Abstract
Bombesin receptor subtype 3 (BRS-3) is a GPCR that is expressed in the CNS, peripheral tissues, and tumors. Our understanding of BRS-3’s role in physiology and pathophysiology is limited because its natural ligand is unknown. In an attempt to identify this ligand, we screened toad skin (Bufo bufo gargarizans Cantor) extracts and identified prostaglandins as putative ligands. In BRS-3–transfected human embryonic kidney (HEK) cells, we found that prostaglandins, with prostaglandin E2 (PGE2) being the most potent, fulfill the pharmacologic criteria of affinity, selectivity, and specificity to be considered as agonists to the BRS-3 receptor. However, PGE2 is unable to activate BRS-3 in different cellular environments. We speculated that EP receptors might be the cause of this cellular selectivity, and we found that EP3 is the receptor primarily responsible for the differential PGE2 effect. Consequently, we reconstituted the HEK environment in Chinese hamster ovary (CHO) cells and found that BRS-3 and EP3 interact to potentiate PGE2 signaling. This potentiating effect is receptor specific, and it occurs only when BRS-3 is paired to EP3. Our study represents an example of functional crosstalk between two distantly related GPCRs and may be of clinical importance for BRS-3–targeted therapies.—Zhang, Y., Liu, Y., Wu, L., Fan, C., Wang, Z., Zhang, X., Alachkar, A., Liang, X., Civelli, O. Receptor-specific crosstalk between prostanoid E receptor 3 and bombesin receptor subtype 3.
Keywords: BRS-3 receptor, EP3 receptor, PGE2, toad skin
Bombesin receptor subtype 3 (BRS-3, also known as BB3) is a GPCR that is present in the CNS and peripheral tissues (1–4). First discovered in 1992 through homology screening approaches, BRS-3 was assigned to the bombesin receptor family because of its sequence similarity to the other mammalian bombesin receptors, the neuromedin B (NMB) receptor (NMB-R, BB1; 47% similarity) and the gastrin-releasing peptide (GRP) receptor (GRP-R, BB2; 51% similarity) (1, 5). Pharmacologic activation studies of BRS-3 and genetic studies using knockout mice have suggested important roles for BRS-3 in the regulation of energy homeostasis, insulin secretion, heart rate, and blood pressure (6–10). In particular, BRS-3 knockout mice have been reported to display late-onset obesity (4, 11) and to show less anxiety (12). Synthetic BRS-3 agonists stimulate sympathetic tone, suggesting a possible role for BRS-3 in regulating the sympathetic nervous system (13). In addition, the BRS-3 receptor has been found to be overexpressed in developing lungs and certain carcinoma cells, indicating that the receptor may play a role in cell growth and proliferation (14–16).
BRS-3 is an orphan GPCR. It does not exhibit high affinity to bombesin or the endogenous peptides of the other mammalian bombesin receptors. Many groups have unsuccessfully attempted to deorphanize BRS-3 using different systems including parabiotic mice (17). BRS-3 seems to be constitutively active (18), and in vertebrate species, BRS-3 could be potently activated by GRP and NMB (19), indicating that there is an endogenous mammalian ligand for BRS-3 from an evolutionary aspect. Because bombesin was originally discovered in the skin of the toad species Bombina bombina and Bombina variegate (20), we hypothesized that toads may express an endogenous BRS-3 ligand. Because BRS-3 is found in cancer cells (14–16), we chose to begin our search using the skin of the toad Bufo bufo gargarizans Cantor, which is widely used in traditional Chinese medicines for treating hepatoma, lung cancer, and carcinoma (21). We applied the reverse pharmacology approach commonly used in the deorphanization of GPCRs (22). We described the surprising result that BRS-3 can be activated by prostaglandins. However, we also found that this activation is cell specific. The cell type selectivity led us to study whether the selective activation is due to crosstalk between EP and BRS-3 receptors. Our results shed light on a previously unappreciated role of the orphan BRS-3 receptor in modifying other receptors’ functions.
MATERIALS AND METHODS
Materials
The skin of the toad B. bufo gargarizans Cantor was purchased from Shandong province and authenticated by the Institute of Medication, Xiyuan Hospital of Chinese Academy of Traditional Chinese Medicine. Acetonitrile for analysis was purchased from Thermo Fisher Scientific (Waltham, MA, USA). Formic acid was obtained from Acros Organics (Morris, NJ, USA). Water for mobile phases was purified on a Milli-Q system (EMD Millipore, Billerica, MA, USA). HPLC-grade methanol was purchased from Shandong Yuwang (Yucheng City, China). Industrial-grade ethanol (95%) was used in the extraction and pretreatment of samples. The acetonitrile used in preparative HPLC was of industry grade. Formic acid used in preparative HPLC was of analytical grade (Supervision of Tianjin Kermel Chemical Reagents, Tianjin, China). Compound 16a, a BRS-3 agonist (23), N1-(2-phenylethyl)-(2R)-2-{[(1S)-1-(benzylcarboxamido)ethyl]carboxamido}-3-([1H]-3-indolyl)propanamide (Supplemental Fig. S1), was purchased from Bachem Americas (Torrance, CA, USA). Bombesin was purchased from Phoenix (Burlingame, CA, USA). Bantag-1, the BRS-3 antagonist (6), was kindly provided by Merck (Kenilworth, NJ, USA). D-Nal-Cys-Tyr-D-Trp-Lys-Val-Cys-Nal-NH2, the NMB-R antagonist (15), was purchased from Bio-Techne (Minneapolis, MN, USA). L-798,106, the EP3 antagonist (24), was purchased from Sigma-Aldrich (St. Louis, MO, USA). Prostaglandins were purchased from Cayman Chemicals (Ann Arbor, MI, USA). Fluo-4 AM was purchased from Molecular Probes (Eugene, OR, USA). Lipofectamine and Lipofectamine 2000 were purchased from Thermo Fisher Scientific.
All GPCRs used in this study were amplified from either a human, mouse, or rat cDNA library (Clontech Laboratories, Mountain View, CA, USA) and cloned into pcDNA 3.1(−) (Thermo Fisher Scientific). The sequences were confirmed by sequencing from both ends and with internal primers by Laragen (Culver City, CA, USA). The isoform of EP3 is variant 6.
Preparation of crude sample
The dried skin of B. bufo gargarizans Cantor (10 kg) was cut into pieces, then extracted with ethanol (95%) (3 × 100 L, 1 h each) at 78°C. The combined extracts were concentrated to about 5 L using a rotary evaporator. The residue (490 g, solid) was diluted to 10 L with water and then was loaded on an Amberlite XAD-4 macroporous resin (30 L, 20–50 mesh) (Rohm and Haas, Philadelphia, PA, USA) column (160 cm × 20 cm i.d.) eluted with water (200 L), 30% ethanol (90 L), and 95% ethanol (90 L). The fraction eluted with 95% ethanol was combined and concentrated to dryness in vacuum, yielding 125 g of residue. An aliquot of residue (75 g) was dissolved in methanol (300 ml) and filtered through a micropore membrane (0.45 μm) as the crude sample.
Purification and identification
The fractionation was carried out on Prep C18 columns (XTerra MS C18, 100 × 19 mm i.d., 5 μm particle size, 120 Å pore size; Waters, Milford, MA, USA) using a parallel 4-channel preparative HPLC system (Waters). The crude sample (75 g) was redissolved in methanol and applied to the column in batches of 250 mg. The mobile phase was composed of 0.1% (v/v) formic acid aqueous solution (mobile phase A) and acetonitrile with 0.1% (v/v) formic acid (mobile phase B). The gradient was starting from 5% B to 25% B over 30 min, then from 25% B to 50% B in 22 min. Fractions were collected automatically at 1 min intervals from the minutes 4 to 52 and denoted as fraction 4 to fraction 52.
The flow rate was 17 ml/min, and the elution was monitored by an MS detector (ZQ2000; MicroMass, Cary, NC, USA). The passive splitter was about 1/3000. Mass scans were acquired in positive ion mode from m/z 300 to 2000.
Fraction 46 was further purified using a homemade Click CD column (4.6 × 250 mm, 5 μm) on a Waters 2695 HPLC system with a fraction collector. The mobile phase was composed of 0.1% (v/v) formic acid aqueous solution (mobile phase A) and acetonitrile with 0.1% (v/v) formic acid (mobile phase B). The gradient was from 5 to 50% B over 70 min. The active fraction was further purified with the same method as fraction 46. The resulting active compound was identified by using various spectral techniques. High-resolution electrospray ionization mass spectrometry data were recorded on a quadrupole time-of-flight system (Waters). NMR spectra were obtained on a Bruker Avance III 600 MHz NMR spectrometer with a cryogenic probe (Bruker BioSpin, Rheinstetten, Germany).
Cell culture and transfection
HEK-mBRS-3 stable cells (a generous gift from Merck) were grown in DMEM with 200 mg/L G418 sulfate and 2 mg/L puromycin supplemented with 10% fetal bovine serum. Chinese hamster ovary (CHO) cells were grown in α-MEM supplemented with 5% fetal bovine serum. HeLa cells were grown in DMEM supplemented with 10% fetal bovine serum. All the cells were cultured at 37°C in a humidified atmosphere of 5% CO2. The cells were transiently transfected with the plasmids encoding the GPCRs used in this report using Lipofectamine reagent according to the manufacturer’s instructions. The experiments were carried out 24 h after transfection.
Ca2+ response monitored by fluorometric imaging plate reader assay
The fluorometric imaging plate reader (FLIPR) assay was performed as previously described (25). Briefly, the GPCR transfected cells were seeded into poly-d-lysine–coated, black-walled, clear-bottom 96-well plates at a density of 80,000 cells per well. Twenty-four hours later, the medium was removed and replaced with 100 μl of dye loading solution (2 μM Fluo-4 AM dissolved in FLIPR buffer, which consists of pluronic acid in 1 × Hanks buffer supplemented with 20 mM HEPES, pH 7.4) for 1 h at 37°C. The cells were then washed 3 times with FLIPR buffer before FLIPR assay. The samples, which were redissolved in DMSO and stored in 96-well drug plates, were diluted with FLIPR buffer and then added into the cells within 4 s automatically. For inhibition experiments, the cells were treated with antagonists for 15 min before the addition of the agonists. The intracellular Ca2+ concentration was monitored by FLIPR (Molecular Devices, Sunnyvale, CA, USA) at 520 nM with an excitation wavelength of 488 nm over 4 min. Some data in the Supplemental Information were acquired by using the FlexStation III Multireader (Molecular Devices).
RESULTS
Identification and characterization of a potential BRS-3 receptor agonist from toad skin extract
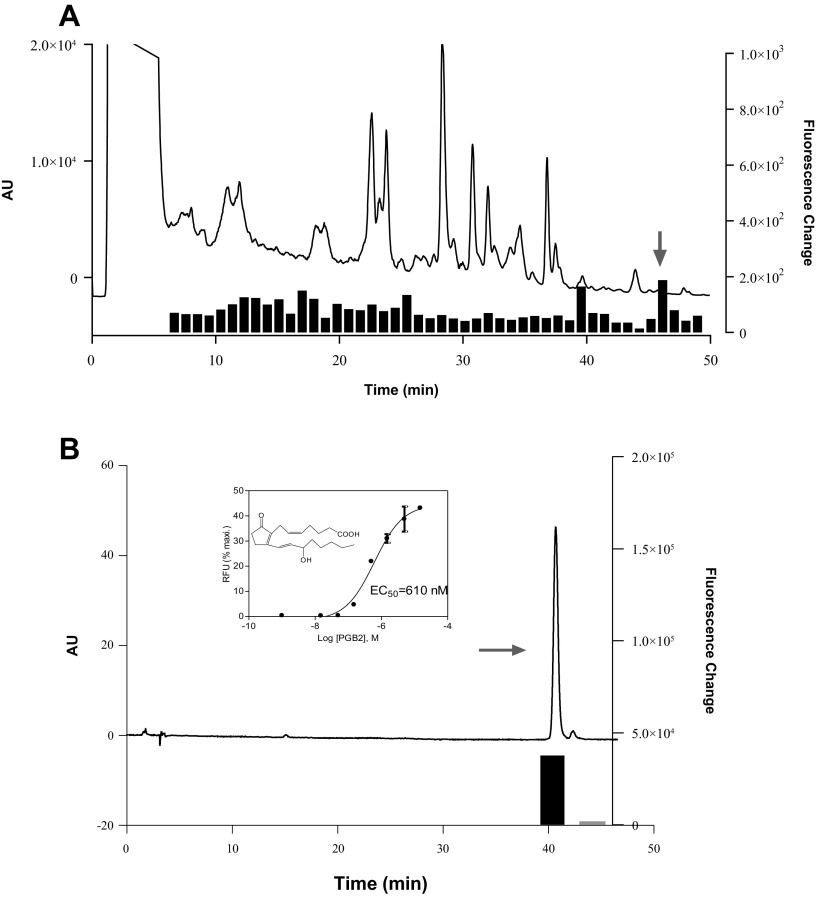
Toad skin extracts were separated by a preparative C18 column into 49 fractions. These fractions were screened on human embryonic kidney (HEK) cells stably expressing the mouse BRS-3 receptor (HEK-mBRS-3). Intracellular Ca2+ changes were monitored using the FLIPR system. A reproducible and robust change in Ca2+ mobilization was observed with fraction 46 in HEK-mBRS-3 but not in parental HEK cells (Fig. 1A). Fraction 46 was further purified by HPLC. Bioguided separation led to the isolation of the active component (Fig. 1B).
Figure 1.
Isolation and characterization of potential BRS-3 ligand from toad skin. A) Elution profile of toad skin extract on C18HCE reverse-phase HPLC column (4.6 × 150 mm, 5 μm) and activities of 48 fractions (1 min) tested for their abilities to induce intracellular Ca2+ mobilization in HEK293 cells expressing BRS-3 receptor. Signal is expressed as ratio of increment obtained by BRS-3–transfected HEK293 cells vs. HEK293 wild-type cells. Elution was performed with linear gradient from 5 to 15% CH3CN in 30 min, then from 15T to 95% CH3CN in 10 min. Flow rate was 1 ml/min. Fraction 46 (arrow) was most active. B) PGB2 purification and structure elucidation. Inset shows structure of PGB2 and its response in BRS-3/HEK monitored by Ca2+ mobilization. Error bars represent sem of triplicate measurements for each point.
The structure of this component was elucidated by mass spectrometry and NMR, and was determined to be prostaglandin B2 (PGB2; structure shown in Fig. 1B, inset). Purified PGB2 induced a dose-dependent Ca2+ change in HEK-mBRS-3 stable cells with an EC50 value of 610 nM (Fig. 1B, inset).
Prostaglandins activate HEK cells expressing BRS-3 receptors
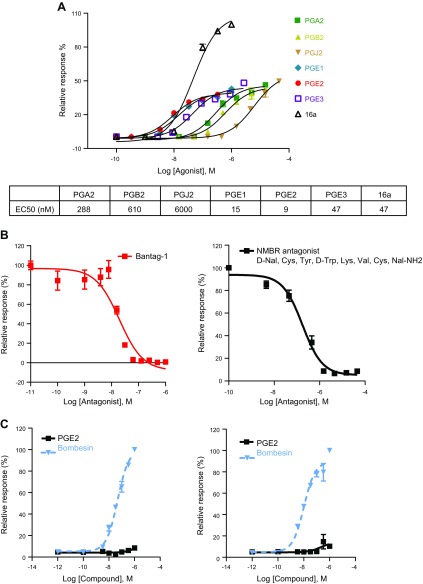
Because PGB2 belongs to a group of lipid compounds derived from C-20 unsaturated fatty acids, we tested prostaglandins and compounds in the prostaglandin biosynthesis pathway for their activities to activate BRS-3 receptors. Seven of 18 compounds were able to activate HEK-mBRS-3 stable cells: PGA2, PGB2, PGJ2, PGF2, PGE2, PGE1, PGE2 and PGE3. We then determined the dose-dependent responses of these 7 compounds. We found that these prostaglandins differ in their potency (Fig. 2A). PGE1, PGE2, and PGE3 were the most potent, with EC50 values of 15, 9, and 47 nM, respectively.
Figure 2.
Prostaglandin activation of BRS-3. A) Prostaglandins dose–response curve in HEK cells stably expressing BRS-3 receptors. BRS-3 agonist 16a was used as positive control. B) Inhibitory effects of BRS-3 antagonists on PGE2 induced Ca2+ mobilization in BRS-3 stable cells. Bantag-1 dose-dependently inhibits PGE2 (5 nM) induced response (left). NMB receptor antagonist D-Nal-Cys-Tyr-D-Trp-Lys-Val-Cys-NaI-NH2 dose-dependently inhibits PGE2 (5 nM) induced response (right). C) No PGE2 induced Ca2+ mobilization in HEK cells expressing NMB (left) and GRP receptors (right). Error bars represent sem of triplicate measurements for each point.
Next, we examined the ability of these 7 prostaglandins to induce intracellular Ca2+ change in HEK cells that are transiently transfected with human, mouse, or rat BRS-3 receptors. The FLIPR results demonstrate that all the 7 compounds induced intracellular Ca2+ release in a dose-dependent manner (Supplemental Fig. S2). These responses are BRS-3 receptor dependent because the same compounds were not able to produce any response in the untransfected HEK cells (data not shown). The calcium mobilization responses to the prostaglandin compounds were similar in BRS-3 transient and stable transfection, indicating that these responses are not linked to the clonal nature of the stably transfected cells. The rank order of the prostaglandin compounds’ potencies is almost identical to that in observed HEK-mBRS-3 stable cells. In the following experiments, we chose to use prostaglandin E2 (PGE2) as our control compound because it displayed the highest relative potency.
The calcium mobilization response of HEK-mBRS-3 to PGE2 was inhibited by bantag-1, a selective BRS-3 antagonist, in a dose-dependent manner (Fig. 2B, left), with an IC50 value of 19.5 nM. The NMB-R antagonist D-Nal-Cys-Tyr-D-Trp-Lys-Val-Cys-NaI-NH2, which is known to inhibit BRS-3 activation (15), was also able to block the response of HEK-mBRS-3 to PGE2, with an IC50 value of 175 nM (Fig. 2B, right). These findings provide evidence for the specificity of the response of BRS-3 receptors to PGE2.
However, PGE2 was unable to induce a response in HEK cells expressing NMB-R (Fig. 2C left) or GRP-R (Fig. 2C right). To verify the expression of these receptors in HEK cells, we examined their responses to bombesin, a potent agonist of NMB-R and GRP-R. Our FLIPR results showed that bombesin elicited a strong calcium response in these cells with EC50 values of 47 and 10 nM, respectively (Fig. 2C), confirming the expression of the NMB-R and GRP-R receptors in HEK cells.
Taken together, these data suggest that the response to PGE2 in HEK cells transfected with BRS-3 is specific and thus points at PGE2 as a possible endogenous ligand for the BRS-3 receptor.
Cell specificity
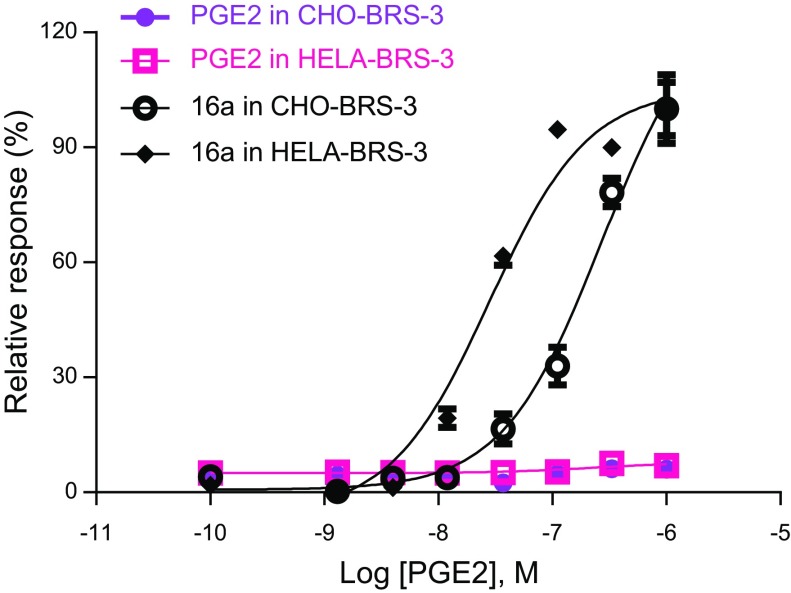
In order to substantiate our results, we examined the PGE2-induced response of BRS-3 receptors in other cell types. BRS-3 receptor was transiently transfected in CHO and HeLa cells, and the calcium mobilization response to PGE2 was monitored. We found that PGE2 was unable to activate BRS-3 in these cell systems (Fig. 3). None of the other prostaglandins induced any detectable Ca2+ response (data not shown). As a positive control experiment, we tested these BRS-3–transfected CHO and HeLa cells for their responses to 16a, a potent BRS-3 receptor agonist (23). As expected, 16a was able to elicit a strong calcium response in these cell systems, thus verifying the expression of the BRS-3 receptors in these cells (Fig. 3).
Figure 3.
No effects of PGE2 may be observed in CHO-BRS-3 or HeLa-BRS-3 cells; 16a was used as positive control.
We expected that HEK cells possess endogenous EP receptors and consequently hypothesized that the response induced by PGE2 as well as other prostaglandins may result from the interaction between the BRS-3 and EP receptors that are expressed endogenously in the HEK but not in the CHO or HeLa cellular environments.
There exist 4 EP receptors, EP1 through EP4. EP1 is coupled to Gαq, EP2 and EP4 are coupled to Gαs, and EP3 is coupled to Gαi/Gαq proteins. We first examined the response to PGE2 in HEK cells transfected with each subtype of EP receptors. Dose–response curves showed that PGE2 induced potent effects on all these EP receptors (Supplemental Fig. S3A). Interestingly, we observed that PGE2 has higher efficacy when EP3 was cotransfected with Gqi3, indicating that EP3 receptors preferentially couple to Gαi protein. In addition, because we have found PGB2 to be the active component in the toad skin extract, we examined whether PGB2 can activate EP3 receptor in HEK-EP3/Gqi3 cells. The results, shown in Supplemental Fig. S3B, indicate that PGB2 dose-dependently induced Ca2+ response, with an EC50 value of 250 nM, which is close to that in HEK-BRS-3 cells (610 nM). The activation by PGB2 in HEK-EP3/Gqi3 cells was blocked by L-798,106, an EP3 selective antagonist (Supplemental Fig. S3C).
We then used FLIPR to determine which of the endogenous EP receptors resulted in activation in HEK cells. We transiently transfected Gα15 or the chimera Gqi3, which allowed us to monitor Gαs- and Gαi-mediated coupling, respectively. We found that PGE2 induced intracellular Ca2+ release when Gα15 or Gqi3 were transfected into HEK cells but not CHO cells (Table 1). Therefore, we postulated that EP3 receptors are most likely the EP receptor recognized in the assay because EP3 receptors are the only EP receptors that are coupled to Gαi/Gαq. Furthermore, the increase of intracellular Ca2+ release was inhibited by L-798,106, a selective EP3 receptor antagonist in Gqi3-transfected HEK cells (Supplemental Fig. S4A). The expression of EP3 receptors in HEK cells is also supported by real-time quantitative PCR (qPCR) analysis (Supplemental Fig. S4B).
TABLE 1.
Calcium responses in HEK, CHO, and HeLa cells
| HEK | CHO | HeLa | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Response | Vector | Vector + Gqi3 | Vector + Gα15 | Vector | Vector + Gqi3 | Vector + Gα15 | Vector | Vector + Gqi3 | Vector + Gα15 |
| 16a | − | − | − | − | − | − | − | − | − |
| PGE2 | − | + | + | − | − | − | − | − | − |
−, no Ca2+ influx observed; +, Ca2+ influx observed.
Reconstitution in CHO cells of responses found in HEK cells
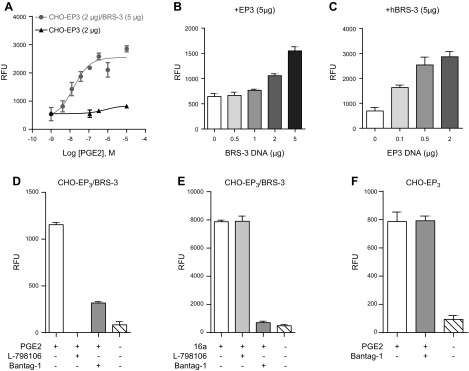
To reconstitute the HEK cellular environment in CHO cells, we carried out a series of experiments where BRS-3 was cotransfected with EP3 receptor cDNAs. We first transfected CHO cells with either EP3 alone or with EP3/BRS-3. As expected, we found that PGE2 was able to induce a strong response in the cells cotransfected with BRS-3 and EP3 (CHO- EP3/BRS-3) and only a minimal one in cells solely transfected with EP3 receptor cDNA (CHO-EP3) (Fig. 4A). We then transfected CHO cells with variable amounts of the cDNAs of EP3 and BRS-3. We found that when EP3 and BRS-3 are coexpressed in CHO cells, the PGE2-induced response correlates positively with the concentration of the EP3 and BRS-3 (Fig. 4B, C). We then tested whether the PGE2-induced responses in CHO cells cotransfected with BRS-3 and EP3 (CHO-EP3/BRS-3) display the distinctive pharmacologic profiles of BRS-3 and of EP3. As expected, both BRS-3 and EP3 antagonists (bantag-1 and L-798,106, respectively) were able to inhibit the PGE2 response, albeit with different efficiencies (Fig. 4D). Furthermore, when the CHO-EP3/BRS-3 cells were activated by the BRS-3 agonist 16a, the response was inhibited by bantag-1 but not by L-798,106 (Fig. 4E). As a control, the PGE2 activation of CHO cells transfected solely with EP3 was not inhibited by bantag-1, demonstrating that bantag-1 does not act directly at the EP3 receptor (Fig. 4F). These data show that the responses to PGE2 in HEK cells expressing BRS-3 receptors can be reproduced in CHO cells, provided that EP3 receptors are expressed in these cells.
Figure 4.
Crosstalk between BRS-3 and EP3 receptors in CHO cells. A) Enhanced response to PGE2 (1 μM) in CHO-EP3 (2 μg)/BRS-3 (5 μg) cells. B, C) Dose–response of BRS-3 (B) and EP3 (C) on PGE2 (1 μM) induced Ca2+ influx in CHO-EP3/BRS-3. D, E) Effects of EP3 (L-798,106, 10 μM; Sigma-Aldrich) and BRS-3 antagonists (bantag-1, 10 μM; Sigma-Aldrich) on PGE2 (1 μM) (D) or 16a (1 μM) (E) induced Ca2+ influx in CHO-EP3 (2 μg)/BRS-3 (5 μg) cells. F) No inhibitory effect of bantag-1 on PGE2 response in CHO-EP3 cells. Error bars represent sem of triplicate measurements for each point.
Specificity of BRS-3/EP3 crosstalk
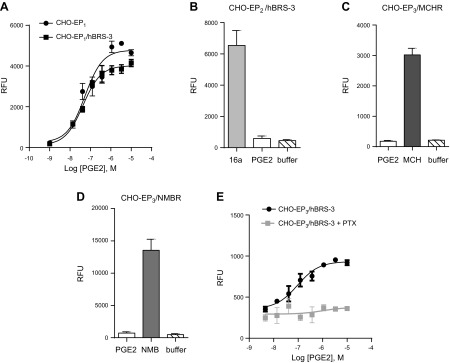
Because our qPCR data also indicated the mRNA expression of EP1 and EP2 occurred in HEK cells (Supplemental Fig. S4B), we cotransfected BRS-3 with EP1 or EP2 receptors in CHO cells. PGE2 was able to induce Ca2+ mobilization in CHO transfected by EP1 (which couples to Gαq), but the coexpression of BRS-3 had no enhancing effect (Fig. 5A). PGE2 did not induce any Ca2+ mobilization in CHO-EP2 or CHO-EP2/BRS-3 cells (Fig. 5B) because EP2 receptors couple to Gαs proteins, the activation of which cannot be monitored in the FLIPR format. These results indicate that it is unlikely that BRS-3 receptors interact with EP1 or EP2 receptors.
Figure 5.
Specificity of EP3/BRS-3 receptor pair and G protein involved. No enhancement of PGE2 induced Ca2+ mobilization in (A) CHO-EP1/hBRS-3 cells, (B) CHO-EP2/hBRS-3 cells, (C) CHO-EP3/MCH, and (D) CHO-EP3/NMB cells. E) Effect of PTX (100 ng/ml) treatment on PGE2 induced Ca2+ mobilization in CHO-EP3/BRS-3. Error bars represent sem of triplicate measurements for each point.
To further examine the specificity of EP3/BRS-3 receptor pairing, we cotransfected the EP3 receptor in CHO cells with a receptor of the BRS-3 receptor family (NMB-R) or with an unrelated peptidergic receptor (melanin-concentrating hormone, or MCH, receptor). Both NMB and MCH receptors are known to couple to Gαq protein and thus intracellular calcium release. As controls, we demonstrated that both CHO-EP3/NMB and CHO-EP3/MCH increased intracellular calcium release upon exposure to MCH (Fig. 5C) or NMB, respectively (Fig. 5D). In contrast, PGE2 could not induce a response in any of these cotransfection cell systems (Fig. 5C, D). Taken together, these data indicate that BRS-3 receptors specifically interact with EP3 receptors.
Furthermore, we wanted to determine whether the blockade of Gαi protein by pertussis toxin (PTX) would affect the response to PGE2 in CHO-EP3/BRS-3 cells. We found that PTX markedly inhibited PGE2-induced response in this cotransfection system (Fig. 5E), indicating that the response to PGE2 in the CHO-EP3/BRS-3 cells is mediated primarily through the activation of Gαi protein. These results suggest that signaling via the Gαi protein pathway is required for PGE2-induced calcium mobilization, and they further support the presence of the endogenous Gαi/Gαq-coupled EP3 receptor and its crosstalk with the BRS-3 receptor in HEK cells.
DISCUSSION
Because bombesin was originally discovered in toad skin, we chose to use this tissue as a potential source for an endogenous ligand of the orphan BRS-3 receptor. Using the reverse pharmacology approach, we identified PGB2 as a putative ligand of BRS-3 and extended this discovery to different prostaglandins. These experiments relied on the use of BRS-3 transfected HEK cells. The rank order of prostaglandin potency was similar to that reported for the EP receptors, PGE2 being the most potent (26, 27).
The activation of BRS-3 by PGE2 was found to be specific being inhibited by bantag-1 and D-Nal-Cys-Tyr-D-Trp-Lys-Val-Cys-NaI-NH2, two synthetic BRS-3 antagonists. The fact that two structurally unrelated antagonists can inhibit the PGE2 response is a strong argument in favor of receptor specificity. NMB-R and GRP-R, the two other receptors that are part of the bombesin receptor family, were not activated by PGE2. These data show that prostaglandins, in particular PGE2, can act as natural agonists to the BRS-3 receptor.
However, when BRS-3 was transfected in CHO or HeLa cells, PGE2 was unable to induce a response pointing at a cellular selectivity. We speculated that an endogenous EP may account for this selectivity, and indeed our further investigation showed that EP3 is the EP receptor that is detected in our experiments in HEK cells. Consequently, we set up to reproduce in CHO cells the environment that equips HEK-BRS-3 cells to respond to PGE2. We found that BRS-3 and EP3 coexpression leads to a dramatic increase of PGE2-mediated Ca2+ response compared to EP3 expression alone. PGE2 efficacy increases with increasing levels of both EP3 and BRS-3 receptors, indicating that the number of both EP3 and BRS-3 receptors determines the extent of PGE2-mediated enhancement. We observed that in CHO-EP3/BRS-3, PGE2 signaling was completely blocked by L-798,106, the selective antagonist of EP3 receptor, and partially inhibited by bantag-1, the BRS-3 selective antagonist (Fig. 4D). These data indicate that practically all of the PGE2 response is carried out by the EP3 receptor, while bantag-1 blocks only the BRS-3–dependent response. We further found that the enhancement of PGE2-mediated signaling was completely abolished by PTX in CHO-EP3/BRS-3, suggesting that this activation is Gαi mediated. It has been reported that EP3 causes Ca2+ increase as a result of Gαi-mediated PLC activation (28). The PGE2-mediated enhancement is BRS-3 specific in that this effect was only observed in the presence of BRS-3 but not two other Gαq-coupled receptors, NMB-R and MCH-R. The data obtained in cell lines coexpressing BRS-3 with EP1 or EP2 receptors show that the enhancement in PGE2 signaling is selective to the EP3/BRS-3 receptors pair. Our results indicate that PGE2 acts directly on the EP3 receptor and that the presence of BRS-3 potentiates this response.
Several possible mechanisms may explain our results and may be addressed in the future. First, it is possible that BRS-3 recruits Gαq proteins to interact with EP3 and leads to the potentiation effect described here. Direct transfer of information between receptors has been documented (29–31). Second, the phenomenon described here may also result from a functional crosstalk, as previously reported for other Gαq- and Gαi-coupled receptor pairs (32). This phenomenon particularly occurs when the receptors share their signaling pathways or when their pathways meet at crossroads at the membrane (33, 34). Third, we should not rule out the possibility of a crosstalk due to receptor clustering in microdomains (35, 36). The exact molecular mechanism of the crosstalk found between BRS-3 and EP3 remains to be studied and clarified. Moreover, our observations were made in a heterologous system, and it is currently unclear whether the interaction of this receptor pair occurs in vivo, either in normal physiology or in pathologic processes. Behavioral studies are also warranted to validate the functional relevance of this receptor pair.
It should be mentioned that a similar phenomenon has been described for adenosine. Adenosine was once considered a potential ligand for growth hormone secretagogue receptor (GHS-R) (37, 38), a ghrelin receptor, but it was later revealed to potentiate the GHS-R–mediated Ca2+ signaling through activating the endogenous adenosine receptor 2B (39, 40). Other studies suggested extensive functional crosstalk between adenosine receptor 2B and GHS-R (41). Indeed, all GPCRs are hydrophobic molecules that have the potential, at least to a certain extent, to interact with one another. This GPCR propensity might complicate the process of identifying the ligand for certain GPCRs when using an overexpressed GPCR cell system. This is particularly important in the deorphanization process because an orphan GPCR may be crosstalking to a known GPCR. Hence, it is of critical importance to use multiple cell systems and assays when identifying a natural ligand of an orphan receptor.
In summary, our results provide strong evidence for the potentiating effect of the orphan BRS-3 receptor on EP3 receptor signaling. Our study represents the first example of functional crosstalk between the orphan GPCR, the BRS-3 receptor, and a member of the large deorphanized rhodopsin-like GPCR family. The implications of the crosstalk between these two receptors should help understand the functional role of the BRS-3 receptor. The possibility that the role of the BRS-3 receptor is to modulate other receptors provides a hypothetical speculation for why a BRS-3 ligand is still unknown. Our study may affect drug development and lead to novel BRS-3 targeted therapies. Meanwhile, studying the crosstalk between orphan GPCR and deorphanized GPCRs may become a new strategy to obtain better insight into the biology of GPCRs with known ligands and functions. Our findings also raise the question of whether some orphan GPCRs may have ligand-independent functions such as the regulation of other GPCRs through heterodimerization or functional crosstalk.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health (NIH) National Institute on Drug Abuse Grant DA024746, and the Eric L. and Lila D. Nelson Chair in Neuropharmacology (to O.C.). This work was also supported by the National Natural Science Foundation of China Key Program 21135005 (to X.L.), and Grant 81402806 (to Y.Z.). The authors declare no conflicts of interest.
Glossary
- BRS-3
bombesin receptor subtype 3
- CHO
Chinese hamster ovary
- FLIPR
fluorometric imaging plate reader
- GHS-R
growth hormone secretagogue receptor
- GRP
gastrin-releasing peptide
- GRP-R
gastrin-releasing peptide receptor
- HEK
human embryonic kidney
- MCH
melanin-concentrating hormone
- NMB
neuromedin B
- NMB-R
neuromedin B receptor
- PGB2
prostaglandin B2
- PGE2
prostaglandin E2
- PTX
pertussis toxin
- qPCR
quantitative PCR
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
X. Liang and O. Civelli conceived, initiated, and directed the project; Y. Zhang, L. Wu, C. Fan, and Z. Wang performed the pharmacologic experiments; Y. Liu and X. Zhang performed the purification of the toad skin; A. Alachkar analyzed the data and edited the article; and Y. Zhang and O. Civelli wrote the article.
REFERENCES
- 1.Fathi Z., Corjay M. H., Shapira H., Wada E., Benya R., Jensen R., Viallet J., Sausville E. A., Battey J. F. (1993) BRS-3: a novel bombesin receptor subtype selectively expressed in testis and lung carcinoma cells. J. Biol. Chem. 268, 5979–5984 [PubMed] [Google Scholar]
- 2.Sano H., Feighner S. D., Hreniuk D. L., Iwaasa H., Sailer A. W., Pan J., Reitman M. L., Kanatani A., Howard A. D., Tan C. P. (2004) Characterization of the bombesin-like peptide receptor family in primates. Genomics 84, 139–146 10.1016/j.ygeno.2004.01.008 [DOI] [PubMed] [Google Scholar]
- 3.Porcher C., Juhem A., Peinnequin A., Bonaz B. (2005) Bombesin receptor subtype-3 is expressed by the enteric nervous system and by interstitial cells of Cajal in the rat gastrointestinal tract. Cell Tissue Res. 320, 21–31 10.1007/s00441-004-1032-1 [DOI] [PubMed] [Google Scholar]
- 4.Ohki-Hamazaki H., Watase K., Yamamoto K., Ogura H., Yamano M., Yamada K., Maeno H., Imaki J., Kikuyama S., Wada E., Wada K. (1997) Mice lacking bombesin receptor subtype-3 develop metabolic defects and obesity. Nature 390, 165–169 10.1038/36568 [DOI] [PubMed] [Google Scholar]
- 5.Jensen R. T., Battey J. F., Spindel E. R., Benya R. V. (2008) International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol. Rev. 60, 1–42 10.1124/pr.107.07108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan X. M., Chen H., Dobbelaar P. H., Dong Y., Fong T. M., Gagen K., Gorski J., He S., Howard A. D., Jian T., Jiang M., Kan Y., Kelly T. M., Kosinski J., Lin L. S., Liu J., Marsh D. J., Metzger J. M., Miller R., Nargund R. P., Palyha O., Shearman L., Shen Z., Stearns R., Strack A. M., Stribling S., Tang Y. S., Wang S. P., White A., Yu H., Reitman M. L. (2010) Regulation of energy homeostasis by bombesin receptor subtype-3: selective receptor agonists for the treatment of obesity. Cell Metab. 11, 101–112 10.1016/j.cmet.2009.12.008 [DOI] [PubMed] [Google Scholar]
- 7.Guan X. M., Metzger J. M., Yang L., Raustad K. A., Wang S. P., Spann S. K., Kosinski J. A., Yu H., Shearman L. P., Faidley T. D., Palyha O., Kan Y., Kelly T. M., Sebhat I., Lin L. S., Dragovic J., Lyons K. A., Craw S., Nargund R. P., Marsh D. J., Strack A. M., Reitman M. L. (2011) Antiobesity effect of MK-5046, a novel bombesin receptor subtype-3 agonist. J. Pharmacol. Exp. Ther. 336, 356–364 10.1124/jpet.110.174763 [DOI] [PubMed] [Google Scholar]
- 8.Feng Y., Guan X. M., Li J., Metzger J. M., Zhu Y., Juhl K., Zhang B. B., Thornberry N. A., Reitman M. L., Zhou Y. P. (2011) Bombesin receptor subtype-3 (BRS-3) regulates glucose-stimulated insulin secretion in pancreatic islets across multiple species. Endocrinology 152, 4106–4115 10.1210/en.2011-1440 [DOI] [PubMed] [Google Scholar]
- 9.Lateef D. M., Xiao C., Brychta R. J., Diedrich A., Schnermann J., Reitman M. L. (2016) Bombesin-like receptor 3 regulates blood pressure and heart rate via a central sympathetic mechanism. Am. J. Physiol. Heart Circ. Physiol. 310, H891–H898 10.1152/ajpheart.00963.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao C., Reitman M. L. (2016) Bombesin-like receptor 3: physiology of a functional orphan. Trends Endocrinol. Metab. 27, 603–605 10.1016/j.tem.2016.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ladenheim E. E., Hamilton N. L., Behles R. R., Bi S., Hampton L. L., Battey J. F., Moran T. H. (2008) Factors contributing to obesity in bombesin receptor subtype-3–deficient mice. Endocrinology 149, 971–978 10.1210/en.2007-1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamada K., Santo-Yamada Y., Wada E., Wada K. (2002) Role of bombesin (BN)-like peptides/receptors in emotional behavior by comparison of three strains of BN-like peptide receptor knockout mice. Mol. Psychiatry 7, 113–117, 116 [DOI] [PubMed] [Google Scholar]
- 13.Reitman M. L., Dishy V., Moreau A., Denney W. S., Liu C., Kraft W. K., Mejia A. V., Matson M. A., Stoch S. A., Wagner J. A., Lai E. (2012) Pharmacokinetics and pharmacodynamics of MK-5046, a bombesin receptor subtype-3 (BRS-3) agonist, in healthy patients. J. Clin. Pharmacol. 52, 1306–1316 10.1177/0091270011419854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou X., Wei L., Harada A., Tatamoto K. (2006) Activation of bombesin receptor subtype-3 stimulates adhesion of lung cancer cells. Lung Cancer 54, 143–148 10.1016/j.lungcan.2006.08.005 [DOI] [PubMed] [Google Scholar]
- 15.Tan Y. R., Qi M. M., Qin X. Q., Xiang Y., Li X., Wang Y., Qu F., Liu H. J., Zhang J. S. (2006) Wound repair and proliferation of bronchial epithelial cells enhanced by bombesin receptor subtype 3 activation. Peptides 27, 1852–1858 10.1016/j.peptides.2005.12.012 [DOI] [PubMed] [Google Scholar]
- 16.Toi-Scott M., Jones C. L., Kane M. A. (1996) Clinical correlates of bombesin-like peptide receptor subtype expression in human lung cancer cells. Lung Cancer 15, 341–354 10.1016/0169-5002(95)00597-8 [DOI] [PubMed] [Google Scholar]
- 17.Lateef D. M., Xiao C., Reitman M. L. (2015) Search for an endogenous bombesin-like receptor 3 (BRS-3) ligand using parabiotic mice. PLoS One 10, e0142637 10.1371/journal.pone.0142637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gbahou F., Holst B., Schwartz T. W. (2010) Molecular basis for agonism in the BB3 receptor: an epitope located on the interface of transmembrane-III, -VI, and -VII. J. Pharmacol. Exp. Ther. 333, 51–59 10.1124/jpet.109.162131 [DOI] [PubMed] [Google Scholar]
- 19.Mo C., Huang L., Cui L., Lv C., Lin D., Song L., Zhu G., Li J., Wang Y. (2017) Characterization of NMB, GRP and their receptors (BRS3, NMBR and GRPR) in chickens. J. Mol. Endocrinol. 59, 61–79 10.1530/JME-17-0020 [DOI] [PubMed] [Google Scholar]
- 20.Anastasi A., Erspamer V., Bucci M. (1971) Isolation and structure of bombesin and alytesin, 2 analogous active peptides from the skin of the European amphibians Bombina and Alytes. Experientia 27, 166–167 10.1007/BF02145873 [DOI] [PubMed] [Google Scholar]
- 21.Zhou B., Wu F., Yuan L., Miao Z., Zhu S. (2015) Is HuaChanSu beneficial in treating advanced non–small-cell lung cancer? Evidence from a meta-analysis of its efficacy combined with chemotherapy. Evid. Based Complement. Alternat. Med. 2015, 408145 10.1155/2015/408145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Civelli O., Nothacker H. P., Saito Y., Wang Z., Lin S. H., Reinscheid R. K. (2001) Novel neurotransmitters as natural ligands of orphan G-protein–coupled receptors. Trends Neurosci. 24, 230–237 10.1016/S0166-2236(00)01763-X [DOI] [PubMed] [Google Scholar]
- 23.Zhang L., Nothacker H. P., Wang Z., Bohn L. M., Civelli O. (2009) Pharmacological characterization of a selective agonist for bombesin receptor subtype-3. Biochem. Biophys. Res. Commun. 387, 283–288 10.1016/j.bbrc.2009.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jugus M. J., Jaworski J. P., Patra P. B., Jin J., Morrow D. M., Laping N. J., Edwards R. M., Thorneloe K. S. (2009) Dual modulation of urinary bladder activity and urine flow by prostanoid EP3 receptors in the conscious rat. Br. J. Pharmacol. 158, 372–381 10.1111/j.1476-5381.2009.00275.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y., Wang C., Wang L., Parks G. S., Zhang X., Guo Z., Ke Y., Li K. W., Kim M. K., Vo B., Borrelli E., Ge G., Yang L., Wang Z., Garcia-Fuster M. J., Luo Z. D., Liang X., Civelli O. (2014) A novel analgesic isolated from a traditional Chinese medicine. Curr. Biol. 24, 117–123 10.1016/j.cub.2013.11.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takano S., Sawano A., Watanabe H., Suzuki T. (1978) A comparison of responses of guinea-pig isolated trachea to six prostaglandins. Prostaglandins 15, 485–489 10.1016/0090-6980(78)90132-6 [DOI] [PubMed] [Google Scholar]
- 27.Kiriyama M., Ushikubi F., Kobayashi T., Hirata M., Sugimoto Y., Narumiya S. (1997) Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br. J. Pharmacol. 122, 217–224 10.1038/sj.bjp.0701367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Irie A., Segi E., Sugimoto Y., Ichikawa A., Negishi M. (1994) Mouse prostaglandin E receptor EP3 subtype mediates calcium signals via Gi in cDNA-transfected Chinese hamster ovary cells. Biochem. Biophys. Res. Commun. 204, 303–309 10.1006/bbrc.1994.2460 [DOI] [PubMed] [Google Scholar]
- 29.Rozenfeld R., Gupta A., Gagnidze K., Lim M. P., Gomes I., Lee-Ramos D., Nieto N., Devi L. A. (2011) AT1R-CB1R heteromerization reveals a new mechanism for the pathogenic properties of angiotensin II. EMBO J. 30, 2350–2363 10.1038/emboj.2011.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ayoub M. A., Levoye A., Delagrange P., Jockers R. (2004) Preferential formation of MT1/MT2 melatonin receptor heterodimers with distinct ligand interaction properties compared with MT2 homodimers. Mol. Pharmacol. 66, 312–321 10.1124/mol.104.000398 [DOI] [PubMed] [Google Scholar]
- 31.Olmo I. G., Ferreira-Vieira T. H., Ribeiro F. M. (2016) Dissecting the signaling pathways involved in the crosstalk between metabotropic glutamate 5 and cannabinoid type 1 receptors. Mol. Pharmacol. 90, 609–619 10.1124/mol.116.104372 [DOI] [PubMed] [Google Scholar]
- 32.Pera T., Penn R. B. (2014) Crosstalk between beta-2–adrenoceptor and muscarinic acetylcholine receptors in the airway. Curr. Opin. Pharmacol. 16, 72–81 10.1016/j.coph.2014.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quitterer U., Lohse M. J. (1999) Crosstalk between Galpha(i)- and Galpha(q)-coupled receptors is mediated by Gbetagamma exchange. Proc. Natl. Acad. Sci. USA 96, 10626–10631 10.1073/pnas.96.19.10626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rives M. L., Vol C., Fukazawa Y., Tinel N., Trinquet E., Ayoub M. A., Shigemoto R., Pin J. P., Prézeau L. (2009) Crosstalk between GABAB and mGlu1a receptors reveals new insight into GPCR signal integration. EMBO J. 28, 2195–2208 10.1038/emboj.2009.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Insel P. A., Head B. P., Patel H. H., Roth D. M., Bundey R. A., Swaney J. S. (2005) Compartmentation of G-protein–coupled receptors and their signalling components in lipid rafts and caveolae. Biochem. Soc. Trans. 33, 1131–1134 10.1042/BST0331131 [DOI] [PubMed] [Google Scholar]
- 36.Sabourin T., Bastien L., Bachvarov D. R., Marceau F. (2002) Agonist-induced translocation of the kinin B(1) receptor to caveolae-related rafts. Mol. Pharmacol. 61, 546–553 10.1124/mol.61.3.546 [DOI] [PubMed] [Google Scholar]
- 37.Tullin S., Hansen B. S., Ankersen M., Møller J., Von Cappelen K. A., Thim L. (2000) Adenosine is an agonist of the growth hormone secretagogue receptor. Endocrinology 141, 3397–3402 10.1210/endo.141.9.7631 [DOI] [PubMed] [Google Scholar]
- 38.Smith R. G., Griffin P. R., Xu Y., Smith A. G., Liu K., Calacay J., Feighner S. D., Pong C., Leong D., Pomés A., Cheng K., Van der Ploeg L. H., Howard A. D., Schaeffer J., Leonard R. J. (2000) Adenosine: a partial agonist of the growth hormone secretagogue receptor. Biochem. Biophys. Res. Commun. 276, 1306–1313 10.1006/bbrc.2000.3610 [DOI] [PubMed] [Google Scholar]
- 39.Carreira M. C., Camiña J. P., Díaz-Rodríguez E., Alvear-Perez R., Llorens-Cortes C., Casanueva F. F. (2006) Adenosine does not bind to the growth hormone secretagogue receptor type-1a (GHS-R1a). J. Endocrinol. 191, 147–157 10.1677/joe.1.06714 [DOI] [PubMed] [Google Scholar]
- 40.Johansson S., Fredholm B. B., Hjort C., Morein T., Kull B., Hu P. S. (2005) Evidence against adenosine analogues being agonists at the growth hormone secretagogue receptor. Biochem. Pharmacol. 70, 598–605 10.1016/j.bcp.2005.05.023 [DOI] [PubMed] [Google Scholar]
- 41.Hermansson N. O., Morgan D. G., Drmota T., Larsson N. (2007) Adenosine is not a direct GHSR agonist—artificial cross-talk between GHSR and adenosine receptor pathways. Acta Physiol. (Oxf.) 190, 77–86 10.1111/j.1365-201X.2007.01691.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.