Abstract
IL-1 signaling is adhesion-restricted in many cell types, but the mechanism that drives it is not defined. We screened for proteins recruited to nascent adhesions in IL-1–treated human fibroblasts with tandem mass tag–mass spectrometry. We used fibronectin bead preparations to enrich 10 actin-associated proteins. There was a 1.2 times log 2-fold enrichment of actin capping protein (ACP) at 30 min after IL-1 stimulation. Knockdown (KD) of ACP by siRNA reduced IL-1–induced ERK activation(by 56%, matrix metalloproteinase-3 (MMP-3) expression by 48%, and MMP-9 expression by 62% (in all reductions, P < 0.01). Confocal or structured illumination microscopy showed that ACP was diffused throughout the cytosol but strongly accumulated at the ruffled border of spreading cells. ACP colocalized with nascent paxillin- and vinculin-containing adhesions at the ruffled border, but not with mature adhesions in the center. ACP KD promoted the formation of large, stable adhesions. Immunoprecipitation and proximity ligation analysis showed that ACP was associated with the IL-1 signal transduction proteins myeloid differentiation factor 88 (MyD88) and IL-1 receptor–associated kinase (IRAK) at the ruffled border of the leading edge. IL-1–induced phospho-ERK and MyD88 or IRAK colocalized at the leading edge. We concluded that ACP is required for recruitment and function of IL-1 signaling complexes in nascent adhesions at the leading edge of the cell.—Wang, Q., Delcorde, J., Tang, T., Downey, G. P., McCulloch, C. A. Regulation of IL-1 signaling through control of focal adhesion assembly.
Keywords: fibroblasts, cell spreading, integrins, cytokine signaling
IL-1 is an important mediator of inflammation and promotes the degradation and remodeling of extracellular matrices (ECMs) in several inflammatory diseases—including rheumatoid arthritis, acute lung injury, Still’s disease, and chronic periodontitis—as a result of its up-regulation of matrix metalloproteinase (MMP) expression (1). Two membrane-bound IL-1 receptors (IL-1Rs) have been identified: type I and type II (IL-1RI and IL-1RII), both of which independently bind IL-1 (2). IL-1RI can generate downstream signals (3, 4) and forms a heterodimer with the IL-1R accessory protein (IL-1RAcP) (5) that enhances the binding affinity of IL-1RI to its ligand. By contrast, IL-1RII is unable to generate signals and appears to function primarily as a decoy receptor (3). Binding of IL-1 to IL-1RI leads to the recruitment of IL-1RAcP (6). The IL-1R signaling complex subsequently recruits myeloid differentiation factor 88 (MyD88), an adaptor protein that binds IL-1R–associated kinase (IRAK1 and IRAK2) (7, 8). IRAK is rapidly phosphorylated, then disengages from MyD88 and associates with TNF-α (7), which then activates a number of signal transduction cascades involving the MEK family members ERK (9), JNK (10), and p38 (11).
Despite extensive investigation, the regulation of IL-1 signaling in stromal cells is not well defined, especially in the early stages of cell anchorage to the ECM, when cells exhibit multiple biologic responses to IL-1 that are dependent on the extent of cell spreading (12). Gingival fibroblasts and chondrocytes are directly involved in the matrix degradation seen in common inflammatory disorders including periodontitis and arthritis. These cells require the formation of focal adhesions (FAs) in vitro in order to generate IL-1–induced signals including tyrosine phosphorylation and activation of FA kinase (13), Ca2+ release from the endoplasmic reticulum (14, 15), and activation of ERK (9, 16). All of these processes are involved in the increased expression of MMPs (17, 18), proteases that degrade matrix proteins and initiate intracellular signaling through processes such as limited proteolytic processing of cell surface receptors. IL-1 also promotes FA remodeling as shown by the enrichment of talin (19), α-actinin (20), paxillin, vinculin (21), and protein tyrosine phosphatase-α in FAs after IL-1 treatment (17, 21).
FAs provide mechanical links that connect actin filaments to the ECM (22). These organelles also serve as platforms for concentrating multiple signaling and scaffolding proteins at sites where integrins cluster and bind to matrix proteins (22). The networks of signaling proteins found in FAs and their interactions are dynamic, mechanosensitive, and complex (23). The complexity of FAs is currently being addressed with a variety of technologies including imaging (24), proteomics (25), and bioinformatics (26), all of which are providing new insights into their composition, organization, and regulation. However, the key mechanisms by which the interactions of molecules within FAs enable cells to respond to environmental signals are not fully defined.
The temporally and spatially precise assembly of actin filaments is crucial to myriad cellular functions (27, 28) and to the formation of subcellular structures including FAs (29). In cytokine signaling, the organization of actin filament networks in FAs is critical to activating IL-1–induced ERK (9). In this phenomenon, high-density actin filament networks in FAs may provide a scaffold (30) that enables the assembly of IL-1R–associated signaling molecules into a functional complex (31). There is also is an apparent functional interdependence of IL-1 signaling on the actin cytoskeleton because IL-1 induces transient cell contraction and reorganization of the actin filament network (32). Currently, little is known about the processes by which IL-1’s signaling machinery is recruited to FAs.
At the ruffled border of the leading edge of migrating cells, actin filaments undergo cycles of assembly and disassembly that enable the extension of the leading lamellipodia and the formation and reorganization of cell adhesions (33). Notably, the structure, function, and remodeling of actin filaments are regulated by actin-binding proteins that enable the creation of a diverse array of actin filament structures through their interactions with actin monomers and filaments. When considering that discrete actin-binding proteins may be essential for regulating the assembly of nascent adhesions and the earliest IL-1 signaling complexes, we designed experiments that used tandem mass tag–mass spectrometry (TMT-MS) to identify actin-binding proteins that are recruited to FAs during the early stages of cell spreading in an IL-1–dependent manner. One of the key proteins identified was actin capping protein (ACP) (34, 35), a ubiquitous, highly conserved heterodimer that regulates actin assembly by capping the fast-growing, barbed ends of actin filaments. ACP is an important functional component in the assembly of various actin structures including the dynamic, branched filament network at the ruffled border of motile cells (36, 37). In this study, we examined the role of ACP in regulating the generation of IL-1 signals in spreading fibroblasts.
MATERIALS AND METHODS
Reagents
Fibronectin (FN), poly-l-lysine, and bovine serum albumin (BSA) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Rabbit polyclonal antibodies to ACP and paxillin were purchased from MilliporeSigma (Burlington, MA, USA). Antibodies to IL-1RAcP, IRAK, and MyD88 were obtained from Abcam (Cambridge, United Kingdom). Rabbit monoclonal antibodies to phospho-p44/42 MAPK and polyclonal antibodies to p44/42 MAPK were obtained from Cell Signaling Technology (Danvers, MA, USA). Recombinant human IL-1β and Mouse Total MMP-3 and Mouse Total MMP-9 Quantikine ELISA kits were purchased from R&D Systems (Minneapolis, MN, USA). On-targetplus Smartpool small interfering RNA (siRNA) targeted at ACP and Dharmafect 1 transfection reagent were purchased from GE Dharmacon (Lafayette, CO, USA). Rat anti-mouse antibody to CD29 (9EG7) was obtained from BD Biosciences (Franklin Lakes, NJ, USA). Goat antibody to mouse integrin α5β1 was purchased from Chemicon (Temecula, CA, USA). Duolink In Situ Probemaker Plus and Minus (DUO92009–92010) proximity ligation assay (PLA) probes as well as Detection Reagents Red (DUO92008) were obtained from MilliporeSigma.
Cell culture
Passages 3–12 of human gingival fibroblasts (HGFs) were cultured at 37°C in minimal essential medium containing 10% fetal bovine serum and antibiotics [124 U/ml penicillin G (Sigma-Aldrich), 50 μg/ml gentamicin SO4− (Wisent Bioproducts), and 0.25 μg/ml Fungizone (Wisent Bioproducts, St-Bruno, QC, Canada)]. Cells were maintained in a humidified incubator gassed with 95% air and 5% CO2, and were passaged with 0.01% trypsin.
Isolation of FAs
FA complexes were prepared from cells incubated with collagen- or BSA-coated magnetite beads after incubation for a specific amount of time as previously described (9). Briefly, cell-bead complexes were scraped into ice-cold cytoskeleton extraction buffer (0.5% Triton X-100, 50 mM NaCl, 300 mM sucrose, 3 mM MgCl2, 20 µg/ml aprotinin, 1 µg/ml leupeptin, 1 µg/ml pepstatin, 1 mM PMSF, and 10 mM PIPES, at pH 6.8). After washing beads 3 times in a Triton X-100 buffer and using magnetic separation to remove nonspecifically bound proteins, the remaining bead-associated (FA) proteins were eluted in Laemmli sample buffer by boiling for 10 min.
siRNA knockdown
HGFs were seeded at 30% confluence 24 h prior to transfection and were incubated with 10 nM scrambled siRNA (AllStars negative control; Qiagen, Hilden, Germany) or On-Targetplus CAPZB siRNA (Dharmacon) and DharmaFECT 1 transfection reagent 1 for 48 h following the manufacturer’s instructions. Cells were trypsinized and replated on FN (10 mg/ml) for 3 h in medium containing 1% serum before stimulating with 20 ng/ml IL-1 or vehicle control in serum-free medium for various time points. Whole cell lysates were collected and protein concentrations were determined by BCA assay. Equal amounts of total proteins from each treatment condition were separated on 10% acrylamide gels and immunoblotted to estimate the effectiveness of capping protein knockdown (KD) and IL-1β–induced ERK activation.
Immunoblotting
Equal amounts of protein (Bradford assay; Bio-Rad, Hercules, CA, USA) were resolved by SDS-PAGE and transferred to nitrocellulose membranes. Membranes were blocked with 5% milk or 0.2% BSA in Tris-buffered saline solution (TBS) for 1 h and then incubated with primary antibodies overnight at 4°C in TBS with 0.1% Tween 20 (BioShop, Burlington, ON, Canada) and 5% milk or 0.2% BSA followed by fluorochrome-conjugated secondary antibodies for 1 h in TBS with 0.1% TWEEN 20 at room temperature. Image Studio (Li-Cor, Lincoln, NE, USA) software was used to analyze immunoblots.
Immunoprecipitation
Cells were lysed in 1% Tris-NaCl-Triton immunoprecipitation buffer (20 mM Tris at pH 7.5, 1% Triton X-100, 0.1% SDS, 150 mM NaCl) containing 1 mM PMSF, 1 mM NaVO3, 10 μg/ml leupeptin, and 10 μg/ml aprotinin. Equal amounts of protein from cleared extracts were immunoprecipitated with the Dynabeads immunoprecipitation protocol (Invitrogen, Carlsbad, CA, USA).
Confocal fluorescence, structured illumination microscopy, and total internal reflection microscopy
HGFs were seeded on FN-coated glass coverslip dishes (MatTek Corporation, Ashland, MA, USA) and treated with either PBS or IL-1β (20 ng/ml) in serum-free medium for 5–60 min. After fixation with 4% paraformaldehyde for 15 min, cells were permeabilized with 0.2% Triton X-100, blocked for 1 h in 0.2% BSA, and stained with either the appropriate antibodies or fluorescent affinity dyes. A TCS SP8 confocal microscope equipped with a ×40 oil-immersion objective lens (Leica Microsystems, Wetzlar, Germany) was used to determine the spatial distribution of proteins of interest. Colocalization of proteins in images was quantified as previously described (38), and data were expressed using the Pearson correlation coefficient, which represents the spatial relation between structures in the 2 fluorescence channels. Structured illumination microscopy (SIM) images were acquired using an Elyra S1 microscope (×63 oil objective lens with a numerical aperture of 1.4; Carl Zeiss, Oberkochen, Germany) and an Andor iXon 897 PLAM camera (Belfast, United Kingdom). Spatial colocalization analysis of SIM images was performed using the particle function of a distribution of ImageJ software (National Institutes of Health, Bethesda, MD, USA) called Fiji, which assigned equal-sized regions of interest to the leading edge of spreading cells. Particles (1–10 pixels) above background staining were counted in regions of interest for each cell (designated as dots). Total internal reflection microscopy (TIRF) (×100 oil-immersion objective lens, LAS AF; Leica Microsystems) was employed to generate fluorescence excitation within a narrow zone (∼100 nm) on the coverslip, which affected fluorescent proteins only on the ventral cell surface and immediately below the plasma membrane.
Proximity ligation assay
We obtained a more detailed assessment of the spatial associations between MyD88 or IRAK and ACP in situ using PLA, which was performed with a Duolink PLA Kit (Sigma-Aldrich). HGFs were plated on FN-coated glass coverslip dishes and treated with either PBS or IL-1β (20 ng/ml) in serum-free medium for 15–30 min. After fixation with 4% paraformaldehyde for 15 min, cells were permeabilized with 0.2% Triton X-100, blocked with Duolink blocking buffer for 30 min at 37°C, and then incubated with conjugated antibodies (Plus and Minus PLA probes) to a suitable concentration in the PLA probe diluent. PLA detection was performed in accordance with the manufacturer’s instructions. Fluorescence signals were detected using a TCS SP8 confocal microscope equipped with a ×40 oil-immersion objective lens (Leica Microsystems).
Real-time quantitative PCR
After IL-1 stimulation in low-serum (1% fetal bovine serum) minimal essential medium, total RNA was extracted from HGFs using an RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. Total RNA samples (1 μg as determined by a Thermo Fisher Scientific NanoDrop ND-1000 spectrophotometer) were reverse-transcribed using the iScript cDNA Synthesis Kit (Bio-Rad) according to the manufacturer’s instructions. Real-time quantitative PCR was performed on a CFX96 Touch real-time PCR detection system according to the product protocol using SsoFast EvaGreen Supermix (Bio-Rad) and validated mouse MMP-3 primers (forward, 5′-TGGGATTCTGTGAGGATGCCTTA-3′; reverse, 5′-AAAGCCCTTCCCATAGTTGCCAGA-3′), MMP-9 primers (forward, 5′-GACATAGACGGCATCCAGTATC-3′; reverse, 5′-GTGGGAGGTATAGTGGGACA-3′) and mouse GAPDH (glyceraldehyde 3-phosphate dehydrogenase) primers (forward, 5′-AATGGTGAAGGTCGGTGTG-3′; reverse, 5′-GTGGAGTCATACTGGAACATGTAG-3′). PCR reactions were performed twice, and all assays were performed in triplicate. The relative expression of the MMP3 and MMP9 genes to the housekeeping gene GAPDH was calculated using the 2−ΔΔCt method.
Measurement of intracellular calcium [Ca2+]i
Cells on cover slips were loaded with 3 μM Fura-2-acetoxymethyl ester (Fura2-AM) for 20 min at 37°C. The nominally calcium-free buffer consisted of a bicarbonate-free medium containing 150 mM NaCl, 5 mM KCI, 10 mM d-glucose, 1 mM MgSO4, 1 mM Na2HPO4, and 20 mM HEPES at pH 7.4 with an osmolarity of 291 milliosmoles. After incubation with Fura-2AM, inspection of cells by fluorescence microscopy demonstrated no vesicular compartmentalization of Fura-2AM, suggesting that the dye loading method permitted measurement of cytosolic [Ca2+]i. Whole-cell [Ca2+]i measurements were obtained using C-Imaging Image Analysis software (Compix, Cranberry Township, PA, USA) with excitation wavelengths of 340 and 380 nm and an emission wavelength of 520 nm. Changes in [Ca2+]i were monitored by the ratio of Fura-2AM fluorescence at 340 and 380 nm.
Mass spectrometry
FA complexes were prepared from cells incubated with FN- or BSA-coated magnetite beads after IL-1 stimulation for varying periods of time. They then were eluted with 50 mM glycine buffer (pH 2.3–2.5). The eluted proteins were dialyzed for 36 h in carbonate buffer (25 mM NH4HCO3 at pH 7.5). Trypsin (1 μg; Roche Diagnostics, Rotkreuz, Switzerland) was added to the sample, which was rotated overnight at 37°C. Subsequently, 0.1% acetic acid was added to the sample, which was then air-dried with an evaporator. Lyophilized samples were analyzed by TMT-MS on a QStar XL Hybrid LC/MS/MS System (Applied Biosystems, Foster City, CA, USA; MDS Sciex, Concord, ON, Canada) at the Hospital for Sick Children SPARC BioCentre (Toronto, ON, Canada). Scaffold 4.0 (Proteome Software, Portland, OR, USA) was used for analyzing search results, calculating P values for each peptide match, matching peptide spectra, and enriching the folding of proteins from FN-coated vs. BSA-coated bead preparations.
Data analysis
All experiments were conducted in quadruplicate and were repeated ≥3 times. For continuous variables, means ± sd were computed. A Student’s t test was used for 2-sample comparisons, and statistical significance was set at a type I error rate of P < 0.05. For multiple comparisons, ANOVA and post hoc Tukey’s test were used.
RESULTS
ACP in IL-1–induced signaling and MMP expression
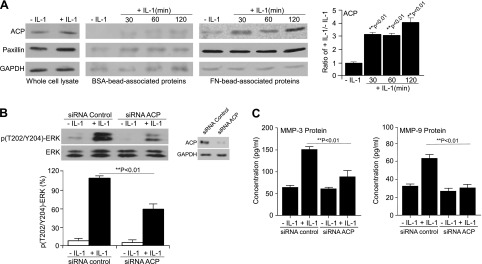
Based on the dynamic interrelation between IL-1 signaling and the remodeling of FAs (19–21), we used TMT-MS to identify cell adhesion proteins that are involved in IL-1 signaling. We identified >200 different proteins in FN-coated bead–associated preparations that model the ligand-bound state of integrins and their associated adhesion proteins (39). Of these, ∼35 proteins were specific to FN-coated bead preparations (Supplemental Table 1), based on comparisons with BSA-coated bead preparations, which represent nonspecific adhesive interactions. The fold enrichment of proteins prepared from FN-coated beads compared with BSA-coated beads and the discovery of adhesion-related proteins as anticipated underlines the validity of this method for the preparation of adhesion-associated fractions. After IL-1 stimulation of FN-specific protein preparations, there was a time-dependent increase in the abundance of 10 actin-associated regulatory proteins (Table 1) including ACP, which was of interest because of its role in regulating actin filament growth (35) at the ruffled border of the cell’s leading edge. The presence of ACP in FN-coated bead–associated protein preparations was confirmed by immunoblotting (Fig. 1A). As a negative control, FN-coated bead–associated preparations were immunoblotted for GAPDH, a cytosolic protein expressed at low levels in FA preparations. As a positive control, FN-coated bead–associated preparations were immunoblotted for the FA-associated protein paxillin; no time-dependent increase in paxillin was detected after IL-1 stimulation (Fig. 1A). ACP KD strongly reduced recruitment of MyD88 and IRAK to FA preparations (Supplemental Fig. 1A). We conducted immunoprecipitation experiments to examine whether IL-1 enhanced the association between ACP and the actin-binding proteins vinculin and paxillin, and between ACP and MyD88 or IRAK. These data showed that IL-1 strongly increased the association between each of these proteins and ACP, whereas immunoprecipitations conducted with control IgG showed low protein signals with no specific changes (Supplemental Fig. 1B).
TABLE 1.
TMT-MS analysis of actin-associated regulatory proteins derived from BSA- and FN-coated beads at indicated times after treatment of HGFs with 20 ng/ml of IL-1β
| Identified protein | Molecular mass (kDa) | Accession no. | Probability score (log 2–fold) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BSA + IL-1 (min) | FN + IL-1 (min) | |||||||||
| 0 | 30 | 60 | 180 | 0 | 30 | 60 | 180 | |||
| Serine/threonine-protein kinase PAK 2 | 58 | Q13177/PAK2 | 0.0 | −4.5 | −0.1 | 0.5 | 0.9 | 1.1 | 1.0 | 1.2 |
| Ras suppressor protein 1 | 32 | Q15404/RSU1 | 0.0 | 0.0 | 0.0 | −0.3 | 0.3 | 0.8 | 0.7 | 0.7 |
| F-ACP subunit β | 31 | P47756/CAPZB | 0.0 | −0.2 | 0.6 | −0.2 | 0.3 | 1.2 | 0.9 | 1.3 |
| Isoform 2 of Protein Flightless-1 | 138 | Q13045-2/FLII | 0.0 | 0.6 | 0.0 | 0.1 | 1.0 | 1.1 | 1.2 | 1.1 |
| Isoform 2 of Rho GTPase-activating protein 17 | 88 | Q68EM7-2/RHG17 | 0.0 | −1.5 | 0.1 | 0.0 | −1.9 | 0.6 | 0.8 | 0.6 |
| Myosin-9 | 227 | P35579/MYH9 | 0.0 | 0.2 | −0.2 | −0.1 | 0.7 | 1.0 | 0.9 | 0.8 |
| Isoform 2 of Filamin-A | 280 | P21333-2/FLNA | 0.0 | 0.0 | 0.0 | 0.1 | 0.6 | 0.9 | 1.0 | 0.8 |
| Actin, α-cardiac muscle 1 | 42 | P68032/ACTC | 0.0 | −0.4 | 0.2 | 0.2 | −0.5 | 0.3 | 0.6 | 1.0 |
| Isoform 2 of Filamin-C | 278 | Q14315-2/FLNC | 0.0 | 0.2 | 0.1 | 0.3 | 0.8 | 0.7 | 0.6 | 0.7 |
| Isoform 5 of Palladin | 128 | Q8WX93-5/PALLD | 0.0 | −0.2 | −0.5 | −0.6 | −0.1 | 0.4 | 0.6 | 0.6 |
Italic text indicates that there was a 1.2 times log 2-fold enrichment of ACP at 30 min after IL-1 stimulation.
Figure 1.
ACP regulates IL-1–induced ERK phosphorylation and MMP-3 and MMP-9 protein expression. A) Whole-cell lysates and FN-coated bead–associated proteins were prepared from HGFs that had been treated with either PBS or IL-1 (20 ng/ml) for indicated time periods. Lysates were immunoblotted for ACP, paxillin, and GAPDH. Densitometry data are expressed as means ± sd. Differences between indicated groups were assessed by ANOVA and post hoc Tukey’s test. B) HGFs were transfected with control siRNA or ACP siRNA for 48 h, and then treated with either PBS or IL-1 (20 ng/ml) for 30 min. Lysates were immunoblotted for p(T202/Y204)-ERK and total ERK. Lysates of control siRNA and ACP siRNA–transfected cells were immunoblotted for ACP to confirm ACP KD; GAPDH was used for loading control. Densitometry data were assessed by ANOVA and are expressed as means ± sd. C) Cells transfected as above were treated with either PBS or IL-1 (20 ng/ml) for 8 h. Culture medium was concentrated 5–10-fold. MMP-3 and MMP-9 proteins were quantified by ELISA.
IL-1–induced signaling can lead to the expression of proteins that degrade the ECM such as MMPs, the expression of which is dependent on MAPK (40). Because ERK activation stimulated by IL-1 is dependent on FAs (14), we examined the role of ACP in IL-1–induced ERK activation and MMP-3/MMP-9 expression in HGFs transfected with siRNA to ACP. Compared with control siRNA, ACP KD by siRNA substantially diminished IL-1–induced ERK phosphorylation (Fig. 1B) and also markedly reduced MMP-3 and MMP-9 expression (Fig. 1C, P < 0.01). These observations indicate that ACP is involved in the regulation of IL-1–induced MAPK signaling, leading to MMP expression in gingival fibroblasts.
We also examined whether other signals downstream of IL-1, including Ca2+ flux and FA kinase activation, are affected by ACP KD. These data (Supplemental Fig. 2) showed that intracellular Ca2+ concentration and FA kinase activation (phosphotyrosine 397) in response to IL-1 are not affected by ACP KD.
ACP associates with nascent paxillin and vinculin
We examined the spatial association between ACP and paxillin or ACP and vinculin using confocal microscopy; SIM, which provides high resolution analysis; and TIRF, which generates fluorescence excitation within a narrow zone (∼100 nm) on the coverslip. These methods were used to assess protein recruitment into nascent FAs.
HGFs were attached to FN-coated coverslips for 15, 30, or 60 min, treated with either PBS or IL-1 for 30 min, and coimmunostained for either ACP and paxillin or ACP and vinculin. ACP was distributed throughout the cytosol but also accumulated at the ruffled border of the leading edge of spreading cells. The extent of colocalization of ACP with paxillin (Fig. 2A) and the colocalization of ACP with vinculin (Fig. 2B) was estimated using Pearson correlation coefficients (38) of confocal images and separately by automated counting (using Fiji’s particle analysis function) of the number of discrete, dual-labeled, superimposed fluorescent dots in SIM images at the cell edge. These data showed a time-dependent colocalization of ACP with nascent paxillin- or vinculin-containing adhesions at the ruffled border of the cell leading edge, but much less so in more mature adhesions located toward the cell center.
Figure 2.
Localization of ACP with paxillin and vinculin. A) HGFs were grown on FN-coated coverslips for 15, 30, or 60 min, treated for 30 min with either PBS or IL-1, and then coimmunostained for ACP and paxillin. Confocal microscopy (top left panel), SIM (lower left panel), and TIRF (top right panel) were used to capture ACP (red) and paxillin (green) in cells spreading on the coverslips. Images of cell leading edges (indicated by white boxes) are displayed in the second row of the confocal microscopy and SIM panels. Original magnification, ∼×4.5 and 6.0. Arrows indicate spatial association between ACP and paxillin at the cell leading edge. The top right panel shows histograms of Pearson correlation coefficients created in ImageJ; data is expressed as means ± sd and was obtained from 30 cells in each of 3 different experiments. Differences between groups were measured by ANOVA and post hoc Tukey’s test. The lower right panel shows a histogram of the mean number of red (ACP staining), green (paxillin staining), and yellow (merged) fluorescent “dots” at the peripheral edge of the cell as determined by Fiji’s particle analysis function. Values from 30 cells were measured in each of 3 independent experiments. B) Identical procedures for cell culture, immunostaining, and image analysis were conducted for ACP and vinculin. For confocal microscopy and SIM analyses, 30 cells were analyzed in each of 3 different experiments.
We examined the effects of ACP depletion on the formation of nascent adhesions. HGFs were transfected with control siRNA or ACP siRNA, grown on FN-coated coverslips for either 30 or 60 min, and coimmunostained for ACP and paxillin. Confocal microscopy (Fig. 3A) showed that, compared with control siRNA, the number of nascent adhesions at the ruffled border of the cell leading edge in ACP siRNA was reduced by ACP KD (Fig. 3A) and the length of adhesions was increased (Fig. 3A). These data indicate that ACP KD results in the formation of larger and presumably more stable adhesions, which underlies the importance of ACP in adhesion remodeling. Similarly designed ACP siRNA experiments were conducted on cells immunostained for ACP and talin (an early FA marker). These data showed reduced numbers of peripheral FAs (P > 0.01) after ACP KD but no change of FA length (Fig. 3B).
Figure 3.
ACP KD affects adhesion formation. HGFs were transfected with control siRNA and ACP siRNA and plated on FN for 30 min in the presence of IL-1 and coimmunostained for ACP and paxillin or talin. A) Confocal microscopy images of immunofluorescence localization of ACP (red) and paxillin (green) in cells spreading on FN. Higher magnification of leading edge of cells is shown in boxed regions. Lysates of control siRNA and ACP siRNA–transfected cells were immunoblotted for ACP to confirm ACP KD. GAPDH was used as a loading control. In the lower left panel, the histogram displays the mean ± sd number of FAs in the cell periphery (<5 μm from the cell membrane) that were immunostained with paxillin; n = 10–12 cells/group. The histogram in the lower left panel shows the means ± sd length of FAs immunostained with paxillin in cell periphery (<5 μm from the cell membrane; n = 10–12 cells/group). FAs were quantified using ImageJ. Differences between groups were measured by ANOVA and post hoc Tukey’s test. B) Identical procedures were conducted for ACP KD and immunostaining for talin, followed by analysis of talin-stained FAs including computation of the means ± sd number of peripheral FAs (left panel) and the means ± sd FA length; n =10–12 cells/group. Differences between groups were measured by ANOVA and post hoc Tukey’s test.
Effect of ACP on the recruitment of IL-1 signaling proteins to FAs
We examined the role of ACP in the recruitment of early IL-1 signaling proteins such as IL-1R1, IL-1RAcP, MyD88, and IRAK to FAs by immunoblotting FN-coated bead preparations (39). After IL-1 treatment, the relative abundance of IL-1R1, IL-1RAcP, MyD88, and IRAK was increased in FA preparations compared with whole cell lysates or non-FA preparations (Supplemental Fig. 1A). We also found that IL-1 enhanced the abundance of paxillin in FA preparations, indicating that IL-1 promotes FA maturation. For all experiments, equal amounts of protein were loaded into each lane. As discussed, GAPDH, a non-FA cytoplasmic protein serving as a control, was expressed at very low levels in FA preparations and there was no difference in its abundance after IL-1 treatment. ACP getting knockdown by siRNA eliminated IL-1–induced recruitment of endogenous IL-1R1, IL-1RAcP, MyD88, and IRAK to FA preparations (Supplemental Fig. 1A).
We examined whether ACP associated with IL-1 signaling proteins through immunoprecipitation. After treating HGFs with IL-1 or PBS, MyD88, IRAK, paxillin and vinculin were immunoprecipitated from FA preparations. The respective immunoprecipitates were immunoblotted for these proteins (i.e., inputs) and for ACP (Supplemental Fig. 1B). Treatment with IL-1 enhanced the association of ACP with MyD88, IRAK, paxillin and vinculin. Immunoprecipitations with control (rabbit) IgG showed no specific associations or enhancements for these same proteins.
ACP associates with MyD88 and IRAK at the cell edge
We examined the spatial colocalization of ACP with MyD88 or IRAK using SIM particle analysis. HGFs were plated on FN for 15, 30 or 60 min, treated with either PBS or IL-1, and coimmunostained for MyD88 and ACP (Fig. 4A) or for IRAK and ACP (Fig. 4C). Protein colocalization at the ruffled border of the leading edge was quantified as described above. SIM analysis showed that IL-1 treatment enhanced the time-dependent spatial association between ACP and MyD88 or IRAK. We extended these spatial colocalization analyses of immunostained ACP and MyD88 or IRAK with an in situ PLA, a well-established antibody-based technique that visualizes protein–protein interactions with sufficient amplification to detect single molecular events but without requiring protein modification (41). This technique has the advantage of providing an in situ snapshot of the proteins of interest because the theoretical maximum distance between the 2 target proteins must be <40 nm to detect a PLA signal according to manufacturer’s instructions. As a negative control, either 1 or both primary antibodies were omitted at a time; we observed very few single fluorescent dots under these conditions (Fig. 4B, D insets). Positive PLA signals manifested as single fluorescent dots over individual cells (Fig. 4B, D). The ACP-MyD88 or ACP-IRAK PLA signals were diffusely distributed over cells, but we observed enhanced colocalization at the ruffled border of the cell leading edge after IL-1 stimulation. We confirmed the validity of this method by examining both a positive control, in which a known ligand for an adhesion receptor (β1 integrin–FN) was imaged, and a negative control (N-cadherin-FN). These results indicate that ACP spatially colocalized with the IL-1 signaling proteins MyD88 or IRAK at the ruffled border after IL-1 stimulation.
Figure 4.
MyD88 is spatially associated with ACP at the leading edge of the cell. A) Left panel: SIM images of immunofluorescence localization of ACP (red) and MyD88 (green) in cells spreading on FN-coated coverslips for 30 min. Cells were treated with either PBS or IL-1. Images of cell leading edges (indicated by white boxes) are displayed in the second row. Arrows indicate spatial association between ACP and MyD88 at the leading edge. Original magnification, ∼×5.0. Right panel: The histogram shows the number of red (ACP staining), green (MyD88 staining), and yellow (merged) fluorescent “dots” at the peripheral edge of cell as determined by Fiji. Values from 30 cells in each of 3 independent experiments are expressed as means ± sd. Comparisons between groups were observed using ANOVA and post hoc Tukey’s test. B) Left panel: representative confocal images of PLA interaction (red dots) between ACP and MyD88 in cells spreading on FN-coated coverslips for 30 min without and with IL-1 stimulation, respectively. Images of the white boxed regions are displayed in the second row. Inset shows HGFs incubated with antibody to MyD88 alone. Original magnification, ∼×9.5. Center panel: The mean ± sd number of fluorescent dots attributable to PLA reaction in the cell periphery (<5 μm from the cell membrane) were quantified using ImageJ; n = 10–12 cells/group. Right panel: representative confocal images of negative control cells (left), in which PLA interactions are not detected using the N-cadherin-FN pair. Positive control cells (right) show PLA interactions detected for a β1 integrin–FN pair. C, D) Results of ACP and IRAK analysis derived from the same procedures shown in A and B, respectively.
MyD88 and IRAK colocalize with nascent adhesions after IL-1 stimulation
To determine whether ACP, MyD88, and IRAK are truly enriched in the ruffled border of the leading edge of the cell, we coimmunostained for ACP and GAPDH in HGFs, which were attached to FN-coated coverslips for 5 or 30 min before being treated with either PBS or IL-1. Confocal imagines and line scanning analysis showed that GAPDH was homogenously distributed from the edge of the cell toward the center whereas ACP strongly accumulated at the ruffled border at the leading edge of the cell (Fig. 5A). We then used confocal microscopy to examine the spatial relations between MyD88 or IRAK and nascent FAs at the ruffled border of the leading edge of spreading cells. HGFs were attached to FN-coated coverslips for 1, 5, 15 or 30 min, treated with either PBS or IL-1 for 30 min, coimmunostained for either MyD88 or IRAK and paxillin, and analyzed by line scanning. MyD88 and IRAK were distributed throughout the cytosol but accumulated and became progressively more colocalized with paxillin after 15 mins (Fig. 5B, C). Thus, IL-1 promotes the accumulation of MyD88 and IRAK in nascent FAs at the ruffled border of the leading edge of the cell.
Figure 5.
IL-1 induces colocalization of MyD88 and IRAK with nascent adhesions. A) Top panels: HGFs were grown on FN-coated coverslips for 5 and 30 min, treated with either PBS or IL-1 for 30 min, and coimmunostained for ACP and GAPDH. Confocal microscopy images of immunofluorescence localization of ACP (red), GAPDH (green), and the two merged (yellow). Bottom panels: ACP and GAPDH staining intensity distribution along line scans perpendicular to the cell leading edge. Each distribution represents the mean of 10–15 intensity line scans obtained from ≥3 cells for each condition, data is expressed as means ± sd. B) Top panels: HGFs were grown on FN-coated coverslips for 1, 5, 15, and 30 min, treated with either PBS or IL-1 for 30 min, and coimmunostained for MyD88 and paxillin. Confocal microscopy images of immunofluorescence localization of MyD88 (red), paxillin (green), and the two merged (yellow). Images of cell leading edges (indicated by white boxes) are inset. Bottom panels: MyD88 and paxillin staining intensity distribution along line scans perpendicular to the cell leading edge. Each distribution represents the mean of 10–15 intensity line scans obtained from ≥3 cells for each condition. Data is expressed as means ± sd. Original magnification, ∼×3.5. C) IL-1 induces colocalization of IRAK with nascent adhesions. Results were derived from the same procedures shown in A and B.
We used double immunostaining and confocal microscopy to localize MyD88, paxillin, IRAK, phospho-ERK, and vinculin, and the spatial relations among them when ACP is knocked down. After ACP KD, the colocalization of MyD88, IRAK, phospho-ERK, and paxillin and their accumulation at the leading edge of cells were markedly diminished (Supplemental Figs. 3–5).
MyD88 and IRAK cluster with IL-1–induced ERK phosphorylation
IL-1 rapidly induces the activation and recruitment of ERK to nascent FAs (20), events that occur downstream of IL-1 signaling complex assembly. We examined the spatial relations between and temporal dynamics of proteins in IL-1 signaling complexes (marked by MyD88 and IRAK) and IL-1–induced downstream signaling events (marked by ERK phosphorylation). HGFs were attached to FN-coated coverslips for 15, 30, or 60 min, incubated with either PBS or IL-1, immunostained for phospho-ERK and either MyD88 or IRAK , imaged by SIM or by confocal microscopy, and analyzed by line scanning. IL-1–induced ERK activation was detected as focal increases of phospho-ERK fluorescence that colocalized with MyD88 (Fig. 6A) or IRAK (Fig. 6B) at the ruffled border of the leading edge of cells. Line scans of coimmunostained cells showed that phospho-ERK and either MyD88 or IRAK exhibited time-dependent peaks in fluorescence at the cell leading edge (Fig. 6A, B), indicating that IL-1 signaling complexes and activated MAPK cluster at the ruffled border of the leading edge of cells.
Figure 6.
MyD88, IRAK, and phospho-ERK are recruited to the leading edge of the cell. A) HGFs were grown on FN-coated coverslips for 15, 30, and 60 min, treated with either PBS or IL-1 for 30 min, and coimmunostained for MyD88 and phospho-ERK. Top left panel: representative SIM immunofluorescence images of MyD88 (red), phospho-ERK (green), and the two merged (yellow). Images of cell leading edges (indicated by white boxes) are displayed in the second row. Arrows indicate spatial association between MyD88 and phospho-ERK at leading edge. Original magnification, ∼×6.5. Top right panel: histogram shows number of red (ACP staining), green (phospho-ERK staining), and yellow (merged) fluorescence dots in peripheral edge per cell using Fiji. Values of 30 cells from 3 independent experiments are expressed as means ± sd. Bottom left panel: representative confocal immunofluorescence images of MyD88 (red) and phospho-ERK (green). Images of cell leading edges (indicated by white boxes) are inset. Original magnification, ∼×4.5. Bottom right panel: line scans of MyD88 and phospho-ERK staining intensity in both control and IL-1–treated cells. Line scans begin outside the cell and cross inward. The cell edge is indicated by the vertical black line. Each distribution represents the mean of 10–15 intensity line scans obtained from ≥3 cells for each condition, data is expressed as means ± sd. B) Cells were coimmunostained for IRAK and phospho-ERK according to the procedures described in A. SIM analysis was conducted using 30 cells from 3 independent experiments, and line scan analyses were conducted by taking 15 line scans from 4 cells for each condition.
DISCUSSION
IL-1 signaling in fibroblasts, chondrocytes, and synoviocytes is dependent on cells adhering tightly to the ECM (13). This process enables the approximation of IL-1Rs with IL-1R–associated proteins in FAs (9, 19), followed by the generation of downstream signals, including ERK activation; the increased expression of MMP-3 and MMP-9; and matrix degradation (17, 42). Our new, major findings are that ACP is enriched in nascent FAs, regulates adhesion remodeling, spatially colocalizes with the IL-1 signal transduction proteins MyD88 and IRAK at the ruffled border of the leading edge of the cell, and is required for IL-1 signal transduction. Thus, IL-1 signaling complexes are concentrated and their formation is regulated early on during cell attachment and spreading on the matrix. These findings highlight the way ACP acts on the ruffled border of spreading cells, which may be relevant to IL-1 signal transduction in anchorage-dependent cells in the rapidly remodeling ECMs of inflamed connective tissues.
FAs are dynamic organelles that assemble at sites of clustered integrin receptors bound to ECM ligands (43). These organelles mature through stages that include focal contacts, FAs, and fibrillar adhesions, each with a distinctive appearance and molecular composition (44). We screened for IL-1–stimulated protein recruitment to nascent adhesions in spreading HGFs. Proteins were isolated with FN- or BSA-coated beads and detected by TMT-MS. Analysis of the FN-coated bead–associated proteins indicates that the proteins identified in these fractions are similar in overall composition to previous studies conducted by us (17, 45–47) and others (26, 48–50), and correlate with other methods for assessment of FA proteins.
TMTs are chemical labels used in MS that enable concurrent identification and quantification of target proteins and, compared with TMT-MS, provide greatly improved signal-to-noise ratios for assessing proteins in cell preparations. We detected ACP only in preparations prepared from FN-coated beads, and its presence increased over time after IL-1 treatment. Notably, depletion of ACP by siRNA substantially diminished IL-1–induced ERK phosphorylation and also markedly reduced MMP-3 and MMP-9 expression. These observations indicate that ACP is involved in the regulation of IL-1–induced signaling in anchorage-dependent cells.
ACP, a ubiquitous, highly conserved heterodimer, tightly caps the fast-growing, barbed end of the actin filament and is an important component in the assembly of various actin structures, including the dynamic branched filament network at the leading edge of spreading cells (36). By examining the spatial association between ACP and either paxillin or vinculin in cells spreading on FN after IL-1 treatment, we found that ACP colocalized with nascent paxillin- and vinculin-containing adhesions at the leading edge, but not with mature adhesions. Further, depletion of ACP affected the size of cell adhesions, suggesting that ACP participates in processes that regulate FA remodeling. These data underline the possibility that the very earliest phases of cell–matrix interactions are contemporaneous with the assembly of IL-1–signaling complexes. Accordingly, we assessed whether IL-1–signaling complexes localized to the sites of cell-spreading machinery.
The proximal, receptor-associated IL-1 signal transduction complex consists of IL-1R, IL-1RAcP, MyD88, IRAK, and TRAF6 (31). ACP KD eliminated recruitment of these molecules to FAs. Immunoprecipitation also showed that ACP associated with IL-1RAcP, MyD88, and IRAK in FA preparations. Moreover, confocal microscopy and SIM analysis of immunostained cells showed that the IL-1 signal transduction proteins MyD88 and IRAK were intimately associated with ACP at the ruffled border of spreading cells after IL-1 stimulation. Using PLA, we confirmed that ACP and either MyD88 or IRAK tightly colocalized at the leading edge of cells after IL-1 treatment. Further, IL-1–induced phospho-ERK and either MyD88 or IRAK spatially colocalize at the leading edges of HGFs in a time-dependent fashion. Thus, ACP and IL-1 signaling complexes collectively regulate IL-1 signaling through cell adhesion to the ECM.
In the early stages of cell spreading, cells are bound to the underlying ECM by nascent integrin and cytoskeletal structures (nascent adhesions), which are regulated by interactions involving a large array of cytoskeletal proteins including actin-binding proteins (51–53). One of these actin-binding proteins, ACP, is enriched at the ruffled border of spreading cells and plays an important role in regulating the structure and function of actin filaments and in the remodeling of nascent adhesions. FAs comprise plaques (<200 nm) that link the ECM to the actin cytoskeleton (54, 55). Three-dimensional superresolution fluorescence microscopy (56, 57) has demonstrated a vertically oriented core region (∼40 nm in diameter) of FAs that consists of multiple proteins organized into specific strata between ligand-bound integrins and actin filaments. These strata comprise a membrane-apposed integrin signaling layer, an intermediate force-transduction layer, and an uppermost actin-regulatory layer (56). Evidently, many intricate molecular machines contribute to the complexity of the molecular composition and dynamics of FAs (58). Accordingly, the 3-dimensional arrangement of molecules in FAs and their interactions with molecules like ACP are likely to play important roles in the regulation of FA dynamics and FA-dependent pathophysiological processes such as IL-1 signaling.
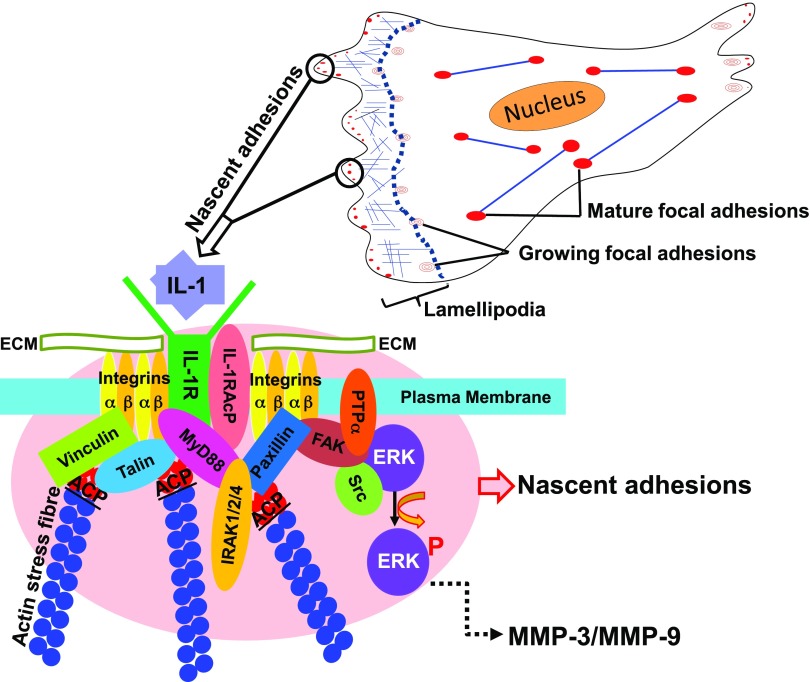
In fibroblasts, chondrocytes, and synoviocytes, sequestration of the upstream IL-1 signaling machinery to FAs is required for IL-1–induced activation of the MAPK ERK and for MMP expression (13–17). FAs are enriched with integrins (59), IL-1Rs (19), the IL-1RAcP, and IRAK (9), all of which are essential for IL-1 signaling. The novelty of the current report is that ACP, a marker of the ruffled border, regulates FA assembly, spatially colocalizes with the IL-1 signaling complex, and enables concentration of IL-1 signaling complexes at the edges of spreading cells. Therefore, ACP is an unexpectedly critical protein in IL-1 signal transduction, which suggests a novel role of cellular leading-edge dynamics in signal generation in response to cytokines (Fig. 7).
Figure 7.
Proposed model for the function of ACP in IL-1 signaling. In spreading cells, nascent adhesions (small red dots) are formed just behind the leading edge of spreading cells. At these sites, ACP regulates the formation of actin filaments in nascent adhesions and the recruitment of effector molecules that regulate IL-1 signaling.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Dr. Jonathan Krieger (SPARC BioCentre, Hospital for Sick Children Toronto, ON, Canada) for performing MS. This work was supported by U.S. National Institutes of Health, National Heart, Lung, and Blood Institute Grant HL132950, and by Canadian Institutes of Health Research (CIHR) Grant MOP-483461. C.A.M. is supported by a Canada Research Chair (Tier 1) in Matrix Dynamics. The authors declare no conflicts of interest.
Glossary
- ACP
actin capping protein
- BSA
bovine serum albumin
- ECM
extracellular matrix
- FA
focal adhesion
- FN
fibronectin
- Fura-2AM
Fura-2-acetoxymethyl ester
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HGF
human gingival fibroblasts
- IL-1R
IL-1 receptor
- IL-1RAcP
IL-1R accessory protein
- IRAK
IL-1R–associated kinase
- KD
knockdown
- MMP
matrix metalloproteinase
- MyD88
myeloid differentiation factor 88
- PLA
proximity ligation assay
- SIM
structured illumination microscopy
- siRNA
small interfering RNA
- TIRF
total internal reflection microscopy
- TMT-MS
tandem mass tag–mass spectrometry
- TBS
Tris-buffered saline solution
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
Q. Wang, G. P. Downey, and C. A. McCulloch designed research and analyzed data; Q. Wang, J. Delcorde, and T. Tang performed research; and Q. Wang and C.A. McCulloch wrote the manuscript.
REFERENCES
- 1.Hönig J., Rordorf-Adam C., Siegmund C., Wiedemann W., Erard F. (1989) Increased interleukin-1 beta (IL-1β) concentration in gingival tissue from periodontitis patients. J. Periodontal Res. 24, 362–367 10.1111/j.1600-0765.1989.tb00883.x [DOI] [PubMed] [Google Scholar]
- 2.Slack J., McMahan C. J., Waugh S., Schooley K., Spriggs M. K., Sims J. E., Dower S. K. (1993) Independent binding of interleukin-1α and interleukin-1β to type I and type II interleukin-1 receptors. J. Biol. Chem. 268, 2513–2524 [PubMed] [Google Scholar]
- 3.Sims J. E., Gayle M. A., Slack J. L., Alderson M. R., Bird T. A., Giri J. G., Colotta F., Re F., Mantovani A., Shanebeck K., Grabstein K. H., Dower S. K. (1993) Interleukin 1 signaling occurs exclusively via the type I receptor. Proc. Natl. Acad. Sci. USA 90, 6155–6159 10.1073/pnas.90.13.6155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curtis B. M., Gallis B., Overell R. W., McMahan C. J., DeRoos P., Ireland R., Eisenman J., Dower S. K., Sims J. E. (1989) T-cell interleukin 1 receptor cDNA expressed in Chinese hamster ovary cells regulates functional responses to interleukin 1. Proc. Natl. Acad. Sci. USA 86, 3045–3049 10.1073/pnas.86.9.3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenfeder S. A., Nunes P., Kwee L., Labow M., Chizzonite R. A., Ju G. (1995) Molecular cloning and characterization of a second subunit of the interleukin 1 receptor complex. J. Biol. Chem. 270, 13757–13765 10.1074/jbc.270.23.13757 [DOI] [PubMed] [Google Scholar]
- 6.Wesche H., Korherr C., Kracht M., Falk W., Resch K., Martin M. U. (1997) The interleukin-1 receptor accessory protein (IL-1RAcP) is essential for IL-1–induced activation of interleukin-1 receptor–associated kinase (IRAK) and stress-activated protein kinases (SAP kinases). J. Biol. Chem. 272, 7727–7731 10.1074/jbc.272.12.7727 [DOI] [PubMed] [Google Scholar]
- 7.Wesche H., Henzel W. J., Shillinglaw W., Li S., Cao Z. (1997) MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity 7, 837–847 10.1016/S1074-7613(00)80402-1 [DOI] [PubMed] [Google Scholar]
- 8.Muzio M., Ni J., Feng P., Dixit V. M. (1997) IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science 278, 1612–1615 10.1126/science.278.5343.1612 [DOI] [PubMed] [Google Scholar]
- 9.MacGillivray M. K., Cruz T. F., McCulloch C. A. G. (2000) The recruitment of the interleukin-1 (IL-1) receptor–associated kinase (IRAK) into focal adhesion complexes is required for IL-1β–induced ERK activation. J. Biol. Chem. 275, 23509–23515 10.1074/jbc.M003186200 [DOI] [PubMed] [Google Scholar]
- 10.Burns K., Martinon F., Esslinger C., Pahl H., Schneider P., Bodmer J.-L., Di Marco F., French L., Tschopp J. (1998) MyD88, an adapter protein involved in interleukin-1 signaling. J. Biol. Chem. 273, 12203–12209 10.1074/jbc.273.20.12203 [DOI] [PubMed] [Google Scholar]
- 11.McDermott E. P., O’Neill L. A. J. (2002) Ras participates in the activation of p38 MAPK by interleukin-1 by associating with IRAK, IRAK2, TRAF6, and TAK-1. J. Biol. Chem. 277, 7808–7815 10.1074/jbc.M108133200 [DOI] [PubMed] [Google Scholar]
- 12.Rajshankar D., Downey G. P., McCulloch C. A. (2012) IL-1β enhances cell adhesion to degraded fibronectin. FASEB J. 26, 4429–4444 10.1096/fj.12-207381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arora P. D., Ma J., Min W., Cruz T., McCulloch C. A. G. (1995) Interleukin-1-induced calcium flux in human fibroblasts is mediated through focal adhesions. J. Biol. Chem. 270, 6042–6049 10.1074/jbc.270.11.6042 [DOI] [PubMed] [Google Scholar]
- 14.Lo Y. Y., Luo L., McCulloch C. A., Cruz T. F. (1998) Requirements of focal adhesions and calcium fluxes for interleukin-1–induced ERK kinase activation and c-fos expression in fibroblasts. J. Biol. Chem. 273, 7059–7065 10.1074/jbc.273.12.7059 [DOI] [PubMed] [Google Scholar]
- 15.Luo L., Cruz T., McCulloch C. (1997) Interleukin 1–induced calcium signalling in chondrocytes requires focal adhesions. Biochem. J. 324, 653–658 10.1042/bj3240653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Q., Downey G. P., Choi C., Kapus A., McCulloch C. A. (2003) IL-1 induced release of Ca2+ from internal stores is dependent on cell-matrix interactions and regulates ERK activation. FASEB J. 17, 1898–1900 10.1096/fj.03-0069fje [DOI] [PubMed] [Google Scholar]
- 17.Wang Q., Rajshankar D., Laschinger C., Talior-Volodarsky I., Wang Y., Downey G. P., McCulloch C. A. (2010) Importance of protein-tyrosine phosphatase-α catalytic domains for interactions with SHP-2 and interleukin-1–induced matrix metalloproteinase-3 expression. J. Biol. Chem. 285, 22308–22317 10.1074/jbc.M110.102426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reunanen N., Westermarck J., Häkkinen L., Holmström T. H., Elo I., Eriksson J. E., Kähäri V.-M. (1998) Enhancement of fibroblast collagenase (matrix metalloproteinase-1) gene expression by ceramide is mediated by extracellular signal-regulated and stress-activated protein kinase pathways. J. Biol. Chem. 273, 5137–5145 10.1074/jbc.273.9.5137 [DOI] [PubMed] [Google Scholar]
- 19.Qwarnström E. E., MacFarlane S. A., Page R. C., Dower S. K. (1991) Interleukin 1 beta induces rapid phosphorylation and redistribution of talin: a possible mechanism for modulation of fibroblast focal adhesion. Proc. Natl. Acad. Sci. USA 88, 1232–1236 10.1073/pnas.88.4.1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrera Abreu M. T., Wang Q., Vachon E., Suzuki T., Chow C.-W., Wang Y., Hong O., Villar J., McCulloch C. A. G., Downey G. P. (2006) Tyrosine phosphatase SHP-2 regulates IL-1 signaling in fibroblasts through focal adhesions. J. Cell. Physiol. 207, 132–143 10.1002/jcp.20544 [DOI] [PubMed] [Google Scholar]
- 21.Wang Q., Herrera Abreu M. T., Siminovitch K., Downey G. P., McCulloch C. A. (2006) Phosphorylation of SHP-2 regulates interactions between the endoplasmic reticulum and focal adhesions to restrict interleukin-1–induced Ca2+ signaling. J. Biol. Chem. 281, 31093–31105 10.1074/jbc.M606392200 [DOI] [PubMed] [Google Scholar]
- 22.Carragher N. O., Frame M. C. (2004) Focal adhesion and actin dynamics: a place where kinases and proteases meet to promote invasion. Trends Cell Biol. 14, 241–249 10.1016/j.tcb.2004.03.011 [DOI] [PubMed] [Google Scholar]
- 23.Sun Z., Guo S. S., Fässler R. (2016) Integrin-mediated mechanotransduction. J. Cell Biol. 215, 445–456 10.1083/jcb.201609037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morimatsu M., Mekhdjian A. H., Chang A. C., Tan S. J., Dunn A. R. (2015) Visualizing the interior architecture of focal adhesions with high-resolution traction maps. Nano Lett. 15, 2220–2228 10.1021/nl5047335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manninen A., Varjosalo M. (2017) A proteomics view on integrin-mediated adhesions. Proteomics 17, 1600022 10.1002/pmic.201600022 [DOI] [PubMed] [Google Scholar]
- 26.Horton E. R., Byron A., Askari J. A., Ng D. H. J., Millon-Frémillon A., Robertson J., Koper E. J., Paul N. R., Warwood S., Knight D., Humphries J. D., Humphries M. J. (2015) Definition of a consensus integrin adhesome and its dynamics during adhesion complex assembly and disassembly. Nat. Cell Biol. 17, 1577–1587 10.1038/ncb3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollard T. D., Cooper J. A. (2009) Actin, a central player in cell shape and movement. Science 326, 1208–1212 10.1126/science.1175862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanchoin L., Boujemaa-Paterski R., Sykes C., Plastino J. (2014) Actin dynamics, architecture, and mechanics in cell motility. Physiol. Rev. 94, 235–263 10.1152/physrev.00018.2013 [DOI] [PubMed] [Google Scholar]
- 29.Livne A., Geiger B. (2016) The inner workings of stress fibers—from contractile machinery to focal adhesions and back. J. Cell Sci. 129, 1293–1304 10.1242/jcs.180927 [DOI] [PubMed] [Google Scholar]
- 30.Murphy-Ullrich J. E. (2001) The de-adhesive activity of matricellular proteins: is intermediate cell adhesion an adaptive state? J. Clin. Invest. 107, 785–790 10.1172/JCI12609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCulloch C. A., Downey G. P., El-Gabalawy H. (2006) Signalling platforms that modulate the inflammatory response: new targets for drug development. Nat. Rev. Drug Discov. 5, 864–876 10.1038/nrd2109 [DOI] [PubMed] [Google Scholar]
- 32.Zhu P., Xiong W., Rodgers G., Qwarnström E. E. (1998) Regulation of interleukin 1 signalling through integrin binding and actin reorganization: disparate effects on NF-κB and stress kinase pathways. Biochem. J. 330, 975–981 10.1042/bj3300975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ponti A., Machacek M., Gupton S. L., Waterman-Storer C. M., Danuser G. (2004) Two distinct actin networks drive the protrusion of migrating cells. Science 305, 1782–1786 10.1126/science.1100533 [DOI] [PubMed] [Google Scholar]
- 34.Shekhar S., Pernier J., Carlier M.-F. (2016) Regulators of actin filament barbed ends at a glance. J. Cell Sci. 129, 1085–1091 10.1242/jcs.179994 [DOI] [PubMed] [Google Scholar]
- 35.Edwards M., Zwolak A., Schafer D. A., Sept D., Dominguez R., Cooper J. A. (2014) Capping protein regulators fine-tune actin assembly dynamics. Nat. Rev. Mol. Cell Biol. 15, 677–689 10.1038/nrm3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooper J. A., Sept D. (2008) New insights into mechanism and regulation of actin capping protein. Int. Rev. Cell Mol. Biol. 267, 183–206 10.1016/S1937-6448(08)00604-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lanier M. H., McConnell P., Cooper J. A. (2016) Cell migration and invadopodia formation require a membrane-binding domain of CARMIL2. J. Biol. Chem. 291, 1076–1091 10.1074/jbc.M115.676882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bolte S., Cordelières F. P. (2006) A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224, 213–232 10.1111/j.1365-2818.2006.01706.x [DOI] [PubMed] [Google Scholar]
- 39.Plopper G. E., McNamee H. P., Dike L. E., Bojanowski K., Ingber D. E. (1995) Convergence of integrin and growth factor receptor signaling pathways within the focal adhesion complex. Mol. Biol. Cell 6, 1349–1365 10.1091/mbc.6.10.1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mengshol J. A., Vincenti M. P., Coon C. I., Barchowsky A., Brinckerhoff C. E. (2000) Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor κB: differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum. 43, 801–811 10.1002/1529-0131(200004)43:4%3c801::AID-ANR10%3e3.0.CO;2-4 [DOI] [PubMed] [Google Scholar]
- 41.Söderberg O., Leuchowius K.-J., Gullberg M., Jarvius M., Weibrecht I., Larsson L.-G., Landegren U. (2008) Characterizing proteins and their interactions in cells and tissues using the in situ proximity ligation assay. Methods 45, 227–232 10.1016/j.ymeth.2008.06.014 [DOI] [PubMed] [Google Scholar]
- 42.Wang Q., Rajshankar D., Branch D. R., Siminovitch K. A., Herrera Abreu M. T., Downey G. P., McCulloch C. A. (2009) Protein-tyrosine phosphatase-α and Src functionally link focal adhesions to the endoplasmic reticulum to mediate interleukin-1–induced Ca2+ signaling. J. Biol. Chem. 284, 20763–20772 10.1074/jbc.M808828200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burridge K., Chrzanowska-Wodnicka M. (1996) Focal adhesions, contractility, and signaling. Annu. Rev. Cell Dev. Biol. 12, 463–519 10.1146/annurev.cellbio.12.1.463 [DOI] [PubMed] [Google Scholar]
- 44.Wehrle-Haller B., Imhof B. A. (2002) The inner lives of focal adhesions. Trends Cell Biol. 12, 382–389 10.1016/S0962-8924(02)02321-8 [DOI] [PubMed] [Google Scholar]
- 45.Wang Q., Wang Y., Downey G. P., Plotnikov S., McCulloch C. A. (2016) A ternary complex comprising FAK, PTPα and IP3 receptor 1 functionally engages focal adhesions and the endoplasmic reticulum to mediate IL-1–induced Ca2+ signalling in fibroblasts. Biochem. J. 473, 397–410 10.1042/BJ20150907 [DOI] [PubMed] [Google Scholar]
- 46.Wang Q., Wang Y., Fritz D., Rajshankar D., Downey G. P., McCulloch C. A. (2014) Interactions of the protein-tyrosine phosphatase-α with the focal adhesion targeting domain of focal adhesion kinase are involved in interleukin-1 signaling in fibroblasts. J. Biol. Chem. 289, 18427–18441 10.1074/jbc.M113.540294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Q., Siminovitch K. A., Downey G. P., McCulloch C. A. (2013) Ras-guanine-nucleotide-releasing factors 1 and 2 interact with PLCγ at focal adhesions to enable IL-1–induced Ca2+ signalling, ERK activation and MMP-3 expression. Biochem. J. 449, 771–782 10.1042/BJ20121170 [DOI] [PubMed] [Google Scholar]
- 48.Geiger T., Zaidel-Bar R. (2012) Opening the floodgates: proteomics and the integrin adhesome. Curr. Opin. Cell Biol. 24, 562–568 10.1016/j.ceb.2012.05.004 [DOI] [PubMed] [Google Scholar]
- 49.Gallegos L., Ng M. R., Brugge J. S. (2011) The myosin-II–responsive focal adhesion proteome: a tour de force? Nat. Cell Biol. 13, 344–346 10.1038/ncb2230 [DOI] [PubMed] [Google Scholar]
- 50.Kuo J.-C., Han X., Hsiao C.-T., Yates J. R., III, Waterman C. M. (2011) Analysis of the myosin-II–responsive focal adhesion proteome reveals a role for β-Pix in negative regulation of focal adhesion maturation. Nat. Cell Biol. 13, 383–393 10.1038/ncb2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iskratsch T., Yu C.-H., Mathur A., Liu S., Stévenin V., Dwyer J., Hone J., Ehler E., Sheetz M. (2013) FHOD1 is needed for directed forces and adhesion maturation during cell spreading and migration. Dev. Cell 27, 545–559 10.1016/j.devcel.2013.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dolat L., Hunyara J. L., Bowen J. R., Karasmanis E. P., Elgawly M., Galkin V. E., Spiliotis E. T. (2014) Septins promote stress fiber–mediated maturation of focal adhesions and renal epithelial motility. J. Cell Biol. 207, 225–235 10.1083/jcb.201405050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kai F., Fawcett J. P., Duncan R. (2015) Synaptopodin-2 induces assembly of peripheral actin bundles and immature focal adhesions to promote lamellipodia formation and prostate cancer cell migration. Oncotarget 6, 11162–11174 10.18632/oncotarget.3578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Franz C. M., Müller D. J. (2005) Analyzing focal adhesion structure by atomic force microscopy. J. Cell Sci. 118, 5315–5323 10.1242/jcs.02653 [DOI] [PubMed] [Google Scholar]
- 55.Zamir E., Geiger B. (2001) Components of cell-matrix adhesions. J. Cell Sci. 114, 3577–3579 [DOI] [PubMed] [Google Scholar]
- 56.Kanchanawong P., Shtengel G., Pasapera A. M., Ramko E. B., Davidson M. W., Hess H. F., Waterman C. M. (2010) Nanoscale architecture of integrin-based cell adhesions. Nature 468, 580–584 10.1038/nature09621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiu C.-L., Gratton E. (2013) Axial super resolution topography of focal adhesion by confocal microscopy. Microsc. Res. Tech. 76, 1070–1078 10.1002/jemt.22267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y.-L. (2007) Flux at focal adhesions: slippage clutch, mechanical gauge, or signal depot. Sci. STKE 2007, pe10 10.1126/stke.3772007pe10 [DOI] [PubMed] [Google Scholar]
- 59.Geiger B., Spatz J. P., Bershadsky A. D. (2009) Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 10, 21–33 10.1038/nrm2593 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.