Abstract
Objectives
The English Bowel Cancer Screening Programme offers biennial guaiac faecal occult blood test (gFOBT) screening to 60–74-year-olds. Participants with positive results are referred for follow-up, but many do not have significant findings. If they remain age eligible, these individuals are reinvited for gFOBT screening. We evaluated the performance of repeat screening in this group.
Methods
We analysed data on programme participants reinvited to gFOBT screening after either previous negative gFOBT (n = 327,542), or positive gFOBT followed by a diagnostic investigation negative for colorectal cancer (CRC) or adenomas requiring surveillance (n = 42,280). Outcomes calculated were uptake, test positivity, yield of CRC, and positive predictive value (PPV) of gFOBT for CRC.
Results
For participants with a previous negative gFOBT, uptake in the subsequent screening round was 87.5%, positivity was 1.3%, yield of CRC was 0.112% of those adequately screened, and the PPV of gFOBT for CRC was 9.1%. After a positive gFOBT and a negative diagnostic investigation, uptake in the repeat screening round was 82.6%, positivity was 11.3%, CRC yield was 0.172% of participants adequately screened, and the PPV of gFOBT for CRC was 1.7%.
Conclusion
With high positivity and low PPV for CRC, the suitability of routine repeat gFOBT screening in two years among individuals with a previous positive test and a negative diagnostic examination needs to be carefully considered.
Keywords: Colorectal cancer, gFOBT, colonoscopy, screening programme, faecal occult blood test
Introduction
The English Bowel Cancer Screening Programme (BCSP) currently offers screening using the guaiac faecal occult blood test (gFOBT), which has been shown in randomized controlled trials to reduce colorectal cancer cause-specific mortality.1 In gFOBT or faecal immunochemical test (FIT) screening programmes, many participants with a positive test do not have cancer or adenomas requiring surveillance. In the BCSP, in line with the British Society of Gastroenterology guidelines, only individuals with intermediate risk adenomas (three or four small, <1 cm diameter adenomas, or at least one adenoma ≥ 1 cm) or high risk adenomas (five or more adenomas, or three or more with at least one ≥ 1 cm) require surveillance following polypectomy.2 In the first round of the BCSP, only 37.4% of 17,518 participants undergoing diagnostic investigation (98.1% had colonoscopy as first investigation performed) for positive gFOBT were diagnosed with colorectal cancer or intermediate/high risk adenomas. For the remaining participants undergoing diagnostic investigation, the outcome was normal colon and rectum in 29.7%, low risk adenomas (one or two adenomas <1 cm diameter) in 15.7%, other abnormal colorectal findings in 11.5%, and missing in 5.8%.3
In the BCSP, individuals with a positive gFOBT who are referred for diagnostic investigation and found to have a normal colon and rectum, low risk adenomas or other abnormal colorectal findings are currently reinvited to gFOBT screening in two years, provided they remain within the eligible age range (60–74). The performance of repeat gFOBT screening in these three groups is not well understood. Given that these individuals have had a colonoscopy, or other diagnostic investigation, negative for colorectal cancer and intermediate/high risk adenomas, positivity and yield of a repeat gFOBT screen after two years may be low. There is, however, a small miss rate of colonoscopy for both large adenomas and cancer.4–6 A repeat gFOBT screen may detect these missed lesions.
A number of studies have previously examined the question of whether further gFOBT or FIT screening is effective in individuals who have had a colonoscopy negative for cancer or adenomas requiring surveillance; however, their conclusions have been contradictory.7–15 Furthermore, these previous studies have been based on smaller datasets and have not examined the effect of age, sex, and previous diagnostic test outcome (normal, low risk adenomas, or other abnormal findings) on efficacy of repeat screening.
This investigation aimed to evaluate the outcomes of repeat gFOBT screening in BCSP participants who previously had a positive gFOBT, followed by a diagnostic investigation negative for intermediate or high risk adenomas and colorectal cancer.
Methods
The BCSP has been described in detail elsewhere.3 From the age of 60–74 (previously 60–69), men and women registered to a general practice are invited by post every two years to complete and return a gFOBT kit.
The gFOBT (Hema-Screen®) includes six windows for two samples from three separate stools. No dietary restrictions are requested of participants. If 5–6 windows of the gFOBT are positive, the test is considered positive. When 1–4 windows test positive, participants are immediately invited to take a second test. If any of the windows is positive on retesting, the gFOBT is considered positive. When no windows are positive on retesting, the participant is promptly invited to take a third gFOBT. Similarly, this test is considered positive if any windows test positive. If all windows are negative on either the first or third kit, the subject is considered normal, and discharged from the screening round.
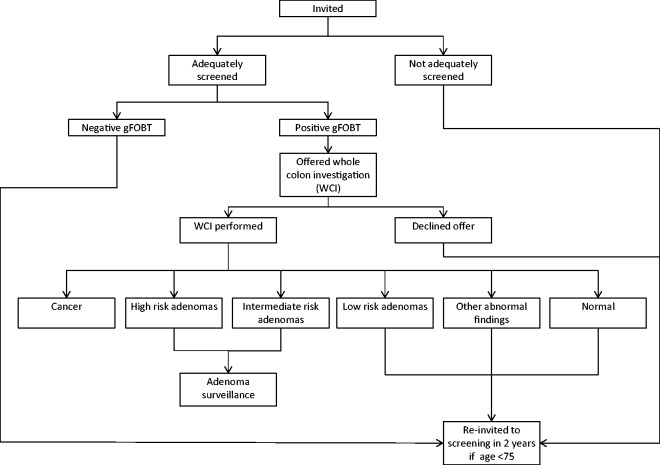
Participants with a positive gFOBT are referred to a specialist screening practitioner (Figure 1). The first line diagnostic investigation for a positive gFOBT is a colonoscopy. In a small proportion of participants (typically < 3%), a colonoscopy is considered inappropriate, and computed tomographic (CT) colonography or barium enema are performed.3 The outcome of diagnostic investigation may be normal colon and rectum, low risk adenomas, intermediate risk adenomas, high risk adenomas, cancer, or other abnormal colorectal findings such as diverticular disease, ulcerative colitis, or haemorrhoids.3 Following polypectomy, participants who had intermediate or high risk adenomas enter colonoscopic surveillance.
Figure 1.
Flow chart of the BCSP screening pathway.
Participants found to have a normal colon and rectum, low risk adenomas or other abnormal colorectal findings at diagnostic investigation are re-invited to gFOBT screening in two years, provided they remain in the eligible age range. We investigated the outcome of repeat gFOBT screening in these people.
We obtained individual-level de-identified data, extracted from the Bowel Cancer Screening System, on 42,280 BCSP participants invited to two consecutive screening rounds between August 2006 and April 2013, where the outcome of the earlier screening round was positive gFOBT and diagnostic investigation negative for cancer and intermediate/high risk adenomas. We compare our findings to aggregate data provided by the BCSP on 327,542 participants invited to a further screening round between January and May 2013 after a negative gFOBT kit in their first screening round.
The key outcome measures included are uptake (participants adequately screened/individuals invited), test positivity (participants with positive gFOBT/participants adequately screened), positive predictive value (PPV) for colorectal cancer (participants with colorectal cancer/participants attending diagnostic investigation following positive gFOBT), and PPV for intermediate or high risk adenomas (participants with intermediate or high risk adenomas/participants attending diagnostic investigation following positive gFOBT). Additionally of interest are yield of colorectal cancer, and yield of intermediate or high risk adenomas, as a percentage of participants adequately screened. Adequately screened participants are defined as those with a definitive positive or negative gFOBT result. Patients were categorized by their most advanced neoplastic finding. For instance, when intermediate or high risk adenomas were detected concurrently with cancer, the outcome was classified as colorectal cancer. Binomial exact 95% confidence intervals (CIs) are presented for each of the key outcomes.
Results
The majority of the 42,280 BCSP participants with a previous positive gFOBT and diagnostic investigation negative for cancer and intermediate/high risk adenomas had been investigated by colonoscopy (97.2%), with the remainder receiving one or more of CT colonography, barium enema, or flexible sigmoidoscopy. The outcome of these diagnostic investigations was normal colon and rectum, low risk adenomas, and other abnormal colorectal findings in 17,979, 11,578, and 12,723 participants, respectively (Table 1).
Table 1.
Outcomes of repeat screening stratified by sex and findings of preceding screening round (n = 369,822).
| Outcome of preceding round |
Outcome of repeat screening round |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Result of gFOBT | Result of diagnostic investigation | Invited | Adequately screened (%) | Positive gFOBT (%) | Attended diagnostic investigation | CRC (PPV, %) | IR or HR adenomas (PPV, %) | Yield among adequately screened |
||
| Group | CRC (%) | IR or HR adenomas (%) | ||||||||
| Overall | Negative | Not applicable | 327,542 | 286,492 (87.5) | 3851 (1.3) | 3521 | 321 (9.1) | 902 (25.6) | 0.112 | 0.315 |
| Positive | No cancer or IR/ HR adenomas | 42,280 | 34,942 (82.6) | 3940 (11.3) | 3572 | 60 (1.7) | 251 (7.0) | 0.172 | 0.718 | |
| Positive | Normal colon & rectum | 17,979 | 14,471 (80.5) | 1590 (11.0) | 1423 | 19 (1.3) | 77 (5.4) | 0.131 | 0.532 | |
| Positive | Low risk adenomas | 11,578 | 10,014 (86.5) | 1100 (11.0) | 1029 | 23 (2.2) | 109 (10.6) | 0.230 | 1.088 | |
| Positive | Other abnormal | 12,723 | 10,457 (82.2) | 1250 (12.0) | 1120 | 18 (1.6) | 65 (5.8) | 0.172 | 0.622 | |
| Men | Negative | Not applicable | 150,863 | 131,426 (87.1) | 2209 (1.7) | 2025 | 207 (10.2) | 614 (30.3) | 0.158 | 0.467 |
| Positive | No cancer or IR/ HR adenomas | 22,730 | 19,069 (83.9) | 2181 (11.4) | 1972 | 34 (1.7) | 170 (8.6) | 0.178 | 0.891 | |
| Positive | Normal colon & rectum | 8665 | 7124 (82.2) | 783 (11.0) | 690 | 9 (1.3) | 49 (7.1) | 0.126 | 0.688 | |
| Positive | Low risk adenomas | 7301 | 6334 (86.8) | 710 (11.2) | 660 | 16 (2.4) | 75 (11.4) | 0.253 | 1.184 | |
| Positive | Other abnormal | 6764 | 5611 (83.0) | 688 (12.3) | 622 | 9 (1.4) | 46 (7.4) | 0.160 | 0.820 | |
| Women | Negative | Not applicable | 176,679 | 155,066 (87.8) | 1642 (1.1) | 1496 | 114 (7.6) | 288 (19.3) | 0.074 | 0.186 |
| Positive | No cancer or IR/ HR adenomas | 19,550 | 15,873 (81.7) | 1759 (11.1) | 1600 | 26 (1.6) | 81 (5.1) | 0.164 | 0.510 | |
| Positive | Normal colon & rectum | 9314 | 7347 (78.9) | 807 (11.0) | 733 | 10 (1.4) | 28 (3.8) | 0.136 | 0.381 | |
| Positive | Low risk adenomas | 4277 | 3680 (86.0) | 390 (10.6) | 369 | 7 (1.9) | 34 (9.2) | 0.190 | 0.924 | |
| Positive | Other abnormal | 5959 | 4846 (81.3) | 562 (11.6) | 498 | 9 (1.8) | 19 (3.8) | 0.186 | 0.392 | |
CRC: colorectal cancer; IR or HR: intermediate or high risk.
Uptake of a repeat gFOBT screen was 82.6% (95% CI: 82.3–83.0%) in participants with a previous diagnostic investigation negative for cancer or intermediate/high risk adenomas (Table 1). In comparison, uptake was 87.5% (95% CI: 87.4–87.6%) in participants invited to a second round of screening following a negative first round gFOBT.
Positivity of gFOBT for participants with a previous diagnostic investigation negative for cancer or intermediate/high risk adenomas was 11.3% (95% CI: 10.9–11.6%). This is much higher than the 1.3% positivity (95% CI: 1.3–1.4%) observed in BCSP participants in a second screening round following a negative gFOBT.
In the repeat screening round for participants with a previous negative diagnostic investigation, the PPV for colorectal cancer was 1.7% (95% CI: 1.3–2.2%) (Table 1). This was considerably lower than the 9.1% (95% CI: 8.2–10.1%) PPV observed following previous negative gFOBT. Similarly, the PPV for intermediate or high risk adenomas was only 7.0% (95% CI: 6.2–7.9%) in participants with a previous negative diagnostic investigation. In contrast, following a previous negative gFOBT, the PPV for intermediate or high risk adenomas was 25.6% (95% CI: 24.2–27.1%).
Though the PPV for colorectal cancer was low in the repeat screening round following positive gFOBT and negative diagnostic investigation, the yield of colorectal cancer, and the yield of intermediate or high risk adenomas, as a proportion of those adequately screened by gFOBT, was higher than after previous negative gFOBT. Yield of colorectal cancer and yield of intermediate or high risk adenomas were 0.172% (95% CI: 0.131–0.221%) and 0.718% (95% CI: 0.632–0.813%) of participants adequately screened, respectively. In comparison, after previous negative gFOBT, the yield of colorectal cancer and yield of intermediate or high risk adenomas were 0.112% (95% CI: 0.100–0.125%) and 0.315% (95% CI: 0.295–0.336%) of participants adequately screened, respectively.
Among the 42,280 individuals with a positive gFOBT followed by a negative diagnostic examination, the repeat screening round identified 60 individuals with colorectal cancer. Data on lesion size were missing for 16 individuals. For the 44 cancers with information on lesion size, median size was 25 mm (interquartile range: 15–35 mm). Of the 60 individuals with cancer, 57 had received a complete colonoscopy in the previous screening round, one had an incomplete colonoscopy, and two had not received a colonoscopy but had received a CT colonography. Among the 58 individuals who underwent colonoscopy, the quality of bowel preparation had been good for 34 (58.6%), adequate for 21 (36.2%), and poor for two individuals (3.4%); this information was missing for one individual.
In analyses stratified by age, sex, or whether the participant was previously diagnosed as having a normal colon and rectum, low risk adenomas, or other abnormal colorectal findings, repeat gFOBT positivity was high and PPV for colorectal cancer was low, in comparison with participants with a previous negative gFOBT (Tables 1 and 2).
Table 2.
Outcomes of repeat screening by age group in participants with a previous positive gFOBT followed by a diagnostic investigation negative for cancer and intermediate/high risk adenomas (n = 42,280).
| Outcome of repeat screening round |
|||||||
|---|---|---|---|---|---|---|---|
| Result of diagnostic investigation in preceding round | Age groupa (years) | Invited | Adequately screened (%) | Positive gFOBT (%) | Attended diagnostic investigation | CRC (PPV, %) | IR or HR adenomas (PPV, %) |
| Normal colon & rectum | 60 to <65 | 6605 | 5261 (79.7) | 546 (10.4) | 480 | 4 (0.8) | 27 (5.6) |
| 65 to <70 | 7937 | 6429 (81.0) | 748 (11.6) | 672 | 9 (1.3) | 36 (5.4) | |
| 70+ | 3437 | 2781 (80.9) | 296 (10.6) | 271 | 6 (2.2) | 14 (5.2) | |
| Low risk adenomas | 60 to <65 | 3686 | 3164 (85.8) | 332 (10.5) | 309 | 8 (2.6) | 30 (9.7) |
| 65 to <70 | 5147 | 4446 (86.4) | 510 (11.5) | 478 | 9 (1.9) | 49 (10.3) | |
| 70+ | 2745 | 2404 (87.6) | 258 (10.7) | 242 | 6 (2.5) | 30 (12.4) | |
| Other abnormal | 60 to <65 | 4341 | 3460 (79.7) | 420 (12.1) | 380 | 2 (0.5) | 24 (6.3) |
| 65 to <70 | 5552 | 4645 (83.7) | 556 (12.0) | 505 | 8 (1.6) | 26 (5.1) | |
| 70+ | 2830 | 2352 (83.1) | 274 (11.7) | 235 | 8 (3.4) | 15 (6.4) | |
CRC: Colorectal cancer; IR or HR: Intermediate or high risk.
Age at initiation of repeat screening round.
The interval between the initial and the repeat gFOBT screen was two years for most participants (97.0%). Excluding participants with an interval between consecutive screening rounds of greater than two years did not affect positivity or PPV substantially (data not shown). Positivity remained 11.0% in participants with a previous diagnostic test outcome of low risk adenomas or normal colon and rectum, and 12.0% in participants with a previous outcome of abnormal colorectal findings. The PPV for colorectal cancer remained at 2.2% and 1.6% in participants with a previous outcome of low risk adenomas and abnormal colorectal findings, respectively, and increased marginally from 1.3% to 1.4% in participants with a previous diagnostic outcome of normal colon and rectum.
Among the 3572 individuals who tested positive again at their repeat gFOBT, and attended another diagnostic investigation, 3261 (91.3%) did not have cancer, or adenomas requiring surveillance, detected; 632 of these 3261 participants were invited for a further gFOBT screening round between August 2006 and April 2013. Uptake of this further gFOBT screen was 88.4% (95% CI: 85.7–90.8%). The positivity in this further round was even higher, at 22.9% (95% CI: 19.5–26.6%), yet upon diagnostic investigation of 115 of the 128 gFOBT positive individuals, no cancers were found, and only four participants had intermediate or high risk adenomas detected (Table 3).
Table 3.
Outcomes of a further screening round after two preceding rounds both with an outcome of positive gFOBT and diagnostic investigation negative for cancer or intermediate/high risk adenomas (n = 632).
| Result of diagnostic investigation in preceding round | Outcome of repeat screening round |
|||||
|---|---|---|---|---|---|---|
| Invited | Adequately screened (%) | Positive gFOBT (%) | Attended diagnostic investigation | CRC | IR or HR adenomas (PPV, %) | |
| No cancer or IR/HR adenomas | 632 | 559 (88.4) | 128 (22.9) | 115 | 0 | 4 (3.5) |
| Normal colon & rectum | 245 | 209 (85.3) | 46 (22.0) | 40 | 0 | 1 (2.5) |
| Low risk adenomas | 128 | 120 (93.8) | 32 (26.7) | 31 | 0 | 3 (9.7) |
| Other abnormal | 259 | 230 (88.8) | 50 (21.7) | 44 | 0 | 0 (0) |
CRC: Colorectal cancer; IR or HR: Intermediate or high risk.
Discussion
Among individuals with a previous positive gFOBT and diagnostic investigation negative for cancer or adenomas requiring surveillance, repeat gFOBT screening positivity was high (11.3%) and the PPV for colorectal cancer (1.7%), or intermediate/high risk adenomas (7.0%), was low. Conversely, among individuals with a previous negative gFOBT, positivity at repeat screening was 1.3%, the PPV for cancer was 9.1%, and the PPV for intermediate/high risk adenomas was 25.6%.
More generally in the BCSP, positivity is lower and PPV for cancer or intermediate/high risk adenomas is higher than reported here after positive gFOBT and negative diagnostic investigation. In a study of 62,099 individuals invited for gFOBT screening in the BCSP Southern Hub, positivity was 1.2% in the first round and 1.7% in the second round of screening.16 The same study reported a PPV for cancer of 10.9% in the first round and 8.4% in the second round.16 Another study reported that among the first 2.1 million individuals ever invited to the BCSP, 17,518 attended a diagnostic investigation following a positive gFOBT, the PPV for cancer was 10.1%, and the PPV for intermediate/high risk adenomas was 27.2%.3
The considerably higher positivity and lower PPV after previous positive gFOBT and negative diagnostic investigation indicates that some individuals are prone to repeat positive gFOBTs in the absence of colorectal cancer or intermediate/high risk adenomas. Further evidence for this comes from the 632 individuals invited to a further gFOBT screening round after two consecutive rounds of positive gFOBT and diagnostic investigation negative for cancer or intermediate/high risk adenomas. Among this group, positivity was even higher, at 22.9%; no cancers were detected, and only four individuals were found to have intermediate or high risk adenomas. The exact cause of repeat positive gFOBTs in the absence of cancer and intermediate or high risk adenomas is unclear. The consistently high positivity and low PPVs across age groups, sex, and outcome of initial investigation (normal, low risk adenomas, or other abnormal) indicates a cause to some extent independent of these factors.
Guaiac faecal occult blood tests detect the peroxidase activity of haem, a component of haemoglobin found in blood in stool. One potential explanation for repeat false-positive gFOBTs is that some participants have an alternative source of chronic gastrointestinal bleeding, such as upper gastrointestinal lesions.17 It is also thought that non-steroidal anti-inflammatory drugs, such as aspirin, may cause increased gastrointestinal bleeding and thereby increase the number of false-positive gFOBTs.18–20 A highly specific non-invasive test for colorectal cancer, which is not based on the detection of occult bleeding, could be very useful for further screening of patients following positive gFOBT and negative colonoscopy. Unfortunately, no such test currently exists.
The consumption of red meat and high-peroxidase fruit and vegetables shortly before testing has also been linked to false-positive gFOBTs.21 In the BCSP, participants are not requested to make any dietary restrictions, given the potential negative effect of this on uptake and uncertain efficacy of dietary restriction in preventing false positivity.3
A number of other studies using smaller datasets have similarly found positivity to be high and PPV for cancer to be low for a repeat gFOBT or FIT screen in participants with a previous negative colonoscopy.7–15 Carrera and colleagues examined the outcomes of repeat gFOBT screening within the Scottish screening pilot among participants with no neoplasia on diagnostic investigation after positive gFOBT.9 In a second round of screening in this group, positivity was 17.4% (157/904 participants) and six participants had cancer (PPV 3.8%). In the third round of screening, 84 individuals who had a positive gFOBT and negative colonoscopy in both the first and second round were invited. Positivity was 25.6% in this group and no cancers or adenomas were detected.
Although the PPV of a repeat screen following positive gFOBT and negative diagnostic investigation was low, the high positivity rate meant that the yield of colorectal cancer and the yield of intermediate/high risk adenomas, as a proportion of participants adequately screened, were higher than after negative gFOBT. However, due to the low PPV, many participants undergoing repeat screening will be subject to additional unnecessary diagnostic investigation, with the accompanying risk of adverse events, such as colonoscopic perforation, and the potential for psychological distress.22–25 Furthermore, additional colonoscopies will increase cost and place additional demand on overburdened endoscopy services.
Missed lesions and incomplete resection are major causes of colorectal cancer detected soon after colonoscopy.26 Ensuring that colonoscopies are of high-quality, with good bowel preparation, cecal intubation, high adenoma detection rates, and complete resection of advanced lesions, is crucial to minimizing occurrences of post-colonoscopy colorectal cancer.
Conclusions
In participants undergoing repeat screening following a previous positive gFOBT and negative colonoscopy, test positivity is high and PPV for colorectal cancer is low. Though colorectal cancers are diagnosed in these participants, it comes at a cost of an increase in the number of colonoscopies needed.
Acknowledgements
We acknowledge Dr Ines Kralj-Hans for her assistance in obtaining the data. Data for this study are based on information collected and quality assured by the PHE National Cancer Registration and Analysis Service. Access to the data was facilitated by the PHE Office for Data Release.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Wendy Atkin has received non-monetary research support from Eiken Chemical Co. Ltd for reagents and lease of a machine for processing immunochemical faecal occult blood tests for the FIT for Follow-Up study.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by The Bobby Moore Fund for Cancer Research UK (grant no. A16894).
References
- 1.Hewitson P, Glasziou P, Watson E, et al. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol 2008; 103: 1541–1549. [DOI] [PubMed] [Google Scholar]
- 2.Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut 2010; 59: 666–689. [DOI] [PubMed] [Google Scholar]
- 3.Logan RF, Patnick J, Nickerson C, et al. Outcomes of the Bowel Cancer Screening Programme (BCSP) in England after the first 1 million tests. Gut 2012; 61: 1439–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenner H, Chang-Claude J, Seiler CM, et al. Interval cancers after negative colonoscopy: population-based case-control study. Gut 2012; 61: 1576–1582. [DOI] [PubMed] [Google Scholar]
- 5.Morris EJ, Rutter MD, Finan PJ, et al. Post-colonoscopy colorectal cancer (PCCRC) rates vary considerably depending on the method used to calculate them: a retrospective observational population-based study of PCCRC in the English National Health Service. Gut 2015; 64: 1248–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rex DK, Cutler CS, Lemmel GT, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology 1997; 112: 24–28. [DOI] [PubMed] [Google Scholar]
- 7.Burke E, Brinchmann K, Philipps L, et al. Endoscopic findings in subjects undergoing multiple index colonoscopies within the BCSP. Gut 2015; 64(S1): A77–A78. [Google Scholar]
- 8.Campari C, Camellini L, Sereni G, et al. Efficacy of FIT 5 years after a negative colonoscopy and a previous positive FIT: Results from a screening cohort. Digest Liver Dis 2015; 47: e145.
- 9.Carrera A, McClements PL, Watling C, et al. Negative screening colonoscopy after a positive guaiac faecal occult blood test: not a contraindication to continued screening. Colorectal Dis 2012; 14: 943–946. [DOI] [PubMed] [Google Scholar]
- 10.Harma C, Chen HC, Raftopoulos S, et al. Effect on previous colonoscopy on yield of colonoscopy for positive faecal occult blood test. J Gastroen Hepatol 2013; 28: 112–113. [Google Scholar]
- 11.Majumdar D, Patnick J, Nickerson C, et al. Continued biennial screening of faecal occult blood test (FOBT) positive and screening colonoscopy negative cohort in English bowel cancer screening programme-is it necessary? Gut 2012; 61(S2): A328–A328. [Google Scholar]
- 12.Nylander D, Ritchie M. Patients with positive faecal occult blood test (FOBt) following previous low risk colonoscopy in the bowel cancer screening programme: should current approach be changed? Gut 2012; 61(S2): A160–A160. [Google Scholar]
- 13.Robertson DJ, Pohl H, Provenzale D. Early Fecal Occult Blood Test (FOBT) after a normal colonoscopy: too much, too soon? Gastroenterology 2009; 136: A29–A29. [Google Scholar]
- 14.Sahnan K, Vaughan-Shaw P, Valori R. Colorectal cancer yield in patients with a previous negative bowel cancer screening programme (BCSP) colonoscopy. Int J Surg 2013; 11: 623–624. [Google Scholar]
- 15.Sehgal V, Stein J, Bloom S, et al. Do patients with a previous normal colonoscopy within the United Kingdom Bowel Cancer Screening Program who subsequently have a positive FOBT require colonoscopy? Gut 2014; 63(S1): A22–A23.
- 16.Lo SH, Halloran S, Snowball J, et al. Colorectal cancer screening uptake over three biennial invitation rounds in the English bowel cancer screening programme. Gut 2015; 64: 282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hisamuddin K, Mowat NA, Phull PS. Endoscopic findings in the upper gastrointestinal tract of faecal occult blood-positive, colonoscopy-negative patients. Dig Liver Dis 2006; 38: 503–507. [DOI] [PubMed] [Google Scholar]
- 18.Lee TJ, Hull MA, Rajasekhar PT, et al. Aspirin users attending for NHS bowel cancer screening have less colorectal neoplasia: chemoprevention or false-positive faecal occult blood testing? Digestion 2012; 85: 278–281. [DOI] [PubMed] [Google Scholar]
- 19.Sawhney MS, McDougall H, Nelson DB, et al. Fecal occult blood test in patients on low-dose aspirin, warfarin, clopidogrel, or non-steroidal anti-inflammatory drugs. Digest Dis Sci 2010; 55: 1637–1642. [DOI] [PubMed] [Google Scholar]
- 20.Ibáñez-Sanz G, Garcia M, Rodríguez-Moranta F, et al. Prescription drugs associated with false-positive results when using faecal immunochemical tests for colorectal cancer screening. Digest Liver Dis 2016; 48: 1249–1254. [DOI] [PubMed] [Google Scholar]
- 21.Konrad G. Dietary interventions for fecal occult blood test screening: systematic review of the literature. Can Fam Phys 2010; 56: 229–238. [PMC free article] [PubMed] [Google Scholar]
- 22.Derbyshire E, Nickerson C, Hungin A, et al. Colonoscopic perforations in the english NHS bowel cancer screening programme (NHSBCSP). Gut 2015; 64(S1): A76–A77. [DOI] [PubMed] [Google Scholar]
- 23.Tam MS and Abbas MA. Perforation following colorectal endoscopy: What happens beyond the endoscopy suite? The Permanente J 2013; 17: 17–21. [DOI] [PMC free article] [PubMed]
- 24.Wardle J, Pope R. The psychological costs of screening for cancer. J Psychosomat Res 1992; 36: 609–624. [DOI] [PubMed] [Google Scholar]
- 25.Denters MJ, Deutekom M, Essink-Bot ML, et al. FIT false-positives in colorectal cancer screening experience psychological distress up to 6 weeks after colonoscopy. Support Care Cancer 2013; 21: 2809–2815. [DOI] [PubMed] [Google Scholar]
- 26.Adler J, Robertson JR. Interval colorectal cancer after colonoscopy: exploring explanations and solutions. Am J Gastroenterol 2015; 110: 1657–1664. [DOI] [PubMed] [Google Scholar]