Abstract
Objective
Non-severe relapses are more common than severe relapses in ANCA-associated vasculitis (AAV), but their clinical course and treatment outcomes remain largely unexamined. We analyzed the outcomes of non-severe relapses in the Rituximab in AAV (RAVE) trial that were treated according to a pre-specified prednisone protocol.
Methods
RAVE was a randomized, double-blind, placebo-controlled trial comparing rituximab (RTX) to cyclophosphamide (CYC) followed by azathioprine (AZA) for remission induction. Patients who experienced non-severe relapses between months 1 and 18 were treated with a prednisone increase without a concomitant change in their non-glucocorticoid immunosuppressants, followed by a taper.
Results
Forty-four patients with a first non-severe relapse were analyzed. In comparison to the 71 patients who maintained relapse-free remission over 18 months, these patients were more likely to have PR3-ANCA, diagnoses of GPA, and relapsing disease at baseline. A prednisone increase led to remission in 35 patients (80%). However, only 13 patients (30%) were able to maintain second remissions through the follow-up period (mean 12.5 months); 31 (70%) relapsed again – 14 with severe disease. The mean time to second relapse was 9.4 months (4.7 RTX, 13.7 CYC/AZA [p<0.01]). Patients who experienced non-severe relapses received more glucocorticoids than those who maintained remission (6.7 grams versus 3.8 grams [p<0.01]).
Conclusion
Treatment of non-severe relapses in AAV with an increase in glucocorticoids is effective in restoring temporary remission in the majority of patients, but recurrent relapses within a relatively short interval are the rule. Alternative treatment approaches for this important subset of patients are needed.
Introduction
Granulomatosis with polyangiitis (GPA, formerly Wegener’s) and microscopic polyangiitis (MPA) are the major forms of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV). Most patients with AAV achieve at least temporary disease remission with induction regimens based on cyclosphosphamide (CYC), rituximab (RTX), or methotrexate (MTX)[1–4]. However, subsequent disease relapses occur in more than half the patients over long-term follow-up [5–7]. The majority of such relapses are non-severe and do not pose immediate threats either to major organ function or the patient’s life [2,8,9]. Despite reports from some clinical trials that non-severe disease relapses are three times more common than severe relapses [2], the clinical course, treatment outcomes, and ultimate implications of such disease relapses remain largely unexamined. Previous prospective trials have either not reported the outcomes of non-severe relapses [1,2,4,5], not differentiated between severe and non-severe relapses [10,11], or not considered patients with one or two recurrent non-severe manifestations of active disease to have experienced a relapse [8,9]. Moreover, the terminology and definitions for non-severe relapses have varied over the past decades, further complicating the interpretation of clinical studies [12,13].
We examined the outcomes of patients with non-severe relapses in the Rituximab in ANCA-associated Vasculitis trial (RAVE) who were treated according to a uniform protocol: a glucocorticoid increase selected at the discretion of the investigator, followed by a defined taper, without a change in non-glucocorticoid immunosuppressants.
Methods
RAVE trial
Details of the RAVE trial design have been published [3,14]. The trial enrolled ANCA-positive patients with GPA or MPA who had severe disease (Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis [BVAS/WG] > 3 or one major item)[15]. Patients were assigned to one of two treatment groups: either 1) CYC (2mg/kg, adjusted for renal insufficiency) for 3–6 months followed by AZA (2 mg/kg) for a total of 18 months; or 2) RTX (four weekly infusions of 375mg/m2) followed by placebo-AZA. Both groups received the same glucocorticoid protocol, tapered to discontinuation by 6 months.
Remission was defined as a BVAS/WG of 0 and complete remission as BVAS/WG of 0 with discontinuation of glucocorticoids. The results of the trial’s primary outcomes, the percentage of patients who achieved and maintained complete remission at 6 and 18 months without additional changes in therapy, have been published [3,16].
Analysis of non-severe relapses
Patients who had an increase in BVAS/WG of three or less and the absence of major BVAS/WG items between months 1 and 18 were included in the analysis. Three patients who had BVAS/WG scores of 4 at relapse were also included because their relapses were considered non-severe by their treating physicians. The disease exacerbations analyzed included “relapses” (n=40), defined as an increase in BVAS/WG following the achievement of remission, and “flares” (n=4), defined as an increase in BVAS/WG before achieving remission. For the purposes of this manuscript, we refer to both “relapses” and “flares” as relapses. Severe relapses were defined as recurrent AAV activity that would have been treated with CYC plus high-dose glucocorticoids under the standard of care that existed at the time the trial began. Patients who had a change in their initially assigned treatment prior to their first non-severe relapse (e.g., crossover to the opposite treatment arm due to a severe relapse) were excluded from the analysis in order to limit the effects of previous therapy on the outcomes of treatments that followed first non-severe relapses.
Treatments and follow-up
Patients with non-severe relapses between months 1 and 18 were treated by increasing prednisone to a dose selected at the discretion of the investigator. The new dose was maintained for 1 month before resumption of a specified taper every 2 weeks as follows: 60 mg, 40 mg, 30 mg, 20 mg, 15 mg, 10 mg, 7.5 mg, 5 mg and 2.5 mg until off.
In order to study clinical events after the first non-severe relapse, patients’ outcome data were analyzed until one of the following occurred: 1) severe relapse, 2) second non-severe relapse, 3) change in non-glucocorticoid immunosuppression, 4) withdrawal from the trial, 5) common closeout date.
Statistical Analysis
Binary outcomes were compared using the Chi-squared or the Fisher’s exact test. Continuous outcomes between treatment arms were compared using the Wilcoxon Rank-Sum test. Wald confidence limits of 95% were calculated for differences in outcomes between treatment arms at the different timepoints. The data sets for these analyses are accessible to readers through TrialShare, a publicly-accessible website developed by the ITN.
Results
Numbers of non-severe relapses
Sixty-seven non-severe relapses occurred in 51 patients between months 1 and 18, compared with a total of 45 severe disease relapses in 41 patients. After excluding 7 patients who did not remain on their originally assigned treatment, 44 patients were analyzed (RTX 23, CYC/AZA 21, p=0.76).
Patient characteristics at first non-severe relapse
Thirty-six patients (82%) were PR3-ANCA-positive and 40 (91%) had GPA (Table 1). Twenty-eight patients (64%) had relapsing disease at study entry. Compared to the subset of patients who achieved and maintained disease remissions through 18 months of follow-up (n = 71), PR3-ANCA positivity at baseline, GPA diagnosis, and history of relapsing disease were each significantly more frequent in the subset of patients who experienced non-severe relapses (Table 2). Among the 44 patients with non-severe relapses, 24 (55%) had all three of these characteristics as compared to 30% of the patients who maintained disease remission (p=0.01).
Table 1.
Characteristics of patients with non-severe relapse
| RTX | CYC/AZA | ALL | |
|---|---|---|---|
| N=23 | N=21 | N=44 | |
| Male | 12 (52%) | 9 (43%) | 21 (48%) |
| PR3-ANCA positive | 18 (78%) | 18 (86%) | 36 (82%) |
| GPA | 20 (87%) | 20 (95%) | 40 (91%) |
| Relapsing disease at study entry | 15 (65%) | 13 (62%) | 28 (64%) |
| Mean time to first non-severe relapse (range, months)* | 7.7 (1.8–15.1) | 7.3 (2.4–16.9) | 7.5 (1.8–16.9) |
| Reached remission before relapse | 20 (87%) | 20 (95%) | 40 (91%) |
| BVAS/WG at relapse | 1.8 | 1.6 | 1.7 |
| Off prednisone at relapse | 14 (61%) | 14 (67%) | 28 (64%) |
| Median prednisone dose at relapse if dose > 0 mg/day (range) | 5.0 (2.5–20) | 5.0 (2.5–15) | 5.0 (2.5–20) |
| Organ involvement | |||
| Constitutional features† | 18 (78%) | 11 (52%) | 29 (66%) |
| Cutaneous involvement | 2 (9%) | 1 (5%) | 3 (7%) |
| Mucous membranes and eyes | 2 (9%) | 5 (24%) | 7 (16%) |
| Mouth ulcers | 1 (4%) | 0 (0%) | 1 (2%) |
| Conjunctivitis/episcleritis | 0 (0%) | 5 (24%) | 5 (11%) |
| Other | 1 (4%) | 0 (0%) | 1 (2%) |
| Ear, nose and throat | 7 (30%) | 4 (19%) | 11 (25%) |
| Bloody nasal discharge | 3 (13%) | 2 (10%) | 5 (11%) |
| Sinus involvement | 2 (9%) | 2 (10%) | 4 (9%) |
| Other | 2 (9%) | 1 (5%) | 3 (7%) |
| Pulmonary involvement | 5 (22%) | 3 (14%) | 8 (18%) |
| Nodules/cavities | 2 (9%) | 1 (5%) | 3 (7%) |
| Endobronchial involvement | 0 (0%) | 2 (10%) | 2 (5%) |
| Other | 3 (13%) | 1 (5%) | 4 (9%) |
| Renal involvement - hematuria | 1 (4%) | 3 (14%) | 4 (9%) |
| Other minor items | 2 (9%) | 0 (0%) | 2 (5%) |
Of the four patients who flared before reaching remission, those flares occurred at 1.8, 2.4 and 3.9 months (in two patients) after study entry.
fevers, arthritis/arthralgias
Table 2.
Baseline characteristics in patients with non-severe relapse compared to patients who reached and maintained remission through 18 months
| Non-severe relapse (n=44) |
Reached and maintained remission (n=71) |
p-value | |
|---|---|---|---|
| GPA | 40 (91%) | 50 (70%) | |
| MPA | 4 (9%) | 21 (30%) | <0.01 |
| PR3 | 36 (82%) | 43 (61%) | |
| MPO | 8 (18%) | 28 (39%) | <0.02 |
| Relapsing disease | 28 (64%) | 29 (41%) | |
| New diagnosis | 16 (36%) | 42 (59%) | <0.02 |
| GPA and PR3 and relapsing disease | 24 (55%) | 21 (30%) | |
| Less than all three factors | 20 (45%) | 50 (70%) | <0.01 |
Forty of 44 patients (91%) achieved remission (BVAS/WG = 0) before experiencing their first non-severe relapse, which occurred on average 7.5 months (range 1.8–16.9) after study entry (RTX 7.7 months versus CYC/AZA 7.3 months, p>0.99). Sixteen of 44 patients (36%) were still receiving prednisone at the time of their first relapse (median 5.0 mg, range 2.5–20.0). Of the 21 patients in the CYC/AZA group, two were on CYC at the time of relapse. Thirty-three patients had an ANCA measured at the first non-severe relapse and 32 had a B-cell measurement. Ten patients (30%) had a negative ANCA (RTX 30%, CYC/AZA 31%) and fourteen patients (44%) had undetectable B-cells (RTX 45%, CYC/AZA 42%). Five of 32 patients (16%) had both negative ANCA and undetectable B-cells at relapse.
The most common manifestations of non-severe relapses were constitutional symptoms; ear, nose, and throat findings; ocular disease; and pulmonary involvement. Only four relapses (9%) were characterized by active kidney disease (hematuria in four patients, red blood cell casts in one). No patient had a serum creatinine rise greater than 30%.
Treatment of first non-severe relapse
After the first non-severe disease relapse, patients’ data were analyzed over an average of 12.5 months (RTX 7.8 versus CYC/AZA 17.6 [p=0.01]; range 0.7–39.2) until a recurrent relapse, change in non-glucocorticoid immunosuppression, or the occurrence of the last study visit, whichever came first.
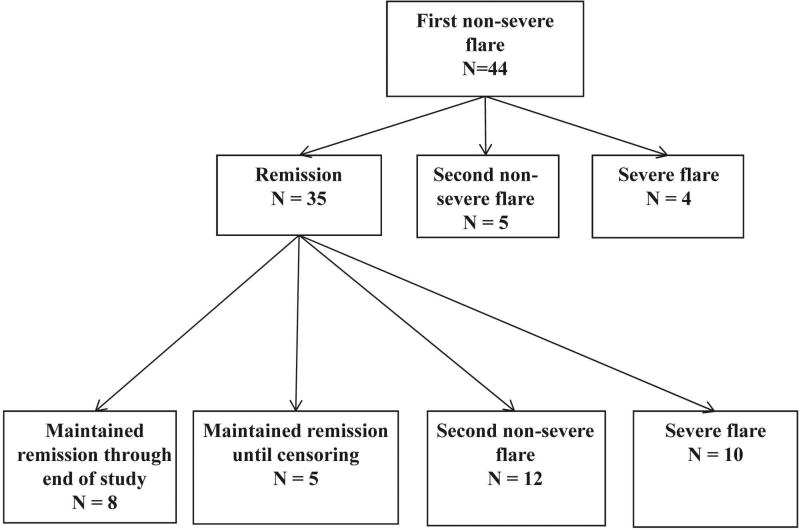
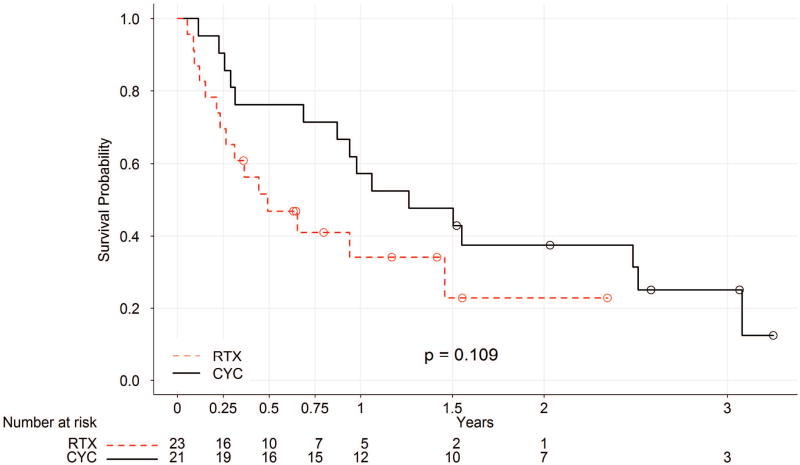
An increase in prednisone led to remission in 35 of 44 patients (80%) (RTX 17 of 23 [74%], CYC/AZA 18 of 21 [86%]; p=0.46) (Table 3). The median prednisone dose used to treat non-severe relapses was 17.5 mg (RTX 11 mg, CYC/AZA 20 mg, range 2.5–80). However, only 13 patients (30%) (8 RTX, 5 CYC/AZA) were able to remain in remission until the conclusion of follow-up (Figures 1 and 2). The remainder had second relapses. Of the 13 patients who maintained remission, five were censored at the 18 month timepoint because they were started on an additional immunosuppressive medication at the discretion of their treating physician (4 in RTX group added AZA and one in CYC/AZA group added RTX).
Table 3.
Outcome after first non-severe relapse
| RTX | CYC/AZA | Total | |
|---|---|---|---|
| N=23 | N=21 | N=44 | |
| Follow-up until next event*† (range, mos) | 7.8 (0.7–28.3) | 17.6 (1.4–39.2) | 12.5 (0.7–39.2) |
| Median prednisone dose for treatment of relapse (range, mg) | 11 (2.5–80) | 20 (4–60) | 17.5 (2.5–80) |
| Reached remission | 17 (74%) | 18 (86%) | 35 (80%) |
| Time to remission (mos) | 2.2 (0.5–4.4) | 2.8 (0.9–4.2) | 2.5 (0.5–4.4) |
| Reached complete remission¥ | 7 (30%) | 11 (52%) | 18 (41%) |
| Time to complete remission (mos) | 3.9 (1.9–6.8) | 6.1 (2.1–12.5) | 5.3 (1.9–12.5) |
| Outcome at end of follow-up | |||
| Non-severe relapse | 10 (44%) | 7 (33%) | 17 (39%) |
| Severe relapse | 5 (22%) | 9 (43%) | 14 (32%) |
| Maintained remission until censoring | 4 (17%) | 0 (0%) | 4 (9%) |
| Maintained remission until end of follow-up | 4 (17%) | 4 (19%) | 8 (18%) |
| Data not available at time of censoring | 0 (0%) | 1 (5%) | 1 (2%) |
| Time to second relapse (mos)‡ | 4.7 (0.7–17.6) | 13.7 (1.4–37.2) | 9.4 (0.7–37.2) |
| Off prednisone at relapse | 5 (22%) | 10 (48%) | 15 (41%) |
| Median prednisone dose at relapse if dose > 0mg (range in mg) | 7.5 (2.5–20) | 7.5 (5–10) | 7.5 (2.5–20) |
Next event was defined as 1) severe relapse 2) non-severe relapse 3) change in non-glucocorticoid immunosuppression (which could occur at the 18 month timepoint at the investigator’s best medical judgment) 4) withdrawal from the trial 5) common closeout date.
Of the four patients who had a non-severe flare before reaching remission all four reached remission and one had a subsequent severe flare.
p < 0.01
p < 0.01
Figure 1.
Outcomes of patients with non-severe relapse treated with glucocorticoids
Figure 2.
Relapse-free survival after initial non-severe relapse
The dose of prednisone used to treat the first non-severe relapse did not appear to influence patient outcomes (Table 4). A similar percentage of patients achieved remission and maintained remission when treated with high-dose prednisone (20mg or greater) as opposed to low-dose prednisone (less than 20mg). Seventy-seven percent of the relapsing patients treated with high-dose prednisone achieved remission and 23% of those patients maintained those remissions for the remainder of follow-up. By comparison, 82% of the relapsing patients treated with low-dose prednisone achieved remission and 36% maintained those remissions. Patients’ likelihood of remaining in remission did not differ according to ANCA type (PR3-ANCA 10 of 36 [28%], MPO-ANCA 3 of 8 [38%]; p=0.68). Of the four patients with MPA, three remained in remission and one had a severe relapse.
Table 4.
Outcomes by dose of prednisone used to treat first non-severe relapse
| High-dose prednisone (20mg or greater) |
Low-dose prednisone (less than 20mg) |
p-value | |
|---|---|---|---|
| N=22 | N=22 | ||
| Mean BVAS/WG at first relapse | 2.0 (1.1) | 1.4 (0.73) | 0.05 |
| Reached remission | 17 (77%) | 18 (82%) | >0.99 |
| Time to remission (mos) | 2.5 (0.5–4.1) | 2.5 (1.0–4.4) | 0.91 |
| Reached complete remission | 8 (36%) | 10 (46%) | 0.54 |
| Time to complete remission (mos) | 6.2 (3.3–12.5) | 4.5 (1.9–9.7) | 0.31 |
| Outcome at end of follow-up | |||
| Non-severe relapse | 8 (36.4%) | 9 (40.9%) | 0.76 |
| Severe relapse | 9 (40.9%) | 5 (22.7%) | 0.20 |
| Time to second relapse (mos) | 9.9 (0.7–30.3) | 8.7 (1.9–37.2) | 0.94 |
Characteristics of second relapses following first non-severe relapses
Of the 31 patients who were not able to reach and maintain remission throughout the follow-up period, 17 experienced a second non-severe relapse (10 RTX, 7 CYC/AZA) and 14 suffered a severe relapse (5 RTX, 9 CYC/AZA). There were no differences in baseline characteristics or organ involvement at first relapse among patients whose second relapse was severe as compared to non-severe (data not shown). Furthermore, the prednisone dose used to treat the first relapse did not appear to impact the likelihood that a second relapse would be severe. Thirty-six percent of patients treated with high-dose prednisone experienced a second non-severe relapse, compared to 41% of those treated with low-dose prednisone. Forty-one percent of patients treated with high-dose prednisone experienced a severe relapse, compared to 23% of those treated with low-dose prednisone. The mean time to second relapse was 9.4 months following the initial relapse (4.7 RTX, 13.7 CYC/AZA [p<0.01]). Sixteen of the 31 patients (52%) were still receiving prednisone at the time of second relapse (median 7.5mg, range 2.5–20mg). Of the 25 patients who had ANCA and B-cell measurements at the time of their second relapses, ANCA was positive or rising in 21 (84%) and B-cells were detectable or reconstituted in 21 patients (84%). Only one patient had a negative ANCA and undetectable B-cells at time of second relapse.
Cumulative prednisone doses and adverse events
Patients who had a non-severe relapse had a greater mean cumulative prednisone dose over the first 18 months of the trial compared to patients who reached the primary endpoint, 6.7g versus 3.8g (p<0.01).
Through the 18-month timepoint, there were no differences in adverse events among patients who experienced non-severe relapses compared to those who achieved the primary endpoint (0.94 versus 0.91 events per patient year, P=0.08). There were 5 severe infections among the 44 relapsing patients and 8 among the 71 patients who achieved the primary endpoint. Damage, as measured by the VDI, increased from trial entry to the first non-severe relapse and again at the end of follow-up (1.2, 1.6, 2.3 respectively). However, the mean VDI increased at a similar rate for patients who reached the primary endpoint at 18 months (1.0 to 2.1, p=0.99).
Discussion
Despite an expansion in the options for remission-induction regimens in AAV over the past two decades, disease relapses remain a therapeutic challenge. Our study is the first to describe the outcomes of patients with non-severe relapses. Although 80% of the patients achieved remission after an increase in their prednisone dose, 70% of such patients went on to experience a second relapse, either non-severe or severe, within an average of six months. Only 13 patients (30%) were able to maintain disease-free remissions until end of follow-up and only 8 (18%) were able to do so until their last study visit. These findings identify an important subset of patients with AAV who are unable to maintain prolonged remission and, as a result, receive higher doses of glucocorticoids. Such patients are more likely to have GPA, be PR3-ANCA-positive, and have relapsing disease.
Because patients with non-severe relapses constitute an important clinical subset, our definition of non-severe relapse should be considered in the context of terminology previously used to characterize non-severe AAV. Carrington first used the term “limited Wegener’s granulomatosis” to describe GPA that primarily involved the lungs as opposed to the kidneys [12]. In 1992, Hoffman et al. reported on the use of MTX and glucocorticoids in GPA patients with mild renal involvement, and such patients were also classified as having “limited” GPA [17]. Several other studies also employed this nomenclature [4,6,18]. Thus, “limited” GPA has often been defined more broadly (particularly in the U.S.) as disease activity that does not pose immediate threats to either major organ function or the patient’s life and does not require treatment with CYC [2].
Several other terms have been employed to describe non-severe relapses. The European Vasculitis Study Group (EUVAS) has used the terms “localized” to define disease confined to the upper or lower respiratory tract without constitutional symptoms and “early systemic” disease to define AAV without progressive end-organ damage and creatinine less than 120 µmol/L (1.35mg/dL) [13]. The “localized” and “early systemic” designations correspond approximately to the “limited” category. Finally, the term “minor relapse” has been used with widely varying definitions in a number of clinical trials, ranging from the occurrence of any BVAS item to the presence of at least three BVAS items [8–10].
Our pre-specified definition of “non-severe” relapse attempts to account for differences in previously used terminology by defining such relapses generally as those with the occurrence of any new disease activity, a BVAS/WG score of three or less, and no major BVAS/WG items. We propose that non-severe relapses be defined in future trials as any new disease activity that does not include a major BVAS/WG item. A standard definition of non-severe flare would facilitate understanding of the clinical course and treatment response of this important patient subgroup. Whether a strict BVAS/WG score of three or less should also be added to the definition to increase standardization further is uncertain, as some patients with a higher BVAS/WG score may be considered by some experts to have non-severe disease, as was the case for three patients in this study. It should be noted that disease manifestations that qualify for a “non-severe” designation may have a profound impact on patients’ prognosis and quality of life. For example, orbital pseudotumors and subglottic stenoses are features of “limited” or “localized” disease, yet often lead to irreversible damage and substantial morbidity.
The high rate of second relapses observed in this subgroup of patients suggests a need for a different treatment paradigm than used in the RAVE trial. Although prednisone was effective in re-inducing remission in 80% of cases, the majority of patients treated in this fashion did not sustain remission after prednisone had been tapered to a lower dose. In clinical practice, the addition or increase in the dose of AZA or MTX has been the standard of care for such disease relapses. B cell depletion may also be a viable approach in such patients [19, 20]. The recently published MAINRITSAN trial demonstrated that RTX, in combination with glucocorticoids, was superior to AZA for prevention of disease relapses, suggesting that alternative regimens for maintenance of disease remission should be considered in AAV [20]. The optimal method for remission induction and maintenance in this setting needs to be evaluated further in clinical trials.
Time to second relapse was shorter in RTX-treated patients in comparison to the CYC/AZA group (4.7 versus 13.7 months, p<0.01). A possible explanation is that patients who were randomized to RTX received placebo after reaching remission rather than AZA. Only four patients in the RTX group had undetectable B-cells at relapse, suggesting that the major effects of RTX had waned by the time of disease relapse. Despite this treatment difference, neither the rates of subsequent relapses nor patients’ ability to reach or maintain remission after a first non-severe relapse differed between the CYC/AZA and RTX groups.
Our results suggest that AZA may prolong time to relapse in patients who have had a previous relapse, but the efficacy of AZA in preventing disease relapses altogether, particularly when glucocorticoids are withdrawn, has not been confirmed. Previous randomized, controlled studies of AZA for remission maintenance enrolled only patients with newly-diagnosed disease [5,10] or maintained low-dose glucocorticoids for at least 18–24 months [5,10,20]. A recent retrospective series demonstrated that longer duration of AZA or MTX therapy reduced the rate of relapse [7]. However, that study only examined newly-diagnosed patients who had remained in remission for at least 18 months [7]. Thus, previous trials have not addressed the specific patient subgroup described in this study. In addition, the MAINRITSAN study demonstrated that despite concomitant glucocorticoid therapy for at least 18 months, 29% of patients treated with AZA experienced a severe relapse within 28 months, further calling into question the efficacy of AZA for maintaining disease remission [20].
The use of low-dose glucocorticoids for remission maintenance remains controversial [21,22]. It is notable that the median prednisone dose among the 44% of patients in this study who suffered second disease relapses while on glucocorticoids was 7.5 mg. These data support the concept that many disease relapses occur in AAV despite continuous prednisone doses higher than those regarded generally as “low-dose”. Although the follow-up period in this study was likely too short to detect differences in treatment-related adverse events or damage, a recent study of patients with rheumatoid arthritis demonstrated that treatment with prednisone 8mg or greater was associated with an increase in death from any cause in a dose-dependent manner, suggesting that the long-term effects of chronic glucocorticoid use in doses required to maintain disease stability in GPA may be substantial [23]. The currently ongoing “The Assessment of Prednisone In Remission” (TAPIR) study may add to our understanding of the utility of low-dose prednisone in maintaining disease remission in AAV [24].
Our study has several limitations. The starting prednisone dose for the treatment of non-severe relapses was not standardized. The fact that the protocol permitted the investigator to select the appropriate starting dose based on clinical judgment, however, accurately reflects the approach used in clinical practice. Moreover, the starting dose did not appear to impact the likelihood of achieving remission or experiencing a second relapse. The majority of patients in this study were PR3-ANCA-positive, a population that is known to be at higher risk for relapse [9, 10, 25–27]. Therefore the generalizability of our findings to MPO-ANCA-positive patients is not certain. Our study also has several strengths, including the protocolized study design, collection of data within the confines of a label-enabling clinical trial with rigorous data monitoring and regulatory agency oversight, and close patient follow-up throughout the study.
In conclusion, treatment of non-severe relapses in AAV with a temporary increase in the glucocorticoid dose restores disease remission in most patients, but recurrent relapses within a relatively short time period remain common. Alternative approaches to the treatment of non-severe relapses, including continuing glucocorticoids indefinitely or the use of B cell depletion in some patients, must be considered.
Acknowledgments
Funding:
This research was performed as a project of the Immune Tolerance Network (NIH Contract N01-AI-15416; Protocol number ITN021AI), an international clinical research consortium headquartered at the University of California San Francisco and supported by the National Institute of Allergy and Infectious Diseases and the Juvenile Diabetes Research Foundation; Genentech, Inc. and Biogen-Idec, Inc. At the Mayo Clinic, the trial was supported by Clinical and Translational Science Award (CTSA) Grant Number 1 UL1 RR024150-01 (National Center for Research Resources; NCRR). At Johns Hopkins, the trial was supported by UL1 RR 025005 (NCRR) and by grants K24 AR049185 (Dr. Stone) and K23 AR052820 (Dr. Seo). At Boston University, the trial was supported by CTSA grant number UL1RR 025771, NIH M01 RR00533, K24 AR02224 (Dr. Merkel), and an Arthritis Foundation Investigator Award (Dr. Monach). At Cleveland Clinic, the trial was supported by UL1TR000439, National Center for Advancing Translational Sciences (NCATS), NIH. ANCA ELISA kits were provided by EUROIMMUN AG (Lübeck, Germany).
References
- 1.Fauci AS, Katz P, Haynes BF, et al. Cyclophosphamide therapy of severe systemic necrotizing vasculitis. N Engl J Med. 1979;301:235–238. doi: 10.1056/NEJM197908023010503. [DOI] [PubMed] [Google Scholar]
- 2.Wegener's Granulomatosis Etanercept Trial (WGET) Research Group. Etanercept plus standard therapy for Wegener's granulomatosis. N Engl J Med. 2005 Jan 27;352(4):351–61. doi: 10.1056/NEJMoa041884. [DOI] [PubMed] [Google Scholar]
- 3.Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363:221–32. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Groot K, Mühler M, Reinhold-Keller E, Paulsen J, Gross WL. Induction of remission in Wegener's granulomatosis with low dose methotrexate. J Rheumatol. 1998 Mar;25(3):492–5. [PubMed] [Google Scholar]
- 5.Hiemstra TF, Walsh M, Mahr A, et al. Mycophenolate mofetil vs azathioprine for remission maintenance in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized controlled trial. JAMA. 2010;304:2381–8. doi: 10.1001/jama.2010.1658. [DOI] [PubMed] [Google Scholar]
- 6.De Groot K, Rasmussen N, Bacon PA, et al. Randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2005 Aug;52(8):2461–9. doi: 10.1002/art.21142. [DOI] [PubMed] [Google Scholar]
- 7.Springer J, Nutter B, Langford CA, Hoffman GS, Villa-Forte A. Granulomatosis with polyangiitis (Wegener's): impact of maintenance therapy duration. Medicine (Baltimore) 2014 Mar;93(2):82–90. doi: 10.1097/MD.0000000000000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Groot K, Harper L, Jayne DR, et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med. 2009;150:670–80. doi: 10.7326/0003-4819-150-10-200905190-00004. [DOI] [PubMed] [Google Scholar]
- 9.Jayne D, Rasmussen N, Andrassy K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med. 2003 Jul 3;349(1):36–44. doi: 10.1056/NEJMoa020286. [DOI] [PubMed] [Google Scholar]
- 10.Pagnoux C, Mahr A, Hamidou MA, et al. Azathioprine or methotrexate maintenance for ANCA-associated vasculitis. N Engl J Med. 2008 Dec 25;359(26):2790–803. doi: 10.1056/NEJMoa0802311. [DOI] [PubMed] [Google Scholar]
- 11.Jones RB, Tervaert JW, Hauser T, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med. 2010 Jul 15;363(3):211–20. doi: 10.1056/NEJMoa0909169. [DOI] [PubMed] [Google Scholar]
- 12.Carrington CB, Liebow AA. Limited forms of angiitis and granulomatosis of Wegener’s type. Am J Med. 1966;41:497–527. doi: 10.1016/0002-9343(66)90214-2. [DOI] [PubMed] [Google Scholar]
- 13.Jayne D. Update on the European Vasculitis Study Group trials. Curr Opin Rheumatol. 2001 Jan;13(1):48–55. doi: 10.1097/00002281-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Specks U, Merkel PA, Hoffman GS, et al. Design of the Rituximab in ANCA-associated Vasculitis (RAVE) Trial. Open Arthritis J. 2011;4:1–18. [Google Scholar]
- 15.Stone JH, Hoffman GS, Merkel PA, et al. A disease-specific activity index for Wegener’sgranulomatosis: modification of the Birmingham Vasculitis Activity Score. Arthritis Rheum. 2001;44:912–20. doi: 10.1002/1529-0131(200104)44:4<912::AID-ANR148>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Specks U, Merkel PA, Seo P, et al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med. 2013 Aug 1;369(5):417–27. doi: 10.1056/NEJMoa1213277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffman GS, Leavitt RY, Kerr GS, Fauci AS. The treatment of Wegener's granulomatosis with glucocorticoids and methotrexate. Arthritis Rheum. 1992 Nov;35(11):1322–9. doi: 10.1002/art.1780351113. [DOI] [PubMed] [Google Scholar]
- 18.Langford CA, Talar-Williams C, Sneller MC. Use of methotrexate and glucocorticoids in the treatment of Wegener's granulomatosis. Long-term renal outcome in patients with glomerulonephritis. Arthritis Rheum. 2000 Aug;43(8):1836–40. doi: 10.1002/1529-0131(200008)43:8<1836::AID-ANR20>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 19.Miloslavsky EM, Specks U, Merkel PA, et al. Rituximab for the treatment of relapses in ANCA-associated vasculitis. Arthritis Rheumatol. 2014 Nov;66(11):3151–9. doi: 10.1002/art.38788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guillevin L1, Pagnoux C, Karras A, et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med. 2014 Nov 6;371(19):1771–80. doi: 10.1056/NEJMoa1404231. [DOI] [PubMed] [Google Scholar]
- 21.McGregor JG, Hogan SL, Hu Y, Jennette CE, Falk RJ, Nachman PH. Glucocorticoids and relapse and infection rates in anti-neutrophil cytoplasmic antibody disease. Clin J Am Soc Nephrol. 2012 Feb;7(2):240–7. doi: 10.2215/CJN.05610611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walsh M, Merkel PA, Mahr A, Jayne D. Effects of duration of glucocorticoid therapy on relapse rate in antineutrophil cytoplasmic antibody-associated vasculitis: A meta-analysis. Arthritis Care Res (Hoboken) 2010 Aug;62(8):1166–73. doi: 10.1002/acr.20176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.del Rincón I, Battafarano DF, Restrepo JF, Erikson JM, Escalante A. Glucocorticoid dose thresholds associated with all-cause and cardiovascular mortality in rheumatoid arthritis. Arthritis Rheumatol. 2014 Feb;66(2):264–72. doi: 10.1002/art.38210. [DOI] [PubMed] [Google Scholar]
- 24.Krischer J, et al. University of South Florida . The Assessment of Prednisone In Remission Trial (TAPIR) - Patient Centric Approach. Bethesda (MD): National Library of Medicine (US); [Accessed 1/8/2015]. ClinicalTrials.gov Available from https://clinicaltrials.gov/ct2/show/NCT01933724. [Google Scholar]
- 25.Franssen C, Gans R, Kallenberg C, Hageluken C, Hoorntje S. Disease spectrum of patients with antineutrophil cytoplasmic autoantibodies of defined specificity: distinct differences between patients with anti-proteinase 3 and anti-myeloperoxidase autoantibodies. J Intern Med. 1998;244:209–16. doi: 10.1046/j.1365-2796.1998.00357.x. [DOI] [PubMed] [Google Scholar]
- 26.Harper L, Morgan MD, Walsh M, Hoglund P, Westman K, Flossmann O. Pulse versus daily oral cyclophosphamide for induction of remission in ANCA-associated vasculitis: long-term follow-up. Ann Rheum Dis. 2012;71:955–60. doi: 10.1136/annrheumdis-2011-200477. [DOI] [PubMed] [Google Scholar]
- Walsh M, Flossmann O, Berden A, Westman K, Hoglund P, Stegeman C, et al. Risk factors for relapse of antineutrophil cytoplasmic antibody–associated vasculitis. Arthritis Rheum. 2012;64:542–8. doi: 10.1002/art.33361. [DOI] [PubMed] [Google Scholar]