Diets, New Brunswick, NJ, USA) or a control diet consisting of 10% kcal/fat (D12450B; Research Diets) for 2 mo. All animal procedures were conducted with the approval of the University Committee on Animal Resources at the University of Rochester School of Medicine and Dentistry.
Micro-CT, histology, and histomorphometry
To compare changes in bone architecture and structural parameters, long bones from hind limbs of the WT and Thy1 KO mice were analyzed with micro-CT. For micro-CT analysis, bones were first dissected from the soft tissue, fixed in 10% neutral-buffered formalin for at least 48 h, and scanned at high resolution (10.5 μm) on a VivaCT40 micro-CT scanner (Scanco Medical, Basserdorf, Switzerland), with an integration time of 300 ms, intensity of 145 μA, and energy of 55 kVp. The 3-dimensional images for all samples were generated with a constant threshold of 280‰ for trabecular bone and 400‰ for cortical bone. Long bones were fixed in 10% neutral-buffered formalin, decalcified in 10% EDTA, and embedded in paraffin. Sections (4 μm thick) were stained with Alcian blue hematoxylin/orange G and tartrate-resistant acidic phosphatase (TRAP) staining. Digital images were generated with a VS120 image system (15) (Olympus USA, Center Valley, PA, USA). Bone surface (BS), bone area (B.A), tissue area (T.A), percentage B.A/T.A (% B.A/T.A), OB number/BS (N.Ob/BS), N.Ob/T.A, osteoclast number/BS (N.Oc/BS), N.Oc/T.A, and adipocyte number/T.A (N.Ad/T.A) in tibial sections were analyzed with VS-110 v.2.3 software (16).
Cell culture, osteoblastogenesis, and adipogenesis
Preosteoblastic MC3T3-E1 cell line and primary bone and bone marrow–derived MSCs and mouse embryonic fibroblasts (MEFs) were cultured in α-MEM culture medium (Thermo Fisher Scientific, Waltham, MA, USA) containing 10% fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA, USA) and 1% antibiotic–antimycotic solution (containing 100 U/ml of penicillin, 100 µg/ml of streptomycin, and 0.25 µg/ml of amphotericin B; MilliporeSigma, Billerica, MA, USA). Preadipocytic 3T3-L1 cells (CL-173; ATCC, American Type Culture Collection, Manassas, VA, USA) were cultured (17). For inducing osteoblastogenesis, MC3T3-E1, 3T3-L1 or bone marrow cells or MEFs were cultured in α-MEM culture medium containing 10% FBS, 50 μg/ml ascorbic acid, and 10 mM β-glycerophosphate (MilliporeSigma) for up to 21 d and mineralized bone nodules were examined by Alizarin red staining. For inducing adipogenesis, 3T3-L1 cells were cultured in adipogenic medium containing 0.5 mM 3-isobutyl-1-methylxanthine, 0.25 μM dexamethasone, and 1 μg/ml insulin (MilliporeSigma) for 2 d, and thereafter fresh adipogenic medium lacking 3-isobutyl-1-methylxanthine was added for another 5 d. MEFs were induced to form adipocytes, with a similar adipogenic medium with the addition of 2 μM rosiglitazone (Cayman, Ann Arbor, MI, USA), and the cells were treated for 10 d.
Real-time quantitative RT-PCR
Total RNA was extracted using an RNeasy kit (Qiagen, Germantown, MD, USA) and cDNAs were synthesized using iSCRIPT cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). Inventoried Applied Biosystems TaqMan Gene Expression Assays were purchased from Thermo Fisher Scientific to quantify mRNA levels for different genes of interest. Amplification was performed with TaqMan Gene Expression Master Mix (Thermo Fisher Scientific) on a StepOnePlus Real-Time PCR System (Thermo Fisher Scientific) (18). Thermal cycling was performed at 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. The constitutively expressed gene, GAPDH, was used as a control for normalization of cDNA levels. The 2−ΔΔCt method was used to semiquantify mRNA levels, according to the manufacturer’s protocol (Thermo Fisher Scientific). Representative data are presented as means ± se of the triplicates or of 4 wells of cell culture.
Western blot analysis
Protein was isolated from 1 to 2 × 106 cells for which cells were lysed in 60 mM Tris (pH 6.8) and 2% SDS, containing 1 time protease inhibitor cocktail (MilliporeSigma). Samples were treated with 4× SDS loading buffer containing 2-ME before use. Total protein (5–40 μg/lane) was subjected to SDS-PAGE. Protein gels were transferred to PVDF membrane (Thermo Fisher Scientific) and probed with the appropriate antibodies. The Thy1 antibody used for Western blot analyses was purchased from R&D Systems (Minneapolis, MN, USA). Blots were developed with the SuperSignal West Pico or Femto chemiluminescent substrate kit (Thermo Fisher Scientific). Signals were detected by Image Lab software for the ChemiDoc XRS+ system (Bio-Rad) or by using Kodak scientific films (Eastman Kodak, Rochester, NY, USA).
Knockdown of gene expression by siRNA
Cells were seeded at 2.5 × 104 or 1 × 105 into 48- or 12-well cell culture plates (Corning, Corning, NY, USA), respectively, and subsequently transiently transfected with 50–200 nM of Thy1 or control small interfering (si)RNA (Thermo Fisher Scientific) using Lipofectamine RNAiMax (Thermo Fisher Scientific) according to the manufacturer’s protocol. Cells were incubated with siRNA for 3–4 d and subjected to osteogenic differentiation as described above. At the start of differentiation, cells were transfected again with 100 nM siRNA.
Statistical analysis
Data are means ± se, and all experiments were performed at least 3 times with similar results. Statistical analyses were performed with Prism statistical software (GraphPad Software, La Jolla, CA, USA). For the analysis of the micro-CT and histomorphometry data, differences between the groups were compared by using unpaired Welch’s t test. For the analysis of real-time qPCR and Western blot data, differences between the groups were compared by using the Wilcoxon signed-rank test or Friedman test followed by Dunn’s post hoc test. Statistical significance was set at P < 0.05.
RESULTS
Thy1 modulates bone homeostasis in obese mice
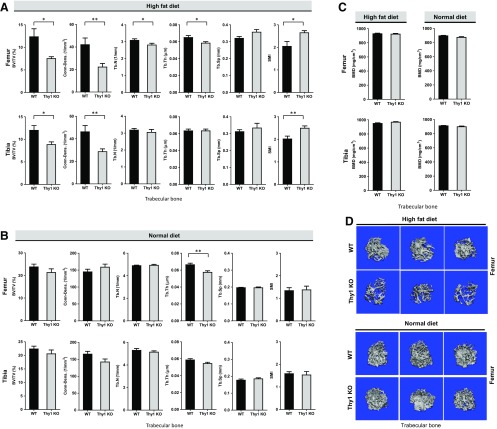
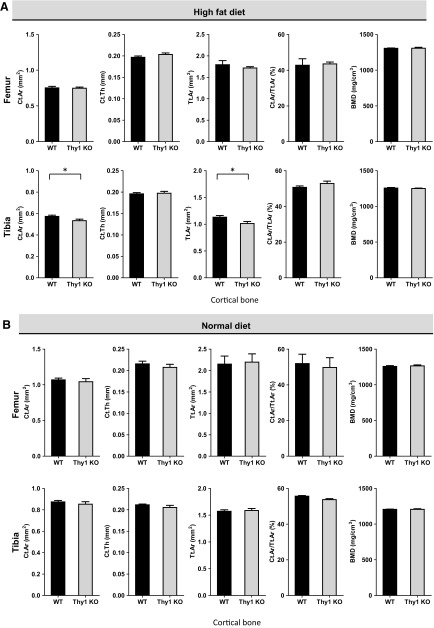
We had discovered that Thy1 deficiency leads to increased obesity in HFD-fed mice (12). To learn whether Thy1 deficiency affects bone homeostasis in obese mice, we analyzed the bone structural parameters in Thy1 KO mice compared with WT mice after consuming an HFD. Morphometric analysis of long bones from Thy1 KO and WT mice fed an HFD using micro-CT revealed significant differences in analyzed key bone structural parameters between WT and Thy1 KO mice (Fig. 1). In particular, a significant reduction in trabecular bone mass was noted especially in obese Thy1 KO mice compared to the obese WT mice (Fig. 1A, D). The most striking differences were apparent in the percentage trabecular bone volume density fraction (% BV/TV) and trabecular bone connective density (Fig. 1A). In femoral trabecular bone of Thy1 KO mice, we observed up to a 50% decrease in bone volume fraction (% BV/TV). The differences in % BV/TV were statistically significant (P < 0.05), and, overall, more than a 45% decrease in trabecular bone connective density was noted in the femurs of KO mice (P < 0.001). We also found similar differences in the tibial bone: BV/TV (P < 0.05) and bone connective density. We also found a reduction in trabecular number (Tb.N) and trabecular thickness (Tb.Th) and a minor increase in trabecular spacing (Tb.Sp) in Thy1 KO obese mice compared to obese WT mice. We also noted that the structural model index (SMI), a measure of the shape of trabeculae (19), was significantly higher (24–30% higher) in obese Thy1 KO mice compared to WT controls reflecting more rod-like morphology of trabeculae in obese Thy1 KO mice compared to relatively more plate-like morphology in the WT mice. We also compared the bone mineral density (BMD) measures obtained from micro-CT analysis of the long bones but did not find any significant differences between the obese WT and Thy1 KO mice (Fig. 1C). In contrast to the notable differences in many key structural parameters of the femoral trabecular bone, no significant differences were noted in femoral cortical bone from obese Thy1 KO and WT mice. However, the tibial cortical bone thickness was significantly decreased in obese Thy1 KO compared to obese WT mice although the magnitude of the differences were comparatively smaller when compared to trabecular bone parameters (Fig. 2A).
Figure 1.
Reduced trabecular bone mass and changes in structural parameters in Thy1-deficient obese mice. A, B) Long bones from HFD (A) or normal diet (B) fed WT and Thy1 KO mice were analyzed with micro-CT to compare changes in bone architecture and structural parameters. BV/TV, trabecular bone connective density (Conn-Dens.), trabecular number (Tb.N), trabecular thickness (Tb.Th), trabecular space (Tb.Sp), and structural model index (SMI) were selected as measures of trabecular femoral and tibial bone mass and structure. C) Measurement of BMD for femur and tibia. D) Representative micro-CT images of the femoral trabecular bone in WT and Thy1 KO mice. Data are means ± se (n = 6 mice/group). *P < 0.05, **P < 0.001 (unpaired Welch’s t test).
Figure 2.
Reduced cortical bone in HFD-fed Thy1-deficient mice. Long bones from normal-diet–fed WT and Thy1 KO mice were analyzed with micro-CT to compare cortical femoral and tibial bones in HFD-fed (A) or normal-diet-fed (B) WT and Thy1 KO mice. Cortical bone area (Ct.Ar), cortical thickness (Ct.Th), total cross-sectional area (Tt.Ar), and cortical bone area fraction (Ct.Ar/Tt.Ar) were selected as measures of cortical bone parameters (n = 6 mice/group). Data are means ± se. *P < 0.05, **P < 0.001 (unpaired Welch’s t test).
We also compared the long bones of normal-diet–fed WT and Thy1 KO mice by using micro-CT, and our analysis revealed modest differences in terms of some of the analyzed bone structural parameters (Fig. 1B). Our micro-CT analysis revealed that trabecular bone area (BV/TV) and Tb.Th in Thy1 KO were modestly lower, compared with WT, whereas other bone structural parameters, such as Tb.N, Tb.Sp, and SMI, were comparable. Unlike the notable difference in trabecular bone connective density of the long bones from HFD-fed obese Thy1 KO and WT mice, in the femur of normal-diet–fed WT and Thy1 KO mice, connective density was shown to be comparable, whereas in the tibia, it appeared to be decreased modestly in the Thy1 KO mice. We did not find any differences in BMD measures of the trabecular bones of obese WT and Thy1 KO mice (Fig. 1C). Finally, there were no significant differences in the analyzed structural parameters of the cortical bone of normal-diet–fed Thy1 KO and WT mice (Fig. 2B). These findings together suggest that the impact of Thy1 deficiency was much more pronounced in the HFD-fed obese mice, whereas, with a normal diet, the differences in the bone structure parameters were not that apparent, although there appeared to be certain modest differences between the Thy1 KO and WT mice.
Thy1 is a positive regulator of OB differentiation
Previous studies of fibroblasts revealed that the absence of Thy1 is associated with increased accumulation of lipid droplets in these cells, providing them a lipofibroblastic phenotype (20). In our recent study, we noted a gradual decrease in Thy1 expression levels during adipogenesis from the adipogenic stem progenitor cells and decreased adipogenesis in cells that overexpress Thy1 (12). Considering the distinct differentiation pathways of adipocytes and OBs from mesenchymal cell progenitors, we hypothesized that the lower trabecular volume and connectivity in obese Thy1 KO mice arises from decreased osteoblastogenesis and OB activity.
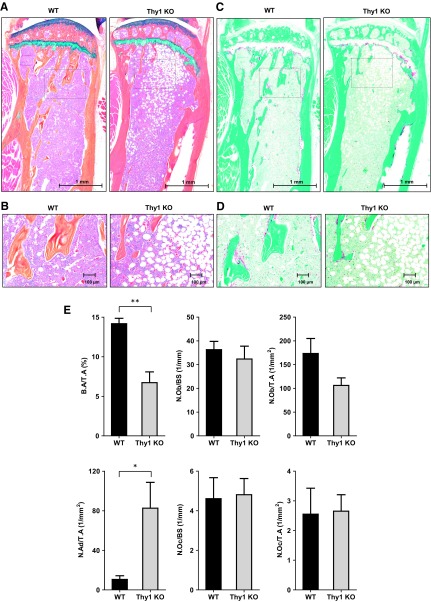
Thus, to determine the cellular mechanisms of Thy1 regulation of bone homeostasis in obese mice, we performed histomorphometric analysis with the tibial sections from the obese WT and Thy1 KO mice. Consistent with micro-CT measurements, we observed decreased % B.A/T.A in the obese Thy1 KO mice compared with obese WT mice (Fig. 3A, E). We further analyzed the number of OBs and OCs over the BS and the T.A. We did not find any statistically significant differences in N.Ob/BS in tibial section from the obese Thy1 KO as compared to WT obese mice (Fig. 3E) but noted a decreased trend in the overall number of OBs over the T.A. In addition, we noted a significant increase in bone marrow adipocytes in the tibial section from the obese Thy1 KO mice compared to obese WT mice (Fig. 3E). On the contrary, we did not find significant differences in N.Oc/BS or N.Oc/T.A in the tibia of the obese Thy1 KO and WT mice (Fig. 3C–E). These data suggest that compromised OB activity related to loss of Thy1 in association with increased adipogenesis in vivo, rather than an altered number of OCs contributing to decreased bone mass in Thy1 KO obese mice.
Figure 3.
Obese Thy1 KO mice demonstrate decreased bone volume. Bones from 4-mo-old Thy1 KO mice and WT littermates were analyzed. A) Representative image of longitudinal sections of the tibia stained with Alcian blue, hematoxylin, orange-G, phloxine-B, eosin. Scale bars, 1 mm. Boxes denote areas shown in higher magnification in the subsequent figure. B) Selected areas (box) from tibial sections shown at higher magnification. Scale bars, 100 μm. C) Representative image of longitudinal sections of the tibia stained with TRAP. Scale bars, 1 mm. D) Selected areas (box) from TRAP stained tibial section shown at higher magnification. Scale bars, 100 μm. E) Histomorphometric analyses of % B.A/T.A, N.Ob/BS, N.Ob/T.A, N.Oc/BS, N.Oc/T.A, and N.Ad/T.A (n = 4–5 mice/group). Data are means ± se. *P < 0.05, **P < 0.001 (unpaired Welch’s t test).
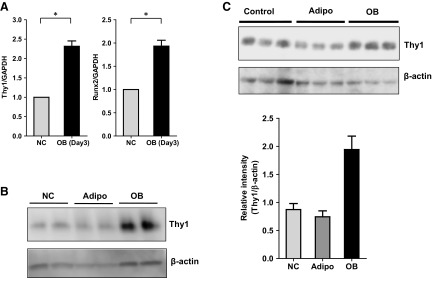
We also performed in vitro experiments with bone-derived osteoblastogenic progenitor cells from WT mice and noted upregulation of Thy1 mRNA expression levels when these cells were cultured in osteogenic media (Fig. 4A) that correlated with increased expression of the key osteogenic gene, Runx2. Runx2 plays essential role in OB differentiation and is known to be upregulated in osteogenic conditions (21). To further investigate the role of Thy1 in OB differentiation, we used the 3T3-L1, MC3T3-E1, and MEFs derived from WT and Thy1 KO mouse embryos as described (12). All 3 cell types can serve as OB progenitor cells for in vitro analyses (21–23). We had reported that Thy1 expression levels in both 3T3-L1 and MEFs decline during adipogenesis (12). When these cell lines were cultured in osteogenic media; however, Thy1 expression was maintained or increased during osteogenic differentiation in 3T3-L1 and MEFs (Fig. 4B, C). Thus, in both 3T3-L1 and MEFs, Thy1 expression declined in adipogenic media and was maintained or induced in osteogenic media, suggesting a regulatory role for Thy1 in adipogenesis and osteogenesis pathways.
Figure 4.
Thy1 is upregulated during osteoblastogenesis. A) Bone-derived mesenchymal stroma cells from WT C57BL/6 mice were cultured in osteogenic conditions for 3 d and expression of OB differentiation marker, Runx2, and Thy1 levels were monitored by real-time qPCR analysis (n = 5). *P < 0.05 (Wilcoxon signed-rank test). B) 3T3-L1 cells were cultured in adipogenic or osteogenic conditions for 7 d, and expression levels of Thy1 protein were compared by Western blot analysis. C) MEFs were cultured in adipogenic or osteogenic conditions for 10 d and protein expression levels of Thy1 protein was compared by Western blot analysis (n = 3). Data are presented as means ± se; Friedman test followed by Dunn’s post hoc test.
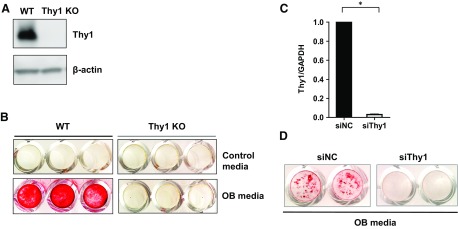
To further examine the importance of Thy1 in osteogenesis, we used Thy1 KO MEFs and Thy1 siRNA. Thy1 KO MEFs show no Thy1 expression compared with WT MEFs, which show robust Thy1 expression (Fig. 5A). These cells were cultured in osteogenic media for 21 d. After 21 d, cells were stained with Alizarin red, and results showed dramatically reduced bone mineralization in Thy KO MEFs compared with control cells cultured under the same conditions (Fig. 5B). Similarly, we studied the impact of siRNA-mediated knockdown of Thy1 in preosteoblastic MC3T3-E1 cells whereby Thy1 siRNA reduced Thy1 expression levels by more than 95% after 3 d (Fig. 5C). Serial cultures of Thy1 siRNA–treated MC3T3-E1 cells in osteogenic conditions demonstrated impaired mineralization activity, as revealed by the reduced Alizarin red staining compared to control cells treated with nonspecific control siRNAs (Fig. 5D). Taken together, these findings support the concept that Thy1 is a critical positive regulator of OB differentiation.
Figure 5.
Thy1 is required for OB differentiation. A) WT and Thy1 KO MEFs were cultured in osteogenic condition for 10 d and Thy1 expression levels were analyzed by Western blot. B) WT and Thy1 KO MEFs were cultured in osteogenic condition for 21 d and thereafter OB differentiation and mineralization were analyzed by Alizarin red staining. C) Thy1 mRNA expression levels in MC3T3-E1 cells treated with scramble siRNA or Thy1 siRNA were monitored by real-time qPCR analysis. Data are means ± se (n = 6). *P < 0.05 (Wilcoxon signed-rank test). D) MC3T3-E1 cells treated with scrambled siRNA or Thy1 siRNA were cultured in osteogenic conditions for 10 d, and OB differentiation and mineralization were analyzed by Alizarin red staining. Images shown are representative of 3 independent experiments.
DISCUSSION
We found that trabecular bone mass and connectivity in the femur and tibia was significantly decreased in obese Thy1-deficient mice compared with obese WT control animals. Specifically, significantly lower BV/TV and lower trabecular connective density were noted in obese Thy1 KO mice. BV/TV serves as a key microstructural parameter of trabecular bone. Similarly, bone connectivity is one of the structural properties of the trabecular bone (24) considered a key component of bone structure (25). Bone with well-connected trabecular networks adds to the overall mechanical strength and protects cortical bone by preventing the retention and accumulation of local micro and macro damage through the diffusion of mechanical forces immediately through the trabecular bone network (17). Bones with a well-developed trabecular bone network have increased overall strength. We also noted the SMI values for the trabecular femoral and tibial bones were more than 20% higher for obese Thy1 KO mice compared to WT controls (Fig. 1A) reflecting rod-like morphology of trabeculae in obese Thy1 KO mice compared to the plate-like morphology in the WT mice. SMI is a measure to quantify the characteristic form of a 3-dimensional described structure in terms of the amount of plates and rods composing the structure (0 for plates and 3 for cylindrical rods) (19). Similar to the BV/TV, the SMI is known to significantly correlate with the apparent modulus and stiffness of bone (26). It is also known that deterioration of cancellous bone structure caused by aging and disease is characterized by a conversion from plate elements to rod elements and thus higher SMI values indicate less overall mechanical strength and higher susceptibility for bending and buckling failure modes (19, 27). Thus, higher SMI values in the case of cancellous bone of the obese Thy1 KO mice suggest bone from these mice are more prone to microdamage and more susceptible to bending and buckling failure modes compared to bones of obese WT mice. Detailed analysis of the cortical bone parameters obtained by micro-CT revealed some minor but statistically significant differences especially in the tibial cortical bone only when the WT and Thy1 KO mice were fed an HFD (Fig. 2A), whereas no such differences were noted when mice were fed a normal diet (Fig. 2B). It is also important to note that according to earlier report, WT C57BL/6 mice fed an HFD demonstrate increased marrow adiposity with only minor changes in the bone structural parameters (28). Thus, our current findings of notable reductions in the key quantitative measures of bone structural parameters in the HFD-fed obese Thy1 KO mice compared to the HFD-fed obese WT mice support the concept that Thy1 is necessary for optimal bone development and homeostasis.
We also demonstrated that Thy1 is a positive regulator of OB differentiation and Thy1 deficiency is associated with a significant decrease in OB differentiation. Our findings from histomorphometric analysis indicated that reduced osteogenesis and not an altered number of OCs contribute to lower trabecular bone mass in obese Thy1 KO mice. Findings from our analysis further suggest reduced OB activity is likely to be the major variable in the altered bone parameters recorded in the obese Thy1 KO mice. In concert with our current findings, it has been noted that Thy1-positive selection of human adipose-derived stromal cells enhances their overall osteogenic capacity (29). Similarly, periosteal cells sorted based on Thy1 expression showed higher proliferative capacity and osteogenic potential in vitro and in vivo compared with unsorted periosteum-derived cell populations (30). Similarly, subodontoblastic dental pulp stem cells expressing higher Thy1 levels on the cell surface showed accelerated induction of alkaline phosphatase activity and mineralized matrix formation and a higher capacity to form hard tissue compared to the cells with lower expression of Thy1 (31). Taken together, these findings coupled with the reports outlined above, establish Thy1 as a positive regulator of OB differentiation that is centrally involved in bone homeostasis, especially in the context of obesity.
Findings from our current study indicated changes in OBs possibly combined with increased adipogenesis and not changes in number of OCs as the major cause of the reduced trabecular bone mass and altered structure in obese Thy1 KO mice, but further mechanistic details remain to be elucidated and will be addressed in future studies. The relationship between the increased number of adipocytes in the bone marrow and how these cells may or not affect bone homeostasis in obese Thy1 KO mice as compared to obese WT mice remains to be determined, and how deficiency of Thy1 alters the fate and type of adipogenesis in bone marrow also requires further study.
The relationship between obesity and bone homeostasis is complex. Obesity has been considered a protective factor against bone loss and osteoporosis (32, 33). Yet, recent studies suggest that increased abdominal fat tissue is a risk factor for decreased BMD and osteoporosis, both in women and in men (34–36). Increased marrow fat content coupled with a reduced number of osteoblastic cells has been noted in patients with osteoporosis (37–39). Increased bone marrow adiposity has also been observed in conditions associated with bone loss, such as obesity, aging, and other pathologies (37, 38). A better understanding of the relationship between obesity, adiposity, and bone homeostasis will provide insights into strategies that restore the normal balance between formation of bone and deposition of fat. Our current observations have established that Thy1 expression is necessary to maintain bone homeostasis, especially in the context of obesity. Based on these findings, modulation of Thy1 may serve as a promising therapeutic strategy to not only control obesity and adipogenesis but also to improve bone health.
In summary, we showed that Thy1 is necessary for OB differentiation in vitro and obese Thy1 KO mice demonstrate decreased volume and connectivity of trabecular bone compared with obese WT mice. Thy1 deficiency is associated with impaired osteoblastogenesis. These data support the hypothesis that Thy1 is a positive regulator of OB differentiation and modulates bone homeostasis, but the mechanisms that underlie these findings remain to be elucidated.
ACKNOWLEDGMENTS
The authors thank Michael Thullen, Sarah Mack, and Christopher Dean (all from the University of Rochester) for excellent technical assistance with micro-CT and histological analysis; and Cheryl Ackert-Bicknell, Edward M. Schwarz, Michael John Zuscik, Jennifer Jonason, and Thomas J. Mariani (all from the University of Rochester) for assistance and helpful scientific discussions. This study was supported by funds from the Lung Biology Strategic Plan High Risk Project Pilot Research Award, a Health Sciences Research Using High Performance Computational Resources (HSCCI) Pilot Grant and a Department of Medicine Research Pilot Projects Award from University of Rochester; and from U.S. National Institutes of Health (NIH), National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants AR0169000, AR063650, and P30AR069655, and National Institute for Translational Sciences Grant 5UL1TR000042-09. The authors declare no conflicts of interest.
Glossary
- Ad.N
adipocyte number
- BMD
bone mineral density
- BS
bone surface
- BV
bone volume
- HFD
high-fat diet
- KO
knockout
- MEFs
mouse embryonic fibroblasts
- micro-CT
micro-computed tomography
- MSC
mesenchymal stem cell
- OB
osteoblast
- Ob.N
osteoblast number
- OC
osteoclast
- Oc.N
osteoclast number
- qPCR
quantitative PCR
- siRNA
small interfering RNA
- SMI
structural model index
- T.A
tissue area
- Tb.N
trabecular number
- Tb.Sp
trabecular spacing
- Tb.Th
trabecular thickness
- TRAP
tartrate-resistant acidic phosphatase
- TV
total volume
- WT
wild type
AUTHOR CONTRIBUTIONS
A. Paine and C. F. Woeller designed the research study; A. Paine, C. F. Woeller, H. Zhang, M. L. Garcia-Hernandez, and N. Huertas performed the experiments; A. Paine, C. F. Woeller, H. Zhang, L. Xing, R. P. Phipps, and C. T. Ritchlin analyzed the data; and A. Paine, C. F. Woeller, L. Xing, R. P. Phipps, and C. T. Ritchlin wrote the paper.
REFERENCES
- 1.Bar-Shavit Z. (2007) The osteoclast: a multinucleated, hematopoietic-origin, bone-resorbing osteoimmune cell. J. Cell. Biochem. 102, 1130–1139 10.1002/jcb.21553 [DOI] [PubMed] [Google Scholar]
- 2.Rodan G. A. (1998) Bone homeostasis. Proc. Natl. Acad. Sci. USA 95, 13361–13362 10.1073/pnas.95.23.13361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Komori T. (2006) Regulation of osteoblast differentiation by transcription factors. J. Cell. Biochem. 99, 1233–1239 10.1002/jcb.20958 [DOI] [PubMed] [Google Scholar]
- 4.Caplan A. I. (1991) Mesenchymal stem cells. J. Orthop. Res. 9, 641–650 10.1002/jor.1100090504 [DOI] [PubMed] [Google Scholar]
- 5.Chen Q., Shou P., Zheng C., Jiang M., Cao G., Yang Q., Cao J., Xie N., Velletri T., Zhang X., Xu C., Zhang L., Yang H., Hou J., Wang Y., Shi Y. (2016) Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ. 23, 1128–1139 10.1038/cdd.2015.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horwitz E. M., Prockop D. J., Fitzpatrick L. A., Koo W. W. K., Gordon P. L., Neel M., Sussman M., Orchard P., Marx J. C., Pyeritz R. E., Brenner M. K. (1999) Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat. Med. 5, 309–313 10.1038/6529 [DOI] [PubMed] [Google Scholar]
- 7.Pino A. M., Rosen C. J., Rodríguez J. P. (2012) In osteoporosis, differentiation of mesenchymal stem cells (MSCs) improves bone marrow adipogenesis. Biol. Res. 45, 279–287 10.4067/S0716-97602012000300009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reif A. E., Allen J. M. (1964) Immunological distinction of Akr thymocytes. Nature 203, 886–887 10.1038/203886a0 [DOI] [PubMed] [Google Scholar]
- 9.Phipps R. P., Penney D. P., Keng P., Quill H., Paxhia A., Derdak S., Felch M. E. (1989) Characterization of two major populations of lung fibroblasts: distinguishing morphology and discordant display of Thy 1 and class II MHC. Am. J. Respir. Cell Mol. Biol. 1, 65–74 10.1165/ajrcmb/1.1.65 [DOI] [PubMed] [Google Scholar]
- 10.McNiece I. (2000) The CD34+Thy1+ cell population: are they all stem cells? Exp. Hematol. 28, 1312–1314 10.1016/S0301-472X(00)00609-3 [DOI] [PubMed] [Google Scholar]
- 11.Rege T. A., Hagood J. S. (2006) Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. FASEB J. 20, 1045–1054 10.1096/fj.05-5460rev [DOI] [PubMed] [Google Scholar]
- 12.Woeller C. F., O’Loughlin C. W., Pollock S. J., Thatcher T. H., Feldon S. E., Phipps R. P. (2015) Thy1 (CD90) controls adipogenesis by regulating activity of the Src family kinase, Fyn. FASEB J. 29, 920–931 10.1096/fj.14-257121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woeller C. F., Flores E., Pollock S. J., Phipps R. P. (2017) Editor’s highlight: Thy1 (CD90) expression is reduced by the environmental chemical tetrabromobisphenol-A to promote adipogenesis through induction of microRNA-103. Toxicol. Sci. 157, 305–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X. D., Qian H. Y., Neff L., Satomura K., Horowitz M. C. (1999) Thy-1 antigen expression by cells in the osteoblast lineage. J. Bone Miner. Res. 14, 362–375 10.1359/jbmr.1999.14.3.362 [DOI] [PubMed] [Google Scholar]
- 15.Zhang L., Chang M., Beck C. A., Schwarz E. M., Boyce B. F. (2016) Analysis of new bone, cartilage, and fibrosis tissue in healing murine allografts using whole slide imaging and a new automated histomorphometric algorithm. Bone Res. 4, 15037. 10.1038/boneres.2015.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi J., Liang Q., Zuscik M., Shen J., Chen D., Xu H., Wang Y. J., Chen Y., Wood R. W., Li J., Boyce B. F., Xing L. (2014) Distribution and alteration of lymphatic vessels in knee joints of normal and osteoarthritic mice. Arthritis Rheumatol. 66, 657–666 10.1002/art.38278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehmann G. M., Woeller C. F., Pollock S. J., O’Loughlin C. W., Gupta S., Feldon S. E., Phipps R. P. (2010) Novel anti-adipogenic activity produced by human fibroblasts. Am. J. Physiol. Cell Physiol. 299, C672–C681 10.1152/ajpcell.00451.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paine A., Kirchner H., Immenschuh S., Oelke M., Blasczyk R., Eiz-Vesper B. (2012) IL-2 upregulates CD86 expression on human CD4(+) and CD8(+) T cells. J. Immunol. 188, 1620–1629 10.4049/jimmunol.1100181 [DOI] [PubMed] [Google Scholar]
- 19.Hildebrand T., Rüegsegger P. (1997) Quantification of bone microarchitecture with the structure model index. Comput. Methods Biomech. Biomed. Engin. 1, 15–23 10.1080/01495739708936692 [DOI] [PubMed] [Google Scholar]
- 20.Koumas L., Smith T. J., Feldon S., Blumberg N., Phipps R. P. (2003) Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. Am. J. Pathol. 163, 1291–1300 10.1016/S0002-9440(10)63488-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi T. (2011) Overexpression of Runx2 and MKP-1 stimulates transdifferentiation of 3T3-L1 preadipocytes into bone-forming osteoblasts in vitro. Calcif. Tissue Int. 88, 336–347 10.1007/s00223-011-9461-9 [DOI] [PubMed] [Google Scholar]
- 22.Quarles L. D., Yohay D. A., Lever L. W., Caton R., Wenstrup R. J. (1992) Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: an in vitro model of osteoblast development. J. Bone Miner. Res. 7, 683–692 10.1002/jbmr.5650070613 [DOI] [PubMed] [Google Scholar]
- 23.Garreta E., Genové E., Borrós S., Semino C. E. (2006) Osteogenic differentiation of mouse embryonic stem cells and mouse embryonic fibroblasts in a three-dimensional self-assembling peptide scaffold. Tissue Eng. 12, 2215–2227 10.1089/ten.2006.12.2215 [DOI] [PubMed] [Google Scholar]
- 24.Rege T. A., Hagood J. S. (2006) Thy-1, a versatile modulator of signaling affecting cellular adhesion, proliferation, survival, and cytokine/growth factor responses. Biochim. Biophys. Acta 1763, 991–999 10.1016/j.bbamcr.2006.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Genant H. K., Jiang Y. (2006) Advanced imaging assessment of bone quality. Ann. N. Y. Acad. Sci. 1068, 410–428 10.1196/annals.1346.038 [DOI] [PubMed] [Google Scholar]
- 26.Issever A. S., Link T. M., Kentenich M., Rogalla P., Schwieger K., Huber M. B., Burghardt A. J., Majumdar S., Diederichs G. (2009) Trabecular bone structure analysis in the osteoporotic spine using a clinical in vivo setup for 64-slice MDCT imaging: comparison to microCT imaging and microFE modeling. J. Bone Miner. Res. 24, 1628–1637 10.1359/jbmr.090311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen H., Zhou X., Fujita H., Onozuka M., Kubo K. Y. (2013) Age-related changes in trabecular and cortical bone microstructure. Int. J. Endocrinol. 2013, 213234. 10.1155/2013/213234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doucette C. R., Horowitz M. C., Berry R., MacDougald O. A., Anunciado-Koza R., Koza R. A., Rosen C. J. (2015) A high fat diet increases bone marrow adipose tissue (MAT) but does not alter trabecular or cortical bone mass in C57BL/6J mice. J. Cell. Physiol. 230, 2032–2037 10.1002/jcp.24954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung M. T., Liu C., Hyun J. S., Lo D. D., Montoro D. T., Hasegawa M., Li S., Sorkin M., Rennert R., Keeney M., Yang F., Quarto N., Longaker M. T., Wan D. C. (2013) CD90 (Thy-1)-positive selection enhances osteogenic capacity of human adipose-derived stromal cells. Tissue Eng. Part A 19, 989–997 10.1089/ten.tea.2012.0370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim Y. K., Nakata H., Yamamoto M., Miyasaka M., Kasugai S., Kuroda S. (2016) Osteogenic potential of mouse periosteum-derived cells sorted for CD90 in vitro and in vivo. Stem Cells Transl. Med. 5, 227–234 10.5966/sctm.2015-0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hosoya A., Hiraga T., Ninomiya T., Yukita A., Yoshiba K., Yoshiba N., Takahashi M., Ito S., Nakamura H. (2012) Thy-1-positive cells in the subodontoblastic layer possess high potential to differentiate into hard tissue-forming cells. Histochem. Cell Biol. 137, 733–742 10.1007/s00418-012-0928-1 [DOI] [PubMed] [Google Scholar]
- 32.Albala C., Yáñez M., Devoto E., Sostin C., Zeballos L., Santos J. L. (1996) Obesity as a protective factor for postmenopausal osteoporosis. Int. J. Obes. Relat. Metab. Disord. 20, 1027–1032 [PubMed] [Google Scholar]
- 33.Ribot C., Trémollières F., Pouillès J. M. (1994) The effect of obesity on postmenopausal bone loss and the risk of osteoporosis. Adv. Nutr. Res. 9, 257–271 [DOI] [PubMed] [Google Scholar]
- 34.Watts N. B.; GLOW Investigators . (2014) Insights from the Global Longitudinal Study of Osteoporosis in Women (GLOW). Nat. Rev. Endocrinol. 10, 412–422 10.1038/nrendo.2014.55 [DOI] [PubMed] [Google Scholar]
- 35.Søgaard A. J., Holvik K., Omsland T. K., Tell G. S., Dahl C., Schei B., Falch J. A., Eisman J. A., Meyer H. E. (2015) Abdominal obesity increases the risk of hip fracture: a population-based study of 43,000 women and men aged 60-79 years followed for 8 years: cohort of Norway. J. Intern. Med. 277, 306–317 10.1111/joim.12230 [DOI] [PubMed] [Google Scholar]
- 36.Compston J. E., Flahive J., Hosmer D. W., Watts N. B., Siris E. S., Silverman S., Saag K. G., Roux C., Rossini M., Pfeilschifter J., Nieves J. W., Netelenbos J. C., March L., LaCroix A. Z., Hooven F. H., Greenspan S. L., Gehlbach S. H., Díez-Pérez A., Cooper C., Chapurlat R. D., Boonen S., Anderson F. A., Jr., Adami S., Adachi J. D., Investigators G.; GLOW Investigators . (2014) Relationship of weight, height, and body mass index with fracture risk at different sites in postmenopausal women: the Global Longitudinal study of Osteoporosis in Women (GLOW). J. Bone Miner. Res. 29, 487–493 10.1002/jbmr.2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meunier P., Aaron J., Edouard C., Vignon G. (1971) Osteoporosis and the replacement of cell populations of the marrow by adipose tissue: a quantitative study of 84 iliac bone biopsies. Clin. Orthop. Relat. Res. 80, 147–154 10.1097/00003086-197110000-00021 [DOI] [PubMed] [Google Scholar]
- 38.Justesen J., Stenderup K., Ebbesen E. N., Mosekilde L., Steiniche T., Kassem M. (2001) Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2, 165–171 10.1023/A:1011513223894 [DOI] [PubMed] [Google Scholar]
- 39.Yeung D. K., Griffith J. F., Antonio G. E., Lee F. K., Woo J., Leung P. C. (2005) Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: a proton MR spectroscopy study. J. Magn. Reson. Imaging 22, 279–285 10.1002/jmri.20367 [DOI] [PubMed] [Google Scholar]