Abstract
Based on genetic models with mutation or deletion of core clock genes, circadian disruption has been implicated in the pathophysiology of metabolic disorders. Thus, we examined whether circadian desynchronization in response to shift work–type schedules is sufficient to compromise metabolic homeostasis and whether inflammatory mediators provide a key link in the mechanism by which alterations of circadian timekeeping contribute to diet-induced metabolic dysregulation. In high-fat diet (HFD)-fed mice, exposure to chronic shifts of the light–dark cycle (12 h advance every 5 d): 1) disrupts photoentrainment of circadian behavior and modulates the period of spleen and macrophage clock gene rhythms; 2) potentiates HFD-induced adipose tissue infiltration and activation of proinflammatory M1 macrophages; 3) amplifies macrophage proinflammatory cytokine expression in adipose tissue and bone marrow–derived macrophages; and 4) exacerbates diet-induced increases in body weight, insulin resistance, and glucose intolerance in the absence of changes in total daily food intake. Thus, complete disruption of circadian rhythmicity or clock gene function as transcription factors is not requisite to the link between circadian and metabolic phenotypes. These findings suggest that macrophage proinflammatory activation and inflammatory signaling are key processes in the physiologic cascade by which dysregulation of circadian rhythmicity exacerbates diet-induced systemic insulin resistance and glucose intolerance.—Kim, S.-M., Neuendorff, N., Alaniz, R. C., Sun, Y., Chapkin, R. S., Earnest, D. J. Shift work cycle-induced alterations of circadian rhythms potentiate the effects of high-fat diet on inflammation and metabolism.
Keywords: macrophage, clock gene, cytokines, metabolic disorders, insulin resistance
Circadian clocks located in peripheral tissues and cells throughout the body regulate daily rhythms that provide for the temporal organization of many local physiologic processes, including inflammation and metabolism. Cell autonomous clocks in immune cells provide for the circadian regulation of the following: 1) their abundance in the circulation; 2) NF-κB, a key mediator of immune responses that modulates the expression of target inflammatory genes; 3) macrophage expression of TLR 4 that regulates innate immunity and LPS responsiveness; and 4) LPS-induced TNF-α and IL-6 release by macrophages (1–3). In mammals, robust circadian rhythms are also observed in various metabolic enzymes and processes. For example, AMPK, a nutrient-sensing protein that increases β-oxidation of fatty acids, is marked by circadian rhythms of mRNA expression and phosphorylation in mouse liver that peak during the subjective day (4), and lipid levels in the circulation similarly fluctuate with a rhythmic peak around midday (5). This precise temporal coordination of tissue- and cell-specific processes is believed to play an important role in the homeostatic regulation of inflammatory responses, metabolism, and other key physiologic processes that govern organismal health.
Using genetic models based on mutation or deletion of core clock genes, recent studies indicate that global and tissue-specific disruption of circadian clock function produces obesity, diabetes, or other signs of metabolic dysregulation. In conjunction with their altered circadian phenotype, Clock mutant mice exhibit many hallmarks of metabolic disease, including hyperphagia, obesity, glucose intolerance, and insulin resistance (6, 7). Furthermore, global and adipocyte-specific circadian disruption caused by deletion of Bmal1 produces a metabolic phenotype characterized by increased body weight and adipose tissue mass, adipocyte hypertrophy, and reduced circulating levels of polyunsaturated fatty acids (8). Similarly, pancreas-specific Bmal1 mutant mice are distinguished by impaired glucose tolerance and reduced insulin secretion (7). Specifically how circadian clock disruption contributes to metabolic disorders is unclear, but the activation of proinflammatory macrophages and inflammatory signaling have been identified as key processes in the physiologic cascade linking clock- and metabolic-dysregulated phenotypes. In this regard, global and myeloid cell–specific clock disruption (Per1ldc/Per2ldc) exacerbates macrophage proinflammatory activation, leading to adipose tissue inflammation and further potentiation of high-fat diet (HFD)-induced increases in body weight, systemic insulin resistance, and hyperglycemia (9). Collectively, these observations suggest that macrophage proinflammatory activation and adipose tissue inflammation are critical factors in the mechanism by which circadian clock disruption potentiates diet-induced metabolic phenotypes.
Because many of the clock genes targeted for global or tissue-specific disruption function as transcription factors that regulate important mediators of inflammatory responses such as TLR 9, chemokine ligand 2, IL-6, and peroxisome proliferator-activated receptor (PPAR)-γ (10–13), it is possible that the amplified inflammatory and metabolic responses observed by using these genetic models are due to the disruption of nonclock (rather than circadian timekeeping) functions of core clock genes. To address this possibility and circumvent the implications of nonclock functions of core clock genes associated with genetic models, the present study used a chronic shift work paradigm to determine whether environmental alteration or desynchronization of circadian rhythms produces inflammatory and metabolic phenotypes similar to those found in mice with clock gene deletions or mutations. Experiments specifically examined the effects of chronic shifts in the light–dark (LD) cycle (12 h every 5 d) on circadian behavior and peripheral clock gene rhythms, macrophage infiltration and polarization, proinflammatory cytokines, and metabolic responses (i.e., body weight, glucose tolerance, insulin resistance) to HFD.
MATERIALS AND METHODS
Animals
This study used male and female mice (C57BL/6J background) that were derived from breeding pairs of homozygous mPer2Luc knock-in mice (generously provided by Dr. Joseph Takahashi, University of Texas Southwestern Medical School, Dallas, TX, USA). In these mice, a luciferase (Luc) gene was fused in-frame to the C terminus of the endogenous mPER2 coding sequence, thus enabling real-time recording of mPER2-driven oscillations via luciferase bioluminescence (14).
Experiments used a chronic LD cycle shift paradigm that has been shown to be effective in desynchronizing circadian rhythms and in exacerbating pathologic outcomes (15, 16). Before experimentation, all animals were fed standard rodent chow (Teklad Rodent Diet; Envigo, Huntingdon, United Kingdom) and maintained under standard LD 12:12 conditions (lights on at 7:00 am). At 5–6 wk of age (22–25 g), mPer2Luc mice were randomly divided into 2 groups and exposed for ∼10 wk either to this fixed LD 12:12 cycle or to a shifted LD 12:12 cycle. In the shifted LD 12:12 cycle, lights-on was advanced by 12 h every 5 d. During exposure to experimental lighting conditions, all fixed and shifted LD mice were fed an HFD (60% fat calories, 20% protein calories, and 20% carbohydrate calories), described previously (17, 18), to examine the effect of environmental disruption of circadian rhythms on obesity-associated metabolic phenotypes in vivo. Some animals were housed individually in cages equipped with running wheels to provide for continuous analysis of the effect of environmental dysregulation on the circadian rhythm of locomotor activity in vivo. Body weight was monitored before and every 10 d during HFD feeding. After completion of the feeding regimen and experimental lighting treatments, mice were euthanized for collection of epididymal adipose tissue and bone marrow cells so as to occur at the same relative time during the LD cycle (i.e., midday or inactive phase) in both fixed and shifted LD mice. Some mice were fasted for 12 or 6 h and used for glucose tolerance and insulin resistance tests.
All animal procedures used in this study were conducted in compliance with Animal Use Protocol 2014-0248 as reviewed and approved by the Institutional Animal Care and Use Committee of Texas A&M University.
Analysis of rhythm in wheel-running activity
Wheel-running activity of fixed and shifted LD mice was continuously recorded; the data were stored in 10-min bins, graphically depicted in actograms, and analyzed by using ClockLab data collection and analysis software (Actimetrics, Wilmette, IL, USA). Entrainment and qualitative parameters of the activity rhythm were measured over the same interval for all animals. The onset of activity for a given cycle was identified as the first bin during which an animal attained 10% of peak running-wheel revolutions. A χ2 periodogram analysis was used to determine the period and amplitude of the activity rhythm during exposure to the fixed or shifted LD cycles.
Glucose tolerance and insulin resistance tests
All metabolic testing was conducted so that it occurred at the same time during the LD cycle in both fixed and shifted LD mice. For the glucose tolerance test, mice were unfed for 12 h and testing was initiated at zeitgeber time 2 (9:00 am) by intraperitoneal injection of d-glucose (2 g/kg body weight). Blood samples were collected from the tail vein immediately before and at 15, 30, 60, and 120 min after the injection. For the insulin resistance test, mice were unfed for 6 h and testing was initiated at zeitgeber time 7 (2:00 pm) by intraperitoneal injection of insulin (1 U/kg body weight). Blood samples were collected from the tail vein immediately before and at 30, 60, and 120 min after the injection.
Isolation of stromal vascular cells from adipose tissue
Epididymal adipose tissue was collected from HFD-fed mPer2Luc mice exposed to fixed or shifted LD cycles, and stromal vascular cells (SVCs) were isolated by using the collagenase digestion method as described previously (18, 19). After digestion and centrifugation, the pelleted adipose tissue SVCs were cultured for 7 d, and cultures were then harvested separately at the same time of day (9:00 am). Adipose tissue SVC samples were independently subjected to fluorescence-activated cell sorting (FACS) analysis, real-time PCR analysis of inflammatory cytokines, and bioluminescence analysis of clock gene rhythms by using established methods (9, 20).
Macrophage differentiation and characterization
Bone marrow cells were isolated from the tibias and femurs of HFD-fed mPer2Luc mice exposed to fixed or shifted LD cycles as previously described (21). After differentiation with DMEM containing 10% fetal bovine serum and 10 ng/ml monocyte CSF for 7 d, bone marrow–derived macrophages (BMDMs) were independently subjected to similar assays of clock gene rhythms, macrophage activation and polarization, and inflammatory cytokine mRNA expression.
Real-time analysis of mPer2Luc in SVC and BMDM cultures
SVCs and BMDM cells from HFD-fed mPer2Luc mice exposed to fixed or shifted LD cycles were cultured in DMEM supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 292 μg/ml glutamine. For bioluminescence analysis, cultures were maintained in serum-free recording medium containing 1 μM forskolin, 25 mM HEPES, 292 µg/ml l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10 μM luciferin (Promega Corporation, Madison, WI, USA) as described previously (20). Individual cultures were sealed airtight with sterile glass coverslips (VWR, Radnor, PA, USA) and sterile silicon grease (Dow Corning, Midland, MI, USA). The temporal patterns of mPER2::LUC bioluminescence were analyzed by using an automated 32-channel luminometer (LumiCycle; Actimetrics) that was maintained within a standard cell culture incubator at 32°C. Bioluminescence from individual cultures was continuously recorded with a photomultiplier tube for ∼70 s at intervals of 10 min. Due to the transient induction of bioluminescence following the medium change at the initiation of this analysis, the first cycle was excluded from data analysis. Using the LumiCycle analysis program (Actimetrics), baseline drift in each raw data set was removed by fitting a polynomial curve with an order equal to 1 less than the number of recorded cycles. Rhythm parameters were determined from baseline-subtracted data by using the damped sine fit and Levenberg-Marquardt algorithm.
FACS analysis
SVCs from epididymal fat pads (n = 4–6) and BMDMs from tibias/femurs (n = 3) of HFD-fed mPer2Luc mice exposed to fixed or shifted LD cycles were labeled with fluorescence-tagged antibodies (anti-F4/80, anti-CD11b for macrophages, anti-CD11c, and anti-CD206 for macrophage activation) as previously described (22, 23). Labeled SVCs and BMDM cells were separately subjected to FACS analyses by using an Accuri flow cytometer (BD Biosciences, San Jose, CA, USA). Briefly, the harvested cells were initially analyzed based on analog measurements of forward-scattered light and side-scattered light. Live cells were then assessed for F4/80 (FITC) and CD11b (PerCP/Cy5.5) expression. Mature macrophages (F4/80+ CD11b+ cells) were then gated for CD11c (PE/Cy7) and CD206 (PE) expression (macrophage polarization). For quantitative analysis, mature macrophages that were positive for CD11c but negative for CD206 were counted as M1 macrophages (F4/80+ CD11b+ CD11c+ CD206– cells).
RNA extraction and real-time PCR
To examine macrophage proinflammatory activation and signaling, the relative expression of proinflammatory cytokine mRNAs was analyzed over the course of 24 h in some SVC and BMDM cultures isolated from HFD-fed mice on fixed or shifted LD cycles. SVC and differentiated BMDM cultures were established as described previously and then exposed for 2 h to a 50% serum shock. SVC cultures were collected 6, 12, 18, or 24 h later, and differentiated BMDMs were treated 6, 12, 18, or 24 h later with PBS or LPS (10 ng/ml) for 30 min before cell harvest. Total cellular RNA was extracted from individual SVC and BMDM cultures by using the PureLink RNA Mini Kit (Ambion, Waltham, MA, USA) according to the manufacturer’s protocols. Relative quantification of IL-6 and TNF-α mRNA abundance was performed with the use of SYBR Green PCR technology (Applied Biosystems, Foster City, CA, USA) as previously described (9, 20). For each sample, real-time PCR analysis of IL-6 or TNF-α mRNA levels was performed on duplicate aliquots by using the cDNA equivalent of 1 ng of total RNA. To control for differences in sample RNA content, β-actin mRNA was amplified with the cDNA equivalent of 1 ng of total RNA from the same samples. The comparative CT method was used to calculate the relative abundance for a given cytokine mRNA by normalization to corresponding β-actin levels in each sample and to a calibrator consisting of pooled cDNA from multiple samples. Values for the ratios of IL-6 or TNF-α/β-actin mRNA signal were adjusted relative to the averages of fixed LD controls, which were arbitrarily set at 100%.
Statistical methods
Independent pooled t tests were performed to determine the significance of LD treatment effects (shifted LD cycle relative to fixed LD controls) on circadian wheel-running behavior, macrophage polarization, and expression of inflammatory cytokines. In each case, differences between fixed and shifted LD groups were considered significant at P < 0.05.
RESULTS
Shifted LD cycles disrupt circadian entrainment of the rhythm in wheel-running activity
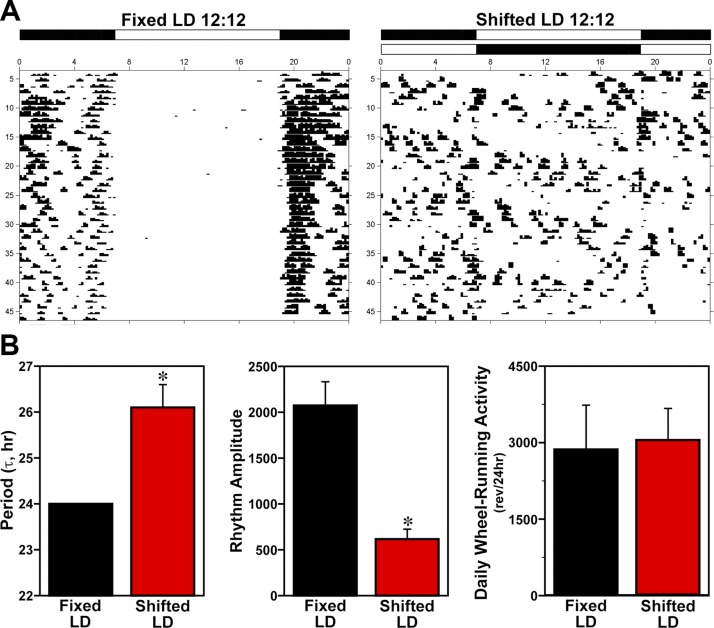
To determine whether environmental manipulations simulating shift work–type schedules induce disturbances or desynchronization of suprachiasmatic nucleus–regulated circadian behavior, wheel-running activity was examined in mice exposed to either fixed or shifted LD cycles for 10 wk. During baseline acclimation by the mice to their running wheels, stable entrainment of the activity rhythm to LD 12:12 was observed in all animals. Throughout the 10-wk exposure to experimental lighting conditions, all HFD-fed male and female mice in the fixed LD treatment group exhibited stable entrainment (Fig. 1A) such that their daily onsets of activity consistently occurred shortly (5–10 min) after lights off (7:00 pm). However, male and female mice exposed to shifted LD cycles were distinguished by desynchronized patterns of wheel-running behavior in which the timing of activity onsets relative to lights-off was highly variable from day to day. Consistent with their desynchronized activity patterns, the period of the activity rhythm in shifted LD mice (τ = 26.1 ± 0.5 h) was significantly (P < 0.05) greater than the 24-h period observed in the fixed LD group as a result of circadian entrainment. In addition, the amplitude of the activity rhythm in shifted LD mice was significantly (P < 0.05) decreased compared with fixed LD controls (Fig. 1B). Despite these alterations in the entrainment and amplitude of the activity rhythm, exposure to shifted LD cycles had no significant effect on the total amount of daily wheel-running activity relative to that observed in fixed LD controls.
Figure 1.
Effects of shifted LD cycles on circadian entrainment and other properties of the rhythm in mouse wheel-running activity. A) Representative daily records of wheel-running activity in mice that were maintained in a fixed LD 12:12 cycle (left) or exposed to a shifted (12 h advance every 5 d) LD 12:12 cycle (right). Actograms are plotted over a 24-h period. The open and closed bars at the top, respectively, signify the timing of the light and dark phase in the fixed and shifted LD 12:12 cycles. B) The period and amplitude of the activity rhythm, and total daily wheel-running activity (revolutions/24 h) of mice exposed to fixed or shifted LD cycles (n = 6). Bars depict mean values (± sem). Asterisks indicate significant differences (P < 0.05) between fixed and shifted LD mice in the period and amplitude of the rhythm in wheel-running activity.
Shifted LD cycles alter clock gene rhythms in lymphoid tissues and cells
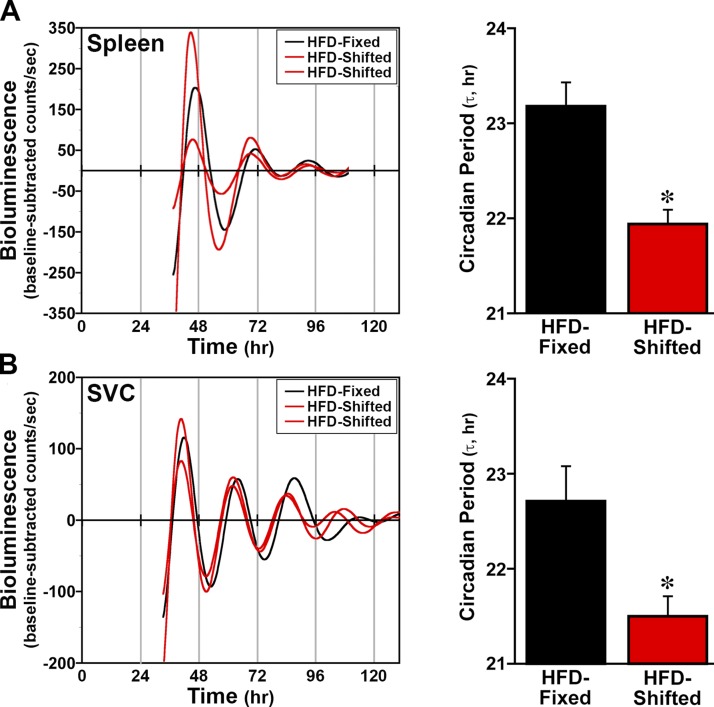
Because lymphoid cells and tissues play an important role in the link between inflammation and metabolism, clock gene oscillations were analyzed in the spleen and adipose tissue SVCs of mPer2Luc mice to determine whether shifted LD cycles also alter circadian properties of peripheral clocks in these tissues/cells. In HFD-fed mice maintained on fixed LD cycles, the spleen, which plays a role in inflammation as well as lipid metabolism in response to HFD (24), displayed robust mPER2::LUC rhythms with a circadian period of 23.2 ± 0.3 h (Fig. 2). Oscillations in mPER2::LUC bioluminescence were also observed in the spleen cultures from HFD-fed mice exposed to shifted LD cycles but the period of these clock gene rhythms was significantly decreased (P < 0.05) by ∼1 h relative to the fixed LD controls. Similarly, the period of PER2::LUC rhythms in SVC cultures from HFD-fed mice on shifted LD cycles (21.47 ± 0.2 h) was significantly decreased (P < 0.05) relative to that observed for SVC rhythms in the fixed LD controls (22.49 ± 0.4 h).
Figure 2.
Effect of shifted LD cycles on clock gene oscillations in lymphoid tissues and cells. Representative temporal patterns of ensemble PER2::LUC bioluminescence recorded from individual cultures of spleen (A) adipose (B) tissue SVCs from HFD-fed mPer2Luc mice that were maintained in fixed (black) or shifted (red) LD 12:12 cycles. Bar graphs depict comparisons of the circadian period (means ± sem) of the PER2::LUC rhythms in spleen (n = 9) and SVC (n = 6) cultures from fixed and shifted LD mice. Asterisks indicate that the period of the PER2::LUC rhythms in cultures from shifted LD mice was significantly decreased (P < 0.05) relative to that observed in lymphoid tissue and cells from fixed LD controls.
Shifted LD cycles exacerbate HFD-induced macrophage proinflammatory activation
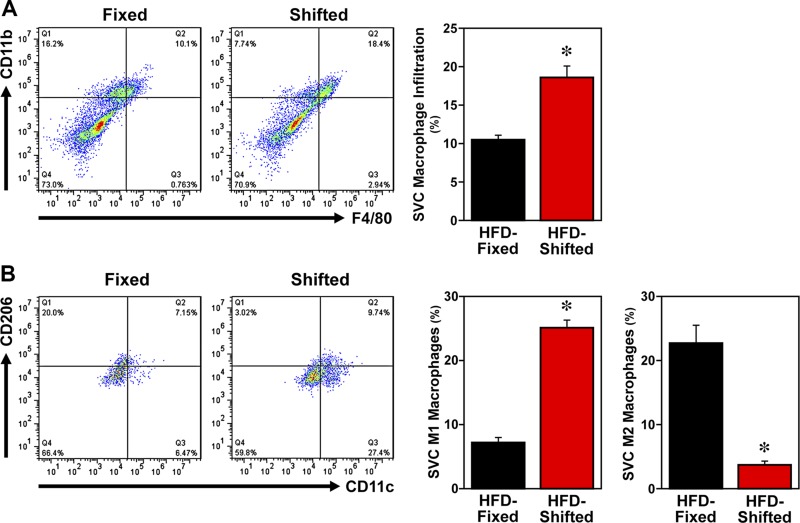
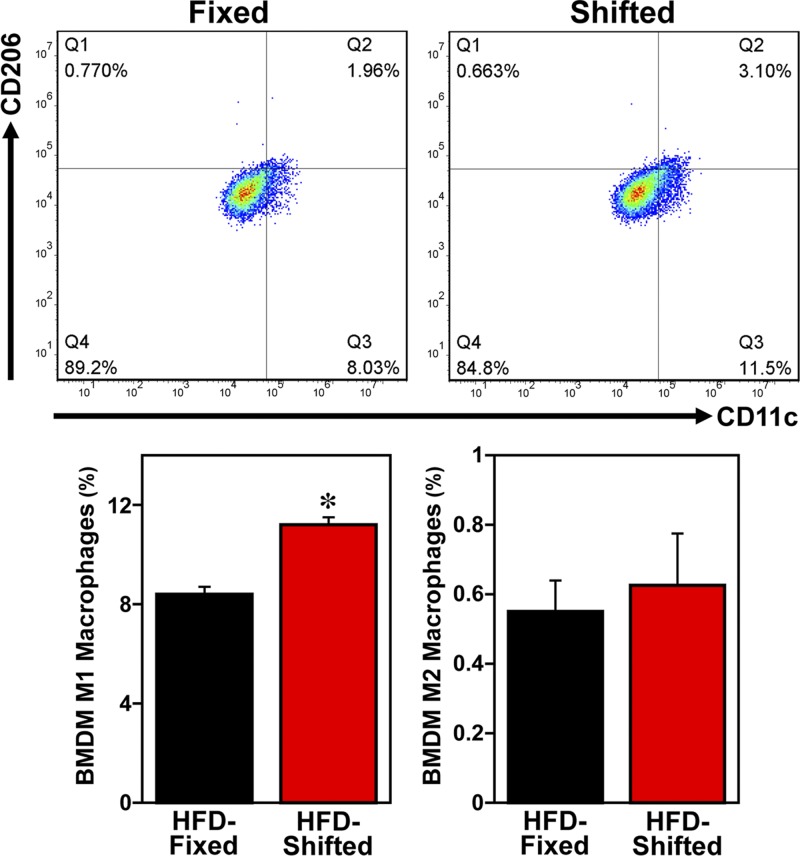
Macrophages are key mediators of inflammation in diet-induced obesity. Because macrophages, similar to other peripheral cells throughout the body, contain circadian clocks that modulate their functional responses to inflammatory challenge (18, 25), we examined the effects of this altered timekeeping in peripheral clocks on macrophage proinflammatory activation and inflammatory responses in HFD-fed mPer2Luc mice exposed to shifted LD cycles. FACS analyses of SVCs (the immune cell–containing fraction of adipose tissue) and BMDMs indicate that the modulation of circadian timekeeping in mice on shifted LD cycles is accompanied by alterations in macrophage inflammatory status. After HFD treatment, mature macrophages (F4/80+ CD11b+ cells) in adipose tissue SVCs from mice exposed to shifted LD cycles were significantly increased (P < 0.05) by 1.4-fold relative to those found in the fixed LD controls (Fig. 3A). Further analysis of the inflammatory status of the mature macrophages revealed that the overall percentage of proinflammatory M1 macrophages (F4/80+ CD11b+ CD11c+ CD206− cells) in adipose tissue SVCs from shifted LD mice was significantly increased (P < 0.05) by 3-fold compared with that observed in fixed LD animals (Fig. 3B). Conversely, exposure to shifted LD cycles induced a significant decrease (P < 0.05) in the percentage of anti-inflammatory M2 macrophages in adipose tissue SVCs relative to fixed LD controls. BMDM cultures from shifted LD mice similarly showed a significant increase (P < 0.05) in the percentage of M1 macrophages relative to that observed in BMDMs from fixed LD controls (Fig. 4). However, the percentage of M2 macrophages in BMDM cultures did not differ between the fixed and shifted LD groups.
Figure 3.
Effect of shifted LD cycles on adipose tissue macrophage infiltration and polarization in HFD-fed mice. FACS analyses of macrophages in epididymal fat pads from HFD-fed mice exposed to fixed or shifted LD 12:12 cycles. Representative scatter plots of adipose tissue SVCs that were quantified for F4/80 and CD11b expression (A) to identify mature macrophages and for F4/80, CD11b, CD11c, and CD206 expression (B) to differentially analyze proinflammatory (M1) macrophages and anti-inflammatory (M2) macrophages. Bar graphs (right panels) depict quantification of the percentages (means ± sem) of mature macrophages (CD11b+ F4/80+ cells), proinflammatory M1 macrophages (F4/80+ CD11b+ CD11c+ CD206− cells), and anti-inflammatory M2 macrophages (F4/80+ CD11b+ CD11c− CD206+ cells) in adipose tissue SVCs from HFD-fed mice on fixed or shifted LD cycles (n = 3). Asterisks indicate significant differences (P < 0.05) between the fixed and shifted LD groups in SVC macrophage infiltration, M1 macrophages, and M2 macrophages.
Figure 4.
Effect of shifted LD cycles on macrophage proinflammatory activation in BMDMs from HFD-fed mice. Representative scatter plots depict FACS analyses of proinflammatory M1 macrophages (F4/80+ CD11b+ CD11c+ CD206− cells) and anti-inflammatory M2 macrophages (F4/80+ CD11b+ CD11c− CD206+ cells) in BMDMs differentiated from the bone marrow cells of HFD-fed mice exposed to fixed or shifted cycles. Bar graphs (right panels) depict quantification of the percentages (means ± sem) of proinflammatory M1 macrophages and anti-inflammatory M2 macrophages in cultured BMDMs from HFD-fed mice on fixed or shifted LD cycles (n = 4). The asterisk indicates that the percentage of proinflammatory M1 macrophages in BMDMs from shifted LD mice was significantly increased (P < 0.05) compared with that in fixed LD controls.
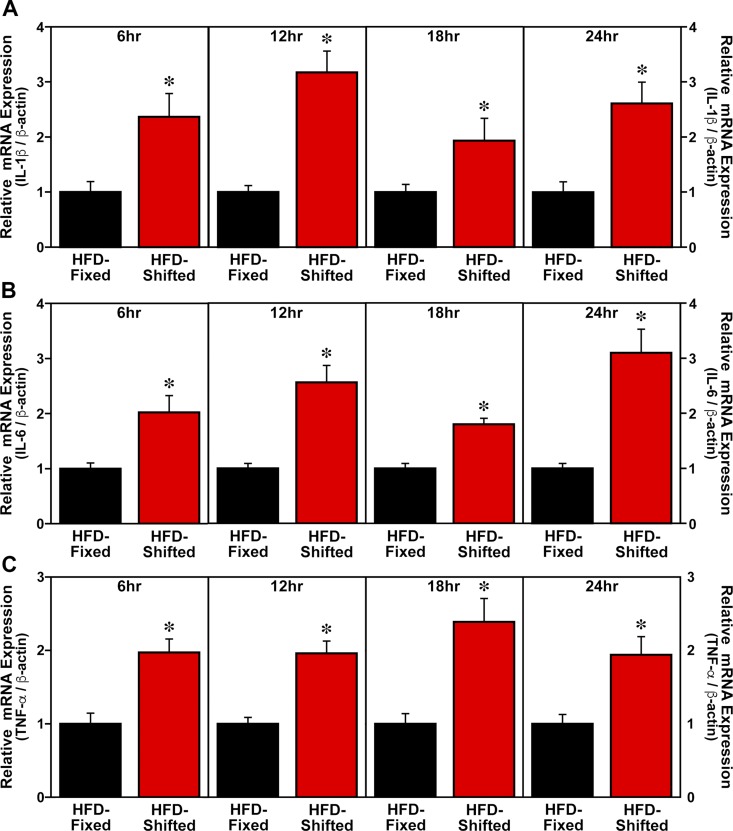
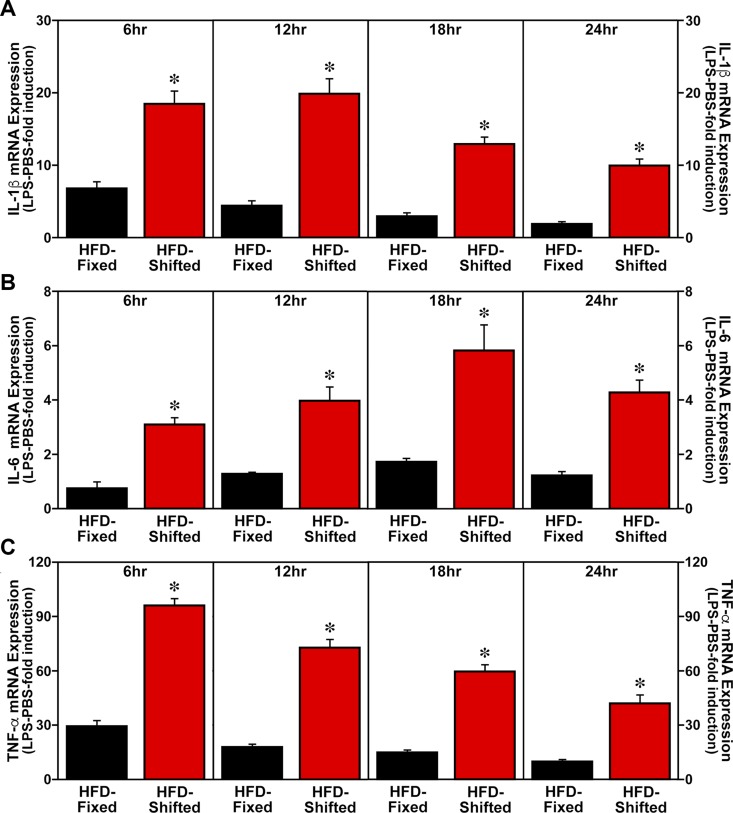
Consistent with its effects in amplifying the HFD-induced activation of proinflammatory M1 macrophages, exposure to shifted LD cycles also potentiated the expression of proinflammatory cytokines in adipose tissue SVCs and LPS-treated BMDM cultures. At all time points throughout the 24-h sampling interval, cultures of adipose tissue SVCs from HFD-fed mice on shifted LD cycles were characterized by IL-1β, IL-6, and TNF-α mRNA levels that were significantly increased (P < 0.05) compared with SVC cytokine expression in fixed LD controls (Fig. 5). SVC levels of IL-1β, IL-6, and TNF-α mRNA were ∼2- to 3-fold greater in HFD-fed mice on shifted LD cycles than in their fixed LD counterparts. In differentiated BMDM cultures from HFD-fed mice on shifted LD cycles, LPS-mediated induction of IL-1β, IL-6, and TNF-α mRNA expression at all analyzed time points (6, 12, 18, and 24 h after serum shock) was significantly increased (P < 0.05) by 3- to 5-fold relative to the levels found in BMDMs from fixed LD controls (Fig. 6).
Figure 5.
Effect of shifted LD cycles on SVC expression of proinflammatory cytokines in HFD-fed mice. SVC cultures were isolated from HFD-fed mice on fixed or shifted LD cycles and collected (n = 3–4) at 6, 12, 18, and 24 h after serum shock administration for real-time PCR analysis of IL-1β (A), IL-6 (B), and TNF-α (C) mRNA levels. Plotted values correspond to the ratios of IL-1β, IL-6, or TNF-α/β-actin mRNA signal (means ± sem) that were adjusted in relation to the average for the fixed LD group. Asterisks denote time-based comparisons in which the relative levels of IL-1β, IL-6, and TNF-α mRNA in SVC cultures from HFD-fed mice on shifted LD cycles were significantly increased (P < 0.05) compared with those found in fixed LD controls.
Figure 6.
Effect of shifted LD cycles on BMDM expression of proinflammatory cytokines in HFD-fed mice. BMDMs from HFD-fed mice on fixed or shifted LD cycles were differentiated and then treated for 30 min with PBS or LPS (10 ng/ml) (n = 6) at 6, 12, 18, and 24 h after serum shock administration. Plotted values correspond to real-time PCR determinations of the ratios of IL-1β (A), IL-6 (B), and TNF-α/β-actin (C) mRNA signal (means ± sem) in LPS-treated BMDMs that were adjusted in relation to the average for PBS-treated cultures from fixed LD controls, which were arbitrarily set as 1. Asterisks denote time-based comparisons in which the relative induction of IL-1β, IL-6, and TNF-α mRNA in LPS-treated BMDM cultures from HFD-fed mice on shifted LD cycles was significantly increased (P < 0.05) compared with the levels found in fixed LD controls.
Shifted LD cycles exacerbate HFD-induced systemic insulin resistance and glucose intolerance
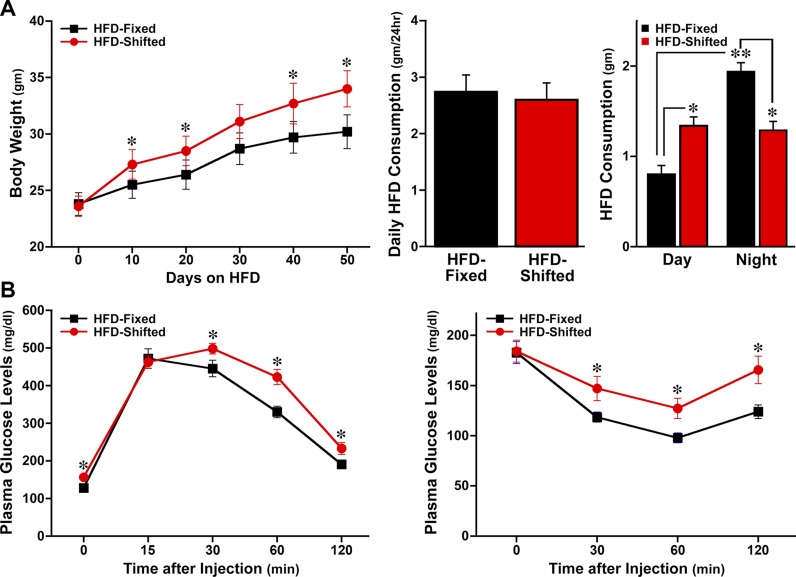
HFD increases macrophage proinflammatory activation (9), and the corresponding induction of inflammatory signaling contributes to the development of insulin resistance and glucose intolerance (26, 27), which are major phenotypes of metabolic disorders. Therefore, we next determined whether the heightened inflammatory status in shifted LD mice was accompanied by corresponding manifestations of metabolic dysregulation. Before HFD feeding, no significant differences were evident in the body weights of fixed and shifted LD mice (Fig. 7A). During the course of HFD treatment, all mice exhibited a progressive increase in body weight, but the HFD-induced gain in body weight was significantly (P < 0.05) greater in shifted LD mice than in fixed LD controls. Importantly, the observed effect of shifted LD on body weight was not associated with a corresponding change in food intake because no significant difference in total daily consumption of HFD was observed between the fixed and shifted LD groups. However, it is noteworthy that food consumption in shifted LD mice was almost equally distributed between day and night, whereas fixed LD mice consumed significantly (P < 0.05) more food during the night than during the day. As a result of these treatment differences in the diurnal distribution of food intake, HFD consumption in shifted LD mice was significantly increased during the daytime and decreased during the night (P < 0.05) relative to that observed in fixed LD controls.
Figure 7.
Effect of shifted LD cycles on the metabolic phenotypes of HFD-fed mice. Graphs depict determinations (mean ± sem) of the following: body weight before and during the HFD feeding regimen, daily food intake, and day-night distribution of HFD consumption (A); and plasma glucose levels in response to glucose tolerance testing (left panel; 2 g/kg) and insulin resistance testing (right panel; 1 U/kg) in HFD-fed mice that were on fixed or shifted LD cycles (n = 11–12) (B). Asterisks denote comparisons in which the body weight and plasma glucose levels in HFD-fed mice on shifted LD cycles were significantly increased (P < 0.05) relative to those found in fixed LD controls. For comparisons of day–night differences in HFD consumption, food intake in fixed LD controls was significantly greater (P < 0.05) during the night than daytime (double asterisks); food intake in shifted LD mice was significantly increased during the daytime but was significantly decreased (P < 0.05) during the night relative to that observed over the same intervals in fixed LD controls (single asterisk).
Consistent with their differential gain of more body weight, HFD-fed mice on shifted LD cycles were characterized by increased severity of systemic glucose intolerance and insulin resistance. For the glucose tolerance and insulin resistance tests, plasma glucose levels at 30, 60, and 120 min after bolus glucose or insulin injection were significantly (P < 0.05) higher in shifted LD mice than in fixed LD controls (Fig. 7B). For all metabolic parameters (i.e., body weight, glucose intolerance, insulin resistance), separate analyses on the basis of sex revealed that statistical differences between fixed and shifted LD groups were fully maintained in both male and female HFD-fed mice. Collectively, these data indicate that environmental alteration of circadian clock function is sufficient to amplify HFD-induced inflammation in concert with metabolic dysregulation and systemic insulin resistance.
DISCUSSION
The link between circadian rhythm disruption and metabolic phenotypes has been predominantly established in studies showing that transgenic mice with genetic mutation or deletion of the clock genes, Clock or Bmal1, are distinguished by glucose intolerance, hyperglycemia, hyperlipidemia, and hypoinsulinemia (6, 7). Similar to the effects of global arrhythmicity observed in Clock∆19/∆19 mutant and Bmal1-deficient mice, our previous studies showed that transplantation of Per1/2-disrupted bone marrow cells into irradiated wild-type mice amplifies the severity of HFD-induced metabolic dysregulation (9). However, it is unclear whether the metabolic-dysregulated phenotypes are caused by the disruption of the circadian timekeeping function or transcriptional regulatory activity of these core clock genes. In the present experiments, chronic 12-h advances in the LD cycle were observed to disrupt photic entrainment of circadian behavior, modulate clock gene rhythms in the spleen and adipose tissue macrophages, and similarly exacerbate HFD-induced increases in body weight, glucose intolerance, and insulin resistance. Consistent with other rodent studies examining the effects of various chronic jet lag or shift work paradigms on diet-induced metabolic pathophysiology (28, 29), these findings suggest that environmental alteration or modulation of circadian rhythms is sufficient to disrupt metabolic homeostasis. Interestingly, the observed effects of shifted LD cycles on metabolism were not accompanied by a corresponding increase in total daily food intake. In this regard, the increased daytime HFD consumption in shifted LD mice may have contributed to the increases in body weight, glucose intolerance, and insulin resistance because the coincidence of HFD feeding during the daytime or inactive phase in rodents has been identified as a key factor in diet-mediated metabolic dysfunction (30, 31). Collectively, these observations have important implications in the interpretation of previous studies using genetic models, suggesting that the systemic metabolic dysregulation is a consequence of the disruption of the circadian timekeeping function of core clock genes, not their role as transcription factors that regulate downstream signaling pathways.
Chronic inflammation is believed to play an important role in diet-associated obesity and metabolic syndrome. In particular, studies on human obesity suggest that chronic activation of proinflammatory signaling pathways induces low-grade inflammation, which in turn leads to decreased insulin sensitivity (32, 33). As such, inflammation may provide a critical link between circadian clock- and metabolic-dysregulated phenotypes. Circadian dysregulation using a chronic jet lag paradigm has been shown to increase inflammation and proinflammatory responses of the innate immune system (34). Furthermore, our previous studies indicate that clock-disrupted (Per1ldc/Per2ldc) macrophages are distinguished by enhanced proinflammatory activation and that myeloid cell–specific Per1ldc/Per2ldc disruption potentiates HFD-induced inflammation and systemic metabolic dysregulation in chimeric mice repopulated with only mutant bone marrow cells (9). In the present study, circadian desynchronization in HFD-fed mice exposed to shifted LD cycles essentially recapitulates the effects of genetic disruption of the core clock mechanism, inducing robust increases in adipose tissue macrophage infiltration and proinflammatory activation in conjunction with the amplification of metabolic phenotypes in diet-induced obesity. Collectively, these findings suggest that macrophage infiltration, proinflammatory activation, and inflammatory signaling may be key processes in the mechanism by which genetic disruption and environmental desynchronization of circadian rhythms hinder metabolic homeostasis.
Although our data and other studies indicate that increased inflammation is a distinctive outcome of genetic disruption and environmental desynchronization of circadian rhythms, how circadian disturbances specifically trigger proinflammatory macrophage activation and inflammatory signaling cascades that lead to systemic metabolic dysregulation remains to be determined. Because chronic jet lag and shift work paradigms alter clock gene expression as well as phase alignment and amplitude of their rhythmic profiles (35–37), it is possible that core or auxiliary feedback loops comprising the circadian clockworks directly mediate the effects of circadian disruption on key elements of inflammatory signaling cascades such as NF-κB, IL-6, and chemokine ligand 2 (10). This speculation is supported by our previous observations indicating that myeloid cell–specific Per1 and Per2 disruption increases NF-κB and JNK1 phosphorylation as well as IL-6 mRNA expression and decreases levels of PPARγ, which promotes anti-inflammatory M2 activation, in macrophages isolated from HFD-fed mice (9). Direct involvement of core or auxiliary clock genes in linking circadian clock dysregulation with the potentiation of diet-induced inflammation is further suggested by studies showing that: 1) CLOCK upregulates NF-κB activity (38) and 2) REV-ERBα modulates macrophage TLR signaling (39) and inhibits proinflammatory IL-6 release (11). Thus, it will be important in future studies to determine whether blockade of inflammatory signaling via NF-κB, IL-6, or up-regulation of PPARγ ameliorates the inductive effects of circadian disruption on diet-associated metabolic dysregulation. Nevertheless, the present data provide primary evidence for the link between circadian clock dysregulation and macrophage proinflammatory activation in diet-induced metabolic dysfunction.
ACKNOWLEDGMENTS
The authors thank Yang-Yi Fan (Texas A&M University) for her assistance with the FACS analysis. This study was supported by the Center for Translational Environmental Health Research (P30ES02351) and U.S. National Institutes of Health, National Cancer Institute Outstanding Investigator Award R35CA197707 (to R.S.C.). The authors declare no conflicts of interest.
Glossary
- BMDM
bone marrow–derived macrophage
- FACS
fluorescence-activated cell sorting
- HFD
high-fat diet
- LD
light–dark
- Luc
luciferase
- Per
period
- PPAR
peroxisome proliferator-activated receptor
- SVC
stromal vascular cell
AUTHOR CONTRIBUTIONS
All authors were responsible for the research design, methods and research, data analysis, and writing of the manuscript; and R. S. Chapkin and D. J. Earnest were responsible for acquisition of funding.
REFERENCES
- 1.Bozek K., Relógio A., Kielbasa S. M., Heine M., Dame C., Kramer A., Herzel H. (2009) Regulation of clock-controlled genes in mammals. PLoS One 4, e4882 10.1371/journal.pone.0004882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keller M., Mazuch J., Abraham U., Eom G. D., Herzog E. D., Volk H. D., Kramer A., Maier B. (2009) A circadian clock in macrophages controls inflammatory immune responses. Proc. Natl. Acad. Sci. USA 106, 21407–21412 10.1073/pnas.0906361106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lange T., Dimitrov S., Born J. (2010) Effects of sleep and circadian rhythm on the human immune system. Ann. N. Y. Acad. Sci. 1193, 48–59 10.1111/j.1749-6632.2009.05300.x [DOI] [PubMed] [Google Scholar]
- 4.Lamia K. A., Sachdeva U. M., DiTacchio L., Williams E. C., Alvarez J. G., Egan D. F., Vasquez D. S., Juguilon H., Panda S., Shaw R. J., Thompson C. B., Evans R. M. (2009) AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science 326, 437–440 10.1126/science.1172156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dallmann R., Viola A. U., Tarokh L., Cajochen C., Brown S. A. (2012) The human circadian metabolome. Proc. Natl. Acad. Sci. USA 109, 2625–2629 10.1073/pnas.1114410109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turek F. W., Joshu C., Kohsaka A., Lin E., Ivanova G., McDearmon E., Laposky A., Losee-Olson S., Easton A., Jensen D. R., Eckel R. H., Takahashi J. S., Bass J. (2005) Obesity and metabolic syndrome in circadian clock mutant mice. Science 308, 1043–1045 10.1126/science.1108750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcheva B., Ramsey K. M., Buhr E. D., Kobayashi Y., Su H., Ko C. H., Ivanova G., Omura C., Mo S., Vitaterna M. H., Lopez J. P., Philipson L. H., Bradfield C. A., Crosby S. D., JeBailey L., Wang X., Takahashi J. S., Bass J. (2010) Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 466, 627–631 10.1038/nature09253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paschos G. K., Ibrahim S., Song W. L., Kunieda T., Grant G., Reyes T. M., Bradfield C. A., Vaughan C. H., Eiden M., Masoodi M., Griffin J. L., Wang F., Lawson J. A., Fitzgerald G. A. (2012) Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat. Med. 18, 1768–1777 10.1038/nm.2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu H., Li H., Woo S. L., Kim S. M., Shende V. R., Neuendorff N., Guo X., Guo T., Qi T., Pei Y., Zhao Y., Hu X., Zhao J., Chen L., Chen L., Ji J. Y., Alaniz R. C., Earnest D. J., Wu C. (2014) Myeloid cell-specific disruption of Period1 and Period2 exacerbates diet-induced inflammation and insulin resistance. J. Biol. Chem. 289, 16374–16388 10.1074/jbc.M113.539601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtis A. M., Bellet M. M., Sassone-Corsi P., O’Neill L. A. (2014) Circadian clock proteins and immunity. Immunity 40, 178–186 10.1016/j.immuni.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 11.Gibbs J. E., Blaikley J., Beesley S., Matthews L., Simpson K. D., Boyce S. H., Farrow S. N., Else K. J., Singh D., Ray D. W., Loudon A. S. (2012) The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc. Natl. Acad. Sci. USA 109, 582–587 10.1073/pnas.1106750109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen K. D., Fentress S. J., Qiu Y., Yun K., Cox J. S., Chawla A. (2013) Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science 341, 1483–1488 10.1126/science.1240636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silver A. C., Arjona A., Walker W. E., Fikrig E. (2012) The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity 36, 251–261 10.1016/j.immuni.2011.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoo S. H., Yamazaki S., Lowrey P. L., Shimomura K., Ko C. H., Buhr E. D., Siepka S. M., Hong H. K., Oh W. J., Yoo O. J., Menaker M., Takahashi J. S. (2004) PERIOD2:LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. USA 101, 5339–5346 10.1073/pnas.0308709101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheer F. A., Hilton M. F., Mantzoros C. S., Shea S. A. (2009) Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 106, 4453–4458 10.1073/pnas.0808180106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Earnest D. J., Neuendorff N., Coffman J., Selvamani A., Sohrabji F. (2016) Sex differences in the impact of shift work schedules on pathological outcomes in an animal model of ischemic stroke. Endocrinology 157, 2836–2843 10.1210/en.2016-1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo X., Xu K., Zhang J., Li H., Zhang W., Wang H., Lange A. J., Chen Y. E., Huo Y., Wu C. (2010) Involvement of inducible 6-phosphofructo-2-kinase in the anti-diabetic effect of peroxisome proliferator-activated receptor gamma activation in mice. J. Biol. Chem. 285, 23711–23720 10.1074/jbc.M110.123174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huo Y., Guo X., Li H., Wang H., Zhang W., Wang Y., Zhou H., Gao Z., Telang S., Chesney J., Chen Y. E., Ye J., Chapkin R. S., Wu C. (2010) Disruption of inducible 6-phosphofructo-2-kinase ameliorates diet-induced adiposity but exacerbates systemic insulin resistance and adipose tissue inflammatory response. J. Biol. Chem. 285, 3713–3721 10.1074/jbc.M109.058446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stienstra R., Duval C., Keshtkar S., van der Laak J., Kersten S., Müller M. (2008) Peroxisome proliferator-activated receptor gamma activation promotes infiltration of alternatively activated macrophages into adipose tissue. J. Biol. Chem. 283, 22620–22627 10.1074/jbc.M710314200 [DOI] [PubMed] [Google Scholar]
- 20.Farnell Y. F., Shende V. R., Neuendorff N., Allen G. C., Earnest D. J. (2011) Immortalized cell lines for real-time analysis of circadian pacemaker and peripheral oscillator properties. Eur. J. Neurosci. 33, 1533–1540 10.1111/j.1460-9568.2011.07629.x [DOI] [PubMed] [Google Scholar]
- 21.Odegaard J. I., Ricardo-Gonzalez R. R., Goforth M. H., Morel C. R., Subramanian V., Mukundan L., Red Eagle A., Vats D., Brombacher F., Ferrante A. W., Chawla A. (2007) Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 447, 1116–1120 10.1038/nature05894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lumeng C. N., Bodzin J. L., Saltiel A. R. (2007) Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 10.1172/JCI29881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prieur X., Mok C. Y., Velagapudi V. R., Núñez V., Fuentes L., Montaner D., Ishikawa K., Camacho A., Barbarroja N., O’Rahilly S., Sethi J. K., Dopazo J., Orešič M., Ricote M., Vidal-Puig A. (2011) Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes 60, 797–809 10.2337/db10-0705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fatouros M., Bourantas K., Bairaktari E., Elisaf M., Tsolas O., Cassioumis D. (1995) Role of the spleen in lipid metabolism. Br. J. Surg. 82, 1675–1677 10.1002/bjs.1800821230 [DOI] [PubMed] [Google Scholar]
- 25.Lumeng C. N., Deyoung S. M., Bodzin J. L., Saltiel A. R. (2007) Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes 56, 16–23 10.2337/db06-1076 [DOI] [PubMed] [Google Scholar]
- 26.Han M. S., Jung D. Y., Morel C., Lakhani S. A., Kim J. K., Flavell R. A., Davis R. J. (2013) JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science 339, 218–222 10.1126/science.1227568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solinas G., Vilcu C., Neels J. G., Bandyopadhyay G. K., Luo J. L., Naugler W., Grivennikov S., Wynshaw-Boris A., Scadeng M., Olefsky J. M., Karin M. (2007) JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 6, 386–397 10.1016/j.cmet.2007.09.011 [DOI] [PubMed] [Google Scholar]
- 28.Iwamoto A., Kawai M., Furuse M., Yasuo S. (2014) Effects of chronic jet lag on the central and peripheral circadian clocks in CBA/N mice. Chronobiol. Int. 31, 189–198 10.3109/07420528.2013.837478 [DOI] [PubMed] [Google Scholar]
- 29.Oike H., Sakurai M., Ippoushi K., Kobori M. (2015) Time-fixed feeding prevents obesity induced by chronic advances of light/dark cycles in mouse models of jet-lag/shift work. Biochem. Biophys. Res. Commun. 465, 556–561 10.1016/j.bbrc.2015.08.059 [DOI] [PubMed] [Google Scholar]
- 30.Arble D. M., Bass J., Laposky A. D., Vitaterna M. H., Turek F. W. (2009) Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 17, 2100–2102 10.1038/oby.2009.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hatori M., Vollmers C., Zarrinpar A., DiTacchio L., Bushong E. A., Gill S., Leblanc M., Chaix A., Joens M., Fitzpatrick J. A., Ellisman M. H., Panda S. (2012) Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 15, 848–860 10.1016/j.cmet.2012.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hotamisligil G. S., Arner P., Caro J. F., Atkinson R. L., Spiegelman B. M. (1995) Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Invest. 95, 2409–2415 10.1172/JCI117936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sartipy P., Loskutoff D. J. (2003) Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 100, 7265–7270 10.1073/pnas.1133870100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castanon-Cervantes O., Wu M., Ehlen J. C., Paul K., Gamble K. L., Johnson R. L., Besing R. C., Menaker M., Gewirtz A. T., Davidson A. J. (2010) Dysregulation of inflammatory responses by chronic circadian disruption. J. Immunol. 185, 5796–5805 10.4049/jimmunol.1001026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reddy A. B., Field M. D., Maywood E. S., Hastings M. H. (2002) Differential resynchronisation of circadian clock gene expression within the suprachiasmatic nuclei of mice subjected to experimental jet lag. J. Neurosci. 22, 7326–7330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Filipski E., Delaunay F., King V. M., Wu M. W., Claustrat B., Gréchez-Cassiau A., Guettier C., Hastings M. H., Francis L. (2004) Effects of chronic jet lag on tumor progression in mice. Cancer Res. 64, 7879–7885 10.1158/0008-5472.CAN-04-0674 [DOI] [PubMed] [Google Scholar]
- 37.Yan L. (2011) Structural and functional changes in the suprachiasmatic nucleus following chronic circadian rhythm perturbation. Neuroscience 183, 99–107 10.1016/j.neuroscience.2011.03.041 [DOI] [PubMed] [Google Scholar]
- 38.Spengler M. L., Kuropatwinski K. K., Comas M., Gasparian A. V., Fedtsova N., Gleiberman A. S., Gitlin I. I., Artemicheva N. M., Deluca K. A., Gudkov A. V., Antoch M. P. (2012) Core circadian protein CLOCK is a positive regulator of NF-κB-mediated transcription. Proc. Natl. Acad. Sci. USA 109, E2457–E2465 10.1073/pnas.1206274109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fontaine C., Rigamonti E., Pourcet B., Duez H., Duhem C., Fruchart J. C., Chinetti-Gbaguidi G., Staels B. (2008) The nuclear receptor Rev-erbalpha is a liver X receptor (LXR) target gene driving a negative feedback loop on select LXR-induced pathways in human macrophages. Mol. Endocrinol. 22, 1797–1811 10.1210/me.2007-0439 [DOI] [PMC free article] [PubMed] [Google Scholar]