Abstract
Chlamydia trachomatis/Neisseria gonorrhoeae assay performance in males is typically determined using post-swab urine, though pre-swab urine is used in practice. We collected swabs and urine from men and used the Cepheid Xpert® CT/NG sample adequacy control to determine the effect of swab collection on urine cellular content. No difference was observed.
Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) are important global public health concerns,1 and over 1.4 million cases of CT and > 350,000 cases of NG infection are reported annually within the United States.2 Screening for at-risk individuals is recommended using highly sensitive nucleic acid amplification tests (NAAT).3 First catch urine (FCU) specimens are a recommended sample for testing in men, as studies of NAAT performance for CT/NG detection have found FCU specimens to be statistically equivalent to, and sometimes superior to, swab specimens in head-to-head comparison using the same assay.3–5 Those studies have typically tested post-swab urine, that is, urine obtained after collection of a urethral swab specimen.3 However, in clinical practice, urethral swabs are rarely performed. To date, the influence of the urethral swab on the cellular content and test performance of post-swab urine specimens remains unknown. The swabbing process may be reasoned to either (i) decrease the cellular content of post-swab urine specimens by removing the majority of urethral cellular content yielding or, conversely, (ii) increase the post-swab urine cellular content by perturbing the urethral epithelium. Given the intracellular nature of both CT and NG, the cellular content may be an important sample quality determinant. If urine cellularity changes as a direct result of swab-related mechanical disruption, the quality of urine collected before, or in the absence of, swabbing may differ from the quality of samples routinely used to evaluate NAAT performance. We designed this study to assess the influence of swabbing on urine cellular content to improve our understanding of the quality of urine samples collected in clinical settings or for studies of assay performance.
The Cepheid Xpert® CT/NG (Sunnydale, CA) is a NAAT approved for CT/NG testing using male urine.6 As a specimen control, the assay also simultaneously performs real-time PCR amplification of the single copy human cellular housekeeping gene hydroxymethylbilane synthase, generating a sample adequacy control cycle threshold (SACCT). This provides assurance that specimens contain adequate cellular material for testing, minimizing false-negative results.7 The SACCT denotes the cycle number at which the hydroxymethylbilane synthase gene was first detected in cell lysates and is inversely proportional to the amount of cellular material present in the analyte (ie, a higher cellular content results in a lower SACCT). In this study, we use the SACCT to determine the influence of the urethral swab on urine cellular content.
Men older than 18 years were recruited from the Jefferson County Department of Health Sexually Transmitted Disease clinic in Birmingham, AL. After informed consent was obtained, men submitted 2 FCU samples in 60 cc urine containers, at least 60 minutes apart. Immediately preceding the second FCU collection, a urethral swab was obtained for Gram stain smear testing using a swabbing technique agreed upon by all study clinicians. We excluded men who were unable to provide 2 urine samples at least 60 minutes apart, refused a urethral swab, or had received antibiotics with CT/NG activity within the past 30 days. This study was approved by the institutional review board of the University of Alabama at Birmingham and the research review committee at Jefferson County Department of Health. The FCU specimens were stored at 4°C and Cepheid Xpert® CT/NG testing performed within 7 days of collection as described in the package insert. Differences in the median SACCT values were assessed using a non-parametric Wilcoxon matched pairs signed rank test or a nonparametric unpaired Mann-Whitney U test and significance reported as P < 0.05 using Prism software (v7.0; Graphpad, San Diego, Calif).
Fifty men were included in this study. Participants were 19 to 60 (median, 25) years of age, 42 (84%) were black and attended the clinic for symptom evaluation (N = 27; 54%), as recent sexual contacts to partners with sexually transmitted infections (N = 9; 18%), or for STI screening (N = 14; 28%). Five men (10%) reported sex with other men. Eleven (22%) of 50 men were infected with CT and/or NG: 5 with CT, 3 with both CT and NG, and 3 with NG.
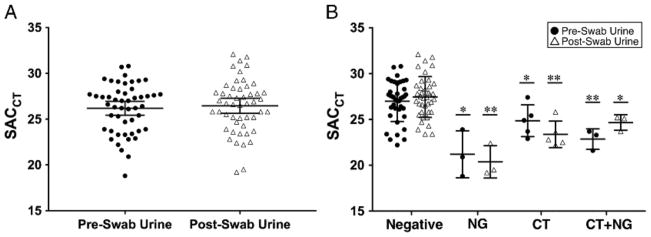
To determine the impact of the urethral swab on urine cellular content, we compared the SACCT in pre-swab versus post-swab FCU specimens. For the entire group, the median SACCT was similar for pre-swab and post-swab urine specimens (26.2 vs 26.5, P = 0.34, Fig. 1A). We then questioned whether the cellular content in urine specimens, and therefore SACCT, may be influenced by CT and/or NG infection. No significant difference in the SACCT was detected among pre-swab and post-swab urine specimens when CT and/or NG infection was present, although our study was not powered to address this question (Fig. 1B). Although outside the primary focus of this study, we observed that CT and/or NG infection resulted in a significant increase in cellular content compared to men without infection (P < 0.05, Fig. 1B).
Figure 1.
A, Comparison of the SACCT in pre-swab versus post-swab urine specimens. No differences in the medians between urine specimens was detected by Wilcoxon matched-pairs rank sum test. B, SACCT in pre-swab and post-swab urine specimens by CT/NG test result. No differences in the medians comparing pre-swab and post-swab urine specimens were detected. Men with CT and/or NG had significantly lower SACCT compared with men without infection. The single and double asterisks denote P < 0.05 and P < 0.01 by Mann–Whitney U test, respectively, compared to negative specimens of the same urine type. The horizontal bar denotes the median and the whiskers denote the 95% confidence interval.
In this study, we used the Cepheid Xpert® CT/NG SACCT to evaluate whether collecting a urethral swab influences the cellular content in post-swab urine compared with pre-swab urine specimens. Despite rationale for a difference (in an unknown direction) in urine cellularity based on the effect of swab collection, we observed no such difference in this study. To our knowledge, this is the first study directly comparing pre-swab and post-swab urine specimens specifically measuring cellular debris. The data suggest that the urethral swab has little influence on the quality of urine specimens. Thus, we can have high confidence that the performance estimates in package inserts based on post-swab urine specimens are likely appropriate approximations of performance for routine urine collection.
Our study was not powered to determine the impact of swab collection on performance of CT/NG detection, and no differences were observed. However, we did identify a significant increase in urine cellular content in specimens with CT and/or NG infection as we would have expected, given that these pathogens are responsible for urethral inflammation. This supports a prior observation that detection of squamous cells in FCU specimens correlates with the presence of leukocytes, suggesting that inflammation may increase cellular shedding of squamous epithelium.8
Our study has several limitations. First, the SACCT cannot differentiate the proportion of epithelial cells or leukocytes in urine specimens. Additionally, we cannot rule out the presence of nucleated cells from sources proximal to the urethra (eg, proximal urethra or bladder) influencing the urine cellular content, although this is unlikely for several reasons; (i) the cellular contribution of mid-stream urine is minimal compared to first-stream urine,9 and (ii) the significant changes in cellular content detected in the presence of CT and/or NG infection suggests the urethra is the predominant source of detectable cellular debris.
In summary, this brief report provides reassurance that the clinical practice of obtaining urine specimens for CT/NG testing, in the absence of any urethral swab collection, provides an adequate specimen type as the cellular content is comparable to post-swab urine specimens.
Acknowledgments
The authors are grateful to Paula Dixon for her assistance with specimen processing.
Source of Funding: B.V.D.P. has received honoraria, consulting fees, or research support from the following sponsors: Abbott Molecular Diagnostics, Atlas Genetics, BD Diagnostics, Beckman Coulter, Great Basin, Scientific, Cepheid, Hologic, Rheonix, and Roche Diagnostics. E.W.H. has received honoraria, research support, or consulting fees from Cepheid, BD Diagnostics, Gen-Probe Hologic, Roche Diagnostics, and Cempra Pharmaceuticals. Reagents and test kits for this study were supplied by Cepheid (Sunnyvale, CA).
Footnotes
Conflicts of Interest: S.J.J. has no conflicts of interest.
References
- 1.World Health Organization. Global incidence and prevalence of selected curable sexually transmitted infections-2008. World Health Organization; 2012. [Accessed March 17, 2014]. Available from: http://www.who.int/reproductivehealth/publications/rtis/stisestimates/en./ [Google Scholar]
- 2.Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2014. Atlanta, U.S: Department of Health and Human Services; 2015. [Google Scholar]
- 3.Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm Rep. 2014;63(RR-02):1–19. [PMC free article] [PubMed] [Google Scholar]
- 4.Martin DH, Cammarata C, Van Der Pol B, et al. Multicenter evaluation of AMPLICOR and automated COBAS AMPLICOR CT/NG tests for Neisseria gonorrhoeae. J Clin Microbiol. 2000;38:3544–3549. doi: 10.1128/jcm.38.10.3544-3549.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaydos CA, Ferrero DV, Papp J. Laboratory aspects of screening men for Chlamydia trachomatis in the new millennium. Sex Transm Dis. 2008;35(11 Suppl):S45–S50. doi: 10.1097/OLQ.0b013e31816d1f6d. [DOI] [PubMed] [Google Scholar]
- 6.Gaydos CA, Van Der Pol B, Jett-Goheen M, et al. Performance of the Cepheid CT/NG Xpert rapid PCR test for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2013;51:1666–1672. doi: 10.1128/JCM.03461-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bristow C, Adachi K, Nielsen-Saines K, et al. Characteristics of the Sample Adequacy Control (SAC) in the Cepheid Xpert® CT/NG Assay in Female Urine Specimens. JMEN. 2014;1:1–5. [Google Scholar]
- 8.Wiggins R, Horner PJ, Whittington K, et al. Quantitative analysis of epithelial cells in urine from men with and without urethritis: implications for studying epithelial: pathogen interactions in vivo. BMC Res Notes. 2009;2:139. doi: 10.1186/1756-0500-2-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manoni F, Gessoni G, Alessio MG, et al. Mid-stream vs. first-voided urine collection by using automated analyzers for particle examination in healthy subjects: an Italian multicenter study. Clin Chem Lab Med. 2012;50:679–684. doi: 10.1515/cclm.2011.823. [DOI] [PubMed] [Google Scholar]