The title crystals are isomorphous, and form centrically stacked planar sheets formed by CN⋯I and NC⋯I short contacts.
Keywords: crystal structure, nitrile, isocyanide, N⋯I contacts, C⋯I contacts
Abstract
The title crystals, C7H2I3N, are isomorphous. Both molecules lie across two crystallographic mirror planes and a twofold axis. The principal supramolecular interaction is centric R 2 2(10) CN/NC⋯I short contacts involving both ortho I atoms, with two contacts bisecting each cyano and isocyano group. These form ribbons along [010] and give rise to a planar sheet structure parallel to (100). All pairs of adjacent sheets have centric stacking, a mode not previously reported for sheets of this type. This study completes the series of homo-2,4,6-trihalobenzonitriles, in which I atoms give the strongest CN⋯X and NC⋯X interactions (X = F, Cl, Br, I).
Chemical context
The strength of cyano–halo interactions tends to increase with increasing polarizability, or the elemental period, of the halogen. Structure-directing CN⋯F interactions are usually not observed (Bond et al., 2001 ▸). In crystals of the other 4-halobenzonitriles (X = Cl, Br, I), parallel or antiparallel 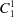 (7) CN⋯X chains dominate the secondary structures (Fig. 1 ▸; Desiraju & Harlow, 1989 ▸). When the halo atom is moved to the 2-position,
(7) CN⋯X chains dominate the secondary structures (Fig. 1 ▸; Desiraju & Harlow, 1989 ▸). When the halo atom is moved to the 2-position, 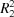 (10) CN⋯X rings can form, usually as inversion dimers. Halogenation at both ortho positions allows the formation of CN⋯X-derived ribbons or sheets. The aforementioned periodic trend is exhibited by the homo-2,4,6-trihalobenzonitriles. No CN⋯F contacts are observed in 2,4,6-trifluorobenzonitrile (F3CN). Instead, each CN group is bisected by two CN⋯H contacts (Fig. 2 ▸
a; Britton, 2008 ▸). In 2,4,6-trichlorobenzonitrile (Cl3CN), half of these have been replaced by CN⋯Cl contacts (Fig. 2 ▸
b; Pink et al., 2000 ▸). In 2,4,6-tribromobenzonitrile (Br3CN), no CN⋯H contacts are found, and each CN group is bisected by two CN⋯Br contacts (Fig. 2 ▸
c; Britton et al., 2016 ▸).
(10) CN⋯X rings can form, usually as inversion dimers. Halogenation at both ortho positions allows the formation of CN⋯X-derived ribbons or sheets. The aforementioned periodic trend is exhibited by the homo-2,4,6-trihalobenzonitriles. No CN⋯F contacts are observed in 2,4,6-trifluorobenzonitrile (F3CN). Instead, each CN group is bisected by two CN⋯H contacts (Fig. 2 ▸
a; Britton, 2008 ▸). In 2,4,6-trichlorobenzonitrile (Cl3CN), half of these have been replaced by CN⋯Cl contacts (Fig. 2 ▸
b; Pink et al., 2000 ▸). In 2,4,6-tribromobenzonitrile (Br3CN), no CN⋯H contacts are found, and each CN group is bisected by two CN⋯Br contacts (Fig. 2 ▸
c; Britton et al., 2016 ▸).
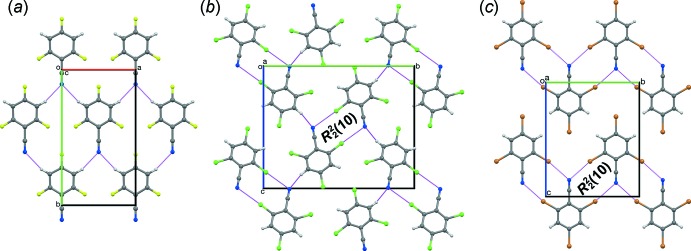
Figure 1.
Several molecules in the crystal of 4-iodobenzonitrile (4ICN), viewed along [001]. Dashed green lines represent CN⋯I short contacts, which collectively form a C(7) chain motif along [010]. All previously reported 4-iodobenzonitriles form similar chains.
Figure 2.
(a) A sheet in a crystal of F3CN, showing two CN⋯H contacts per CN group, viewed along [001]; (b) A sheet in a crystal of Cl3CN, showing one CN⋯H and one CN⋯Cl contact per CN group, viewed along [100]; (c) A sheet in the Z = 8 polytype of Br3CN, showing two CN⋯Br contacts per CN group, viewed along [100]. Dashed magenta lines represent short contacts.
Database survey
No entries were found in the most recent update of the Cambridge Structural Database (Version 5.37, May 2017; Groom et al., 2016 ▸) that have I atoms at both ortho positions of a benzonitrile. Four of the five crystalline 2-iodobenzonitriles have CN⋯I contacts (Britton, 2001 ▸, 2004 ▸; Ketels et al., 2017 ▸; Lam & Britton, 1974 ▸); the fifth is a cyano alcohol that forms O—H⋯NC hydrogen bonds (Salvati et al., 2008 ▸). The 3-iodo analogs do not pack as efficiently. Three of the four examples feature I⋯I contacts (Britton, 2006 ▸; Merz, 2006 ▸); packing in the fourth example is directed by hydrogen bonding between acetamido groups (Garden et al., 2007 ▸). All five reported 4-iodobenzonitriles form C(7) CN⋯I chains (Fig. 1 ▸; Bond et al., 2001 ▸; Britton, 2004 ▸; Desiraju & Harlow, 1989 ▸; Gleason & Britton, 1978 ▸). It is pertinent to determine the crystal structure of 2,4,6-triiodobenzonitrile (I3CN) to complete the series of homo-2,4,6-trihalobenzonitriles, and to determine whether the primary packing interaction is CN⋯I-derived C(7) chains, 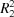 (10) rings, or another motif. 2,4,6-Triiodophenyl isocyanide (I3NC) is included to contribute to the library of corresponding halogenated nitrile-isocyanide crystal pairs.
(10) rings, or another motif. 2,4,6-Triiodophenyl isocyanide (I3NC) is included to contribute to the library of corresponding halogenated nitrile-isocyanide crystal pairs.
Structural commentary
Molecules of I3CN and I3NC (Fig. 3 ▸) lie about a twofold axis and two orthogonal vertical mirror planes. Thus, they have crystallographically-imposed C2v symmetry and are planar, with the para I atom (I4; I14) collinear with the CN and NC groups. All of the aryl bond angles are roughly 120°. The ortho I atoms (I2, I2′; I12, I12′) are scissored slightly toward the ipso C atom (C1; C11), which is probably caused by the intermolecular CN⋯I and NC⋯I short contacts. The bond lengths are typical for their respective functional groups.
Figure 3.
The molecular structures of (a) I3CN and (b) I3NC, with atom labeling and displacement ellipsoids at the 50% probability level. Unlabeled atoms are generated by the symmetry operation (1 − x,  − y, z).
− y, z).
Supramolecular features
Crystals of I3CN and I3NC are isomorphous. The CN and NC groups are bisected by C7≡N7⋯I2 and N17≡C17⋯I12 contacts (Table 1 ▸), forming ribbons of 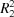 (10) rings parallel to (100) along [010]. Adjacent ribbons translate along [001]. The resulting planar sheet structure (Fig. 4 ▸) matches that observed in Br3CN and the corresponding isocyanide (Br3NC) (Britton et al., 2016 ▸), and the 4-chloro (Britton, 2005 ▸) and 4-nitro (Noland & Tritch, 2017 ▸) analogs of Br3CN. In crystals of I3CN and I3NC, all pairs of adjacent sheets have centric stacking along [100] (Fig. 5 ▸), with molecules stacked about a glide plane and an inversion center. In the polytypes of Br3CN and Br3NC, adjacent sheets had combinations of centric and translational stacking, but not solely centric stacking. The 4-chloro analog had translational stacking. The 4-nitro analog had glide stacking, with no inversion center between stacked molecules. Thus, the all-centric stacking of I3CN and I3NC can be regarded as a new polytype in this series.
(10) rings parallel to (100) along [010]. Adjacent ribbons translate along [001]. The resulting planar sheet structure (Fig. 4 ▸) matches that observed in Br3CN and the corresponding isocyanide (Br3NC) (Britton et al., 2016 ▸), and the 4-chloro (Britton, 2005 ▸) and 4-nitro (Noland & Tritch, 2017 ▸) analogs of Br3CN. In crystals of I3CN and I3NC, all pairs of adjacent sheets have centric stacking along [100] (Fig. 5 ▸), with molecules stacked about a glide plane and an inversion center. In the polytypes of Br3CN and Br3NC, adjacent sheets had combinations of centric and translational stacking, but not solely centric stacking. The 4-chloro analog had translational stacking. The 4-nitro analog had glide stacking, with no inversion center between stacked molecules. Thus, the all-centric stacking of I3CN and I3NC can be regarded as a new polytype in this series.
Table 1. Contact geometry (Å, °) for I3CN and I3NC.
| A≡B⋯I | A≡B | B⋯I | A≡B⋯I |
|---|---|---|---|
| C7≡N7⋯I2i | 1.151 (3) | 3.074 (2) | 132.85 (3) |
| N17≡C17⋯I12i | 1.164 (3) | 3.106 (2) | 134.18 (3) |
Symmetry code: (i) −x + 1, y −  , −z + 2
, −z + 2
Figure 4.
A space-filling drawing of the sheet structure of I3NC, viewed along [100].
Figure 5.
Two adjacent sheets in I3NC, viewed along [100], illustrating the centric stacking mode. Dashed magenta lines represent short contacts in the front layer. Molecules in the rear layer are drawn with smaller balls and sticks, lower opacity, and green dashed lines representing short contacts.
The mean CN⋯X contact lengths can be compared for X = Cl, Br, and I (Table 2 ▸). For 4-chlorobenzonitrile (4ClCN), 4-bromobenzonitrile (4BrCN), and 4-iodobenzonitrile (4ICN) (Table 2 ▸, col. 2), the contact distance decreases with increasing halogen size, highlighting the increase in contact strength (Desiraju & Harlow, 1989 ▸). This trend is essentially mirrored among 2,4,6-trihalobenzonitriles (Table 2 ▸, col. 3), although the contact distance in I3CN is 0.01 Å larger than in Br3CN. The N⋯X non-bonded contact radii are listed (Table 2 ▸, col. 4; Rowland & Taylor, 1996 ▸). The ‘shortness’ of contacts in 2,4,6-trihalobenzonitriles is expressed as the ratios of contact radii to the respective contact distances (Table 2 ▸, col. 5). A similar comparison of NC⋯X contact lengths in the corresponding trihalo isocyanides also shows decreasing contact length with increasing halogen size (Table 3 ▸, col. 2). The NC⋯X contacts have slightly greater shortness (Table 3 ▸, col. 4) than the corresponding CN⋯X contacts. The N17≡C17⋯I12 contacts in I3NC are the strongest cyano/isocyano–halo interactions in this series.
Table 2. Mean CN⋯X contact lengths (Å) in 4-halobenzonitriles (4XCN) and 2,4,6-trihalobenzonitriles (X3CN).
| X | 4XCN | X3CN | r [N + X] (Å) | [r/X3CN] |
|---|---|---|---|---|
| Cl | 3.370 (4) | 3.153 (2) | 3.35 | 1.06 |
| Br | 3.249 (5) | 3.064 (4) | 3.46 | 1.13 |
| I | 3.127 (4) | 3.074 (2) | 3.61 | 1.17 |
Table 3. Mean NC⋯X contact lengths (Å) in 2,4,6-trihalophenyl isocyanides (X3NC).
| X | X3NC | r [C + X] | [r/X3NC] |
|---|---|---|---|
| Cl | 3.245 (3) | 3.49 | 1.08 |
| Br | 3.151 (4) | 3.60 | 1.14 |
| I | 3.106 (2) | 3.75 | 1.21 |
Synthesis and crystallization
2,4,6-Triiodoaniline (I3NH2), adapted from the work of Jackson & Whitmore (1915 ▸): Aniline (1.0 mL) and hydrochloric acid (0.7 M, 850 mL) were combined and stirred in a round-bottomed flask. Iodine monochloride (8.2 g) was placed in a separate flask and then warmed to 315 K. The two flasks were connected with a glass bridge. A slow stream of nitrogen was passed through the headspace in the second vessel so that the iodine monochloride was gradually swept into the first vessel over 2–4 d. After the transfer was complete, the reaction mixture was neutralized with NaHCO3 solution, followed by reduction of excess iodine by washing with Na2S2O3 solution. Dichloromethane (approx. 100 mL) was added, with stirring, until nearly all solids had dissolved. The organic portion was filtered through silica gel (3 cm H × 4 cm D), and then the filter was washed with dichloromethane (3 × 20 mL). The filtrate was placed in a loosely-covered beaker. After most of the dichloromethane had evaporated, beige needles were collected by suction filtration (4.48 g, 89%). M.p. 459–460 K (lit. 459); 1H NMR (300 MHz, CDCl3) δ 7.864 (s, 2H), 4.658 (s, 2H); 13C NMR (75 MHz, (CD3)2SO) δ 147.0 (1C), 145.4 (2C), 83.0 (2C), 78.8 (1C); IR (KBr, cm−1) 3417, 3056, 2987, 1632, 1422, 1265, 741, 704; MS (EI, m/z) [M]+ calculated for C6H4I3N 470.7472, found 470.7470.
2,4,6-Triiodobenzonitrile (I3CN), was prepared from I3NH2 (101 mg; Fig. 6 ▸) based on the Sandmeyer procedure described by Britton et al. (2016 ▸). Ethyl acetate (20 mL), toluene-4-sulfonic acid monohydrate (77 mg), and isoamyl nitrite (60 µL) were used in place of water, acetic and sulfuric acids, and sodium nitrite, respectively. The desired chromatographic fraction (Rf = 0.44 in 4:1 hexane–ethyl acetate) was concentrated on a rotary evaporator, giving a beige powder (67 mg, 65%). M.p. 517–518 K; 1H NMR (500 MHz, (CD3)2SO) δ 8.431 (s, 2H, H3); 13C NMR (126 MHz, CD2Cl2) δ 147.5 (2C, C3), 127.3 (1C, C1), 120.7 (1C, C7), 101.1 (1C, C4), 99.6 (2C, C2); IR (NaCl, cm−1) 3070, 2227, 1532, 1359, 1206, 1081, 861, 787, 706; MS (ESI, m/z) [M + Na]+ calculated for C7H2I3N 503.7213, found 503.7216.
Figure 6.
The synthesis of I3CN and I3NC.
2,4,6-Triiodoformanilide (I3FA) was prepared from I3NH2 (1.01 g) according to the formylation procedure described by Britton et al. (2016 ▸), with 1,2-dichloroethane (10 mL and 100 mL) in place of tetrahydrofuran, giving white needles (962 mg, 90%). M.p. 557–558 K; 1H NMR (300 MHz, (CD3)2SO; 2 conformers observed) δ 10.089 (s, 1H; major), 9.655 (s, 1H; minor), 8.303 (s, 1H; major), 8.278 (s, 2H; minor), 8.233 (s, 2H; major), 7.978 (s, 1H; minor); 13C NMR (126 MHz, (CD3)2SO; 2 conformers observed) δ 164.4 (1C; minor), 159.4 (1C; major), 146.4 (2C; minor), 146.1 (2C; major), 141.0 (1C; major), 140.4 (1C; minor), 102.4 (2C; minor), 101.9 (2C; major), 95.9 (1C; minor), 95.6 (1C; major); IR (NaCl, cm−1) 3221, 3076, 2919, 1637, 1490, 1380, 1232, 1143, 857, 794, 703, 682; MS (ESI, m/z) [M - H]− calculated for C7H4I3NO 497.7354, found 497.7365.
2,4,6-Triiodophenyl isocyanide (I3NC) was prepared from I3FA (397 mg) according to the dehydration procedure described by Britton et al. (2016 ▸), giving a white powder (330 mg, 86%). M.p. 467–468 K; 1H NMR (300 MHz, CDCl3) 8.198 (s, 2H, H13); 13C NMR (126 MHz, (CD3)2SO) δ 170.0 (1C, C17), 146.2 (2C, C13), 133.8 (1C, C11), 98.8 (1C, C14), 97.7 (2C, C12); IR (KBr, cm−1) 3073, 3037, 2920, 2126, 1529, 1079, 861, 704; MS (ESI, m/z) [M - H]− calculated for C7H2I3N 479.7249, found 479.7226.
Crystallization: Crystals of I3CN and I3NC were prepared by slow evaporation of acetonitrile solutions at ambient temperature, followed by decantation and then washing with pentane.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 4 ▸. A direct-methods solution was calculated, followed by full-matrix least squares/difference-Fourier cycles. All H atoms were placed in calculated positions (C—H = 0.95 Å) and refined as riding atoms with U iso(H) set to 1.2U eq(C).
Table 4. Experimental details.
| I3CN | I3NC | |
|---|---|---|
| Crystal data | ||
| Chemical formula | C7H2I3N | C7H2I3N |
| M r | 480.80 | 480.80 |
| Crystal system, space group | Orthorhombic, I m m a | Orthorhombic, I m m a |
| Temperature (K) | 100 | 100 |
| a, b, c (Å) | 7.0593 (4), 10.5346 (5), 13.0658 (6) | 7.0552 (3), 10.4947 (5), 13.1557 (5) |
| V (Å3) | 971.66 (8) | 974.08 (7) |
| Z | 4 | 4 |
| Radiation type | Mo Kα | Mo Kα |
| μ (mm−1) | 9.59 | 9.56 |
| Crystal size (mm) | 0.12 × 0.10 × 0.10 | 0.15 × 0.09 × 0.07 |
| Data collection | ||
| Diffractometer | Bruker VENTURE PHOTON-II | Bruker VENTURE PHOTON-II |
| Absorption correction | Multi-scan (SADABS; Sheldrick, 1996 ▸) | Multi-scan (SADABS; Sheldrick, 1996 ▸) |
| T min, T max | 0.281, 0.344 | 0.251, 0.344 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 8436, 1303, 1261 | 13040, 1314, 1267 |
| R int | 0.024 | 0.026 |
| (sin θ/λ)max (Å−1) | 0.835 | 0.834 |
| Refinement | ||
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.013, 0.030, 1.18 | 0.011, 0.024, 1.14 |
| No. of reflections | 1303 | 1314 |
| No. of parameters | 40 | 40 |
| H-atom treatment | H-atom parameters constrained | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.89, −0.60 | 0.72, −0.48 |
Supplementary Material
Crystal structure: contains datablock(s) I3CN, I3NC. DOI: 10.1107/S2056989017018217/lh5864sup1.cif
Structure factors: contains datablock(s) I3CN. DOI: 10.1107/S2056989017018217/lh5864I3CNsup2.hkl
Structure factors: contains datablock(s) I3NC. DOI: 10.1107/S2056989017018217/lh5864I3NCsup3.hkl
Supporting information file. DOI: 10.1107/S2056989017018217/lh5864I3CNsup4.cml
Supporting information file. DOI: 10.1107/S2056989017018217/lh5864I3NCsup5.cml
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
The authors thank Victor G. Young, Jr. (X-Ray Crystallographic Laboratory, University of Minnesota) for assistance with the crystallographic determination, and the Wayland E. Noland Research Fellowship Fund at the University of Minnesota Foundation for generous financial support of this project. This work was taken in large part from the PhD thesis of KJT (Tritch, 2017 ▸).
supplementary crystallographic information
2,4,6-Triiodobenzonitrile (I3CN). Crystal data
| C7H2I3N | Dx = 3.287 Mg m−3 |
| Mr = 480.80 | Melting point: 517 K |
| Orthorhombic, Imma | Mo Kα radiation, λ = 0.71073 Å |
| a = 7.0593 (4) Å | Cell parameters from 2721 reflections |
| b = 10.5346 (5) Å | θ = 2.5–36.4° |
| c = 13.0658 (6) Å | µ = 9.59 mm−1 |
| V = 971.66 (8) Å3 | T = 100 K |
| Z = 4 | Square bipyramid, colorless |
| F(000) = 840 | 0.12 × 0.10 × 0.10 mm |
2,4,6-Triiodobenzonitrile (I3CN). Data collection
| Bruker VENTURE PHOTON-II diffractometer | 1261 reflections with I > 2σ(I) |
| Radiation source: micro-focus | Rint = 0.024 |
| φ and ω scans | θmax = 36.4°, θmin = 2.5° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | h = −9→11 |
| Tmin = 0.281, Tmax = 0.344 | k = −17→17 |
| 8436 measured reflections | l = −21→21 |
| 1303 independent reflections |
2,4,6-Triiodobenzonitrile (I3CN). Refinement
| Refinement on F2 | Hydrogen site location: inferred from neighbouring sites |
| Least-squares matrix: full | H-atom parameters constrained |
| R[F2 > 2σ(F2)] = 0.013 | w = 1/[σ2(Fo2) + (0.0078P)2 + 1.2371P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.030 | (Δ/σ)max = 0.001 |
| S = 1.18 | Δρmax = 0.89 e Å−3 |
| 1303 reflections | Δρmin = −0.60 e Å−3 |
| 40 parameters | Extinction correction: SHELXL2014 (Sheldrick, 2015b), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 0 restraints | Extinction coefficient: 0.00199 (9) |
2,4,6-Triiodobenzonitrile (I3CN). Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
2,4,6-Triiodobenzonitrile (I3CN). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| I2 | 0.5000 | 0.46395 (2) | 0.83491 (2) | 0.01267 (4) | |
| I4 | 0.5000 | 0.7500 | 0.43429 (2) | 0.01359 (4) | |
| N7 | 0.5000 | 0.7500 | 1.00506 (18) | 0.0167 (4) | |
| C1 | 0.5000 | 0.7500 | 0.80692 (18) | 0.0103 (4) | |
| C2 | 0.5000 | 0.63456 (15) | 0.75302 (13) | 0.0105 (3) | |
| C3 | 0.5000 | 0.63428 (16) | 0.64671 (13) | 0.0117 (3) | |
| H3 | 0.5000 | 0.5565 | 0.6100 | 0.014* | |
| C4 | 0.5000 | 0.7500 | 0.59458 (19) | 0.0113 (4) | |
| C7 | 0.5000 | 0.7500 | 0.9170 (2) | 0.0130 (4) |
2,4,6-Triiodobenzonitrile (I3CN). Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| I2 | 0.01764 (6) | 0.00914 (5) | 0.01122 (5) | 0.000 | 0.000 | 0.00198 (3) |
| I4 | 0.01801 (8) | 0.01463 (7) | 0.00812 (6) | 0.000 | 0.000 | 0.000 |
| N7 | 0.0218 (11) | 0.0137 (9) | 0.0145 (9) | 0.000 | 0.000 | 0.000 |
| C1 | 0.0104 (9) | 0.0113 (9) | 0.0094 (8) | 0.000 | 0.000 | 0.000 |
| C2 | 0.0123 (6) | 0.0088 (6) | 0.0104 (6) | 0.000 | 0.000 | 0.0017 (5) |
| C3 | 0.0157 (7) | 0.0095 (6) | 0.0099 (6) | 0.000 | 0.000 | 0.0002 (5) |
| C4 | 0.0121 (9) | 0.0115 (9) | 0.0104 (9) | 0.000 | 0.000 | 0.000 |
| C7 | 0.0137 (10) | 0.0120 (9) | 0.0133 (10) | 0.000 | 0.000 | 0.000 |
2,4,6-Triiodobenzonitrile (I3CN). Geometric parameters (Å, º)
| I2—C2 | 2.0916 (16) | C1—C7 | 1.438 (3) |
| I4—C4 | 2.094 (2) | C2—C3 | 1.389 (2) |
| N7—C7 | 1.151 (3) | C3—C4 | 1.396 (2) |
| C1—C2 | 1.405 (2) | C3—H3 | 0.9500 |
| C1—C2i | 1.405 (2) | C4—C3i | 1.396 (2) |
| C2—C1—C2i | 119.9 (2) | C2—C3—H3 | 120.5 |
| C2—C1—C7 | 120.07 (11) | C4—C3—H3 | 120.5 |
| C2i—C1—C7 | 120.07 (11) | C3i—C4—C3 | 121.6 (2) |
| C3—C2—C1 | 120.19 (16) | C3i—C4—I4 | 119.19 (11) |
| C3—C2—I2 | 120.65 (12) | C3—C4—I4 | 119.19 (11) |
| C1—C2—I2 | 119.16 (13) | N7—C7—C1 | 180.0 |
| C2—C3—C4 | 119.07 (16) | ||
| C2i—C1—C2—C3 | 0.000 (1) | C1—C2—C3—C4 | 0.000 (1) |
| C7—C1—C2—C3 | 180.000 (1) | I2—C2—C3—C4 | 180.000 (1) |
| C2i—C1—C2—I2 | 180.000 (1) | C2—C3—C4—C3i | 0.000 (1) |
| C7—C1—C2—I2 | 0.000 (1) | C2—C3—C4—I4 | 180.000 (1) |
Symmetry code: (i) −x+1, −y+3/2, z.
2,4,6-Triiodophenyl isocyanide (I3NC). Crystal data
| C7H2I3N | Dx = 3.279 Mg m−3 |
| Mr = 480.80 | Melting point: 467 K |
| Orthorhombic, Imma | Mo Kα radiation, λ = 0.71073 Å |
| a = 7.0552 (3) Å | Cell parameters from 2833 reflections |
| b = 10.4947 (5) Å | θ = 2.5–36.3° |
| c = 13.1557 (5) Å | µ = 9.56 mm−1 |
| V = 974.08 (7) Å3 | T = 100 K |
| Z = 4 | Block, colourless |
| F(000) = 840 | 0.14 × 0.09 × 0.07 mm |
2,4,6-Triiodophenyl isocyanide (I3NC). Data collection
| Bruker VENTURE PHOTON-II diffractometer | 1267 reflections with I > 2σ(I) |
| Radiation source: micro-focus | Rint = 0.026 |
| φ and ω scans | θmax = 36.3°, θmin = 2.5° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | h = −11→11 |
| Tmin = 0.251, Tmax = 0.344 | k = −17→12 |
| 13040 measured reflections | l = −21→21 |
| 1314 independent reflections |
2,4,6-Triiodophenyl isocyanide (I3NC). Refinement
| Refinement on F2 | Hydrogen site location: inferred from neighbouring sites |
| Least-squares matrix: full | H-atom parameters constrained |
| R[F2 > 2σ(F2)] = 0.011 | w = 1/[σ2(Fo2) + (0.0044P)2 + 1.2499P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.024 | (Δ/σ)max = 0.001 |
| S = 1.14 | Δρmax = 0.72 e Å−3 |
| 1314 reflections | Δρmin = −0.48 e Å−3 |
| 40 parameters | Extinction correction: SHELXL2014 (Sheldrick, 2015b), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 0 restraints | Extinction coefficient: 0.00312 (10) |
2,4,6-Triiodophenyl isocyanide (I3NC). Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
2,4,6-Triiodophenyl isocyanide (I3NC). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| I12 | 0.5000 | 0.46223 (2) | 0.83472 (2) | 0.01190 (3) | |
| I14 | 0.5000 | 0.7500 | 0.43715 (2) | 0.01245 (4) | |
| N17 | 0.5000 | 0.7500 | 0.91223 (14) | 0.0120 (3) | |
| C11 | 0.5000 | 0.7500 | 0.80681 (15) | 0.0098 (3) | |
| C12 | 0.5000 | 0.63389 (13) | 0.75389 (11) | 0.0102 (2) | |
| C13 | 0.5000 | 0.63411 (14) | 0.64790 (11) | 0.0112 (2) | |
| H13 | 0.5000 | 0.5561 | 0.6113 | 0.013* | |
| C14 | 0.5000 | 0.7500 | 0.59640 (15) | 0.0108 (3) | |
| C17 | 0.5000 | 0.7500 | 1.00074 (17) | 0.0150 (4) |
2,4,6-Triiodophenyl isocyanide (I3NC). Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| I12 | 0.01610 (5) | 0.00910 (4) | 0.01051 (4) | 0.000 | 0.000 | 0.00184 (3) |
| I14 | 0.01561 (6) | 0.01413 (6) | 0.00761 (5) | 0.000 | 0.000 | 0.000 |
| N17 | 0.0134 (7) | 0.0125 (7) | 0.0102 (7) | 0.000 | 0.000 | 0.000 |
| C11 | 0.0106 (8) | 0.0106 (7) | 0.0081 (7) | 0.000 | 0.000 | 0.000 |
| C12 | 0.0121 (5) | 0.0086 (5) | 0.0098 (5) | 0.000 | 0.000 | 0.0012 (4) |
| C13 | 0.0138 (6) | 0.0096 (5) | 0.0101 (5) | 0.000 | 0.000 | 0.0000 (4) |
| C14 | 0.0124 (8) | 0.0116 (8) | 0.0086 (7) | 0.000 | 0.000 | 0.000 |
| C17 | 0.0174 (9) | 0.0148 (9) | 0.0127 (8) | 0.000 | 0.000 | 0.000 |
2,4,6-Triiodophenyl isocyanide (I3NC). Geometric parameters (Å, º)
| I12—C12 | 2.0920 (14) | C11—C12 | 1.4035 (17) |
| I14—C14 | 2.095 (2) | C12—C13 | 1.394 (2) |
| N17—C17 | 1.164 (3) | C13—C14 | 1.3922 (17) |
| N17—C11 | 1.387 (3) | C13—H13 | 0.9500 |
| C11—C12i | 1.4035 (17) | C14—C13i | 1.3922 (17) |
| C17—N17—C11 | 180.0 | C14—C13—C12 | 119.21 (14) |
| N17—C11—C12i | 119.75 (9) | C14—C13—H13 | 120.4 |
| N17—C11—C12 | 119.74 (9) | C12—C13—H13 | 120.4 |
| C12i—C11—C12 | 120.51 (18) | C13i—C14—C13 | 121.76 (18) |
| C13—C12—C11 | 119.65 (13) | C13i—C14—I14 | 119.12 (9) |
| C13—C12—I12 | 120.65 (10) | C13—C14—I14 | 119.12 (9) |
| C11—C12—I12 | 119.70 (11) | ||
| N17—C11—C12—C13 | 180.000 (1) | C11—C12—C13—C14 | 0.000 (1) |
| C12i—C11—C12—C13 | 0.000 (1) | I12—C12—C13—C14 | 180.000 (1) |
| N17—C11—C12—I12 | 0.000 (1) | C12—C13—C14—C13i | 0.000 (1) |
| C12i—C11—C12—I12 | 180.000 (1) | C12—C13—C14—I14 | 180.000 (1) |
Symmetry code: (i) −x+1, −y+3/2, z.
References
- Bond, A. D., Davies, J. E., Griffiths, J. & Rawson, J. M. (2001). Acta Cryst. E57, o231–o233.
- Britton, D. (2001). Acta Cryst. E57, o702–o704.
- Britton, D. (2004). Acta Cryst. E60, o184–o186.
- Britton, D. (2005). Acta Cryst. E61, o1726–o1727.
- Britton, D. (2006). Acta Cryst. B62, 109–117. [DOI] [PubMed]
- Britton, D. (2008). Acta Cryst. C64, o583–o585. [DOI] [PubMed]
- Britton, D., Noland, W. E. & Tritch, K. J. (2016). Acta Cryst. E72, 178–183. [DOI] [PMC free article] [PubMed]
- Bruker (2012). APEX3 and SAINT. Bruker AXS, Inc., Madison, WI, USA.
- Desiraju, G. R. & Harlow, R. L. (1989). J. Am. Chem. Soc. 111, 6757–6764.
- Garden, S. J., Custódio, C. de A., Wardell, J. L., Low, J. N. & Glidewell, C. (2007). Acta Cryst. C63, o408–o410. [DOI] [PubMed]
- Gleason, W. B. & Britton, D. (1978). Cryst. Struct. Commun. 7, 365–370.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Jackson, C. L. & Whitmore, F. C. (1915). J. Am. Chem. Soc. 37, 1522–1537.
- Ketels, M., Konrad, D. B., Karaghiosoff, K., Trauner, D. & Knochel, P. (2017). Org. Lett. 19, 1666–1669. [DOI] [PubMed]
- Lam, S. & Britton, D. (1974). Acta Cryst. B30, 1119–1120.
- Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J. & Wood, P. A. (2008). J. Appl. Cryst. 41, 466–470.
- Merz, K. (2006). Cryst. Growth Des. 6, 1615–1619.
- Noland, W. E. & Tritch, K. J. (2017). IUCrData, 2, x171617.
- Pink, M., Britton, D., Noland, W. E. & Pinnow, M. J. (2000). Acta Cryst. C56, 1271–1273. [DOI] [PubMed]
- Rowland, R. S. & Taylor, R. (1996). J. Phys. Chem. 100, 7384–7391.
- Salvati, M. E., Balog, A., Shan, W., Rampulla, R., Giese, S., Mitt, T., Furch, J. A., Vite, G. D., Attar, R. M., Jure-Kunkel, M., Geng, J., Rizzo, C. A., Gottardis, M. M., Krystek, S. R., Gougoutas, J., Galella, M. A., Obermeier, M., Fura, A. & Chandrasena, G. (2008). Bioorg. Med. Chem. Lett. 18, 1910–1915. [DOI] [PubMed]
- Sheldrick, G. M. (1996). SADABS. University of Göttingen, Germany.
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Tritch, K. J. (2017). PhD thesis, University of Minnesota, Minneapolis, MN, USA.
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I3CN, I3NC. DOI: 10.1107/S2056989017018217/lh5864sup1.cif
Structure factors: contains datablock(s) I3CN. DOI: 10.1107/S2056989017018217/lh5864I3CNsup2.hkl
Structure factors: contains datablock(s) I3NC. DOI: 10.1107/S2056989017018217/lh5864I3NCsup3.hkl
Supporting information file. DOI: 10.1107/S2056989017018217/lh5864I3CNsup4.cml
Supporting information file. DOI: 10.1107/S2056989017018217/lh5864I3NCsup5.cml
Additional supporting information: crystallographic information; 3D view; checkCIF report