Abstract
We sought to investigate safety of axitinib or sorafenib in renal cell carcinoma (RCC) patients and compare toxicity of these two vascular endothelial growth factor receptor inhibitors. Databases of PubMed and Embase were searched. We included phase II and III prospective trials, as well as retrospective studies, in which patients diagnosed with RCC were treated with axitinib or sorafenib monotherapy at a starting dose of 5 mg and 400 mg twice daily, respectively. The overall incidence of high grade hypertension, fatigue, gastrointestinal toxicity and hand-foot syndrome, along with their 95% confidence intervals (CI), were calculated using fixed- or random- effects model according to heterogeneity test results. A total of 26 trials, including 4790 patients, were included in our meta-analysis. Among them, 6 arms were related to axitinib and 22 were associated with sorafenib. The incidences of hypertension (24.9% vs. 7.9%), fatigue (8.2% vs. 6.6%), and gastrointestinal toxicity (17.6% vs. 11.3%) were higher in patients receiving axitinib versus those receiving sorafenib, while the incidence of hand-foot syndrome was lower in patients receiving axitinib versus those receiving sorafenib (9.5% vs. 13.3%). In conclusion, axitinib showed noticeably higher risks of toxicity versus sorafenib. Close monitoring and effective measures for adverse events are recommended during therapy.
Keywords: axitinib, sorafenib, safety, renal cell carcinoma, meta-analysis
Introduction
Renal cell carcinoma (RCC) accounts for 2-3% of all malignant diseases in adults worldwide[1]. It was surmised that about 63,000 new cases and 14,000 deaths associated with RCC occurred in the USA in 2016[2]. Therapeutic options for this chemotherapy-refractory disease have been constantly updated according to availability of targeted drugs over the past few years. Sorafenib and axitinib are two representative drugs targeting vascular endothelial growth factor receptor (VEGFR) which were approved by the US Food and Drug Administration (FDA) in 2005 and 2012, respectively[3– 28].
Sorafenib is a tyrosine kinase inhibitor (TKI) that targets molecules involved in tumor cell proliferation and angiogenesis, such as VEGFR-2, VEGFR-3, platelet-derived growth factor (PDGF) receptor-b, c-KIT and FLT-3[10, 29]. Axitinib, a second generation TKI, is more potent and selective for VEGFR 1-3[30]. The efficacy of axitinib and sorafenib have been demonstrated and compared in two phase III clinical trials[31]; however, the result of safety still remains to be defined given the limited sample size and follow-up time. This meta-analysis included available studies of axitinib and sorafenib monotherapy for patients with RCC, and collected safety related data. In this study, we aimed to compare safety and toxicity of axitinib and sorafenib so as to provide evidence for clinical and policy decision-making.
Patients and methods
Search criteria
Trials meeting the following criteria were enrolled: patients were diagnosed with cytologically or pathologically proven advanced/metastatic RCC. Therapy in either arm must be axitinib or sorafenib monotherapy at a starting dosage of 5 mg and 400 mg twice daily, respectively. Prior anticancer therapies including radiotherapy, nephrectomy, interferons and interleukins were permitted. Toxicity data were recorded according to version II or III of the Common Terminology Criteria for Adverse Events (CTCAE) of National Cancer Institute. Trials including concomitant interventions were excluded.
Search strategy
Databases of PubMed and Embase were reviewed with the following terms: ('sorafenib' OR 'axitinib') AND ('renal cell carcinoma'). Studies which were conducted on or before October 2016 and published only in English were included. This study not only focused on phase II and III clinical trials, but also some retrospective studies, in which axitinib or sorafenib monotherapy was implemented. Phase I trials were not considered given multiple dose levels and limited number of cases. The latest one was adopted if more than one article was found with the same trial. To guarantee that we did not miss any eligible study, related articles from reference list of each study were also retrieved. Further scanning was conducted to determine whether the study was suitable for final analysis.
Data extraction
Two investigators assessed the eligibility of all the articles independently. The trials were identified through the first author and the year of publication, and divergences were resolved by consensus to ensure the accuracy. Then, trial phase, the number of treated patients, the type and dosage of drugs used in the experimental and control arm, median age and proportion of the male gender were extracted. Toxicity data (grade 3/4 adverse events) recorded in the eligible studies were retrieved, extracted, reorganized and assessed, respectively.
Statistical methods
For each study, the rate of patients with hypertension, fatigue, diarrhea, decreased appetite, nausea, vomiting and hand-foot syndrome, as well as their 95% confidence intervals (CI), were calculated. To test statistical heterogeneity between studies, the Cochran's Q test was performed. If Pheterogeneity<0.1 or I2>50%, heterogeneity would be considered to be statistically significant and then data was analyzed through random effects model. Otherwise, a fixed- effects model was applied. Publication bias was estimated using Egger test. Sensitivity analysis was conducted by removing one trial each time to assess the robustness of the finding. Statistical analysis and forest plots were performed using the Comprehensive Meta Analysis version 2 software (Biostat, Englewood, NJ, USA).
Results
Study selection
A total of 1,232 articles on axitinib and 4,433 articles on sorafenib were identified initially from the database and both first and second line treatments were enrolled. Among these, 1,280 were found to be duplicated. After reviewing titles and abstracts, 4,240 subjects were excluded because they were review articles, comments, case reports, pharmacokinetic research or early phase studies (Fig. 1). Afterwards, the remaining 145 papers were retrieved for precise browse. Moreover, 119 of the 145 articles were excluded because their results originated from the same patient population in the same trial. Finally, a total of 26 studies were included in this meta-analysis.
Fig.1.
Flowchart of study selection procedure
Study characteristics
Among the trials, a total of 15 trials had only a single arm with axitinib (4 trials) or sorafenib (11 trials). Different kinds of comparators, such as placebo[9], IFN-α-2a[12], temsirolimus[13], and sunitinib[17, 26], were observed in the remaining 11 trials. In two phase III trials[3– 4], axitinib and sorafenib arms were used in the same trial, which resulted in the number of arms exceeding the number of trials in our final analysis. Two phase III trials and four phase II trials regarding axitinib were adopted finally, and the number for sorafenib in each phase reached 6 and 9, respectively. In addition, three retrospective studies and four articles lack of information concerning phase were also enrolled. Their baseline characteristics are listed in Table 1. The number of patients diagnosed with RCC contained in this meta-analysis reached 4,790 and most of them had received previous therapy like cytokine or nephrectomy. Almost all the patients were over 18 years old, with the median age ranging from 52 to 67 years. A significantly higher proportion of the males were observed in each trial, compared with the females. In the arms of patients treated with axitinib, dose escalation was allowed universally, which was nearly reverse in arms of sorafenib except a few trials[12, 16, 22, 27].
Tab.1.
Characterisics of trials included in the meta-analysis
| Study | Phase | Prior therapy | Age [median(range)] | Male | Dose escalation | Treatment arms | Patients included |
|---|---|---|---|---|---|---|---|
| Motzer et al. 2013[ 3] | 3 | Sunitinib, bevacizumab plus interferon alfa, temsirolimus, cytokines | 61(20-82) 61(22-80) |
265(73%) 258(71%) |
Yes No |
Axitinib 5 mg b.i.d Sorafenib 400 mg b.i.d |
359 355 |
| Hutson et al. 2013[ 4] | 3 | None | 58(23-83) 58(20-77) |
134(70%) 74(77%) |
Yes No |
Axitinib 5 mg b.i.d Sorafenib 400 mg b.i.d |
189 96 |
| Rini et al. 2009[ 5] | 2 | Sorafenib | 60(35-77) | 42(67.7%) | Yes | Axitinib 5 mg b.i.d | 62 |
| Eto et al. 2014[ 6] | 2 | Cytokine | 63(34-80) | 44(69%) | Yes | Axitinib 5 mg b.i.d | 64 |
| Rixe et al. 2007[ 7] | 2 | Cytokine | 59(35-85) | 40(77%) | Yes | Axitinib 5 mg b.i.d | 52 |
| Rini et al. 2013[ 8] | 2 | None | 62(28-87) | 143(67%) | Yes | Axitinib 5 mg b.i.d | 213 |
| Escudier et al. 2009[ 9] | 3 | Cytokine | 58 (19–86) 59 (29–84) |
315 (70% 340 (75%) |
No | Sorafenib 400 mg b.i.d Placebo |
452 451 |
| Ratain et al. 2006[ 10] | 2 | Cytokine | 58(23-83) | 149(74%) | No | Sorafenib 400 mg b.i.d | 202 |
| Naito et al. 2011[ 11] | 2 | Cytokine | 63(30-83) | 100(77.5%) | No | Sorafenib 400 mg b.i.d | 131 |
| Escudier et al. 2009[ 12] | 2 | None | 62(34-78) 62.5(18-80) |
65(67%) 52(56.5%) |
Yes | Sorafenib 400 mg b.i.d IFN-α-2a 9 million U 3 times weekly |
97 90 |
| Hutson et al. 2014[ 13] | 3 | Sunitinib | 61(21-80) 60(19-82) |
192(24%) 193(25%) |
No | Sorafenib 400 mg b.i.d Temsirolimus 25 mg once weekly |
252 249 |
| Suzuki et al. 2014[ 14] | Retrospective | Cytokine | 67(31-84) | 83(74.8%) | No | Sorafenib 400 mg b.i.d | 110 |
| Tafreshi et al. 2014[ 15] | NR | Sunitinib, Temsirolimus, Pazopanib | 60(34-83) | 35(75%) | No | Sorafenib 400 mg b.i.d | 47 |
| Garcia et al. 2010[ 16] | 2 | Bevacizumab, Sunitinib | 64(49-79) | 34(72%) | Yes | Sorafenib 400 mg b.i.d | 47 |
| Zhao et al. 2013[ 17] | Retrospective | None | 57(46-67) 52(41-62) |
18 15 |
No | Sorafenib 400 mg b.i.d Sunitinib 50 mg daily |
20 23 |
| Beck et al. 2011[ 18] | NR | Cytokine | 62(18-84) | 858(75%) | No | Sorafenib 400 mg b.i.d | 1,145 |
| Procopio et al. 2011[ 19] | 2 | None | 62(52-69) 64(57-69) |
43(69%) 52(79%) |
No | Sorafenib 400 mg b.i.d Sorafenib plus IL-2 |
62 66 |
| Motzer et al. 2013[ 20] | 3 | VEGF-targeted, rapamycin-targeted | 59(23-85) 59(23-83) |
189(74%) 185(71%) |
No | Sorafenib 400 mg b.i.d Tivozanib 1.5 mg once daily |
257 259 |
| Jonasch et al. 2010[ 21] | 2 | None | 62.4(45-83) 60.7(43-81) |
32(80%) 29(72.5%) |
No | sorafenib 400 mg b.i.d Sorafenib+ IFN | 40 40 |
| Amato et al. 2012[ 22] | 2 | Cytokine | 62.5(42-78) | 37(84%) | Yes | Sorafenib 400 mg b.i.d | 45 |
| Laber et al. 2009[ 23] | NR | Cytokine | 64(55-82) | 10(71.4%) | No | Sorafenib 400 mg b.i.d | 14 |
| Motzer et al. 2014[ 24] | 3 | Cytokine, VEGF-targeted, mTOR inhibitor | 62(18-81) 61(29-89) |
219(77%) 213(75%) |
No | Sorafenib 400 mg b.i.d Dovitinib |
284 280 |
| Yang et al. 2012[ 25] | NR | Cytokine | (18-80) | 21(70%) | No | Sorafenib 400 mg b.i.d | 30 |
| Park et al. 2012[ 26] | Retrospective | None | 62(26-85) 56.5(17-86) |
35(71%) 161(73%) |
No | Sorafenib 400 mg b.i.d Sunitinib 50 mg once daily |
49 220 |
| Wang et al. 2014[ 27] | 2 | None | 53(24-81) | 33(80%) | Yes | Sorafenib 400, 600, 800 mg b.i.d | 41 |
| Hainsworth et al. 2013[ 28] | 2 | Bevacizumab, Sunitinib | 62(44-86) | 52(69%) | No | Sorafenib 400 mg b.i.d | 75 |
NR, not reported; IFN-α-2a, interferon alfa-2a; IL-2, interleukin-2; IFN, interferon.
Incidence of adverse events
Hypertension
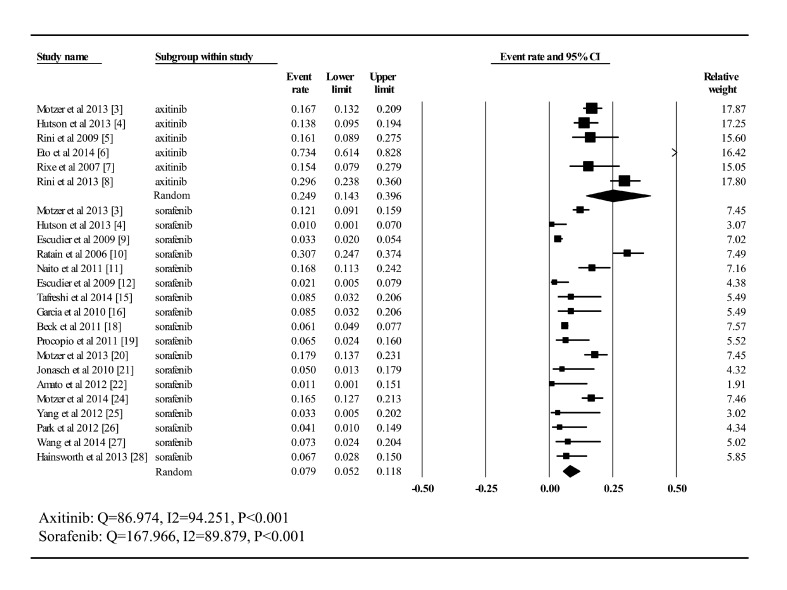
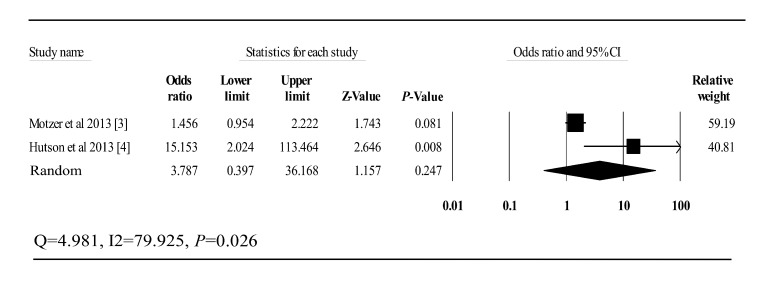
All the six clinical trials related to axitinib, including a total of 939 patients, had data of high grade hypertension available for analysis (Fig. 2). The incidence remained stable with slight fluctuation at around 2% among most trials except a Japanese phase II study, which dramatically jumped to 73.4%. As for sorafenib, the number of trials providing data on high-grade hypertension was 18 and the proportion ranged from 0% to 30.7%. The unique trial reporting no hypertension patients was an intrapatient dose escalation study[22]. The summary incidence of high-grade hypertension in 3455 patients receiving sorafenib was estimated as 7.9% (CI: 5.2%–11.8%), compared with 24.9% (CI: 14.3%–39.6%) for axitinib, after using the random-effects model for analysis (Q= 86.974, I2= 94.251, P<0.001; Q= 167.966, I2= 89.879, P<0.001). In addition, two phase III trials which involve both axitinib and sorafenib monotherapy arm were found during selection process. Thus, an extra analysis was conducted for these two studies and odds ratio (95% CI) for high grade hypertension was 3.787 (0.397–36.168) (Fig. 3).
Fig.2.
Incidence of high-grade hypertension to axitinib and sorafenib
Fig.3.
Odds ratio of axitinib and sorafenib for high-grade hypertension in two phase III trials
Fatigue
As shown in Fig. 4, information regarding high-grade fatigue was available in all six trials associated with axitinib and the incidence fluctuated between 5.3% and 16.1%. Taking it into consideration that heterogeneity had been proved to be statistically significant (Q= 10.326, I2= 51.576, P = 0.067), the random-effects model was adopted to compute the summary proportion (8.2%, CI: 5.2%-12.8%). Among the trials of patients treated with sorafenib, only one study lacked high-grade fatigue data[17]. The largest incidence (25%) was revealed in a phase II study comparing sorafenib monotherapy with combination therapy with sorafenib and low-dose interferon alfa. Similarly, forest plot was performed using the random-effects model (Q= 73.388, I2= 72.748, P<0.001), and the summary rate (6.6%; CI: 5.0%–8.6%) was slightly lower than that hypertension.
Fig.4.
Incidence of high-grade fatigue to axitinib and sorafenib
Gastrointestinal toxicity
According to data extracted in our meta-analysis, gastrointestinal toxicity was universal in almost every trial. The summary incidence of high-grade diarrhea, decreased appetite, nausea and vomiting during treatment with axitinib or sorafenib is presented in Table 2, and the possibility for patients diagnosed with these adverse events after receiving axitinib was obviously larger than that in sorafenib arms.
Tab.2.
Summary incidence of gastrointestinal toxicity
| Axitinib (summary incidence) | Sorafenib (summary incidence) | ||
|---|---|---|---|
| Diarrhea | 9.8% (CI: 8.1%-12.0%) | 5.9% (CI: 4.5%-7.8%) | |
| Decreased appetite | 3.5% (CI: 2.4%-4.9%) | 2.8% (CI: 2.2%-3.4%) | |
| Nausea | 2.3% (CI: 1.4%-3.6%) | 1.4% (CI: 0.8%-2.4%) | |
| Vomiting | 2.0% (CI: 1.1%-3.3%) | 1.2% (CI: 0.9%-1.8%) |
Hand-foot syndrome
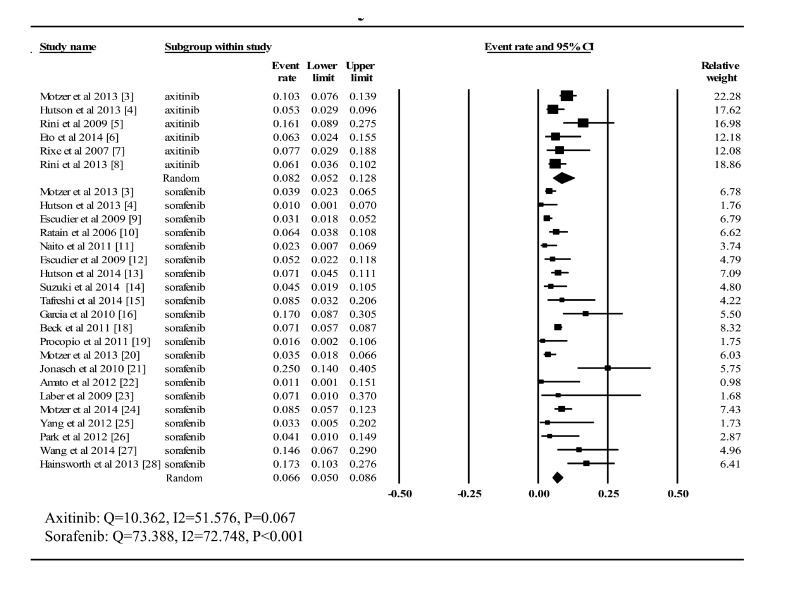
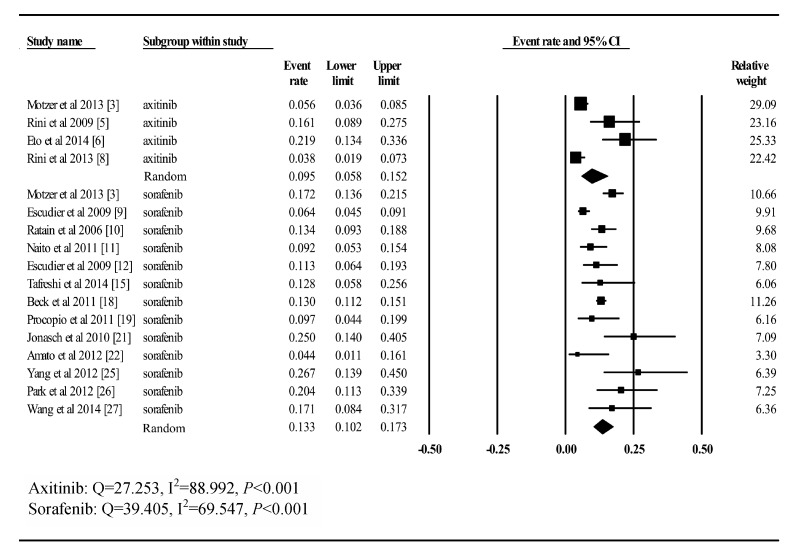
A total of 16 trials, including 698 patients treated with axitinib and 2696 patients treated with sorafenib, provided toxicity data on high-grade hand-foot syndrome in our meta-analysis. Using the random-effects model (Q= 27.253, I2= 88.992, P<0.001; Q= 39.405, I2= 69.547, P<0.001), the incidences in summary were 9.5% (CI: 5.8%–15.2%) for sorafenib and 13.3% (CI: 10.2%–17.3%) for axitinib (Fig. 5).
Fig.5.
Incidence of high-grade hand-foot syndrome to axitinib and sorafenib
Publication bias
Publication bias was not detected for the incidence of each high grade safety effect except for decreased appetite in the sorafenib group (Egger's test: P = 0.012).
Sensitivity analysis
Sensitivity analysis indicated that for all the adverse events reported in this meta-analysis, no trial interrupted the robustness of the whole research seriously exept the trial from Eto et al.[6] for the occurence of hypertension. The summary incidence went down from 0.249 to 0.182 after removing this trial.
Discussion
The toxicity (e.g. hypertension, gastrointestinal effects and hand-foot syndrome) related to VEGFR inhibitors has been previously reported in several systematic reviews[32– 33]. However, the results from most previous reviews evaluated safety effects of combination therapy. Therefore, we conducted a meta-analysis here, where only studies with axitinib or sorafenib monotherapy were enrolled.
Axitinib has been demonstrated to prolong progression free survival (PFS) (axitinib vs. sorafenib, median PFS 6.7 vs. 4.7 months) in a phase III study[31]. However, its toxicity in causing hypertension should not been ignored. In this meta analysis, the incidence of high grade hypertension for patients receiving axitinib tripled compared to that for sorafenib (24.9% vs. 7.9%). Mostly, hypertension originates from anti-VEGF activities[32]. VEGF plays an essential role in promoting endothelial cell proliferation, as well as its survival. Conversely, once VEGF is inhibited, peripheral resistance will trend to ascend given endothelial cell damage and dysfunction[34– 35]. Besides, another mechanism concerning the occurrence of hypertension is considered to be attenuated nitric oxide (NO) production on the surface of different types of vessels[36]. Actually, NO is a vasodilator, and the decrease of NO synthesis may promote vasoconstriction, which will then lead to increased blood pressure. Interestingly, the results from a pharmacokinetic and pharmacodynamic analysis revealed that the increase of diastolic blood pressure can predict favorable PFS and overall survival[37]. Moreover, treatment of hypertension during axitinib experiment would not undermine the efficacy of drugs. Though the association between hypertension and efficacy has been revealed, further research about how they interact with each other still remains to be done.
In addition to hypertension, fatigue and gastrointestinal toxicity like diarrhea, decreased appetite, nausea and vomiting were also common events observed in studies of VEGFR inhibitors. Generally, therapy was generally not suspended if the above events occurred. With the help of dietary intervention or combination therapy, symptoms can be controlled and mitigated. For elderly patients, if high grade diarrhea or vomiting is not controlled well, worse effects like dehydration may occur[38]. Furthermore, it has been reported that treatment-related diarrhea can prolong the duration of multikinase therapy, reduce the mobility and compromise quality of life[39]. As a result, clinical guidelines for managing tumor treatment-related gastrointestinal adverse events should be well conducted.
It is reported that patients receiving axitinib were less likely to suffer from hand food skin reaction (HFSR), compared to patients with sorafenib (9.5% vs. 13.3%). Early in 2007, HFSR was found to be the most evident dermatologic adverse event in patients treated with sunitinib and sorafenib[40– 41]. HFSR was also observed in axitinib treated patients in recent years. The reason for the high incidence of HFSR in sorafenib patients may be that simultaneous inhibition of VEGFR and PDGFR will interrupt normal vascularity, which is indispensable during the repair of fibroblasts and endothelial cells[42– 43]. Interestingly, when VEGFR or PDGFR is separately inhibited with imatinib or some molecules antibodies[44– 45], HFSR is not common. However, axitinib, a specific VEGFR inhibitor, is reported to have comparable incidence here. Actually, the mechanism for this is still not clear, and thus the potential impact of axitinib on PDGFR and VEGFR was originally underestimated. In addition, hypertension due to axitinib may result in vasoconstriction in the sensitive skin[46]. Though HFSR seems to be general for patients treated with sorafenib or axitinib, some precautions, such as removing hyperkeratotic areas prophylactically, wearing soft shoes, avoiding exercises prone to increase friction on the palms and soles[47] and use of urea[48], may be undertaken.
It is important to mention that a couple of limitations still existed in this meta-analysis. First, most studies involved were conducted in institutions from different countries. As a result, potential bias may exist in reporting adverse events. Secondly, we included both prospective and retrospective trials in this analysis, and data was collected during various periods of the study. Moreover, the requirements for dose escalation are not consistent between trials. All of these would increase heterogeneity among the included studies. Thirdly, studies here were conducted in patients only with adequate organ function. Therefore, incidence and its 95% CI calculated in the article may not be applicable to overall population.
In conclusion, axitinib showed noticeably higher risks of toxicity versus sorafenib. Our results indicate that strict monitoring and effective management should be conducted to prevent severe safety effects during therapy with sorafenib and axitinib.
Acknowledgments
The work was supported by grants from the National Natural Science Foundation of China (81773554 to H.Yu), and the National Natural Science Foundation of China Grant for Young Scientists (81302512 to J. Bai).
Contributor Information
Fei Qin, Department of Biostatistics, School of Public Health, Nanjing Medical University, Nanjing, Jiangsu 211166, China..
Hao Yu, Department of Biostatistics, School of Public Health, Nanjing Medical University, Nanjing, Jiangsu 211166, China..
Chang-Rong Xu, Department of Biostatistics, School of Public Health, Nanjing Medical University, Nanjing, Jiangsu 211166, China..
Hui-Hui Chen, Department of Biostatistics, School of Public Health, Nanjing Medical University, Nanjing, Jiangsu 211166, China..
Jian-Ling Bai, Email: jbai@njmu.edu.cn.
References
- 1. Rini BI, Campbell SC, Escudier B. Renal cell carcinoma[J]. Lancet, 2009, 373(9669): 1119–1132 . [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics[J]. CA Cancer J Clin, 2016, 66(1): 7–30 . [DOI] [PubMed] [Google Scholar]
- 3. Motzer RJ, Escudier B, Tomczak P, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial[J]. Lancet Oncol, 2013, 14(6): 552–562 . [DOI] [PubMed] [Google Scholar]
- 4. Hutson TE, Lesovoy V, Al-Shukri S, et al. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomised open-label phase 3 trial[J]. Lancet Oncol, 2013, 14(13): 1287–1294 . [DOI] [PubMed] [Google Scholar]
- 5. Rini BI, Wilding G, Hudes G, et al. Phase II study of axitinib in sorafenib-refractory metastatic renal cell carcinoma[J]. J Clin Oncol, 2009, 27(27): 4462–4468 . [DOI] [PubMed] [Google Scholar]
- 6. Eto M, Uemura H, Tomita Y, et al. Overall survival and final efficacy and safety results from a Japanese phase II study of axitinib in cytokine-refractory metastatic renal cell carcinoma[J]. Cancer Sci, 2014, 105(12): 1576–1583 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rixe O, Bukowski RM, Michaelson MD, et al. Axitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer: a phase II study[J]. Lancet Oncol, 2007, 8(11): 975–984 . [DOI] [PubMed] [Google Scholar]
- 8. Rini BI, Melichar B, Ueda T, et al. Axitinib with or without dose titration for first-line metastatic renal-cell carcinoma: a randomised double-blind phase 2 trial[J]. Lancet Oncol, 2013, 14(12): 1233–1242 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Escudier B, Eisen T, Stadler WM, et al. Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial[J]. J Clin Oncol, 2009, 27(20): 3312–3318 . [DOI] [PubMed] [Google Scholar]
- 10. Ratain MJ, Eisen T, Stadler WM, et al. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma[J]. J Clin Oncol, 2006, 24(16): 2505–2512 . [DOI] [PubMed] [Google Scholar]
- 11. Naito S, Tsukamoto T, Murai M, et al. Overall survival and good tolerability of long-term use of sorafenib after cytokine treatment: final results of a phase II trial of sorafenib in Japanese patients with metastatic renal cell carcinoma[J]. BJU Int, 2011, 108(11): 1813–1819 . [DOI] [PubMed] [Google Scholar]
- 12. Escudier B, Szczylik C, Hutson TE, et al. Randomized phase II trial of first-line treatment with sorafenib versus interferon Alfa-2a in patients with metastatic renal cell carcinoma[J]. J Clin Oncol, 2009, 27(8): 1280–1289 . [DOI] [PubMed] [Google Scholar]
- 13. Hutson TE, Escudier B, Esteban E, et al. Randomized phase III trial of temsirolimus versus sorafenib as second-line therapy after sunitinib in patients with metastatic renal cell carcinoma[J]. J Clin Oncol, 2014, 32(8): 760–767 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suzuki H, Suzuki T, Ishizuka O, et al. Efficacy and safety of advanced renal cell carcinoma patients treated with sorafenib: roles of cytokine pretreatment[J]. Int J Clin Oncol, 2014, 19(4): 686–692 . [DOI] [PubMed] [Google Scholar]
- 15. Tafreshi A, Thientosapol E, Liew MS, et al. Efficacy of sorafenib in advanced renal cell carcinoma independent of prior treatment, histology or prognostic group[J]. Asia Pac J Clin Oncol, 2014, 10(1): 60–65 . [DOI] [PubMed] [Google Scholar]
- 16. Garcia JA, Hutson TE, Elson P, et al. Sorafenib in patients with metastatic renal cell carcinoma refractory to either sunitinib or bevacizumab[J]. Cancer, 2010, 116(23): 5383–5390 . [DOI] [PubMed] [Google Scholar]
- 17. Zhao J, Zhu Y, Zhang C, et al. Sorafenib or sunitinib as postoperative adjuvant therapy for Chinese patients with locally advanced clear cell renal cell carcinoma at high risk for disease recurrence[J]. Urol Oncol, 2013, 31(8): 1800–1805 . [DOI] [PubMed] [Google Scholar]
- 18. Beck J, Procopio G, Bajetta E, et al. Final results of the European Advanced Renal Cell Carcinoma Sorafenib (EU-ARCCS) expanded-access study: a large open-label study in diverse community settings[J]. Ann Oncol, 2011, 22(8): 1812–1823 . [DOI] [PubMed] [Google Scholar]
- 19. Procopio G, Verzoni E, Bracarda S, et al. Sorafenib with interleukin-2 vs sorafenib alone in metastatic renal cell carcinoma: the ROSORC trial[J]. Br J Cancer, 2011, 104(8): 1256–1261 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Motzer RJ, Nosov D, Eisen T, et al. Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: results from a phase III trial[J]. J Clin Oncol, 2013, 31(30): 3791–3799 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jonasch E, Corn P, Pagliaro LC, et al. Upfront, randomized, phase 2 trial of sorafenib versus sorafenib and low-dose interferon alfa in patients with advanced renal cell carcinoma: clinical and biomarker analysis[J]. Cancer, 2010, 116(1): 57–65 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Amato R, Zhai J, Willis J, et al. A phase II trial of intrapatient dose-escalated sorafenib in patients with metastatic renal cell carcinoma[J]. Clin Genitourin Cancer, 2012, 10(3): 153–158 . [DOI] [PubMed] [Google Scholar]
- 23. Laber DA, Mushtaq M. Compassionate use of sorafenib in patients with advanced renal cell cancer[J]. Clin Genitourin Cancer, 2009, 7(1): 34–38 . [DOI] [PubMed] [Google Scholar]
- 24. Motzer RJ, Porta C, Vogelzang NJ, et al. Dovitinib versus sorafenib for third-line targeted treatment of patients with metastatic renal cell carcinoma: an open-label, randomised phase 3 trial[J]. Lancet Oncol, 2014, 15(3): 286–296 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang L, Shi L, Fu Q, et al. Efficacy and safety of sorafenib in advanced renal cell carcinoma patients: Results from a long-term study[J]. Oncol Lett, 2012, 3(4): 935–939 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park SJ, Lee JL, Park I, et al. Comparative efficacy of sunitinib versus sorafenib as first-line treatment for patients with metastatic renal cell carcinoma[J]. Chemotherapy, 2012, 58(6): 468–474 . [DOI] [PubMed] [Google Scholar]
- 27. Wang HK, Zhang HL, Zhu Y, et al. A Phase II trial of dosage escalation of sorafenib in Asian patients with metastatic renal cell carcinoma[J]. Future Oncol, 2014, 10(12): 1941–1951 . [DOI] [PubMed] [Google Scholar]
- 28. Hainsworth JD, Waterhouse DM, Penley WC, et al. Sorafenib and everolimus in advanced clear cell renal carcinoma: a phase I/II trial of the SCRI Oncology Research Consortium[J]. Cancer Invest, 2013, 31(5): 323–329 . [DOI] [PubMed] [Google Scholar]
- 29. Rini BI. Vascular endothelial growth factor-targeted therapy in metastatic renal cell carcinoma[J]. Cancer, 2009, 115(10 Suppl): 2306–2312 . [DOI] [PubMed] [Google Scholar]
- 30. Hu-Lowe DD, Zou HY, Grazzini ML, et al. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3[J]. Clin Cancer Res, 2008, 14(22): 7272–7283 . [DOI] [PubMed] [Google Scholar]
- 31. Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial[J]. Lancet, 2011, 378(9807): 1931–1939 . [DOI] [PubMed] [Google Scholar]
- 32. Qi WX, He AN, Shen Z, et al. Incidence and risk of hypertension with a novel multi-targeted kinase inhibitor axitinib in cancer patients: a systematic review and meta-analysis[J]. Br J Clin Pharmacol, 2013, 76(3): 348–357 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tan Q, Wang W, Long Y, et al. Therapeutic effects and associated adverse events of multikinase inhibitors in metastatic renal cell carcinoma: A meta-analysis[J]. Exp Ther Med, 2015, 9(6): 2275–2280 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maitland ML, Bakris GL, Black HR, et al. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors[J]. J Natl Cancer Inst, 2010, 102(9): 596–604 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer[J]. Br J Cancer, 2007, 96(12): 1788–1795 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Robinson ES, Khankin EV, Choueiri TK, et al. Suppression of the nitric oxide pathway in metastatic renal cell carcinoma patients receiving vascular endothelial growth factor-signaling inhibitors[J]. Hypertension, 2010, 56(6): 1131–1136 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rini BI, Garrett M, Poland B, et al. Axitinib in metastatic renal cell carcinoma: results of a pharmacokinetic and pharmacodynamic analysis[J]. J Clin Pharmacol, 2013, 53(5): 491–504 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. O’Brien BE, Kaklamani VG, Benson AB 3rd. The assessment and management of cancer treatment-related diarrhea[J]. Clin Colorectal Cancer, 2005, 4(6): 375–381., discussion 382–383. [DOI] [PubMed] [Google Scholar]
- 39. Bellmunt J, Eisen T, Fishman M, et al. Experience with sorafenib and adverse event management[J]. Crit Rev Oncol Hematol, 2011, 78(1): 24–32 . [DOI] [PubMed] [Google Scholar]
- 40. Motzer RJ, Michaelson MD, Rosenberg J, et al. Sunitinib efficacy against advanced renal cell carcinoma[J]. J Urol, 2007, 178(5): 1883–1887 . [DOI] [PubMed] [Google Scholar]
- 41. Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma[J]. N Engl J Med, 2007, 356(2): 125–134 . [DOI] [PubMed] [Google Scholar]
- 42. Lacouture ME, Reilly LM, Gerami P, et al. Hand foot skin reaction in cancer patients treated with the multikinase inhibitors sorafenib and sunitinib[J]. Ann Oncol, 2008, 19(11): 1955–1961 . [DOI] [PubMed] [Google Scholar]
- 43. Balagula Y, Wu S, Su X, et al. The risk of hand foot skin reaction to pazopanib, a novel multikinase inhibitor: a systematic review of literature and meta-analysis[J]. Invest New Drugs, 2012, 30(4): 1773–1781 . [DOI] [PubMed] [Google Scholar]
- 44. Breccia M, Carmosino I, Russo E, et al. Early and tardive skin adverse events in chronic myeloid leukaemia patients treated with imatinib[J]. Eur J Haematol, 2005, 74(2): 121–123 . [DOI] [PubMed] [Google Scholar]
- 45. Schenone S, Bondavalli F, Botta M. Antiangiogenic agents: an update on small molecule VEGFR inhibitors[J]. Curr Med Chem, 2007, 14(23): 2495–2516 . [DOI] [PubMed] [Google Scholar]
- 46. Fischer A, Wu S, Ho AL, et al. The risk of hand-foot skin reaction to axitinib, a novel VEGF inhibitor: a systematic review of literature and meta-analysis[J]. Invest New Drugs, 2013, 31(3): 787–797 . [DOI] [PubMed] [Google Scholar]
- 47. Anderson R, Jatoi A, Robert C, et al. Search for evidence-based approaches for the prevention and palliation of hand-foot skin reaction (HFSR) caused by the multikinase inhibitors (MKIs)[J]. Oncologist, 2009, 14(3): 291–302 . [DOI] [PubMed] [Google Scholar]
- 48. Ren ZG, Zhu KS, Kang HY, et al. Randomized controlled trial of the prophylactic effect of urea-based cream on sorafenib-associated hand-foot skin reactions in patients with advanced hepatocellular carcinoma[J]. [J]. J Clin Oncol, 2015, 33(8):894–900. [DOI] [PubMed] [Google Scholar]