Abstract
No clinically approved therapies are currently available that prevent the onset of photoreceptor death in retinal degeneration. Signaling between retinal neurons is regulated by the release and uptake of neurotransmitters, wherein GABA is the main inhibitory neurotransmitter. In this work, novel 3-chloropropiophenone derivatives and the clinical anticonvulsants tiagabine and vigabatrin were tested to modulate GABA signaling and protect against light-induced retinal degeneration. Abca4−/−Rdh8−/− mice, an accelerated model of retinal degeneration, were exposed to intense light after prophylactic injections of one of these compounds. Imaging and functional assessments of the retina indicated that these compounds successfully protected photoreceptor cells from degeneration to maintain a full-visual-field response. Furthermore, these compounds demonstrated a strong safety profile in wild-type mice and did not compromise visual function or damage the retina, despite repeated administration. These results indicate that modulating inhibitory GABA signaling can offer prophylactic protection against light-induced retinal degeneration.—Schur, R. M., Gao, S., Yu, G., Chen, Y., Maeda, A., Palczewski, K., Lu, Z.-R. New GABA modulators protect photoreceptor cells from light-induced degeneration in mouse models.
Keywords: anticonvulsant, GABA aminotransferase, retinal degeneration
Age-related macular degeneration (AMD) is a leading cause of blindness in the elderly population (1). Currently, no treatments are available for individuals with dry AMD or other types of nonexudative retinal dystrophies, such as Stargardt disease. Early in the pathogenesis of retinal degeneration, drusen accumulate under the retina and alterations appear in the retinal pigment epithelium (RPE). Aberrant processing of retinoids in the visual cycle causes buildup of toxic byproducts of this pathway, such as all-trans retinal, which can also condense into bisretinoids and accumulate lipofuscin within the lysosomal compartments of the RPE (2). Toxic retinoid byproducts place continuous oxidative stress on these cells, fueling chronic inflammation. Ultimately, these processes trigger apoptosis of both photoreceptors and the RPE, leading to large areas of cell death in the form of geographic atrophy (3, 4). New treatment strategies for dry AMD focus on preventing the loss of RPE and photoreceptor cells (5). These approaches include antioxidant supplementation (6, 7); visual cycle modulators that slow the visual cycle to limit lipofuscin formation (8–11); and neuroprotection with growth factors (12, 13), antiamyloid-β therapies (14), and serotonin receptor agonists (15–17). Unfortunately, many of these compounds have failed in clinical trials or caused visual system toxicity (18–20). New approaches are needed to safely prevent retinal degeneration.
Communication between retinal neurons is dominated by the neurotransmitter-mediated chemical signaling (21, 22) that occurs at the synaptic terminals in the outer and inner plexiform layers. Excitatory signaling progresses vertically through the retina and is mediated primarily by glutamate at photoreceptor–bipolar cell and bipolar–ganglion cell junctions. Inhibitory signaling progresses laterally via horizontal cells and amacrine cells and is primarily mediated by GABA. Extracellular availability of neurotransmitters is further regulated by uptake and recycling in Müller glia (23). Additional neuroactive amino acids, including glutamate, glutamine, aspartate, taurine, and dopamine, are crucial for the metabolism of neurotransmitters and provide pools of monocarboxylates that feed into the tricarboxylic acid (TCA) cycle (22, 24, 25).
Cellular processes that regulate neurotransmitter availability and metabolism are disrupted during retinal degeneration. Glial cells, that sequester neurotransmitters to reduce their extracellular availability, become activated and undergo gliosis in response to retinal degeneration (23). Furthermore, cellular metabolism, fueled by the TCA cycle that obtains its monocarboxylate substrates from neurotransmitter pools via the GABA shunt, is affected during degeneration, resulting in oxidative and nonoxidative stress (26, 27). In conjunction with these processes, concentrations of neuroactive amino acids are also sensitive to exposure to light (28) and retinal degeneration (27, 29–33). Thus, we suspect that pharmacologic manipulation of retinal neurotransmitter function protects the retina under degenerative conditions.
GABA modulatory drugs are clinically available as anticonvulsants. In patients with epilepsy, overexcitation of neurons causes seizures, which are controlled pharmacologically by decreasing excitatory signaling and increasing inhibitory signaling (34). Glutamate excitotoxicity contributes to retinal degeneration during ischemic damage that has been implicated in the pathogenesis of diseases such as glaucoma and retinal ischemia (35), and reports have demonstrated that anticonvulsants can prevent excitotoxic damage to neural ganglion cells in the inner retina. For example, neuroprotection against NMDA- and monosodium glutamate–induced excitotoxicity in ganglion cells has been reported for clinically validated anticonvulsants, including tiagabine (TGB) (36), vigabatrin (VGB), topiramate (37), and valproic acid (38, 39), as well as endogenous neuroprotective agents, such as taurine (40) and pituitary adenylate cyclase–activating polypeptide (41, 42). Several of the agents that act on neurotransmitter pathways, including pregabalin (43), valproic acid (44), and tauroursodeoxycholic acid (45, 46), also have prevented photoreceptor loss in genetic models of retinal degeneration.
In this work, GABA modulatory compounds were evaluated for prevention of photoreceptor loss in mouse models of light-induced retinal degeneration. Clinical anticonvulsants and novel 3-chloropropiophenone derivatives provided structural and functional protection of the retina in a light-sensitive Abca4−/−Rdh8−/− mouse model of retinal degeneration. Moreover, the chloride derivatives demonstrated a safe profile and effective protection against light-induced retinal degeneration in wild-type BALB/c mice.
MATERIALS AND METHODS
Materials
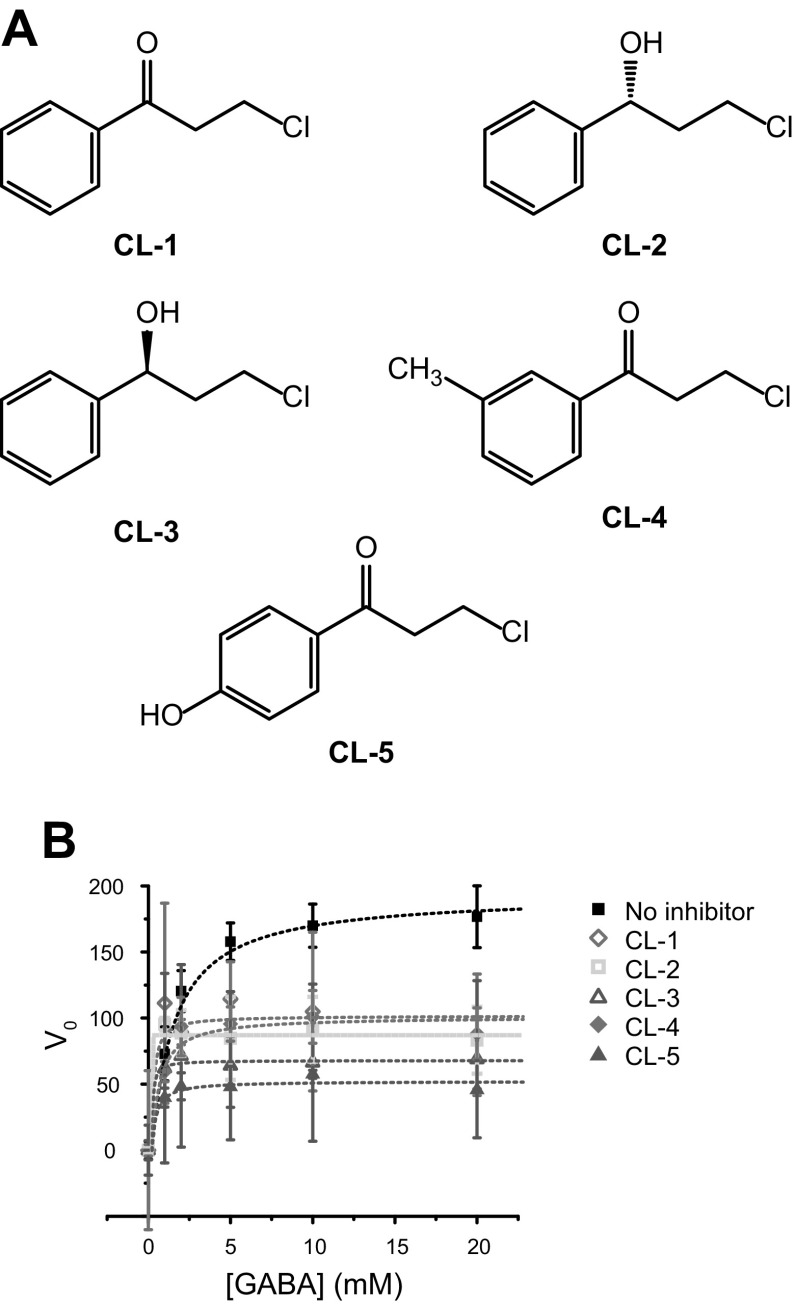
The chemical names, abbreviations, and doses of the chloride derivatives are listed in Table 1, and their structures are shown in Fig. 1A. CL-1, -2, and -3 were purchased from MilliporeSigma (Billerica, MA, USA); CL-4 was purchased from Oakwood Chemical (Estill, SC, USA); and CL-5, TGB, and VGB were from Toronto Research Chemicals (Toronto, ON, Canada).
TABLE 1.
Compounds tested for protection from light-induced retinal degeneration in Abca4−/−Rdh8−/− double-knockout mice
| No. | Compound name | Dose males (mg) | Dose females (mg) |
|---|---|---|---|
| CL-1 | 3-Chloropropiophenone | 0.2 | 1 |
| CL-2 | (R)(+)3-Chloro-1-phenyl-1-propanol | 0.2 | 1 |
| CL-3 | (S)(−)3-Chloro-1-phenyl-1-propanol | 0.2 | 1 |
| CL-4 | 3-Chloro-1-(3-methylphenyl)-1-oxopropanone | 0.2 | 0.5 |
| CL-5 | 3-Chloro-1-(4-hydroxyphenyl)-propan-1-one | 0.5 | 1 |
Figure 1.
Chloride derivatives for inhibition of GABA-AT. A) Structures of the chloride derivatives. B) Michaelis-Menten kinetics of GABase in the presence of various concentrations of GABA substrate and 10 µM chloride derivatives. V0 plotted as a function of GABA substrate concentration.
Enzyme studies
All materials for enzyme studies were purchased from MilliporeSigma. Enzyme conditions were modified from a published protocol (47, 48). Experiments were conducted at 25°C in Tris-HCl buffer (80 mM, pH 9.0), containing 750 mM sodium sulfate, 10 mM 2-ME, 2 mM α-ketoglutarate, 1.4 mM NAD+, 300 µg/ml GABase [1:1 mixture of GABA-aminotransferase (AT) and succinic semialdehyde dehydrogenase (SSADH)], and various concentrations of GABA. Chloride derivatives were dissolved in DMSO and added to the reaction at a final concentration of 10 μM (1% v/v DMSO) and were mixed in a 96-well plate (100 µl/well). Experiments were performed in triplicate. NAD+→NADH turnover was monitored spectrophotometrically every 30 s for 30 min (λex = 340 nm, λem = 460 nm). To measure Michaelis-Menten parameters, the GABA concentration was varied from 0 to 20 mM, and V0 was calculated from the slope of the first 10 min of enzymatic turnover. Enzyme kinetics were fit to the Michaelis-Menten equation (MatLab; MathWorks, Natick, MA, USA) (Eq. 1):
 |
Spectrophotometric measurements were acquired in clear 96-well plates (Corning Costar; Thermo Fisher Scientific, Waltham, MA, USA) in a SpectraMax M5 plate reader with fluorescence output (Molecular Devices, CA, USA).
Animals
Male and female Abca4−/−Rdh8−/− (RRID:MGI:4410294) double-knockout mice were generated (43), and both male and female mice were genotyped, using published protocols, to ensure homozygosity for the Leu450 allele of Rpe65 (49) and the absence of Crb1/rd8 and Pde6/rd1 mutations (50). This mouse model is light sensitive, and retinal degeneration can be accelerated by exposure to intense light (2, 51, 52). Male wild-type BALB/c (RRID:IMSR_JAX:000651) and C57BL/6 (RRID:IMSR_JAX:000664) mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). All animal interventions were initiated when mice were 4 wk old. The animals were housed and bred in the Animal Resource Center at Case Western Reserve University, and were cared for according to an approved protocol by the Case Western Reserve University Institutional Animal Care and Use Committee and in compliance with recommendations from the American Veterinary Medical Association Panel on Euthanasia and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Treatment experiments
For assessment of treatment efficacy, test compounds dissolved in 60 µl DMSO were administered by intraperitoneal injection into 4-wk-old mice 30 min before light exposure. Pilot dose-determination experiments were performed with injections ranging from 0.1 mg (6.67 mg/kg) to 2 mg (133.3 mg/kg) per mouse. Vehicle control animals were given 60 μl DMSO by the same procedures as were used for the drug-treated mice. After the application of 1% tropicamide for pupil dilation, the animals were placed in a white bucket. Abca4−/−Rdh8−/− mice were illuminated at 10,000 lux for either 30 min (males) or 45 min (females; Supplemental Fig. 1) (n = 5 mice/group; males and females evaluated separately). BALB/c mice were illuminated at 5000 lux for 2 h (males, n = 3/group). No more than 2 mice were placed in each bucket during the exposure to light.
Optical coherence tomography
Images of mouse retinal structures were obtained in vivo with ultra–high-resolution spectral domain–optical coherence tomography (OCT) (Envisu C2200; Bioptigen, Irvine, CA, USA) (53). Images were acquired 3 d after treatment and exposure to light, with follow-up images acquired after 8 d. Before imaging, mice were anesthetized by injection of a cocktail (10 µl/g body weight, i.p.) of ketamine (6 mg/ml) and xylazine (0.44 mg/ml) in PBS buffer [10 mM sodium phosphate (pH 7.2) and 100 mM NaCl], and treated with 1% tropicamide for pupil dilation. Twenty images acquired in the B-scan mode were registered and averaged in MatLab to construct a final spectral domain–OCT image.
Scanning laser ophthalmoscopy
In vivo whole-fundus and autofluorescence imaging of mouse retinas was performed by scanning laser ophthalmoscopic (SLO) imaging in the autofluorescence mode (54) (HRAII; Heidelberg Engineering, Heidelberg, Germany) 7 d after treatment and exposure to light. Mice were anesthetized and pupils were dilated, according to the protocol used for OCT imaging. The number of autofluorescent spots in each image was counted manually.
Electroretinograms
Electroretinograms (ERGs) were acquired (55) 10 d after the treatment and exposure to light. Mice were anesthetized and dark adapted for at least 2 d before ERG recording, which was also performed in a dark room. Three electrodes were placed on the animal: a contact lens electrode on the eye, a reference electrode underneath the skin between the ears, and a ground electrode underneath the skin of the tail. ERGs were recorded with the universal electrophysiologic system UTAS E-3000 (LKC Technologies, Gaithersburg, MD, USA). Light intensity, calibrated by the manufacturer, was computer controlled. The mice were placed in a Ganzfeld dome, and photopic and scotopic responses to flash stimuli were obtained from both eyes simultaneously. A- and b-wave amplitudes were calculated in MatLab.
Histology
Mice were euthanized after ERG experiments. Whole eyes were harvested, fixed in a solution of 4% paraformaldehyde and 1% glutaraldehyde overnight, and processed and embedded in paraffin in a Tissue-Tek VIP automatic processor (Sakura Finetek, Torrance, CA, USA). Sections, 5-µm thick, were cut and stained with hematoxylin and eosin (H&E). Stained slides were imaged on a BV100 bright-field microscope (Olympus, Center Valley, PA, USA).
Safety experiment
Male albino (BALB/c) 4-wk-old mice were injected daily for 28 d with 0.2 mg (10 mg/kg, i.p.) CL-4 or -5 dissolved in a mixture of 1:1 PBS:ethanol or vehicle control (n = 5/group). Body weight was measured daily after the injection. Mice were housed in a 12-h light–dark cyclic environment for the first 26 d. OCT images were acquired on d 26. Mice were then transferred to a dark room for 2 d and dark adapted before ERG recording on d 28, and all subsequent animal handling was performed in the dark. Mice were euthanized after ERG testing, and their eyes were collected and prepared for histology as previously described.
Data and statistical analyses
OCT images and ERG traces were visualized and analyzed in MatLab. From the OCT images, the outer nuclear layer (ONL) thickness was quantified at 150, 300, 450, and 600 µm from the optic nerve head (ONH) in the temporal–nasal and superior–inferior directions. A- and b-waves were calculated from ERG traces by using a custom MatLab script. Statistical analyses were performed using a Student’s t test, and statistical significance was set at P ≤ 0.05.
RESULTS
GABA-AT inhibition
Before in vivo testing for therapeutic efficacy, the inhibitory activity of the chloride derivatives on GABA-AT was first verified by an in vitro enzyme assay (Fig. 1B). Breakdown of GABA by GABA-AT was detected via the reporter enzyme SSADH, which couples the GABA-AT–catalyzed reaction to the fluorescent product of NAD+→NADH (λem = 460 nm). GABA-AT inhibition has been demonstrated for CL-5 (56). The chloride derivatives listed in Table 1 exhibited significant inhibition of GABA metabolism, as demonstrated with a commercially available GABase enzyme system (MilliporeSigma). Inhibition of the GABase system reduces the fluorescence output by lowering NADH accumulation during the reaction. The enzyme inhibition was monitored with various concentrations of the GABA substrate and 10 μM of test compound. Relative to the vehicle, all the chloride derivatives reduced the Vmax in the GABase assay, as shown in Table 2. CL-5 evidenced the greatest inhibition, followed by CL-3, -2, -4, and -1.
TABLE 2.
Enzyme kinetic parameters calculated from Michaelis-Menten kinetics
| Chloride derivative | Vmax | Km |
|---|---|---|
| No inhibitor | 193.7 | 1.37 |
| CL-1 | 101.7 | 0.13 |
| CL-2 | 86.67 | 0.02 |
| CL-3 | 67.97 | 0.06 |
| CL-4 | 101.1 | 0.47 |
| CL-5 | 52.21 | 0.26 |
Retinal protection by chloride derivatives
Before treatment efficacy studies, differences in susceptibility to light-induced retinal degeneration were observed in male and female mice. Abca4−/−Rdh8−/− male and female mice were injected with vehicle control (DMSO), and retinal degeneration was evaluated in response to exposure to light for different times at a constant intensity (10,000 lux) (Supplemental Fig. 1). In male mice, OCT images acquired 3 d after exposure to light demonstrated that 30 and 45 min of exposure caused ONL thinning and therefore photoreceptor degeneration. By contrast, OCT images of female mice demonstrated ONL thinning after 45 and 60 min of exposure, but not after 30 min of exposure. These results suggest that the male mice were more susceptible than female mice to retinal degeneration and must be evaluated separately for retinal protection therapies. Based on these results, the lowest amount of light exposure that would cause photoreceptor degeneration was used for subsequent therapeutic efficacy studies (30 min for males, 45 min for females).
To determine the dose range necessary for retinal protection, we performed pilot experiments independently in male and female mice for each compound. OCT imaging was performed 3 d after treatment and exposure to light to evaluate the extent of degeneration (Supplemental Fig. 2). For CL-1, -2, and -3, retinal protection was achieved by a dose of 0.2 mg (13.3 mg/kg) in male mice and 1 mg (66.7 mg/kg) in female mice. For CL-4, retinal protection was achieved with a dose of 0.2 mg (13.3 mg/kg) in males and 0.5 mg (33.3 mg/kg) in females. For CL-5, a dose of 0.5 mg (33.3 mg/kg) was necessary for retinal protection in males and 1 mg (66.7 mg/kg) for females. These lowest effective dosages were selected for further animal studies.
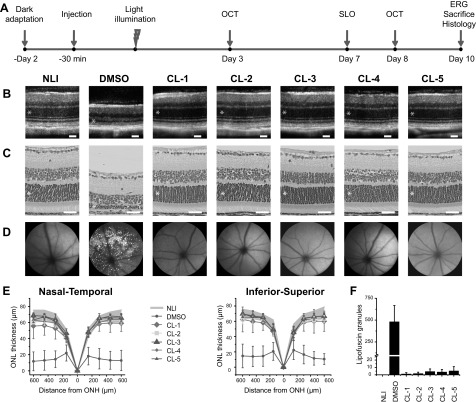
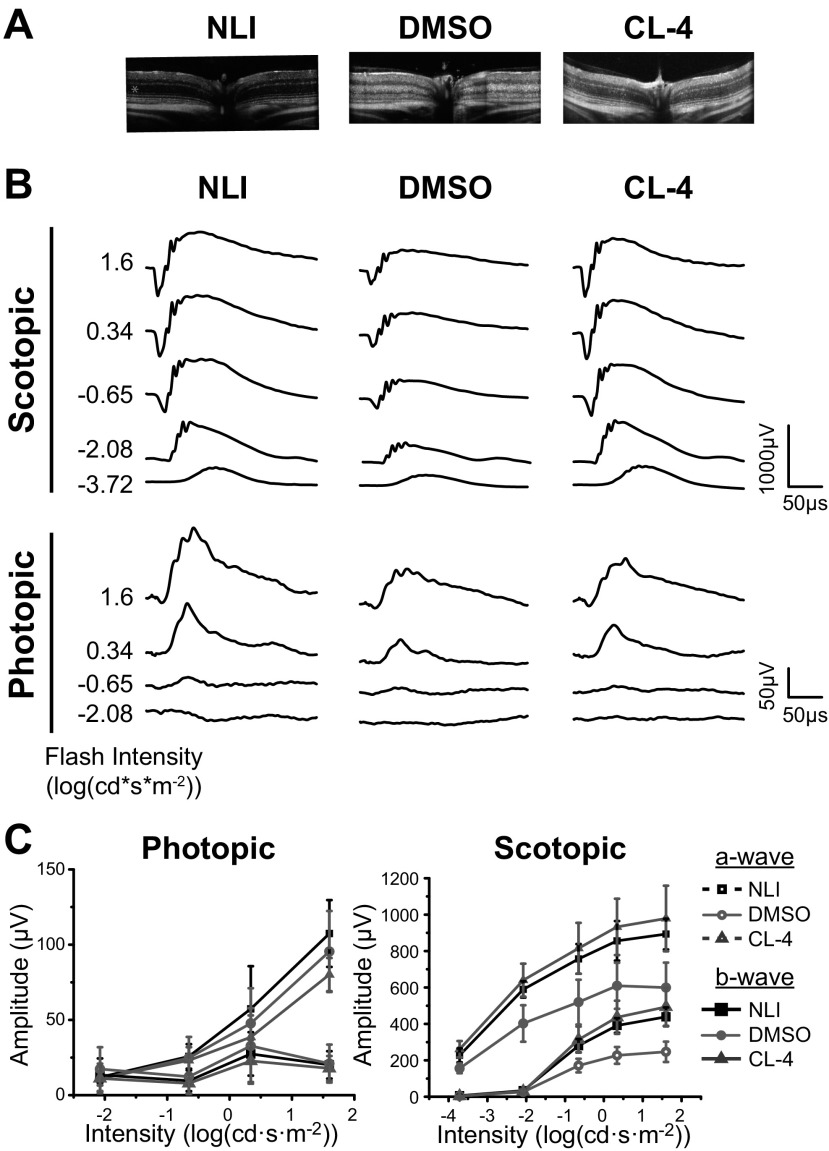
All the chloride derivatives showed effective retinal protection against light-induced degeneration, as revealed by OCT and histology evaluations (Fig. 2). The OCT images correlated well with the corresponding histologic images of the retina’s integrity and ONL thickness in all groups. Negative controls pretreated with DMSO before exposure to light demonstrated significant reductions in the thickness of the ONL containing the photoreceptor nuclei vs. the ONL of mice in the no-light-illumination (NLI) group (Fig. 2E). In contrast, all the layers of the retina remained intact, and the ONL thickness was similar to that of NLI mice in each of the treatment groups.
Figure 2.
Preservation of retinal structure in Abca4−/−Rdh8−/− mice pretreated with chloride derivatives before exposure to light. A) Experimental schedule for treatment, light exposure, and evaluations. B) OCT images of retinal cross-sections. C) H&E staining of retinal tissue slices. D) SLO images of autofluorescent granules in the subretinal space. E) ONL thickness measured from OCT images in the nasal–temporal and inferior–superior directions. NLI control data are shown as gray shading. P < 0.05, DMSO vs. NLI group. F) Counts of autofluorescent granules from SLO images. P < 1E5 chloride-treated vs. DMSO-treated eyes. B, C) Asterisks denote the ONL. Error bars ± sd. Scale bars, 50 µm.
The ability of the chloride derivatives to protect the retina from oxidative damage was also tested. In DMSO-pretreated control mice, SLO images revealed bright autofluorescent granules that were highly concentrated and dispersed throughout the subretinal space (480 ± 187 spots/eye), indicating widespread retinal damage (Fig. 2D). By contrast, SLO images of NLI controls and mice pretreated with the chloride derivatives mainly lacked autofluorescent granules in the RPE, supporting the OCT assessments indicating protection from cellular damage during exposure to intense light (Fig. 2F). In the treated mice, a small number of autofluorescent granules (mean, 1–6 spots/eye;) were visible, mainly at the optic nerve site in the dark center of the image.
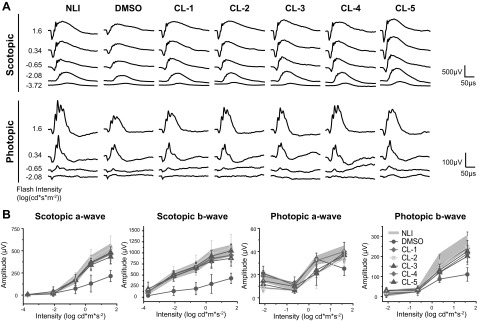
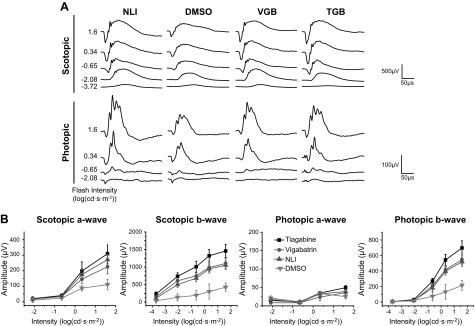
Visual function was also assessed, and in agreement with the imaging results, full protection of the retina was found in all treatment groups. Representative ERG recordings are shown in Fig. 3A. NLI control mice produced strong responses to light flashes during ERG testing, whereas the DMSO control mice produced comparatively weak responses. The a- and b-wave amplitudes, corresponding to the photoreceptor and inner retinal light responses, respectively, were quantified from these traces (Fig. 3B). Against a dark background, scotopic a- and b-wave ERG amplitudes in the chloride-treated mice were similar to those in the NLI controls, but significantly higher than the amplitudes in the DMSO-treated mice. Against a light background, photopic b-wave ERG amplitudes of treated groups were slightly lower than in the NLI controls, but were significantly higher than those in the DMSO-treated group. Photopic a-wave responses were similar across all groups.
Figure 3.
Preservation of visual function in Abca4−/−Rdh8−/− mice pretreated with chloride derivatives before exposure to light. A) Representative traces of ERG responses under scotopic and photopic conditions. B) Scotopic and photopic a- and b-waves quantified from ERG recordings. No illumination control data shaded between error bars. Error bars ± sd. P < 0.001, scotopic ERGs; P < 0.01, photopic ERGs, in chloride-treated vs. DMSO-treated mice.
Retinal protection by clinical anticonvulsants
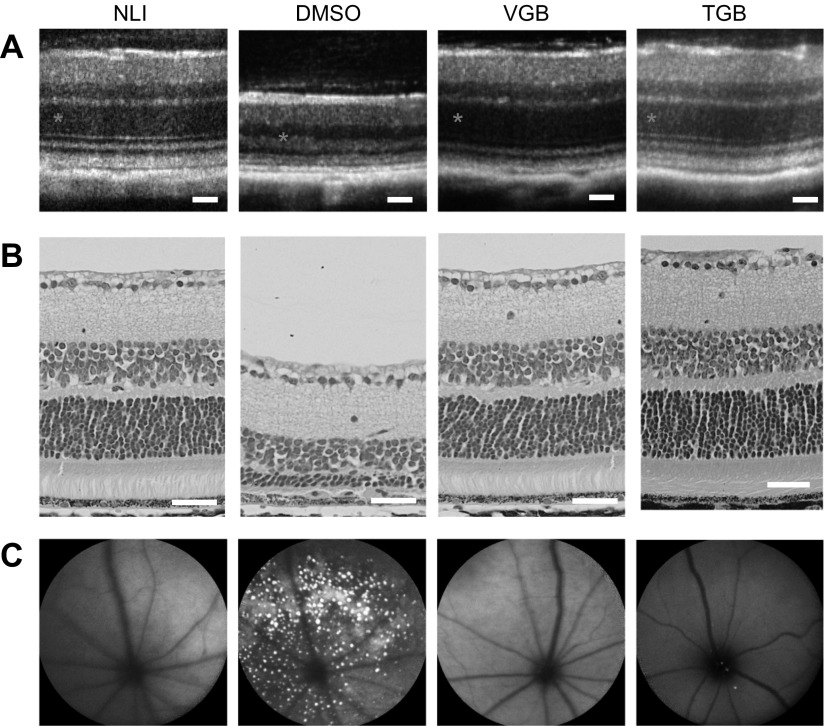
Two clinically available anticonvulsant agents were also tested for their ability to protect the retina from light-induced damage. TGB and VGB, both of which promote GABAergic signaling, protected retinal structure and function during exposure to intense light (Fig. 4). OCT and histology assessments demonstrated that a thick ONL was preserved in animals pretreated with 0.05 mg TGB (3.33 mg/kg) or 1 mg VGB (66.7 mg/kg) before exposure to light. These OCT results were supported by SLO images which revealed few autofluorescent granules in the subretinal space. Finally, ERG recordings indicated that the visual response was preserved in mice pretreated with TGB or VGB (Fig. 5).
Figure 4.
Preservation of retinal structure in Abca4−/−Rdh8−/− mice pretreated with clinical anticonvulsants before light exposure. A) OCT images of retinal cross-sections. B) H&E staining of retinal tissue slices. Asterisks denote the ONL. C) SLO images of autofluorescent granules in the subretinal space. Images from control animals are repeated from Fig. 2 for clarity. Scale bars, 50 µm.
Figure 5.
Preservation of retinal structure in Abca4−/−Rdh8−/− mice pretreated with clinical anticonvulsants before exposure to light. A) Representative traces of ERG responses under scotopic and photopic conditions. B) Scotopic a-waves and photopic b-waves quantified from ERG recordings. Recordings from control animals are repeated from Fig. 2 for comparison. Error bars, ±sd.
Retinal protection in wild-type mice
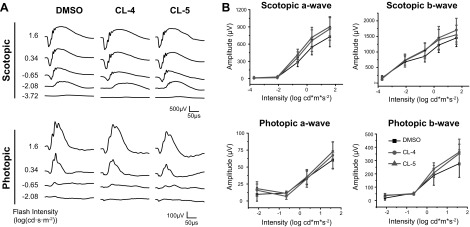
To demonstrate the effectiveness of the chloride derivatives in a wild-type model of light-induced degeneration, the efficacy of pretreatment with CL-4 was tested in BALB/c mice. Similar to the Abca4−/−Rdh8−/− double-knockout model, BALB/c mice undergo retinal degeneration in response exposure to intense light (10). Retinal protection was evident in the albino BALB/c mice pretreated with 0.2 mg CL-4 (Fig. 6). OCT images also revealed an ONL with a thickness similar to that of NLI controls. By contrast, OCT images of control mice pretreated with DMSO demonstrated an inflamed, thinner, degraded ONL caused by exposure to light. ERG recordings demonstrated decreased scotopic a- and b-wave amplitudes in DMSO-treated controls, whereas mice treated with CL-4 produced a full response.
Figure 6.
Retinal protection in wild-type BALB/c mice pretreated with CL-4 before exposure to light. A) OCT images of NLI, DMSO-treated, and CL-4 treated mice. Asterisks denote the ONL. Representative ERG traces (B) and quantified a- and b-waves (C). Error bars ± sd.
Safety
Toxicity to the retina was assessed for the 2 leading chloride derivatives, CL-4 and -5, after single and repeated doses in wild-type mice. To determine whether the chloride derivatives compromised visual function, full-field ERG recordings were collected 30 min after the administration of a single high dose (1 mg; 66.7 mg/kg) of CL-4 or -5 in C57BL/6 mice. ERG responses in both drug treatment groups were similar to responses in vehicle-injected controls, and no significant differences in a- and b-wave amplitudes were measured under photopic or scotopic conditions (Fig. 7).
Figure 7.
Safety profiles of single injections of a high-dose (1 mg/mouse) of CL-4 or -5 in C57BL/6 mice. Representative full-field ERG traces (A) and quantified a- and b-wave amplitudes (B) under photopic and scotopic conditions recorded 30 min after injections. Error bars ± sd.
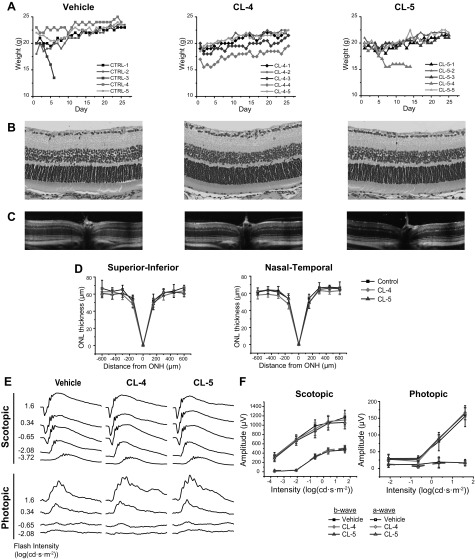
Repeated doses of CL-4 and -5 for 28 d had no deleterious effects on the retina or the overall well-being of the BALB/c mice. Body weight measurements recorded daily over the course of this study are shown in Fig. 8A. With the exception of 2 animals, DMSO control and drug-treated mice exhibited a modest weight gain during the study. One animal from the DMSO group rapidly lost body weight after d 3 and was euthanized at d 6. Likewise, 1 CL-5–treated mouse lost weight but stabilized by d 7; however, this animal was euthanized on d 15 because of its weak appearance. All other mice maintained a healthy weight during the study period.
Figure 8.
Safety profiles after daily administration of chloride derivatives by IP injection in male BALB/c mice for 28 d. A) Body weights of mice injected with vehicle, 0.2 mg CL-4, or 0.2 mg CL-5 for the course of the experiment. B) H&E staining of retinas harvested at d 28 of injections. C, D) In vivo OCT images (C) and quantified ONL thickness (D) of retinal cross-sections imaged at d 26 of injections. E, F) Representative ERG traces (E) and quantified a- and b-wave amplitudes (F) measured at d 28 of injections. ONH, optical nerve head. Error bars, ±sd.
OCT images taken on d 26 indicated that the structure of the retina remained intact during treatment (Fig. 8C, D). The ONL containing photoreceptor nuclei was of a normal thickness (∼60 μm), with no significant differences between treatment groups and the controls. Histologic images of retinal cross-sections collected at the conclusion of the study are shown in Fig. 8B. Histologic findings were in agreement with the OCT images and revealed fully preserved retinal structures in control and treatment groups. Full-field ERG recordings taken after 28 d of injections are shown in Fig. 8E, F. In both treatment groups and the control group, no significant differences were measured in a- or b-wave amplitudes. The ERG responses in the animals agreed with the maintenance of retinal structure observed in OCT and histology images.
DISCUSSION
There have been substantial efforts to develop effective therapeutics for preventing nonexudative retinal degenerative diseases. These include sequestration of toxic all-trans retinal by primary amines (43, 57); increasing clearance of retinoid metabolites with visual cycle inhibitors (10, 11); modulating GPCR signaling (54); and the use of neuroprotective agents (58), antioxidants (6), and gene therapy (59, 60). Many of these approaches have been successful in mice. However, in human clinical trials, inhibitors have slowed the visual cycle (61, 62) and antioxidants failed to prevent the onset of early symptoms of AMD. Neuroprotective agents, such as ciliary neurotrophic factor are in ongoing clinical trials, but their need for local administration by direct intraocular injection is invasive and not well suited for repeated administration. Complex approaches, such as gene or cell-based therapies, present many hurdles to production and clinical use, when compared to systemic administration of small-molecule drugs.
We present a library of small-molecule compounds that efficiently protect the retina from light-induced damage and can be administered repeatedly without causing any apparent damage to the retina. Most of the chloride compounds generated similar effects in in vitro enzyme assays and in in vivo treatment efficacy experiments. The 2 chloride derivatives with functional groups on the phenyl ring of the parent compound, CL-4 and -5, were outliers in their efficacy. Specifically, CL-4 showed highest in vivo efficacy, whereas CL-5 showed the highest in vitro efficacy, yet the lowest in vivo efficacy. We attribute this discrepancy to biodistribution of the compounds, where we anticipate that the less polar methylated CL-4 experiences better distribution than the more polar hydroxylated CL-5. In addition, we observed higher sensitivity to light-induced damage in male mice vs. female mice. In future work, pharmacokinetic studies may aid in understanding the relationship of doses and distribution with extent of degeneration.
In this work, the potential effectiveness of several novel and clinically validated agonists of GABA signaling were evaluated for their protection of photoreceptors from light-induced retinal degeneration. GABA is the main inhibitory neurotransmitter in the retina where it plays a dual role in modulating the transmission of visual signals through the retina while shunting substrates into the TCA cycle for cellular metabolism. GABA is released by horizontal cells, whereby it regulates synaptic communication between photoreceptor and bipolar cells in the outer plexiform layer. GABA is also released by amacrine cells to regulate bipolar–ganglion cell communication in the inner plexiform layer and is recycled by Müller cells that span the length of the retina. GABA metabolism feeds into the TCA cycle for cellular metabolism via GABA-AT–related breakdown of GABA into succinic semialdehyde and then into succinate (32).
Specifically, the use of new GABA-AT inhibitors and clinical anticonvulsants was explored as an alternative strategy for retinal protection against light-induced degeneration. Anticonvulsants act on a spectrum of targets to inhibit excitatory neurotransmitter action and protect neural cells from excitotoxic damage. These drugs act on glutamate pathways (e.g., felbamate and topiramate), ion channels (e.g., benzodiazepines, gabapentin, and phenytoin), and GABA pathways (e.g., VGB, TGB, and valproic acid) (34). Anticonvulsants have demonstrated neuroprotective effects on retinal ganglion cells that prevent excitotoxic damage during ischemic injury (36, 37, 39, 40). Models of excitotoxic retinal damage typically involve inner retinal disease, such as glaucoma, in which the ganglion cell layer is damaged by overexcitation of NMDA or kainic acid receptors, which can be counterbalanced by pharmacologic supplementation of inhibitory signaling.
When considering the translational potential of anticonvulsant used to prevent retinal degeneration, safety is a primary concern. Many anticonvulsants have the side effect of visual impairment, although this adverse effect differs in severity (63, 64). Whereas most of these drugs have side effects that are infrequent and reversible, VGB, a mechanism-based inactivator of GABA-AT, is known to cause irreversible visual field defects after repeated administration in animals (65, 66) and in patients (67, 68). TGB has less severe clinical side effects, such as altered color perception, and these effects are reversible once drug treatment is terminated. Because of these safety concerns, it was necessary to determine whether administration of our chloride derivatives would elicit similar adverse effects on the retina. ERG recordings after single or repeated doses of the lead chloride derivatives did not perturb retinal function. Furthermore, OCT and histologic images of retinal structure showed no signs of the degeneration or plasticity that were observed with VGB (65, 69). These promising results suggest that these chloride derivatives do not pose an acute risk to the retina. Future, comprehensive safety studies are necessary to obtain a full profile of their potential adverse effects.
In this model of light-induced retinal degeneration, a pathologic underpinning for excitotoxic damage remains to be elucidated. In one rodent model of retinitis pigmentosa, increased levels of GABA were found to accompany retinal degeneration (32). However, others have shown no difference in quantity or distribution of GABA as the retina degenerates (27, 28). Indeed, strict quantification of the amount of the various neurotransmitters in the retina does not necessarily correlate with their signaling activity, because of the complex metabolic interplay and rapid uptake by glial cells. However, there is precedent in the literature that implicates the GABAergic pathway in retinal damage. In patients with AMD, genome-wide association studies have identified mutations in SSADH, the enzyme following GABA-AT in the GABA shunt to the TCA cycle (70). Valproic acid, a clinically used anticonvulsant that increases GABAergic signaling, has entered clinical trials for treatment of retinitis pigmentosa with the promise of promoting correct folding of rhodopsin mutants in the dominant form of this disease (38, 39, 44). These trials have generated mixed results, with conflicting reports of visual acuity improvement in some patients, whereas others experienced visual toxicity related to the treatment (19, 71). Serotonin receptor (5-HT1A) agonists, a class of drugs also known to possess neuroprotective functions against NMDA excitotoxicity, have also demonstrated a protective effect against light-induced retinal degeneration in animals (15, 72). Unfortunately, a recent phase III clinical trial, the Geographic Atrophy Treatment Evaluation (GATE) trial, showed no improvement in delaying lesion growth in patients with geographic atrophy secondary to AMD (20). However, this failure of drug efficacy may have been caused by factors other than the therapeutic strategy, such as the advanced stage of disease or transport problems with the topical formulation. Because of failures of neuromodulatory drugs to ameliorate AMD progression, future work should include a rigorous study of the mechanism of retinal protection by these drugs. Appropriate stage of disease, clinical endpoints for efficacy, and in vitro and animal models must be established before these drugs can achieve their translational potential.
In summary, this work demonstrates that modulation of inhibitory neurotransmitters in the retina could provide a new strategy for neuroprotection from light-induced damage. 3-Chloropropiophenone derivatives, a new class of GABA-AT inhibitors, effectively protected retinal structure and function in Abca4−/−Rdh8−/− mice that were treated before exposure to intense light. Clinical anticonvulsants that act on the GABA shunt were also shown to provide retinal protection in this model. Furthermore, the lead chloride derivative CL-4 replicated this protective effect in wild-type BALB/c mice. Preliminary evaluations revealed excellent safety profiles with little systemic or visual toxicity after single or repeated doses with the 2 lead compounds.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Dr. Timothy Kern (Department of Pharmacology, Case Western Reserve University) for valuable insights and critical discussions of this research work, Mr. Da Sun (Center for Biomolecular Engineering, Case Western Reserve University) for assistance in in vivo experiments and data analysis, and Dr. Amita Vaidya (Center for Biomolecular Engineering, Case Western Reserve University) for a critical reading of the manuscript. Z.-R.L. is the M. Frank and Margaret Domiter Rudy Professor of Biomedical Engineering, and K.P. is the John H. Hord Professor of Pharmacology. This work was supported, in part, by U.S. National Institutes of Health (NIH) National Eye Institute Grants EY027283 (to K.P.) and EY024864; and the NIH National Institute of Biomedical Imaging and Bioengineering Interdisciplinary Biomedical Training Program Grant T32EB007509 (to R.M.S.) administered by the Department of Biomedical Engineering, Case Western Reserve University. This report is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors declare no conflicts of interest.
Glossary
- AMD
age-related macular degeneration
- ERG
electroretinography
- GABA-AT
GABA aminotransferase
- NLI
no light illumination
- OCT
optical coherence tomography
- ONH
optic nerve head
- ONL
outer nuclear layer
- RPE
retinal pigment epithelium
- SLO
scanning laser ophthalmoscopy
- SSADH
succinic semialdehyde dehydrogenase
- TCA
tricarboxylic acid
- TGB
tiagabine
- VGB
vigabatrin
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
R. M. Schur was involved in all aspects of this work, including study design and execution and data analysis; S. Gao and Z.-R. Lu conceived the project; S. Gao, G. Yu, and Y. Chen performed pilot experiments; S. Gao performed ERG examinations; A. Maeda and K. Palczewski provided assistance with animal models and in vivo assays; R. M. Schur and Z.-R. Lu wrote the paper; and all authors contributed to the study design and manuscript preparation and have read and approved the final version.
REFERENCES
- 1.Fritsche L. G., Fariss R. N., Stambolian D., Abecasis G. R., Curcio C. A., Swaroop A. (2014) Age-related macular degeneration: genetics and biology coming together. Annu. Rev. Genomics Hum. Genet. 15, 151–171 10.1146/annurev-genom-090413-025610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maeda A., Maeda T., Golczak M., Palczewski K. (2008) Retinopathy in mice induced by disrupted all-trans-retinal clearance. J. Biol. Chem. 283, 26684–26693 10.1074/jbc.M804505200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winkler B. S., Boulton M. E., Gottsch J. D., Sternberg P. (1999) Oxidative damage and age-related macular degeneration. Mol. Vis. 5, 32. [PMC free article] [PubMed] [Google Scholar]
- 4.Ambati J., Fowler B. J. (2012) Mechanisms of age-related macular degeneration. Neuron 75, 26–39 10.1016/j.neuron.2012.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damico F. M., Gasparin F., Scolari M. R., Pedral L. S., Takahashi B. S. (2012) New approaches and potential treatments for dry age-related macular degeneration. Arq. Bras. Oftalmol. 75, 71–76 10.1590/S0004-27492012000100016 [DOI] [PubMed] [Google Scholar]
- 6.Age-Related Eye Disease Study Research Group . (2001) A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 119, 1417–1436 10.1001/archopht.119.10.1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Age-Related Eye Disease Study 2 Research Group . (2013) Lutein+zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial [published correction in JAMA (2013) 310, 208]. JAMA 309, 2005–2015 10.1001/jama.2013.4997 [DOI] [PubMed] [Google Scholar]
- 8.Travis G. H., Golczak M., Moise A. R., Palczewski K. (2007) Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu. Rev. Pharmacol. Toxicol. 47, 469–512 10.1146/annurev.pharmtox.47.120505.105225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sparrow J. R. (2016) Vitamin A-aldehyde adducts: AMD risk and targeted therapeutics. Proc. Natl. Acad. Sci. USA 113, 4564–4569 10.1073/pnas.1600474113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maeda A., Maeda T., Golczak M., Imanishi Y., Leahy P., Kubota R., Palczewski K. (2006) Effects of potent inhibitors of the retinoid cycle on visual function and photoreceptor protection from light damage in mice. Mol. Pharmacol. 70, 1220–1229 10.1124/mol.106.026823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bavik C., Henry S. H., Zhang Y., Mitts K., McGinn T., Budzynski E., Pashko A., Lieu K. L., Zhong S., Blumberg B., Kuksa V., Orme M., Scott I., Fawzi A., Kubota R. (2015) Visual cycle modulation as an approach toward preservation of retinal integrity. PLoS One 10, e0124940 10.1371/journal.pone.0124940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu D. Y., Cringle S., Valter K., Walsh N., Lee D., Stone J. (2004) Photoreceptor death, trophic factor expression, retinal oxygen status, and photoreceptor function in the P23H rat. Invest. Ophthalmol. Vis. Sci. 45, 2013–2019 10.1167/iovs.03-0845 [DOI] [PubMed] [Google Scholar]
- 13.Cayouette M., Behn D., Sendtner M., Lachapelle P., Gravel C. (1998) Intraocular gene transfer of ciliary neurotrophic factor prevents death and increases responsiveness of rod photoreceptors in the retinal degeneration slow mouse. J. Neurosci. 18, 9282–9293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louzada P. R., Paula Lima A. C., Mendonca-Silva D. L., Noël F., De Mello F. G., Ferreira S. T. (2004) Taurine prevents the neurotoxicity of beta-amyloid and glutamate receptor agonists: activation of GABA receptors and possible implications for Alzheimer’s disease and other neurological disorders. FASEB J. 18, 511–518 10.1096/fj.03-0739com [DOI] [PubMed] [Google Scholar]
- 15.Collier R. J., Patel Y., Martin E. A., Dembinska O., Hellberg M., Krueger D. S., Kapin M. A., Romano C. (2011) Agonists at the serotonin receptor (5-HT(1A)) protect the retina from severe photo-oxidative stress. Invest. Ophthalmol. Vis. Sci. 52, 2118–2126 10.1167/iovs.10-6304 [DOI] [PubMed] [Google Scholar]
- 16.Tullis B. E., Ryals R. C., Coyner A. S., Gale M. J., Nicholson A., Ku C., Regis D., Sinha W., Datta S., Wen Y., Yang P., Pennesi M. E. (2015) Sarpogrelate, a 5-HT2A receptor antagonist, protects the retina from light-induced retinopathy. Invest. Ophthalmol. Vis. Sci. 56, 4560–4569 10.1167/iovs.15-16378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coyner A. S., Ryals R. C., Ku C. A., Fischer C. M., Patel R. C., Datta S., Yang P., Wen Y., Hen R., Pennesi M. E. (2016) Retinal neuroprotective effects of flibanserin, an FDA-approved dual serotonin receptor agonist-antagonist. PLoS One 11, e0159776 10.1371/journal.pone.0159776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kubota R., Boman N. L., David R., Mallikaarjun S., Patil S., Birch D. (2012) Safety and effect on rod function of ACU-4429, a novel small-molecule visual cycle modulator. Retina 32, 183–188 10.1097/IAE.0b013e318217369e [DOI] [PubMed] [Google Scholar]
- 19.Bhalla S., Joshi D., Bhullar S., Kasuga D., Park Y., Kay C. N. (2013) Long-term follow-up for efficacy and safety of treatment of retinitis pigmentosa with valproic acid. Br. J. Ophthalmol. 97, 895–899 10.1136/bjophthalmol-2013-303084 [DOI] [PubMed] [Google Scholar]
- 20.Jaffe G. J., Schmitz-Valckenberg S., Boyer D., Heier J., Wolf-Schnurrbusch U., Staurenghi G., Schmidt-Erfurth U., Holz F. G. (2015) Randomized trial to evaluate tandospirone in geographic atrophy secondary to age-related macular degeneration: the GATE study. Am. J. Ophthalmol. 160, 1226–1234 10.1016/j.ajo.2015.08.024 [DOI] [PubMed] [Google Scholar]
- 21.Yang X. L. (2004) Characterization of receptors for glutamate and GABA in retinal neurons. Prog. Neurobiol. 73, 127–150 10.1016/j.pneurobio.2004.04.002 [DOI] [PubMed] [Google Scholar]
- 22.Kalloniatis M., Tomisich G. (1999) Amino acid neurochemistry of the vertebrate retina. Prog. Retin. Eye Res. 18, 811–866 10.1016/S1350-9462(98)00036-6 [DOI] [PubMed] [Google Scholar]
- 23.Bringmann A., Pannicke T., Grosche J., Francke M., Wiedemann P., Skatchkov S. N., Osborne N. N., Reichenbach A. (2006) Müller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 25, 397–424 10.1016/j.preteyeres.2006.05.003 [DOI] [PubMed] [Google Scholar]
- 24.Bui B. V., Kalloniatis M., Vingrys A. J. (2004) Retinal function loss after monocarboxylate transport inhibition. Invest. Ophthalmol. Vis. Sci. 45, 584–593 10.1167/iovs.03-0695 [DOI] [PubMed] [Google Scholar]
- 25.Miya-Coreixas V. S., Maggesissi Santos R., Carpi Santos R., Gardino P. F., Calaza K. (2013) Regulation of GABA content by glucose in the chick retina. Exp. Eye Res. 115, 206–215 10.1016/j.exer.2013.07.026 [DOI] [PubMed] [Google Scholar]
- 26.Jaiswal M., Haelterman N. A., Sandoval H., Xiong B., Donti T., Kalsotra A., Yamamoto S., Cooper T. A., Graham B. H., Bellen H. J. (2015) Impaired mitochondrial energy production causes light-induced photoreceptor degeneration independent of oxidative stress. PLoS Biol. 13, e1002197 10.1371/journal.pbio.1002197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okada M., Okuma Y., Osumi Y., Nishihara M., Yokotani K., Ueno H. (2000) Neurotransmitter contents in the retina of RCS rat. Graefes Arch. Clin. Exp. Ophthalmol. 238, 998–1001 10.1007/s004170000215 [DOI] [PubMed] [Google Scholar]
- 28.Wasowicz M., Morice C., Ferrari P., Callebert J., Versaux-Botteri C. (2002) Long-term effects of light damage on the retina of albino and pigmented rats. Invest. Ophthalmol. Vis. Sci. 43, 813–820 [PubMed] [Google Scholar]
- 29.Ehinger B., Narfström K., Nilsson S. E., van Veen T. (1991) Photoreceptor degeneration and loss of immunoreactive GABA in the Abyssinian cat retina. Exp. Eye Res. 52, 17–25 10.1016/0014-4835(91)90124-W [DOI] [PubMed] [Google Scholar]
- 30.Fletcher E. L., Kalloniatis M. (1997) Neurochemical development of the degenerating rat retina. J. Comp. Neurol. 388, 1–22 10.1002/(SICI)1096-9861(19971110)388:1%3c1::AID-CNE1%3e3.0.CO;2-5 [DOI] [PubMed] [Google Scholar]
- 31.Fariss R. N., Li Z. Y., Milam A. H. (2000) Abnormalities in rod photoreceptors, amacrine cells, and horizontal cells in human retinas with retinitis pigmentosa. Am. J. Ophthalmol. 129, 215–223 [DOI] [PubMed] [Google Scholar]
- 32.Fletcher E. L. (2000) Alterations in neurochemistry during retinal degeneration. Microsc. Res. Tech. 50, 89–102 10.1002/1097-0029(20000715)50:2%3c89::AID-JEMT1%3e3.0.CO;2-9 [DOI] [PubMed] [Google Scholar]
- 33.Srivastava P., Sinha-Mahapatra S. K., Ghosh A., Srivastava I., Dhingra N. K. (2015) Differential alterations in the expression of neurotransmitter receptors in inner retina following loss of photoreceptors in rd1 mouse. PLoS One 10, e0123896 10.1371/journal.pone.0123896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogawski M. A., Löscher W. (2004) The neurobiology of antiepileptic drugs. Nat. Rev. Neurosci. 5, 553–564 10.1038/nrn1430 [DOI] [PubMed] [Google Scholar]
- 35.Ishikawa M. (2013) Abnormalities in glutamate metabolism and excitotoxicity in the retinal diseases. Scientifica (Cairo) 2013, 528940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pisani F., Costa C., Caccamo D., Mazzon E., Gorgone G., Oteri G., Calabresi P., Ientile R. (2006) Tiagabine and vigabatrin reduce the severity of NMDA-induced excitotoxicity in chick retina. Exp. Brain Res. 171, 511–515 10.1007/s00221-005-0298-1 [DOI] [PubMed] [Google Scholar]
- 37.Yoneda S., Tanaka E., Goto W., Ota T., Hara H. (2003) Topiramate reduces excitotoxic and ischemic injury in the rat retina. Brain Res. 967, 257–266 10.1016/S0006-8993(03)02270-4 [DOI] [PubMed] [Google Scholar]
- 38.Biermann J., Grieshaber P., Goebel U., Martin G., Thanos S., Di Giovanni S., Lagrèze W. A. (2010) Valproic acid-mediated neuroprotection and regeneration in injured retinal ganglion cells. Invest. Ophthalmol. Vis. Sci. 51, 526–534 10.1167/iovs.09-3903 [DOI] [PubMed] [Google Scholar]
- 39.Kimura A., Namekata K., Guo X., Noro T., Harada C., Harada T. (2015) Valproic acid prevents NMDA-induced retinal ganglion cell death via stimulation of neuronal TrkB receptor signaling. Am. J. Pathol. 185, 756–764 10.1016/j.ajpath.2014.11.005 [DOI] [PubMed] [Google Scholar]
- 40.Froger N., Moutsimilli L., Cadetti L., Jammoul F., Wang Q. P., Fan Y., Gaucher D., Rosolen S. G., Neveux N., Cynober L., Sahel J. A., Picaud S. (2014) Taurine: the comeback of a neutraceutical in the prevention of retinal degenerations. Prog. Retin. Eye Res. 41, 44–63 10.1016/j.preteyeres.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 41.Varga B., Szabadfi K., Kiss P., Fabian E., Tamas A., Griecs M., Gabriel R., Reglodi D., Kemeny-Beke A., Pamer Z., Biro Z., Tosaki A., Atlasz T., Juhasz B. (2011) PACAP improves functional outcome in excitotoxic retinal lesion: an electroretinographic study. J. Mol. Neurosci. 43, 44–50 10.1007/s12031-010-9406-1 [DOI] [PubMed] [Google Scholar]
- 42.Atlasz T., Szabadfi K., Kiss P., Babai N., Koszegi Z., Tamas A., Reglodi D., Gabriel R. (2008) PACAP-mediated neuroprotection of neurochemically identified cell types in MSG-induced retinal degeneration. J. Mol. Neurosci. 36, 97–104 10.1007/s12031-008-9059-5 [DOI] [PubMed] [Google Scholar]
- 43.Maeda A., Golczak M., Chen Y., Okano K., Kohno H., Shiose S., Ishikawa K., Harte W., Palczewska G., Maeda T., Palczewski K. (2011) Primary amines protect against retinal degeneration in mouse models of retinopathies. Nat. Chem. Biol. 8, 170–178 10.1038/nchembio.759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mitton K. P., Guzman A. E., Deshpande M., Byrd D., DeLooff C., Mkoyan K., Zlojutro P., Wallace A., Metcalf B., Laux K., Sotzen J., Tran T. (2014) Different effects of valproic acid on photoreceptor loss in Rd1 and Rd10 retinal degeneration mice. Mol. Vis. 20, 1527–1544 [PMC free article] [PubMed] [Google Scholar]
- 45.Fernández-Sánchez L., Lax P., Pinilla I., Martín-Nieto J., Cuenca N. (2011) Tauroursodeoxycholic acid prevents retinal degeneration in transgenic P23H rats. Invest. Ophthalmol. Vis. Sci. 52, 4998–5008 10.1167/iovs.11-7496 [DOI] [PubMed] [Google Scholar]
- 46.Fernández-Sánchez L., Lax P., Noailles A., Angulo A., Maneu V., Cuenca N. (2015) Natural compounds from saffron and bear bile prevent vision loss and retinal degeneration. Molecules 20, 13875–13893 10.3390/molecules200813875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsukatani T., Higuchi T., Matsumoto K. (2005) Enzyme-based microtiter plate assay for γ-aminobutyric acid: application to the screening of γ-aminobutyric acid-producing lactic acid bacteria. Anal. Chim. Acta 540, 293–297 10.1016/j.aca.2005.03.056 [DOI] [Google Scholar]
- 48.O’Byrne C. P., Feehily C., Ham R., Karatzas K. A. (2011) A modified rapid enzymatic microtiter plate assay for the quantification of intracellular γ-aminobutyric acid and succinate semialdehyde in bacterial cells. J. Microbiol. Methods 84, 137–139 10.1016/j.mimet.2010.10.017 [DOI] [PubMed] [Google Scholar]
- 49.Danciger M., Lyon J., Worrill D., Hoffman S., Lem J., Reme C. E., Wenzel A., Grimm C. (2004) New retinal light damage QTL in mice with the light-sensitive RPE65 LEU variant. Mamm. Genome 15, 277–283 10.1007/s00335-003-2336-2 [DOI] [PubMed] [Google Scholar]
- 50.Mattapallil M. J., Wawrousek E. F., Chan C. C., Zhao H., Roychoudhury J., Ferguson T. A., Caspi R. R. (2012) The Rd8 mutation of the Crb1 gene is present in vendor lines of C57BL/6N mice and embryonic stem cells, and confounds ocular induced mutant phenotypes. Invest. Ophthalmol. Vis. Sci. 53, 2921–2927 10.1167/iovs.12-9662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maeda A., Maeda T., Golczak M., Chou S., Desai A., Hoppel C. L., Matsuyama S., Palczewski K. (2009) Involvement of all-trans-retinal in acute light-induced retinopathy of mice. J. Biol. Chem. 284, 15173–15183 10.1074/jbc.M900322200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kohno H., Chen Y., Kevany B. M., Pearlman E., Miyagi M., Maeda T., Palczewski K., Maeda A. (2013) Photoreceptor proteins initiate microglial activation via toll-like receptor 4 in retinal degeneration mediated by all-trans-retinal. J. Biol. Chem. 288, 15326–15341 10.1074/jbc.M112.448712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schur R. M., Sheng L., Sahu B., Yu G., Gao S., Yu X., Maeda A., Palczewski K., Lu Z. R. (2015) Manganese-enhanced MRI for preclinical evaluation of retinal degeneration treatments. Invest. Ophthalmol. Vis. Sci. 56, 4936–4942 10.1167/iovs.15-16522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Y., Palczewska G., Mustafi D., Golczak M., Dong Z., Sawada O., Maeda T., Maeda A., Palczewski K. (2013) Systems pharmacology identifies drug targets for Stargardt disease-associated retinal degeneration. J. Clin. Invest. 123, 5119–5134 10.1172/JCI69076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maeda T., Van Hooser J. P., Driessen C. A., Filipek S., Janssen J. J., Palczewski K. (2003) Evaluation of the role of the retinal G protein-coupled receptor (RGR) in the vertebrate retina in vivo. J. Neurochem. 85, 944–956 10.1046/j.1471-4159.2003.01741.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tao Y. H., Xu H. B., Yang X. L. (2009) Inactivation of GABA transaminase by 3-chloro-1-(4-hydroxyphenyl)propan-1-one. Bioorg. Med. Chem. Lett. 19, 731–734 10.1016/j.bmcl.2008.12.033 [DOI] [PubMed] [Google Scholar]
- 57.Zhang J., Dong Z., Mundla S. R., Hu X. E., Seibel W., Papoian R., Palczewski K., Golczak M. (2015) Expansion of first-in-class drug candidates that sequester toxic all-trans-retinal and prevent light-induced retinal degeneration. Mol. Pharmacol. 87, 477–491 10.1124/mol.114.096560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wenzel A., Grimm C., Samardzija M., Remé C. E. (2005) Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog. Retin. Eye Res. 24, 275–306 10.1016/j.preteyeres.2004.08.002 [DOI] [PubMed] [Google Scholar]
- 59.Lenis T. L., Sarfare S., Jiang Z., Lloyd M. B., Bok D., Radu R. A. (2017) Complement modulation in the retinal pigment epithelium rescues photoreceptor degeneration in a mouse model of Stargardt disease. Proc. Natl. Acad. Sci. USA 114, 3987–3992 10.1073/pnas.1620299114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun D., Sahu B., Gao S., Schur R. M., Vaidya A. M., Maeda A., Palczewski K., Lu Z. R. (2017) Targeted multifunctional lipid ECO plasmid DNA nanoparticles as efficient non-viral gene therapy for leber’s congenital amaurosis. Mol. Ther. Nucleic Acids 7, 42–52 10.1016/j.omtn.2017.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kubota R., Al-Fayoumi S., Mallikaarjun S., Patil S., Bavik C., Chandler J. W. (2014) Phase 1, dose-ranging study of emixustat hydrochloride (ACU-4429), a novel visual cycle modulator, in healthy volunteers. Retina 34, 603–609 10.1097/01.iae.0000434565.80060.f8 [DOI] [PubMed] [Google Scholar]
- 62.Dugel P. U., Novack R. L., Csaky K. G., Richmond P. P., Birch D. G., Kubota R. (2015) Phase ii, randomized, placebo-controlled, 90-day study of emixustat hydrochloride in geographic atrophy associated with dry age-related macular degeneration. Retina 35, 1173–1183 10.1097/IAE.0000000000000606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hilton E. J., Hosking S. L., Betts T. (2004) The effect of antiepileptic drugs on visual performance. Seizure 13, 113–128 10.1016/S1059-1311(03)00082-7 [DOI] [PubMed] [Google Scholar]
- 64.Verrotti A., Manco R., Matricardi S., Franzoni E., Chiarelli F. (2007) Antiepileptic drugs and visual function. Pediatr. Neurol. 36, 353–360 10.1016/j.pediatrneurol.2007.03.001 [DOI] [PubMed] [Google Scholar]
- 65.Duboc A., Hanoteau N., Simonutti M., Rudolf G., Nehlig A., Sahel J. A., Picaud S. (2004) Vigabatrin, the GABA-transaminase inhibitor, damages cone photoreceptors in rats. Ann. Neurol. 55, 695–705 10.1002/ana.20081 [DOI] [PubMed] [Google Scholar]
- 66.Wang Q. P., Jammoul F., Duboc A., Gong J., Simonutti M., Dubus E., Craft C. M., Ye W., Sahel J. A., Picaud S. (2008) Treatment of epilepsy: the GABA-transaminase inhibitor, vigabatrin, induces neuronal plasticity in the mouse retina. Eur. J. Neurosci. 27, 2177–2187 10.1111/j.1460-9568.2008.06175.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Malmgren K., Ben-Menachem E., Frisén L. (2001) Vigabatrin visual toxicity: evolution and dose dependence. Epilepsia 42, 609–615 10.1046/j.1528-1157.2001.28600.x [DOI] [PubMed] [Google Scholar]
- 68.Maguire M. J., Hemming K., Wild J. M., Hutton J. L., Marson A. G. (2010) Prevalence of visual field loss following exposure to vigabatrin therapy: a systematic review. Epilepsia 51, 2423–2431 10.1111/j.1528-1167.2010.02772.x [DOI] [PubMed] [Google Scholar]
- 69.Akula J. D., Noonan E. R., Di Nardo A., Favazza T. L., Zhang N., Sahin M., Hansen R. M., Fulton A. B. (2015) Vigabatrin can enhance electroretinographic responses in pigmented and albino rats. Doc. Ophthalmol. 131, 1–11 10.1007/s10633-015-9491-0 [DOI] [PubMed] [Google Scholar]
- 70.SanGiovanni J. P., Rosen R., Kaushal S. (2014) Application and interpretation of genome-wide association (GWA) studies for informing pharmacogenomic research: examples from the field of age-related macular degeneration. Curr. Mol. Med. 14, 814–832 10.2174/1566524014666140811113606 [DOI] [PubMed] [Google Scholar]
- 71.Tilz C., Wang-Tilz Y., Jünemann A., Stefan H., Michelson G. (2007) Visual field defect during therapy with valproic-acid. Eur. J. Neurol. 14, 929–932 10.1111/j.1468-1331.2007.01524.x [DOI] [PubMed] [Google Scholar]
- 72.Collier R. J., Wang Y., Smith S. S., Martin E., Ornberg R., Rhoades K., Romano C. (2011) Complement deposition and microglial activation in the outer retina in light-induced retinopathy: inhibition by a 5-HT1A agonist. Invest. Ophthalmol. Vis. Sci. 52, 8108–8116 10.1167/iovs.10-6418 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.