Abstract
Despite much information on their catalytic properties and gene regulation, we actually know very little of what matrix metalloproteinases (MMPs) do in tissues. The catalytic activity of these enzymes has been implicated to function in normal lung biology by participating in branching morphogenesis, homeostasis, and repair, among other events. Overexpression of MMPs, however, has also been blamed for much of the tissue destruction associated with lung inflammation and disease. Beyond their role in the turnover and degradation of extracellular matrix proteins, MMPs also process, activate, and deactivate a variety of soluble factors, and seldom is it readily apparent by presence alone if a specific proteinase in an inflammatory setting is contributing to a reparative or disease process. An important goal of MMP research will be to identify the actual substrates upon which specific enzymes act. This information, in turn, will lead to a clearer understanding of how these extracellular proteinases function in lung development, repair, and disease.
Keywords: cystic fibrosis, emphysema, epithelium, innate defense, wound repair
Introduction
MMPs comprise a large family of extracellular enzymes that share common structural features, particularly those regions involved in the regulation of proteolytic activity. MMPs, or matrixins, are a subgroup of the much larger metalloproteinase superfamily, which also includes astacin, ADAM, and ADAMTS proteinases, among others [1,2]. Twenty-three different vertebrate MMPs have been cloned to date (Table 1), and additional members continue to be identified.
Table 1.
Matrix metalloproteinases (MMPs)
| MMP designation* | Common name (other or previous names) |
| MMP-1 | Collagenase-1 (fibroblast collagenase, interstitial |
| collagenase) | |
| MMP-2 | Gelatinase-A (72 kDa gelatinase, 72 kDa type IV |
| collagenase) | |
| MMP-3 | Stromelysin-1 (transin-1) |
| MMP-7 | Matrilysin (PUMP) |
| MMP-8 | Collagenase-2 (neutrophil collagenase) |
| MMP-9 | Gelatinase-B (92 kDa gelatinase, 92 kDa type IV |
| collagenase) | |
| MMP-10 | Stromelysin-2 (transin-2) |
| MMP-11 | Stromelysin-3 |
| MMP-12 | Macrophage metalloelastase |
| MMP-13 | Collagenase-3 (rat collagenase) |
| MMP-14 | MT1-MMP (membrane-type MMP) |
| MMP-15 | MT2-MMP |
| MMP-16 | MT3-MMP |
| MMP-17 | MT4-MMP |
| MMP-18 | Collagenase-4† |
| MMP-19 | |
| MMP-20 | Enamelysin |
| MMP-21 | |
| MMP-22 | |
| MMP-23 | CA-MMP |
| MMP-24 | MT5-MMP |
| MMP-25 | Leukolysin (MT6-MMP) |
| MMP-26 | Endometase (matrilysin-2) |
*There is no MMP-4, MMP-5, or MMP-6. After matrilysin was discovered and designated as MMP-7, MMP-4 to MMP-6 were determined to be either MMP-2 or MMP-3. †Collagenase-4 was isolated from Xenopus. A mammalian homolog has not been found.
Numerous studies have assessed the mechanisms controlling MMP expression in lung tissue and lung-derived cells, and to localize the sites of enzyme expression in various diseases. We will not summarize everything that is known about MMPs in the lung, as there are simply too many papers to summarize in a succinct review. We shall instead discuss general and emerging concepts of MMP biology. We will also summarize work on two enzymes, matrilysin and macrophage metalloelastase, as examples of how MMPs can function either beneficially in lung repair or destructively in lung disease.
General features of MMPs
To be classified as a MMP, a protein must have at least two conserved motifs, namely the pro-domain and the catalytic domain. The pro-domain of a typical MMP is about 80 amino acids, and all MMPs, except MMP-21 and CA-MMP [3], contain the consensus sequence PRCXXPD. The catalytic domain contains an active site, Zn2+, that binds three conserved histidines in the sequence HEXXHXXGXXHS/TXXXXXXM, which also contains a conserved methionine to the carboxy side of the zinc-binding site. In the inactive state, the conserved cysteine residue in the pro-domain provides the fourth coordination site for the catalytic zinc ion, and disruption of this bond is necessary for enzyme activity.
In addition to the two conserved regions, MMPs have a variety of specialized domains that contribute to substrate specificity and recognition or interaction with other proteins or molecules. With the exception of matrilysin, CA-MMP, and endometase/matrilysin-2 [3,4,5,6,7], MMPs have a hinge region, which is often proline rich, and a hemopexin-like C-terminal domain. Four of the six membrane-type MMPs (MT1, MT2, MT3, and MT5) have transmembrane and cytosolic domains, whereas MT4-MMP and leukolysin (MT6-MMP) also have C-terminal hydrophobic extensions that act as a glycosylphosphatidylinositol anchoring signal [3,8,9]. The two gelatinases (MMP-2 and MMP-9) have gelatin-binding domains, and CA-MMP has a novel cysteine array motif and an immunoglobulin-like C2-type fold domain [3,10]. MMPs also share a similar gene arrangement, suggesting that they were generated by duplications of an ancestor gene. At least eight of the known human MMP genes (MMP-1, MMP-3, MMP-7, MMP-8, MMP-10, MMP-12, MMP-13, and MMP-20) are clustered on chromosome 11 at 11q21–23 [11]. Other known MMP genes are scattered along chromosomes 1, 8, 12, 14, 16, 20, and 22.
Many of the secreted MMPs, such as collagenase-1 and -3, stromelysins 1, 2, and 3, and gelatinase-B, are not expressed in normal, healthy, resting tissues or their production and activity are maintained at nearly undetectable levels. In contrast, some level of MMP expression is seen in any repair or remodeling process, in any diseased or inflamed tissue, and in essentially any cell type grown in culture. Although the qualitative pattern and quantitative levels of MMPs vary among tissues, diseases, tumors, inflammatory conditions, and cell lines, a reasonably safe generalization is that activated cells express MMPs. This is neither a controversial nor a surprising conclusion. After all, in inflammation, cancer, repair, and other remodeling processes, the matrix is turned over, cells are migrating, and secreted factors are processed, and these, among other events, are the functions attributed to MMPs. Some MMPs, including matrilysin, endometase/matrilysin-2, leukolysin, MT5-MMP, and MMP-19, are expressed in healthy tissues [7,12,13,14], implying roles in tissue homeostasis.
Functions and substrates
As their name suggests, MMPs are thought to be responsible for the turnover and degradation of connective tissue proteins, a function that is clearly performed by several family members. Indeed, cancer patients (including some with lung carcinomas) enrolled in clinical studies to assess the chemotherapeutic effects of a broad-acting synthetic metalloproteinase inhibitor developed reversible skin thickening and joint contractures [15,16]. These sclerotic side effects have been interpreted to indicate that some MMPs, directly or indirectly, are required for the normal catalysis of connective tissue. Although numerous biochemical studies have demonstrated that almost all MMPs can cleave or degrade some protein component(s) of the extracellular matrix, an ability to act on connective tissue protein is not a requirement for membership into the matrix metalloproteinase family. For example, human stromelysin-3 and CA-MMP have no known extracellular matrix substrates [3,17]. As for most protein families, structural features are thus more relevant than a presumed common activity in assigning members to this family [18].
It is becoming increasingly clear that matrix degradation is neither the sole nor common function of these enzymes [18]. MMPs are, after all, proteinases, and most proteinases can act on a wide variety of proteins. Indeed, several reports from past years have suggested or demonstrated that various MMPs can modulate the activity of a variety of non-matrix proteins. For example, matrilysin activates the pro-form of α-defensins [19], a class of secreted antimicrobial peptides (see later), and various MMPs can inactivate the serpin α1-antiproteinase inhibitor [20,21,22]. Several MMPs, such as collagenase-1, gelatinase-A, stromelysin-1, matrilysin, and stromelysin-3, among others, directly modulate the activity of several growth factors and chemokines, such as transforming growth factor (TGF)-β1, tumor necrosis factor (TNF)-α, insulin-like growth factor (IGF)-1, epidermal growth factors, fibroblast growth factors (FGFs), and monocyte chemoattractant protein (MCP)-3 [23,24,25,26,27,28,29]. In addition, fragments of matrix proteins released by MMP-mediated proteolysis can act as chemoattractants for distant cells. MMPs should thus not be viewed solely as proteinases of matrix catalysis, but as extracellular processing enzymes critically involved in cell–cell and cell–matrix signaling.
The use of genetically defined animal models has allowed investigators to uncover specific and, at times, unexpected functions of MMPs (Table 2). All of the MMPs targeted to date (Table 2), except MT1-MMP, show no or only a minor phenotype in unchallenged mice, indicating that these enzymes do not serve vital functions in development or homeostasis. The responses of MMP knockout mice to a variety of challenges, in contrast, indicate that these enzymes do serve specific roles in tissue repair, immunity, angiogenesis, host defense, inflammation, and tumor progression, among others (Table 2). It thus seems that several MMPs, at least those that have been knocked out, have evolved to respond to the many insults we experience in our extra-uterine existence.
Table 2.
Phenotype of matrix metalloproteinase (MMP) knockout mice
| Phenotype | |||
| Gene | Lethal | Unchallenged | Challenged* |
| Gelatinase-A (MMP-2) | No | • None [56] | • Reduction in angiogenesis and tumor growth [57] |
| Stromelysin-1 (MMP-3) | No | • None [58] | • Impaired wound contraction [59] |
| • Reduced contact hypersensitivity response [60] | |||
| • Accelerated arthritis [58] | |||
| Matrilysin (MMP-7) | No | • Lack of activated antimicrobial peptides [19] | ✓ Inability to repair mucosal epithelial wounds [39] |
| • Reduced ability to kill pathogenic bacteria [19] | |||
| • Reduced tumorigenesis [61] | |||
| Gelatinase-B (MMP-9) | No | • Transient slowing of long bone growth | ✓ Normal neutrophil extravasation [63] |
| secondary to reduced angiogenesis [62] | ✓ Lack of alveolar brochiolization in fibrosis [45] | ||
| • Resistant to induced blister formation [22,64] | |||
| • Persistent contact hypersensitivity response [60] | |||
| • Protection against aortic aneurysm formation [65] | |||
| • Reduced ventricular enlargement and rupture | |||
| postmyocardial infarction [66,67] | |||
| • Delayed tumor progression and reduced metastases [68] | |||
| Stromelysin-3 (MMP-11) | No | • None [69] | • Less chemically induced tumors and reduced tumor |
| cell implantation [69] | |||
| • Accelerated and enhanced neointimal formation [70] | |||
| Macrophage | No | • None [53] | ✓ Reduced elastolytic capacity of macrophages [53] |
| metalloelastase (MMP-12) | ✓ Protection against smoking-induced | ||
| emphysema [54] | |||
| • Reduced ability of macrophages to migrate through | |||
| matrix [53] | |||
| MT1-MMP (MMP-14) | Yes | • Severe skeletal abnormalities [71] | • Not yet assessed |
*✓, Assessed in lung.
As for all secreted proteinases, the catalytic activity of MMPs is regulated at four points — gene expression, compartmentalization, enzyme activation, and enzyme inactivation — and is further controlled by substrate availability and affinities. Although each MMP is often described as having a limited substrate specificity, individual enzymes can act on many different proteins, and the spectrum of substrates among many enzymes is actually quite similar. For example, gelatinase-A, gelatinase-B, stromelysin-1, stromelysin-2, matrilysin, macrophage metalloelastase, and collagenase-3 can each degrade non-fibrillar collagens, basement membrane components, fibrillins, fibronectin, and more. Distinct from other MMPs, collagenase-1 and collagenase-2 seem to have a very defined substrate spectrum, being limited to the fibrillar collagens, types I, II, and III. Aside from the collagenases, the set of substrates that overlap among MMPs is more impressive than that of selective substrates.
In a setting such as inflammation, in which essentially all MMPs are present, the shared substrate potential among enzymes would seemingly permit biochemical redundancy. Two processes, however, can hone substrate selectivity: enzyme affinity and compartmentalization. Kinetic studies have demonstrated that specific enzymes degrade some substrates more efficiently than others. For example, both gelatinase-A and gelatinase-B act on cleaved collagen better than other MMPs [30], matrilysin is a more potent proteoglycanse than stromelysin-1 or gelatinase-B [31], macrophage metalloelastase is the most elastolytic enzyme of the MMP family [32], and only the collagenases can cleave native fibrillar collagens. In a complex tissue environment, which contains many types of matrix proteins and many other potential substrates, selectivity of MMP catalysis may thus be directed, in part, by the concentration of a preferred substrate relative to that of other potential substrates within range of a secreted MMP.
Compartmentalization
Where in the tissue environment and by which cells a MMP is expressed and released are equally, if not more important, considerations in regulating the specificity of proteolysis than the affinity of the enzymesubstrate interaction. For example, in a test tube, matrilysin inactivates α1-antiproteinase inhibitor much more efficiently than does gelatinase-B. However, in tissue, at least in inflamed dermis, this serpin is selectively cleaved by neutrophil-derived gelatinase-B [22]. Matrilysin, an epithelial cell product, tends to be released lumenally away from the matrix (see later). An important concept is that cells do not indiscriminately release proteases. Rather, proteinases, such as MMPs, are secreted and anchored to the cell membrane, thereby targeting their catalytic activity to specific substrates within the pericellular space. Specific cell–MMP interactions have been reported in recent years, such as the binding of gelatinase-A to the integrin αvβ3 [33], binding of gelatinase-B to CD44 [28], and binding of matrilysin to surface proteoglycans [34]. Pro-gelatinase-A also interacts with tissue inhibitor of metalloproteinases (TIMP)-2 and MT1-MMP on the cell surface, and this trimeric complex is essential for activation of this gelatinase [35,36]. It is likely that other MMPs are also attached to cells via specific interaction to membrane proteins, and determining these anchors will lead to identifying activation mechanisms and pericellular substrates.
Cells also rely on surface receptors to 'sniff out' the presence and location of specific substrates. For matrix substrates, integrinligand contacts provide an unambiguous signal informing the cell of which protein it has encountered and, hence, which proteinase is needed and to where the enzyme should be delivered and released. A clear example of this type of spatial regulation is seen with collagenase-1 in human cutaneous wounds. Collagenase-1 is induced in basal epidermal cells (keratinocytes), in response to injury, as the cells move off the basement membrane and contact type I collagen in the underlying dermis [37]. Only basal keratinocytes in contact with dermal type I collagen express collagenase-1, and this inductive response is specifically controlled by the collagen-binding integrin α2β1, which also directs secretion of the enzyme to the points of cell–matrix contact [38]. This example demonstrates that expression and activity of a specific MMP can be confined to a specific location in an activated tissue (the superficial plane of a denuded epithelium) and to a specific stage of repair (re-epithelialization).
Matrilysin
As already stated, matrilysin, the smallest (28 kDa) of the known MMPs, is expressed by non-injured, non-inflamed exocrine and mucosal epithelium in most, if not all, adult tissues. In adult human lung, whether normal or diseased, matrilysin is expressed by epithelial cells lining peribronchial glands and conducting airways [39]. The expression of matrilysin in non-injured, normal epithelium suggests that this enzyme serves a common homeostatic function among epithelia, and several observations implicate a role for matrilysin in innate immunity among epithelia. All tissues in which matrilysin is 'constitutively' expressed are open to the environment and, hence, are vulnerable to bacterial exposure, and matrilysin is prominently upregulated in tissues with a heavy bacterial load, such as in lungs with cystic fibrosis (CF) (see later). Both immunohistochemical and cell culture studies have also demonstrated that the matrilysin protein produced by non-injured epithelium is released into the airway lumen [39,40], indicating that this MMP acts on non-matrix substrates. Indeed, matrilysin is responsible for the activation of prodefensins in the small intestine [19].
A common role of the mucosal epithelium is to function as an active barrier against the external environment, and the secretion of antibiotic peptides by epithelial cells appears to be an important component of innate immunity. The α- and β-defensins comprise a family of cationic peptides that kill bacteria by membrane disruption [41]. The pro-segment of α-defensin precursors maintains them in an inactive state, and proteolysis at some point in the secretion pathway is needed to remove the pro-domain. In the mouse small intestine, matrilysin is co-expressed and co-sorted with pro-α-defensins in Paneth cells, which are specialized epithelial cells that secrete defensins and other antimicrobial molecules, and this intimate compartmentalization of proteinase and potential substrate suggests that matrilysin activates these peptides in the secretion pathway. Indeed, in matrilysin knockout mice, pro-α-defensins are not processed to their active forms by matrilysin, and deficiency of this enzyme results in impaired bacteriocidal activity in vitro and in vivo [19]. Because of a lack of defensin activation, matrilysin null mice cannot effectively kill pathogenic Escherichia coli and are themselves killed by doses of Salmonella typhimurium that are not lethal to wild-type mice. Matrilysin thus functions in mucosal immunity by regulating the activity antimicrobial peptides, a function that this MMP may also serve in the lung.
Because of the role of matrilysin in innate defense, it became reasonable to hypothesize that the interaction of bacteria with host cells regulates enzyme expression. Bacteria are, after all, an indirect 'substrate' of matrilysin, and the presence of substrates often regulates MMP production. Indeed, matrilysin is strongly induced (>50-fold) in human and murine mucosal epithelial tissues, including intact human and mouse airway, by bacterial exposure [40]. This induction is remarkably potent and sensitive, requiring relatively short exposure and less than 10 bacteria per epithelial cell in the initial inoculum, and is not mediated by lipopolysaccharide. Large amounts of matrilysin protein, in both its zymogen and activated forms, are released from infected airway epithelial cells and from normal human trachea (Fig. 1). In addition, bacteria-mediated stimulation of matrilysin is restricted to mucosal epithelial cells. Bacterial exposure does not affect the expression of other MMPs examined and does not influence matrilysin expression in other cell types, namely macrophages, fibroblasts, and keratinocytes.
Figure 1.
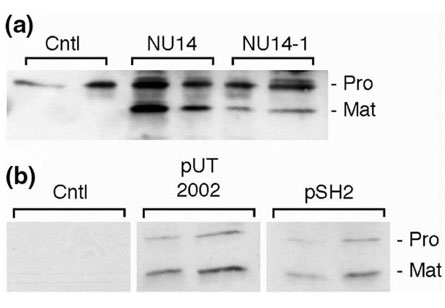
Ex vivo infection of human tracheal explants and infection of human tracheal epithelial cells. (a) Pieces (1 cm3) of freshly isolated normal adult human trachea were infected with the Escherichia coli isolates NU14 (fimH+) and NU14-1 (fimH-) for 90 min and incubated for 24 h in fresh media containing 50 μg/ml gentamicin as previously described [40]. NU14 is an E. coli strain isolated from a patient with cystitis and expresses FimH-containing type 1 pili. NU14-1 is a fimH- mutant in which a chloramphenicol cassette was recombined into the fimH gene in the chromosome of NU14 [25]. FimH is a mannose-binding adhesin that mediates the interaction of type 1-piliated bacteria with mannose-containing glycoproteins on eukaryotic cell surfaces. Released and activated matrilysin was detected by western analysis. (b) Human tracheal primary epithelial cells were infected with the type 1 piliated recombinant strains ORN103/pSH2 (fimH+) and ORN103/pUT2002 (fimH-) for 90 min, and allowed to condition fresh media for 48 h. Matrilysin secretion was assessed by immunoblotting. (Reproduced from [40]; copyright permission of The Rockefeller University Press.)
The widespread production of matrilysin in conducting airway epithelium may be initially induced and subsequently sustained by continual, low-level bacterial exposure. Consistent with this idea, matrilysin is seen in adult tissues but is not detected in developed glandular epithelium in utero [42] or in fetal or perinatal mouse tissues [43]. Furthermore, matrilysin is not produced at detectable levels in adult germ-free mice but is expressed in mice with a conventional microflora and in exgerm-free mice colonized with just one species of commensal bacteria [40]. Bacterial exposure thus seems to be the physiologic signal that regulates matrilysin expression in intact epithelium.
Although matrilysin is clearly regulated by bacterial exposure, it is possible that production of this enzyme is also induced in response to injury. Because injury provides an opportunity for infection and infection can lead to injury, some of the epithelial responses associated with either of these events may actually be a key component of both responses. Expression of matrilysin is indeed seen in airway and alveolar epithelial cells at sites of overt damage in emphysema, in fibrotic lungs with diffuse alveolar damage, in inflamed lungs of patients with idiopathic pneumonia syndrome following bone marrow transplantation, and most prominently in lungs from patients with CF [39]. Because bacterial colonization may be associated with sites of airway injury, especially in the CF samples, it was not clear if injury alone can induce matrilysin expression. A prominent signal for matrilysin protein is, however, seen in migrating epithelial cells in isolated human tracheas that are injured under aseptic conditions (Fig. 2).
Figure 2.
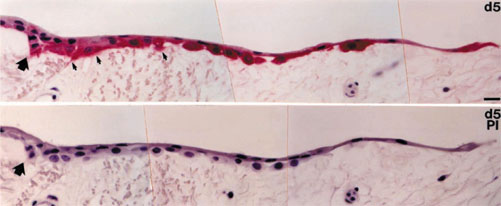
Expression of matrilysin in injured tracheal explants. Sections of a segment of normal human trachea were incubated in culture medium at 37°C for 5 days (d5) before fixation and immunohistochemistry for matrilysin protein [39]. The edge of the biopsy and the margin of basement membrane, which is the clear area underlying the epithelium, is marked by the large arrow. By 5 days postplating, the epithelial cells have migrated progressively and intense staining for matrilysin is observed in these migrating cells, especially those in close contact with the underlying matrix. Release of matrilysin towards the matrix was seen in association with some cells (small arrows). No signal was seen in sections processed with preimmune serum (PI). Bar = 20 μm. (Reproduced from [39]; copyright permission of The American Society for Clinical Investigation.)
These observations demonstrate that matrilysin is induced by injury and is prominently expressed by migrating epithelial cells. Although we do not yet know what process or factor induces matrilysin in epithelial cells at the wound edge, candidates would include a loss of specific cell–cell contacts, changes in cytoskeleton, or establishment of new cell–matrix interactions. Furthermore, in the absence of bacteria, wound-induced matrilysin is released basally towards the underlying matrix, suggesting that matrilysin may facilitate cell migration by acting on an extracellular protein. The regulated, vectorial release (ie compartmentalization) of a MMP would thus allow the proteinase to act on spatial distinct substrates, thereby serving different functions.
A functional role for matrilysin in airway re-epithelialization was demonstrated by repair of injured tracheas from gene-targeted mice [39]. As in human tissue, matrilysin is expressed in airway epithelial cells that had migrated over the cut edges of dissected mouse tracheas [39]. In tracheas from wild-type mice, re-epithelialization progresses rapidly; however, wounds in tracheas from matrilysin null mice show no evidence of epithelial migration. Matrilysin-deficient mice have in fact shown the most severe wound-repair defect among the MMP knockout mice generated to date (Table 2). However, the mechanism of how matrilysin facilities repair is not known. It may be required to loosen cell–matrix and cell–cell contacts, as has been suggested for other MMPs in other epithelial repair/migration models [38,44]. Furthermore, matrilysin may not be the only MMP involved in repair of airway epithelial wounds. Gelatinase-B is also expressed by injured epithelial cells in distal airways, in response to bleomycin instillation, and deficiency of this MMP leads to excessive bronchiolization [45], suggesting a role in either cell migration or differentiation. In addition, Legrand et al have demonstrated that the activity of gelatinase-B is required for the migration of isolated airway epithelial cells over a matrix substratum [46]. Several MMPs may thus act concurrently on different substrates to facilitate repair. These observations also demonstrate important reparative roles for MMPs but, as is discussed later, overexpression or chronic expression of proteinases may lead to tissue damage.
Macrophage metalloelastase
The progressive structural damage associated with emphysema and other forms of chronic obstructive pulmonary disease (COPD) is due to degradation of selective extracellular matrix components. To determine which proteinases are involved in the development of COPD, one must focus on proteinases that can degrade elastin, a highly proteinase-resistant polymer that normally lasts the human lifespan [47]. The elastin content of the lung parenchyma is decreased in emphysema, while the collagen content is increased [48]. Some studies have proposed that collagenases, such as collagenase-1, contribute to airway enlargement [49], but the accumulation of collagen at sites of emphysematous damage strongly indicates that lung collagenases are acting elsewhere. Elastic fibers in the emphysematous lung are ultrastructurally disorganized and fragmented. Instillation of elastases, but not collagenases, into the lungs of experimental animals causes emphysema, further implicating elastolytic enzymes in human disease.
Several MMPs are produced in human emphysematous lung [50,51]. Furthermore, several studies using transgenic and gene-targeted mice lend support to the role of MMPs in emphysema. For example, induced overexpression of IL-13 results in production of several MMPs, leading to emphysema [52]. Among these MMPs, metalloelastase was a reasonably guilty culprit in the breakdown of alveolar elastin. As already mentioned, this enzyme is a potent elastase, and macrophages from metalloelastase null mice cannot degrade elastin or many other matrix substrates [53].
Exposing proteinase null mice to cigarette smoke provides a highly controlled model to assess the role of specific MMPs in emphysema. Long-term exposure of wild-type mice to cigarette smoke leads to inflammatory cell recruitment followed by alveolar space enlargement that is quite similar to the lesions that develop in humans. Mice deficient in metalloelastase, however, are markedly protected from development of emphysema due to long-term smoke exposure [54]. Interestingly, metalloelastase null mice failed to recruit monocytes into their lungs in response to cigarette smoke. Because metalloelastase and most other MMPs are only expressed upon differentiation of monocytes to macrophages, it appeared unlikely that monocytes require metalloelastase for transvascular migration. Cigarette smoke may thus induce constitutive macrophages, which are present in lungs of metalloelastase knockout mice, to produce metalloelastase that in turn cleaves elastin, thereby generating fragments chemotactic for monocytes. This positive feedback loop would perpetuate macrophage accumulation, leading to progressive and chronic lung destruction. Consistent with this idea, experiments performed more than 20 years ago by Senior et al demonstrated that proteolytically generated elastin fragments mediate monocyte chemotaxis [55]. Gene targeting is merely reinforcing this as a major in vivo mechanism of macrophage accumulation in a chronic inflammatory condition.
There are probably several proteinases and inflammatory cells involved in the development of emphysema in humans. The major lesson to be gained from murine models of cigarette smoke-induced emphysema is not that metalloelastase is the sole proteinase responsible for human disease, but that macrophages have the capacity to cause emphysema upon recruitment and activation by cigarette smoke. The predominant macrophage proteinase in mice is metalloelastase; human macrophages probably have a broader spectrum of MMPs (including metalloelastase). Collagenase-1, -2, and -3 also undoubtedly contribute to loss of the airspace; yet, in the small airspace, collagen deposition predominates, leading to airway obstruction complicating simple categorization of collagenases as 'bad guys'.
Moreover, multiple enzymes from different families may cooperate in tissue remodeling at the same time and place. For example, metalloelastase, as well as other MMPs, degrades α1-antiproteinase inhibitor, and neutrophil elastase, a serine proteinase, degrades TIMPs. These enzymes, by neutralizing each other's natural inhibitors, can thus amplify overall proteolytic activity. This concept was shown in vivo in studies by Liu et al [22]. They demonstrated, using a murine model of bullous pemphigoid, that skin blisters form as a result of neutrophil MMP-9 degrading α1-antiproteinase inhibitor, thus allowing unopposed neutrophil elastase activity, which degrades hemidesmosomal proteins. We have not been able to determine a role for MMP-9 in emphysema; however, metalloelastase degrades α1-antiproteinase inhibitor, in part explaining why metalloelastase mice are totally protected from smoke-induced emphysema, whereas neutrophil elastase mice are only partially protected.
Conclusion
Several studies have shown that excessive levels of many MMPs are present in chronically inflamed tissues throughout the body. These observations have led to the often held concept that excessive proteolysis damages tissues and impairs healing. Unregulated, excessive proteolysis is indeed probably responsible for the destruction of matrix proteins in emphysema, as well as in arthritis, aneurysms, and other conditions of structural tissues. It seems presumptuous, however, to conclude that all proteinases expressed in diseased tissue contribute to disease pathogenesis. Based on the mechanisms uncovered in matrilysin and MMP-9 null mice, expression of these MMPs in injured airway cells may reflect beneficial roles in re-epithelialization and mucosal defense against bacteria. Because MMPs can both breakdown and activate proteins, we cannot conclude by their presence alone whether a specific proteinase in an inflammatory or injury setting is contributing to a reparative or disease process.
Acknowledgments
Acknowledgements
Supported by grants from the NIH (HL29594, HL54853, and HL56419).
References
- Woessner JF., Jr The matrix metalloproteinase family. In Matrix Metalloproteinases Edited by Parks WC, Mecham RP New York: Academic Press, Inc, 1998. pp. 1–14.
- Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- Velasco G, Pendas AM, Fueyo A, Knauper V, Murphy G, Lopez-Otin C. Cloning and characterization of human MMP-23, a new matrix metalloproteinase predominantly expressed in reproductive tissues and lacking conserved domains in other family members. J Biol Chem. 1999;274:4570–4576. doi: 10.1074/jbc.274.8.4570. [DOI] [PubMed] [Google Scholar]
- Wilson CL, Matrisian LM. Matrilysin. In Matrix Metalloproteinases Edited by Parks WC, Mecham RP New York: Academic Press, Inc, 1998. pp. 149–184.
- Park HI, Ni J, Gerkema FE, Liu D, Belozerov VE, Sang QX. Identification and characterization of human endometase (matrix metalloproteinase-26) from endometrial tumor. J Biol Chem. 2000;275:20540–20544. doi: 10.1074/jbc.M002349200. [DOI] [PubMed] [Google Scholar]
- Uria JA, Lopez-Otin C. Matrilysin-2, a new matrix metalloproteinase expressed in human tumors and showing the minimal domain organization required for secretion, latency, and activity. Cancer Res. 2000;60:4745–4751. [PubMed] [Google Scholar]
- de Coignac AB, Elson G, Delneste Y, Magistrelli G, Jeannin P, Aubry JP, Berthier O, Schmitt D, Bonnefoy JY, Gauchat JF. Cloning of MMP-26. A novel matrilysin-like proteinase. Eur J Biochem. 2000;267:3323–3329. doi: 10.1046/j.1432-1327.2000.01363.x. [DOI] [PubMed] [Google Scholar]
- Kojima S, Itoh Y, Matsumoto S, Masuho Y, Seiki M. Membrane-type 6 matrix metalloproteinase (MT6-MMP, MMP-25) is the second glycosyl-phosphatidylinositol (GPI)-anchored MMP. FEBS Lett. 2000;480:142–148. doi: 10.1016/S0014-5793(00)01919-0. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Kajita M, Kinoh H, Mori H, Okada A, Seiki M. Membrane type 4 matrix metalloproteinase (MT4-MMP, MMP-17) is a glycosylphosphatidylinositol-anchored proteinase. J Biol Chem. 1999;274:34260–34266. doi: 10.1074/jbc.274.48.34260. [DOI] [PubMed] [Google Scholar]
- Pei D. CA-MMP: a matrix metalloproteinase with a novel cysteine array, but without the classic cysteine switch. FEBS Lett. 1999;457:262–270. doi: 10.1016/S0014-5793(99)01046-7. [DOI] [PubMed] [Google Scholar]
- Shapiro SD. Matrix metalloproteinase degradation of extracellular matrix: biological consequences. Curr Opin Cell Biol. 1998;10:602–608. doi: 10.1016/S0955-0674(98)80035-5. [DOI] [PubMed] [Google Scholar]
- Cossins J, Dudgeon TJ, Catlin G, Gearing AJ, Clements JM. Identification of MMP-18, a putative novel human matrix metalloproteinase. Biochem Biophys Res Commun. 1996;228:494–498. doi: 10.1006/bbrc.1996.1688. [DOI] [PubMed] [Google Scholar]
- Pei D. Identification and characterization of the fifth membrane-type matrix metalloproteinase MT5-MMP. J Biol Chem. 1999;274:8925–8932. doi: 10.1074/jbc.274.13.8925. [DOI] [PubMed] [Google Scholar]
- Pei D. Leukolysin/MMP25/MT6-MMP: a novel matrix metalloproteinase specifically expressed in the leukocyte lineage. Cell Res. 1999;9:291–303. doi: 10.1038/sj.cr.7290028. [DOI] [PubMed] [Google Scholar]
- Tierney GM, Griffin NR, Stuart RC, Kasem H, Lynch KP, Lury JT, Brown PD, Millar AW, Steele RJ, Parsons SL. A pilot study of the safety and effects of the matrix metalloproteinase inhibitor marimastat in gastric cancer. Eur J Cancer. 1999;35:563–568. doi: 10.1016/S0959-8049(99)00007-6. [DOI] [PubMed] [Google Scholar]
- Steward W. Marimastat (BB2516): current status of development. Cancer Chemother Pharmacol. 1999;43 (suppl):S56–S60. doi: 10.1007/s002800051099. [DOI] [PubMed] [Google Scholar]
- Mucha A, Cuniasse P, Kannan R, Beau F, Yiotakis A, Basset P, Dive V. Membrane type-1 matrix metalloprotease and stromelysin-3 cleave more efficiently synthetic substrates containing unusual amino acids in their P1' positions. J Biol Chem. 1998;273:2763–2768. doi: 10.1074/jbc.273.5.2763. [DOI] [PubMed] [Google Scholar]
- Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 2000;14:2123–2133. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- Wilson CL, Ouellette AJ, Satchell DP, Ayabe T, López-Boado YS, Stratman JL, Hultgren SJ, Matrisian LM, Parks WC. Regulation of intestinal α-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999;286:113–117. doi: 10.1016/S0009-2614(98)00088-8. [DOI] [PubMed] [Google Scholar]
- Sires UI, Murphy G, Baragi VM, Fliszar CJ, Welgus HG, Senior RM. Matrilysin is much more efficient than other metalloproteinases in the proteolytic inactivation of α-1 antitrypsin. Biochem Biophys Res Commun. 1994;204:613–620. doi: 10.1006/bbrc.1994.2503. [DOI] [PubMed] [Google Scholar]
- Pei D, Majmudar G, Weiss SJ. Hydrolytic inactivation of a breast carcinoma cell-derived serpin by human stromelysin-3. J Biol Chem. 1994;269:25849–25855. [PubMed] [Google Scholar]
- Liu Z, Zhou X, Shapiro SD, Shipley JM, Diaz LA, Senior RM, Werb Z. The serpin α1-proteinase inhibitor is a critical substrate for gelatinase B/MMP-9 in vivo. Cell. 2000;102:647–655. doi: 10.1016/s0092-8674(00)00087-8. [DOI] [PubMed] [Google Scholar]
- Haro H, Crawford HC, Fingleton B, Shinomiya K, Spengler DM, Matrisian LM. Matrix metalloproteinase-7-dependent release of tumor necrosis factor-alpha in a model of herniated disc resorption. J Clin Invest. 2000;105:143–150. doi: 10.1172/JCI7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi E, Fridman R, Miao HQ, Ma YS, Yayon A, Vlodavsky I. Matrix metalloproteinase 2 releases active soluble ectodomain of fibroblast growth factor receptor 1. Proc Natl Acad Sci USA. 1996;93:7069–7074. doi: 10.1073/pnas.93.14.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Raab G, Moses MA, Fernandez CA, Klagsbrun M. Matrix metalloproteinase-3 releases active heparin-binding EGF-like growth factor by cleavage at a specific juxtamembrane site. J Biol Chem. 1997;272:31730–31737. doi: 10.1074/jbc.272.50.31730. [DOI] [PubMed] [Google Scholar]
- Gearing AJH, Beckett P, Christodoulou M, Churchill M, Clements J, Davidson AH, Drummond AH, Galloway WA, Gilbert R, Gordon JL, Leber TM, Mangan M, Miller K, Nayee P, Owen K, Patel S, Thomas W, Wells G, Wood LM, Woolley K. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature. 1994;370:555–557. doi: 10.1038/370555a0. [DOI] [PubMed] [Google Scholar]
- McGeehan MG, Becherer JD, Bast RCJ, Boyer CM, Champion B, Connolly KM, Conway JG, Furdon P, Karp S, Kidao S. Regulation of tumour necrosis factor-alpha processing by a metalloproteinase inhibitor. Nature. 1994;370:558–560. doi: 10.1038/370558a0. [DOI] [PubMed] [Google Scholar]
- Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- McQuibban GA, Gong J-H, Tam EM, McCulloch CAG, Clark-Lewis I, Overall CM. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science. 2000;289:1202–1206. doi: 10.1126/science.289.5482.1202. [DOI] [PubMed] [Google Scholar]
- Mackay AR, Hartzler JL, Pelina MD, Thorgeirsson UP. Studies on the ability of 65-kDa and 92-kDa tumor cell gelatinases to degrade type IV collagen. J Biol Chem. 1990;265:21929–21934. [PubMed] [Google Scholar]
- Halpert I, Roby JD, Sires UI, Potter-Perigo S, Wight TN, Welgus HG, Shapiro SD, Wickline SA, Parks WC. Matrilysin is expressed by lipid-laden macrophages at sites of potential rupture in atherosclerotic lesions and localizes to areas of versican deposition, a proteoglycan substrate for the enzyme. Proc Natl Acad Sci USA. 1996;93:9748–9753. doi: 10.1073/pnas.93.18.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro SD. Elastolytic metalloproteinases produced by human mononuclear phagocytes. Potential roles in destructive lung disease. Am J Respir Crit Care Med. 1994;150:S160–S164. doi: 10.1164/ajrccm/150.6_Pt_2.S160. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell. 1996;85:683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- Yu WH, Woessner JF., Jr Heparan sulfate proteoglycans as extracellular docking molecules for matrilysin (matrix metalloproteinase 7). J Biol Chem. 2000;275:4183–4191. doi: 10.1074/jbc.275.6.4183. [DOI] [PubMed] [Google Scholar]
- Wang Z, Juttermann R, Soloway PD. TIMP-2 is required for efficient activation of proMMP-2 in vivo. J Biol Chem. 2000;275:26411–26415. doi: 10.1074/jbc.M001270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina JJ, Yamada S, Caterina NC, Longenecker G, Holmback K, Shi J, Yermovsky AE, Engler JA, Birkedal-Hansen H. Inactivating mutation of the mouse tissue inhibitor of metalloproteinases-2 (TIMP-2) gene alters proMMP-2 activation. J Biol Chem. 2000;275:26416–26422. doi: 10.1074/jbc.M001271200. [DOI] [PubMed] [Google Scholar]
- Saarialho-Kere UK, Kovacs SO, Pentland AP, Olerud J, Welgus HG, Parks WC. Cell–matrix interactions modulate interstitial collagenase expression by human keratinocytes actively involved in wound healing. J Clin Invest. 1993;92:2858–2866. doi: 10.1172/JCI116906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilcher BK, Dumin JA, Sudbeck BD, Krane SM, Welgus HG, Parks WC. The activity of collagenase-1 is required for keratinocyte migration on a type I collagen matrix. J Cell Biol. 1997;137:1445–1457. doi: 10.1083/jcb.137.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmore SE, Saarialho-Kere UK, Roby JD, Wilson CL, Matrisian LM, Welgus HG, Parks WC. Matrilysin expression and function in airway epithelium. J Clin Invest. 1998;102:1321–1331. doi: 10.1172/JCI1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Boado YS, Wilson CL, Hooper LV, Gordon JI, Hultgren SJ, Parks WC. Bacterial exposure induces and activates matrilysin in mucosal epithelial cells. J Cell Biol. 2000;148:1305–1315. doi: 10.1083/jcb.148.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T. Immunology: defensins and host defense. Science. 1999;286:420–421. doi: 10.1126/science.286.5439.420. [DOI] [PubMed] [Google Scholar]
- Karelina TV, Goldberg GI, Eisen AZ. Matrilysin (PUMP) correlates with dermal invasion during appendageal development and cutaneous neoplasia. J Invest Dermatol. 1994;103:482–487. doi: 10.1111/1523-1747.ep12395596. [DOI] [PubMed] [Google Scholar]
- Wilson CL, Heppner KJ, Rudolph LA, Matrisian LM. The metalloproteinase matrilysin is preferentially expressed by epithelial cells in a tissue-restricted pattern in the mouse. Mol Biol Cell. 1995;6:851–869. doi: 10.1091/mbc.6.7.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol. 1997;139:1861–1872. doi: 10.1083/jcb.139.7.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betsuyaku T, Fukuda Y, Parks WC, Shipley JM, Senior RM. Gelatinase B is required for alveolar bronchiolization after intratracheal bleomycin. Am J Pathol. 2000;157:525–535. doi: 10.1016/S0002-9440(10)64563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand C, Gilles C, Zahm J-M, Polette M, Buisson A-C, Kaplan H, Birembaut P, Tournier J-M. Airway epithelial cell migration dynamics: MMP-9 role in cell–extracellular matrix remodeling. J Cell Biol. 1999;146:517–529. doi: 10.1083/jcb.146.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro SD, Endicott SK, Province MA, Pierce JA, Campbell EJ. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weaponsrelated radiocarbon. J Clin Invest. 1991;87:1828–1834. doi: 10.1172/JCI115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer RR, Crapo JD. Spatial distribution of collagen and elastin fibers in the lungs. J Appl Physiol. 1990;69:756–765. doi: 10.1152/jappl.1990.69.2.756. [DOI] [PubMed] [Google Scholar]
- D'Armiento J, Dalal SS, Okada Y, Berg RA, Chada K. Collagenase expression in the lungs of transgenic mice causes pulmonary emphysema. Cell. 1992;71:955–961. doi: 10.1016/0092-8674(92)90391-o. [DOI] [PubMed] [Google Scholar]
- Finlay GA, O'Driscoll LR, Russell KJ, D'Arcy EM, Masterson JB, FitzGerald MX, O'Connor CM. Matrix metalloproteinase expression and production by alveolar macrophages in emphysema. Am J Respir Crit Care Med. 1997;156:240–247. doi: 10.1164/ajrccm.156.1.9612018. [DOI] [PubMed] [Google Scholar]
- Ohnishi K, Takagi M, Kurokawa Y, Satomi S, Konttinen YT. Matrix metalloproteinase-mediated extracellular matrix protein degradation in human pulmonary emphysema. Lab Invest. 1998;78:1077–1087. [PubMed] [Google Scholar]
- Zheng T, Zhu Z, Wang Z, Homer RJ, Ma B, Riese RJ, Chapman HA, Shapiro SD, Elias JA. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. J Clin Invest. 2000;106:1081–1093. doi: 10.1172/JCI10458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley JM, Wesselschmidt RL, Kobayashi DK, Ley TJ, Shapiro SD. Metalloelastase is required for macrophage-mediated proteolysis and matrix invasion in mice. Proc Natl Acad Sci USA. 1996;93:3942–3946. doi: 10.1073/pnas.93.9.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema. Science. 1997;277:2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- Senior RM, Griffin GL, Mecham RP. Chemotactic activity of elastin-derived peptides. J Clin Invest. 1980;66:859–862. doi: 10.1172/JCI109926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Ikeda T, Gomi H, Nakao S, Suzuki T, Itohara S. Unaltered secretion of beta-amyloid precursor protein in gelatinase A (matrix metalloproteinase 2)-deficient mice. J Biol Chem. 1997;272:22389–22392. doi: 10.1074/jbc.272.36.22389. [DOI] [PubMed] [Google Scholar]
- Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048–1051. [PubMed] [Google Scholar]
- Mudgett JS, Hutchinson NI, Chartrain NA, Forsyth AJ, McDonnell J, Singer II, Bayne EK, Flanagan J, Kawka D, Shen CF. Susceptibility of stromelysin 1-deficient mice to collagen-induced arthritis and cartilage destruction. Arthritis Rheum. 1998;41:110–121. doi: 10.1002/1529-0131(199801)41:1<110::AID-ART14>3.3.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Bullard KM, Lund L, Mudgett JS, Mellin TN, Hunt TK, Murphy B, Ronan J, Werb Z, Banda MJ. Impaired wound contraction in stromelysin-1-deficient mice. Ann Surg. 1999;230:260–265. doi: 10.1097/00000658-199908000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Qin X, Mudgett JS, Ferguson TA, Senior RM, Welgus HG. Matrix metalloproteinase deficiencies affect contact hypersensitivity: stromelysin-1 deficiency prevents the response and gelatinase B deficiency prolongs the response. Proc Natl Acad Sci USA. 1999;96:6885–6889. doi: 10.1073/pnas.96.12.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CL, Heppner KJ, Labosky PA, Hogan BLM, Matrisian LM. Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc Natl Acad Sci USA. 1997;94:1402–1407. doi: 10.1073/pnas.94.4.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betsuyaku T, Shipley JM, Liu Z, Senior RM. Neutrophil emigration in the lungs, peritoneum, and skin does not require gelatinase B. Am J Respir Cell Mol Biol. 1999;20:1303–1309. doi: 10.1165/ajrcmb.20.6.3558. [DOI] [PubMed] [Google Scholar]
- Liu Z, Shipley JM, Vu TH, Zhou X, Diaz LA, Werb Z, Senior RM. Gelatinase B-deficient mice are resistant to experimental bullous pemphigoid. J Exp Med. 1998;188:475–482. doi: 10.1084/jem.188.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyo R, Lee JK, Shipley JM, Curci JA, Mao D, Ziporin SJ, Ennis TL, Shapiro SD, Senior RM, Thompson RW. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000;105:1641–1649. doi: 10.1172/JCI8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymans S, Luttun A, Nuyens D, Theilmeier G, Creemers E, Moons L, Dyspersin GD, Cleutjens JP, Shipley M, Angellilo A, Levi M, Nube O, Baker A, Keshet E, Lupu F, Herbert JM, Smits JF, Shapiro SD, Baes M, Borgers M, Collen D, Daemen MJ, Carmeliet P. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat Med. 1999;5:1135–1142. doi: 10.1038/13459. [DOI] [PubMed] [Google Scholar]
- Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, Schoen FJ, Kelly RA, Werb Z, Libby P, Lee RT. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest. 2000;106:55–62. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson R, Lefebvre O, Noel A, Fahime ME, Chenard MP, Wendling C, Kebers F, LeMeur M, Dierich A, Foidart JM, Basset P, Rio MC. In vivo evidence that the stromelysin-3 metalloproteinase contributes in a paracrine manner to epithelial cell malignancy. J Cell Biol. 1998;140:1535–1541. doi: 10.1083/jcb.140.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijnen HR, Van Hoef B, Vanlinthout I, Verstreken M, Rio MC, Collen D. Accelerated neointima formation after vascular injury in mice with stromelysin-3 (MMP-11) gene inactivation. Arterioscler Thromb Vasc Biol. 1999;19:2863–2870. doi: 10.1161/01.atv.19.12.2863. [DOI] [PubMed] [Google Scholar]
- Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, Ward JM, Birkedal-Hansen H. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]


