Abstract
Recent advances and review of literature
Robot-assisted orthopaedic surgery is gaining momentum and being gradually adopted and incorporated into our routine practice. With recent innovations in surgical applications of robots, newer techniques are developed and its applications rapidly expanding in orthopaedics. This article reviews the current state of robotics and the development of future robotic technology for Trauma and Orthopaedics.
Materials and methods
A comprehensive analysis of the English literature was performed using Elton B Stephens Co (EBSCO, Birmingham, Alabama) hosted Med-line, CINAHL, PEDro, Cochrane and PubMed databases between 1966 and February 2018. All articles with full text were retrieved and their bibliographies hand-searched for further references. Non-indexed materials on the worldwide web and the System for Information on Grey Literature in Europe (SIGLE) were also included in the review. Initial search yielded 15,204 studies, which were assessed based on relevance, duplication and inclusion and exclusion criteria (Table 1, Fig 1). Eighty-two studies were included in the final analysis and their findings are narrated by subspecialty.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Robot-assisted/performed orthopaedic surgery | Orthopaedic surgeries not using fully active robots including computer navigated surgeries Expert opinion, letters to editor and case reports Experimental studies on animals |
Figure 1.
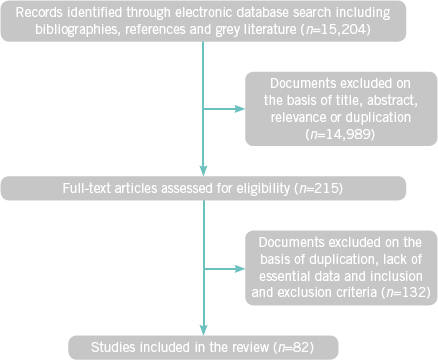
Flow diagram
Review
Musculoskeletal problems account for more than 25% of all surgical interventions in the NHS and account for more than £4.76 billion of NHS spending each year.1–3 With the increase in ageing population and childhood obesity, this is set to rise significantly during the next ten years.3,4 Getting It Right First Time (GIRFT)4 and the available literature has stressed the need for conducting orthopaedic procedures with precision and accuracy to obtain reliable and reproducible outcomes.
Orthopaedics is gradually adopting and incorporating robotic surgery in its armamentarium to help surgeons to achieve the aforementioned goal by improving the spatial accuracy.
Knee
The total number of knee replacements has increased from 13,546 in 2003 to 98,147 in 2016 and during the same period the number of knee revisions has increased from 630 to 5,932 in England, Wales and Northern Ireland.5 The majority of the revisions (>60%) are for malalignment, implant wear/fracture and aseptic loosening. Robotic surgery has the potential to reduce these complications and increase the survivorship of the implants.5
Unicompartmental knee arthroplasty
Between eight and ten per cent of knee replacements in UK are unicompartmental knee arthroplasties (UKA) and this is predicted to grow.6 UKA is more conservative than total knee arthroplasty (TKA) and has a better kinematics, lower perioperative morbidity, earlier functional recovery and better functional outcome than TKA.7 Despite these advantages, UKA have a higher revision rate than TKA and the reasons are multifaceted.6 Component alignment is a significant factor for the survivorship of the implant and indirectly influenced the ligamentous imbalance and properties of the implant.8,9 Accomplishing near-normal coronal plane alignment is a significant factor that influences the long-term results and ultimately the survival of UKA.9–11 Studies have shown that 40%–60% of UKA using conventional technique are outliers beyond the 2° of desired alignment, even in experienced hands.12,13 The problems become worse in patients having minimally invasive UKA.14,15 With computer navigation, the outliers (2° of desired alignment) are less than conventional technique (15%) and the results obtained from robot-assisted UKA are impressive.12,13,16 This was proven in a study by Karia et al where 16 inexperienced surgeons were randomised to constrained robot-assisted or conventional unicompartmental knee replacement on dry bones for a period of 3 weeks.17 In the three weeks, the surgical time decreased in both groups; however, the rotational and translational errors were lower in the robot-assisted group, which suggests robots reduce errors irrespective of experience.
A prospective randomised control trial (RCT) assessed the accuracy of implant position and limb alignment in 62 patients who had UKA implanted by the MAKO Robotic Interactive Orthopaedic Arm (RIO) system vs 58 patients who had a conventional surgical procedure. All the patients had an Oxford Phase-3 unicompartmental knee replacement with traditional instrumentation. The accuracy of component positioning is significantly better in the robotic group, with regard to the femoral component sagittal position (57% compared with 26%, p=0.0008), femoral component coronal position (70% compared with 28%, p=0.0001), femoral component axial position (53% compared with 31%, p=0.0163), tibial component sagittal position (80% compared with 22%, p=0.0001), and tibial component axial position (48% compared with 19%, p=0.0009).16 An earlier prospective double-blinded RCT compared conventional and robot-assisted UKA using the Acrobot system (The Acrobot Co Ltd, London, UK), which had better results than the MAKO RIO system (Cobb et al). The Acrobot group achieved coronal alignment within 2° of the planned position in all their UKAs, with a mean of 0.65° (-1.6° to 0.3°; standard deviation [SD] 0.59). However, in the conventional group only 40% achieved this level of accuracy, with a mean of -0.84° (-4.2° to 4.2°; SD 2.75).13 A recent multicentre prospective study assessed 909 knees (robotic-assisted medial UKA) at an average follow-up of 2.5 years (range: 22–52 months). 92% of the patients were either very satisfied or satisfied with their knee function – with a survivorship of 96–98.8%.18
Lonner et al evaluated the precision and accuracy of Navio PFS (NAVIO system, Blue Belt Technologies, Plymouth, MN) – a semi-autonomous robot, unlike Mako/Acrobat, which are autonomous robots.19 In 25 cadaveric specimens the ‘planned’ and ‘actual’ angular, translational, and rotational positions of the components were assessed. The RMS angular errors were 1.42°–2.34° for the 3 directions for the femoral implant and 1.95°–2.60° for the 3 directions of the tibial implant. The RMS translational errors were 0.92–1.61mm for the femoral implant and 0.97–1.67mm for the tibial implant. However, the authors stressed the advantages of low radiation dose, less conversion to conventional surgery, and no soft-tissue injuries on using this system. Yet there is no peer-reviewed publication in the literature to support these findings from cadaveric lab.
Various studies have emphasised that the accuracy of robot-assisted UKAs is better with regard to the tibial slope and valgus-varus alignment.19–22 Robot-assisted UKA allowed accurate soft-tissue balancing and helped restore natural knee kinematics, with positive implications for implant survival and functional outcomes.23
An experimental study by Wolf et al using an image-free system mini-bone attached robotic system (MBAR) could resect the bone more accurately and precisely than mechanical guides/freehand-cutting in patella-femoral arthroplasty (PFA).24 The system performs the planning intraoperatively in the robot coordinate system and thus eliminating the need for external tracking systems in the operating room. Their study recommended that PFA could be done with a small incision and less operating time. Turktas et al retrospectively analysed 30 knees that had robotically-assisted PFA.25 At a mean follow-up of 15.9 months, the patients made significant functional improvement. The robotic technique had numerous advantages, which include a smaller incision, faster rehabilitation, preservation of bone stock, and implantation without malalignment.
Total knee arthroplasty (TKA)
Robot-assisted TKA is believed by some surgeons to improve implant positioning, preserving bone and to protect soft tissues.26 Yang et al27 retrospectively compared 71 robotic TKAs with 42 conventional TKAs at a mean follow-up of 10 years. Although the clinical outcomes and long-term survival rates were similar between the two groups, the robotic TKA group had significantly fewer (p<0.001) postoperative leg alignment outliers (femoral coronal inclination, tibial coronal inclination, femoral sagittal inclination, tibial sagittal inclination, and mechanical axis) and fewer radio-lucent lines than the conventional TKA group. The mechanical axis improved from 9.0° varus to 1.9° varus in the robotic TKA group, and from 10.0° varus to 2.9° varus in the conventional TKA group. Long-term follow-up studies following TKA has shown that limb malalignment influenced survivorship and outcomes.28 A prospective RCT analysed the quality-of-life (QoL) measures and functional outcome between robotic-assisted and conventional TKA.29 Although the robot-assisted group had a higher rate of complications, they had better SF-36 QoL measures, with significant differences in SF-36 vitality (p=0.03), role emotional (p=0.02) and a larger proportion of patients achieving SF-36 vitality MCID (48.4 vs 13.8 %, p=0.009). However, no significant differences in KSS, OKS or satisfaction/expectation were noted between the groups.
Song et al30 compared the outcome in patients who had bilateral simultaneous TKA with robotic assistance on one joint and a conventional surgical technique on the other. Although the mean mechanical axis (9.1° vs 10.9°) and balanced flexion-extension gaps (27 vs 23) are better in the robotic group, neither these nor the outcome scores were significantly different (p>0.05). In the study by Decking et al,31 CT scans were used preoperatively and postoperatively to assess the mechanical axis. Their results showed excellent accuracy of angular component placement in all planes and a mean deviation from the mechanical axis of only 0.2° (95% confidence intervals [CI] –0.1° to 0.5°). The accurate component positioning is demonstrated in other similar studies.32–35
Recent studies have demonstrated that robots are effective in restoring mechanical axis in complex knee replacements.36–38 Marchand et al36 corrected 64% of patients (82 knees) with severe varus deformity (7° or greater) and 100% of severe valgus (7 knees) deformity to neutral (mean 2°, range 0–3°). The remaining patients with severe varus deformity were corrected within a couple of degrees of neutral. Kim et al analysed 32 patients with haemophilia at a mean follow-up of 5 years. In spite of severe deformities and flexion contractures, the HKA axis was achieved within a range of 0 ± 3° in 30 knees (93.8%).37 Similar results were obtained in patients with severe varus and valgus deformity.38
Hip
Total hip arthroplasty (THA)
Robots have been used clinically used for THA for the past 20 years. ROBODOC (Integrated Surgical Systems, Davis, California) was introduced in 1992 to improve outcomes in uncemented THA by reducing technical errors.39 Bargar et al40 analysed patients from two US Food and Drug Administration RCTs (1994–1998 and 2001–2006) at a mean follow-up of 14 years, comparing traditional vs robotic THA. There was no statistically significant difference in probability of a revision for wear or loosening in either group (χ2=1.80; p=0.179). However, the robot-assisted group had statistically significant higher HSQ pain and Harris pain scores and lower Western Ontario and McMaster Universities Osteoarthritis Index scores.
Bukowski et al41 compared 100 primary THA in each arm (robotic vs traditional) in a retrospective cohort study. There was no statistically significant difference between the groups with regard to operative time (R 131 ± 23 min vs T122 ± 29 min, respectively, p=0.012), blood loss (R374 ± 133 ml vs T423 ± 186 ml, p= 0.035), and overall complication rates (p=0.101). However, the robotic-assisted THA demonstrated significantly higher mean postoperative mHHS (92.1 ± 10.5 vs 86.1 ± 16.2, p=0.002), mean UCLA scores (6.3 ± 1.8 vs 5.8 ± 1.7, p=0.033) and there were no significant differences in SF-12 or WOMAC scores. Besides, the robotic group had a significantly higher proportion of patients with mHHS scores between 90 to 100 points (75% vs 61%, p=0.034) and a lower percentage with scores <70 points (6% vs 19%, p=0.005).
Illgen et al42 assessed the results of a single surgeon series – conventional THAs in his early careerr (2000) vs 100 conventional THAs in his late career (2011) vs consecutive 100 robot-assisted THAs (2012). The ace-tabular component placement within Lewinnek safe zone was 30% early in his career, 45% in his late career and 77% in the robot-assisted group. The accuracy increased in robot-assisted THA by 71% in the first year of use. The dislocation rate was 5% with early THA, 3% in the late THA, and 0% in the robotic cohort within the first two years postoperatively.
Positioning of the acetabulum component affects dislocation rates, component impingement, bearing surface wear rates and need for revision surgery. Kamara et al43 compared acetabulur component placement using radiographs in three groups in a retrospective cohort study. The first 100 fluoroscopically guided direct anterior THAs (FA) vs first 100 robotic–assisted posterior THAs (RP) vs the last 100 manual posterior (MP) THAs done by each surgeon (200 THAs). Seventy-six per cent of MP THAs were within the surgeons’ target zone (inclination, 30°–50°; anteversion, 10°–30°) compared with 84% of FA THAs and 97% of RP THAs.
Variances were lower for acetabulum inclination and anteversion in RP THAs (14.0 and 19.5) as compared with the MP (37.5 and 56.3) and FA (24.5 and 54.6) groups. These differences were statistically significant (p<.01). The study by Tsai et al44 showed that robot-assisted THA has higher precision than manual THA and has the potential to restore native hip geometry. Similar results were achieved by Ellmallah et al;45 99% of their 224 patients achieved preoperatively determined radiographic targets for cup placements with robot assisted THAs. A matched-pair controlled study by Domb et al46 100% (50 hips) of the robotic group had acetabular component placed in the safe zone described by Lewinnek et al47 compared with 80% of conventional THAs (p=0.001). Gupta et al48 studied 105 patients with BMI (body mass index) <30 (n=59), BMI 30–35 (n=34) and BMI >35 (n=12) that had robotic THAs. There was no statistical difference between the groups in regards to the acetabular inclination (p=0.43) or version (p=0.95). They concluded that robotic THA is safe in obese patients and could provide accurate and reproducible placement of the acetabular cup within safe zones. Domb et al49 assessed 175 patients for femoral version after robotic THA. All the patients had native femoral version corrected toward a target of 15°, irrespective of the approach or BMI.
A multicentre study reported a statistically significant improvement in femoral component fit and position (p=0.02, alignment; p=0.01, axial seating). The mean operating time was >240 minutes to start with; however, this was later reduced to 90 minutes as a centre’s experience increased.50 The Limb length discrepancy and varus–valgus stem orientation were improved with robotic THA. Honl et al51 reported 18% conversion rate to conventional THA, significantly longer operating times and significantly higher complication rate with respect to dislocation, heterotopic ossification, rupture of the gluteus medius tendon and revision surgery. Schulz et al had a complication rate of 9.3% and stressed that technology required improvement before widespread use. Nishihara et al52 studied the clinical and radiographic results of uncemented THA using conventional hand-rasping vs robotic milling. The robotic group superior fit the implant with no intraoperative femoral fractures; however, the hand-rasping group suffered from undersizing of the stem, higher vertical seating and unexpected femoral anteversion. The robot-assisted group had significantly superior Merle d’Aubigné hip scores at two years. Nakamura et al53 evaluated the benefits of robotic cementless femoral component implantation vs conventional implantation. At a minimum follow-up of five years the robot-assisted group had more precise implant position, less limb length discrepancy and less stress-shielding in the proximal femur. The results did not change with short-stem implants (metaphyseal fit) – the robotic THAs had showed superior results in terms of stem alignment and leg-length equality than conventional THA.54,55 Studies in the English literature have consistently showed that the THA component position is improved with robotic-assisted THAs56–61 and the risks of systemic embolisation from femur preparation is less with robotic femur preparation.62
In a matched-pair controlled study, the acetabular cup size of robotic THAs are significantly smaller (p<0.02) than the manual THAs.63 Thus robotic THAs could preserve bone stock and could be of immense benefit in revision cases to preserve the remaining acetabulum.
Hip arthroscopy
Hip arthroscopy is a technically demanding procedure and is predominantly used for femoroace-tabular impingement (FAI) or labral pathology. The use of robots for hip joint visualisation is still at an experimental stage;64–67 however, promising results are shown in dry bone models with accurate bone resection in Cam type FAI.66 Park et al compared the freehand resection vs robotic resection of Cam type FAI in saw bones (8 in each group).67 The predetermined desired arc of resection (117.7) was analysed with laser scanner. Freehand resection led to statistically significant (p<0.0001) mean arc of resection error and over-resection with every specimen (p<0.01). This study emphasised the need for robotic-assisted femoral osteochondroplasty when compared with the conventional freehand technique, which is used currently. The haptic three-armed da Vinci® standard surgical system (Intuitive Surgical, Sunnyvale, California) could overcome the technical concerns that are associated with this procedure. Kather et al65 in his cadaveric study showed the technical feasibility of hip arthroscopy using existing laparoscopic instrumentation. In his study he viewed all parts of the joint, performed labral manipulation and resected the limbus and plica in two cadavers.
Spine
The robots are extensively used in spine surgery for the placement of pedicle screws. There have been a couple of studies evaluating the role of robots in anterior and posterior surgical exposure of the vertebra.68,69 Two types of robots were used for spinal instrumentation and are SpineAssist robot (Mazor Surgical Technologies, Caesar-ea, Israel) and ROSA robot (Medtech). Two randomised controlled trials recently evaluated the safety, accuracy and precision of the pedicle screw instrumentation.70,71 Both demonstrated that the precision and accuracy of screw placements were better than the freehand technique. The study by Hyun et al showed that minimally invasive spinal fusions could be performed with less radiation exposure (3.5 vs 13.3 seconds per screw in robot vs freehand technique [p<0.001]) and the average length of stay was 6.8 vs 9.4 days in robot compared with freehand technique (p=0.020).71 Sukovich et al72 reported that 96% of pedicle screws were placed within 1mm of their planned trajectory, with instrumentation performed at thoracic, lumbar and sacral levels.
In a retrospective multicentre analysis of 3,271 robot-assisted pedical screws, 3,204 were placed in a clinically acceptable position. Except for transient neurological deficit in four patients, there were no major complications.73 Similar success stories were reported in various articles.74–83 In patients with spinal metastasis, pedicle screw placement in the thoracolumbar spine can be performed effectively and safely using robot-guided assistance.84 In patients with low-grade spondylolisthesis, robotic pedicle screw placement has a reduced rate of revision surgery compared with placing the screw using navigation or freehand technique.85
Shoulder
Use of robotics in shoulder surgery is in its nascent stage. Robotic shoulder arthroscopy was performed using a four-armed da Vinci® surgical system in two cadavers in both beach chair and lateral decubitus position. Though there were limitations in instrumentation, the authors could access biceps, labrum, supraspinatus, infraspinatus, rotator interval, subscapularis, all glenohumeral ligaments and the coracoid process. The cadavers were dissected after the procedure to evaluate instrument placement, and demonstrated correct placement with no neurovascular injury.86
Foot and ankle
Wiewiorski et al87 evaluated the accuracy of CT-guided robotically (INNOMOTION robotic-assistance device [Innomedic, Herxheim, Germany]) assisted infiltration technique for diagnostic injections in foot and ankle orthopaedics. All injections in tibiotalar, talonavicular, tarsometatarsal, subtalar and calcaneocuboid joints were successful. This was confirmed by contrast visualisation and pain relief. Taking into account radiation exposure and the absence of a comparative group, the benefits of robot-guided foot and ankle injection when compared with radiologically guided injection are far from clear.
Trauma and general orthopaedics
A semi-automated telerobotic surgical system called Trauma Pod is in the experimental stage of development to save the lives of critically injured patients on the battlefield.88 The robot is supplied with autonomous robotic arms that can act as circulating and scrub nurses. The robot could automatically change tools and supply delivery is performed as fast as when performed manually by nurses. In addition, tracking and counting of the supplies is performed automatically. The authors demonstrated in a simulated patient that a surgeon could perform bowel anastomosis and shunt placement in major vessels via teleoperating, and that the process could support intraoperative CT scanning.
The role of robots in percutaneous reduction of fractures has been extensively investigated.89–91 Experiments in cadavers showed promising results, with fracture reduction accuracy of about 1mm and 1.5°. The surgeon reduced the fracture in a 3D virtual environment and the robot manipulator reduced the fragments accurately. The surgeon took 95 seconds on an average to reduce 80 fractures virtually using the navigation system and the robotic arms took an average of about 75 seconds to physically reduce the fracture. The entire reduction procedure was accomplished in about three minutes.90 In a similar study a robot-assisted fracture surgery (RAFS) system was tested on 9 cadaver specimens and was able to reduce 7 out of 9 distal femur fractures (T- and Y-shape 33-C1) with acceptable accuracy (≈1mm, ≈5°).91 This technology could help develop minimally invasive fracture surgeries. Robots have been used to identify the entry point of intramedullary nailing and apply distal locking bolts in cadavers.92,93
The da Vinci® system has been used to safely identify, dissect and repair nerves in Brachial plexus injuries.94,95 Mantovani et al94, through an endoscopic approach in two fresh human cadavers, successfully dissected brachial plexuses and performed nerve graft with minimal dissection. Garcia et al95 performed brachial plexus surgery in three patients and the authors concluded that the robotic surgery will help with tremor filtration, motion-scaling and ergonomics.
Wang et al96 randomised 30 patients requiring posterior pelvic ring stabilisation to either freehand or robot-assisted (TiRobot™ [TINAVI MedicalTechnologies Co, Ltd, Beijing, China]). Of the 45 sacro-iliac joint (S1 and S2) screws, 22 were done freehand and 23 robot-assisted. There was no significant difference between the operation time; however, the robot-assisted group had less radiation exposure. The accuracy of screw placement was graded as excellent and good in 100% of robot-assisted group and 95% in the freehand group.
Discussion
Innovative robotic surgical applications and techniques are being developed and reported every day in Trauma and Orthopaedics. Increased use will eventually fuel the discovery of newer applications of robotic systems. The surgical outcome in Trauma and Orthopaedics is directionally proportionate to the precision and accuracy of the surgery, and robot-assisted systems has huge potential in helping surgeons achieve this. Although there were plenty of prospective randomised controlled trials published in the literature to support the use of robots in Trauma and Orthopaedics (Table 2), numerous factors have to be taken into consideration before widespread implementation and use.
Table 2.
Prospective Randomised control trials. C= Conventional R= Robotic
| Study/year/Procedure | Robot used | Sample size/mean follow-up | Results | Major complications |
|---|---|---|---|---|
| Cobb et al13, 2006 UKA | Acrobot System (The Acrobot Co Ltd, London, UK) | C-14; R-13 18 weeks |
Radiological alignment is better than conventional technique; Functional outcome is similar in both groups | None |
| Bell et al16, 2016 UKA | MAKO Robotic Interactive Orthopedic (RIO) Arm (Stryker, Mahwah, NJ, USA) | C-58; R-62 | Radiological alignment is better than conventional technique; | None |
| Liow et al29 2017 TKA | ROBODOC system (Integrated Surgical Systems, Sacramento, CA) | C-29; R-31 | Subtle improvement in quality–of–life scores in robotic group | None |
| Park et al101, 2007 TKA | ROBODOC system (Integrated Surgical Systems, Sacramento, CA) | C-30; R-32 44–52 months |
Radiological alignment is better than conventional technique Functional outcome is similar in both groups | 1 each of patellar tendon rupture, dislocation of the patella, postoperative supracondylar fracture, patellar fracture, and peroneal injury in early cases where robots were used. None in conventional. |
| Song et al30, 2011 TKA | ROBODOC system (Integrated Surgical Systems, Sacramento, CA) | C-30; R-30 Minimum 12 months |
Simultaneous bilateral surgery Radiological alignment is better than conventional technique Functional outcome is similar in both groups |
None |
| Bargar et al50 THA USA – Prospective, randomised controlled trial Germany – Case series | ROBODOC; Integrated Surgical Systems, Davis, CA | C-65; R-69 24 months Germany R-900 |
Radiological alignment is better than conventional technique Functional outcome is similar in both groups Germany Improvement in both radiological and functional outcome |
No difference between the groups |
| Bargar et al40, 2017 THA | ROBODOC; Integrated Surgical Systems, Davis, CA | 14 years follow-up of Bargar et al50 | Functional outcome and radiological alignment is better in Robot group | None |
| Lim et al55, 2016 THA (Short stem) | ROBODOC; Integrated Surgical Systems, Davis, CA | C-27; R-27 24 months |
Radiological alignment is better than conventional technique Functional outcome is similar in both groups |
2 fractures in Manual rasping group |
| Nishihara et al19 Prospective, RCT | ROBODOC; Integrated Surgical Systems, Davis, CA | C-78; R-78 2.3 years |
Functional and radiological outcome is better in robotic group | Intraoperative femoral fractures C5; R0 |
| Kim et al70, 2017 Pedicle screw–spine | SpineAssist (Mazor Surgical Technologies Ltd, Caesarea, Israel) | C-41; R-41 | Similar accuracy (99.4%) | 13 (15.9%) violations with the freehand technique, none with robots |
| Hyun et al71, 2017 Pedicle screw–spine | SpineAssist (Mazor Surgical Technologies Ltd, Caesarea, Israel) | C-30; R-30 | Accuracy R -100% C -98.6% | None |
| Ringel et al74, 2012 Pedicle screw–spine | SpineAssist (Mazor Surgical Technologies Ltd, Caesarea, Israel) | C-30; R-30 | Accuracy R -85% C -93% | Ten robotic screws require intraoperative conversion to the free hand application. One freehand screw needed a secondary revision. |
| Wang et al96, 2017 Pelvis | TiRobot™ (TINAVI Medical Technologies Co, Ltd, Beijing, China) | C-22; R-23 | Accuracy R -100% C -95% | None |
The costs associated with the use are a major issue, especially in this economic climate of financial pressures in the NHS. The costs of a robotic system ranges from USD 400,000 (GBP 29,000) for a NAVIO orthopaedic system, USD 700, 000 (GBP 502,000) for a MAKO Rio system and for advanced da Vinci® systems the costs could be around USD 2.8 million (GBP 2 million). In addition, for each procedure the costs for consumables could be around USD 2,000 (GBP 1,400) and the annual maintanence fees for robots could cost GBP 140,000.97,98 Studies based on the North American healthcare system have shown that using this system would lead to breaking even in two years.99 A more detailed Markov Decision Analysis to evaluate the costs, outcomes, and incremental cost-effectiveness has shown that the robot-assisted UKA is cost-effective if the centres perform more than 94 cases a year.100 Low- and medium-volume centres should continue with the conventional technique, unless there is enough evidence in the literature to prove that robots reduce revision rates by better component placement and soft-tissue balancing.
The costs are bound to reduce with widespread use and more healthcare industries providing these services. Trehan et al98 revealed that some of these companies operate with a profit margin of 40.3%, which is remarkably high when compared with other industries in the sector. Patented technologies, a high barrier to entry, and bigger companies buying the competitors make the competition unhealthy. The UK (unlike US) has a universal healthcare system in the form of the NHS, which places us in a better position to negotiate with the companies to reduce the costs.
Serious complications were reported in earlier studies in knee replacements101 (patellar tendon rupture, fracture or dislocation of the patella, supracondylar fracture and peroneal nerve injury) and hip replacements58 (increased blood loss, dislocation, revision rate and heterotopic ossification). Any new technique introduced will have a steep learning curve and studies have shown that the operation time, blood loss, and complication rates were more in the early stages of a surgeon’s learning curve. However, with the increase in use of the procedures and training opportunities, the recent RCTs have demonstrated that the robotic–assisted surgeries were as safe as – sometimes better than – the conventional procedures. In spine procedures, robots have shown that soft–tissue dissection and radiation exposure could be reduced in deformed spines.102 As the use of robots could be fraught with issues related to hardware/software, surgeons performing robotic surgery should be competent enough to proceed with conventional surgery.50,75
From the patients’ perspective, the majority think that robotic arthroplasty is more accurate, technically easier to perform, and involves less operating time than conventional surgery.103 The acceptance rate was high in one group of 100 patients in an arthroplasty clinic – although only 12% knew about the system, 80% were happy to have robotic surgery. This review has highlighted the fact that the current problems are to do with economic viability, training and good-quality data rather than safety issues. As suggested in an earlier review,104 a national registry to record these procedures and guidelines, which provides adequate training to the future orthopaedic surgeons is the need of the hour.
Conclusion
Robotic surgery is here to stay and will occupy a key place in the future of Trauma and Orthopaedics. Significant progression has been made in the use of robots in the past few years. Current literature suggests that the robotics are safe and as effective as conventional surgery in UKA, TKA, THA and pedicle screw insertion in the spine. Although some studies show that the robots are better than the conventional technique in achieving limb alignment, reduce operation time and blood loss, more good-quality research is needed form independent centres to confirm the same. The role of robots in other orthopaedic subspecialties is still in its nascent stage. Training future surgeons on cadaveric or simulation systems could reduce the complications rate and steepness of the learning curve. The costs associated with the implant used could be lowered by negotiation, encouraging more commercial ventures and increased use in high-volume centres. Establishment of a national registry is essential to monitor revision rate, complications and patient satisfaction.
References
- 1.Arthritis Research UK State of Musculoskeletal Health 2017. https://www.arthritisresearchuk.org/arthritis–information/ data–and–statistics/state–of–musculoskeletal–health.aspx (cited March 2018). [Google Scholar]
- 2.NHS England 2013/14 CCG programme budgeting benchmarking tool. https://www.england.nhs.uk/resources/resources–for–ccgs/prog–budgeting/ (cited March 2018).
- 3.NHS England Musculoskeletal conditions. Why is it important? https://www.england.nhs.uk/ourwork/ltc–op–eolc/ltc–eolc/our– work–on–long–term–conditions/si–areas/musculoskeletal/ (cited 18 February 2018).
- 4.Getting It Right First Time. http://gettingitrightfirsttime.co.uk (last accessed 8 February 2018).
- 5.National Joint Registry http://www.njrcentre.org.uk/njrcentre/default.aspx (last accessed 8 February 2018).
- 6.Riddle DL, Jiranek WA, McGlynn FJ. Yearly incidence of unicompartmental knee arthroplasty in the United States. J Arthroplasty 2008; (3): 408–412. [DOI] [PubMed] [Google Scholar]
- 7.Noticewala M, Geller J, Lee J, Macaulay W. Unicompartmental knee arthroplasty relieves pain and improves function more than total knee arthroplasty. J Arthroplasty 2012; : 99–105. [DOI] [PubMed] [Google Scholar]
- 8.Hernigou P, Deschamps G. Alignment influences wear in the knee after medial unicompartmental arthroplasty. Clin Orthop Relat Res 2004; (423): 161–165. [DOI] [PubMed] [Google Scholar]
- 9.Collier MB, Eickmann TH, Sukezaki F, et al. Patient, implant, and alignment factors associated with revision of medial compartment unicondylar arthroplasty. J Arthroplasty 2006; : 108–115. [DOI] [PubMed] [Google Scholar]
- 10.Kim KT, Lee S, Kim TW, et al. The influence of postoperative tibiofemoral alignment on the clinical results of unicompartmental knee arthroplasty. Knee Surg Relat Res 2012; : 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collier MB, Engh CA Jr, McAuley JP, Engh GA. Factors associated with the loss of thickness of polyethylene tibial bearings after knee arthroplasty. J Bone Joint Surg [Am] 2007; : 1,306–1,314. [DOI] [PubMed] [Google Scholar]
- 12.Keene G, Simpson D, Kalairajah Y. Limb alignment in computer-assisted minimally-invasive unicompartmental knee replacement. J Bone Joint Surg Br 2006; : 44–48. [DOI] [PubMed] [Google Scholar]
- 13.Cobb J, Henckel J, Gomes P, et al. Hands-on robotic unicompartmental knee replacement: a prospective, randomised controlled study of the acrobat system. J Bone Joint Surg [Br] 2006; : 188–197. [DOI] [PubMed] [Google Scholar]
- 14.Fisher DA, Watts M, Davis KE. Implant position in knee surgery: A comparison of minimally invasive, open unicompartmental, and total knee arthroplasty. J Arthroplasty 2003; ; 7: 2–8. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton WG, Collier MB, Tarabee E, et al. Incidence and reasons for reoperation after minimally invasive unicompartmental knee arthroplasty. J Arthroplasty 2006; : 98–107. [DOI] [PubMed] [Google Scholar]
- 16.Bell SW, Anthony I, Jones B, et al. Improved accuracy of component positioning with robotic-assisted unicompartmental knee arthroplasty: data from a prospective, randomized controlled study. J Bone Joint Surg Am 2016; (8): 627–635. [DOI] [PubMed] [Google Scholar]
- 17.Karia M, Masjedi M, Andrews B, et al. Robotic assistance enables inexperienced surgeons to perform unicompartmental knee arthroplasties on dry bone models with accuracy superior to conventional methods. Adv Orthop 2013; 481039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearle AD, van der List JP, Lee L, et al. Survivorship and patient satisfaction of robotic-assisted medial unicompartmental knee arthroplasty at a minimum two-year follow-up. Knee 2017; (2): 419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res 2010; : 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coon TM. Integrating robotic technology into the operating room. Am J Orthop (Belle Mead NJ) 2009; : 7–9. [PubMed] [Google Scholar]
- 21.Pearle AD, O’Loughlin PF, Kendoff DO. . Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty 2010; : 230–237. [DOI] [PubMed] [Google Scholar]
- 22.Lonner JH, Smith JR, Picard F, et al. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res 2015; : 206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plate JF, Mofidi A, Mannava S, et al. Achieving accurate ligament balancing using roboticassisted unicompartmental knee arthroplasty. Adv Orthop 2013; : 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolf A, Jaramaz B, Lisien B, DiGioia AM. MBARS: mini bone-attached robotic system for joint arthroplasty. Int J Med Robot 2005; (2): 101–121. [DOI] [PubMed] [Google Scholar]
- 25.Turktas U, Piskin A, Poehling GG. Short-term outcomes of robotically assisted patellafemoral arthroplasty. Int Orthop 2016; (5): 919–924. [DOI] [PubMed] [Google Scholar]
- 26.Khlopas A, Chughtai M, Hampp EL, et al. Robotic-arm assisted total knee arthroplasty demonstrated soft tissue protection. Surg Technol Int 2017; : 441–446. [PubMed] [Google Scholar]
- 27.Yang HY, Seon JK, Shin YJ, et al. Robotic total knee arthroplasty with a cruciate-retaining implant: a 10-year follow-up study. Clin Orthop Surg 2017; (2): 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez JA, Bhende H, Ranawat CS. Total condylar knee replacement: a 20-year followup study. Clin Orthop Relat Res 2001; : 10–7. [PubMed] [Google Scholar]
- 29.Liow MHL, Goh GS, Wong MK, et al. Roboticassisted total knee arthroplasty may lead to improvement in quality-of-life measures: a 2-year follow-up of a prospective randomized trial. Knee Surg Sports Traumatol Arthrosc 2017; : 2,942–2,951. [DOI] [PubMed] [Google Scholar]
- 30.Song EK, Seon JK, Park SJ, et al. Simultaneous bilateral total knee arthroplasty with robotic and conventional techniques: a prospective, randomized study. Knee Surg Sports Traumatol Arthrosc 2011; : 1,069–1,076. [DOI] [PubMed] [Google Scholar]
- 31.Decking J, Theis C, Achenbach T, et al. Robotic total knee arthroplasty: the accuracy of CT-based component placement. Acta Orthop Scand 2004; : 573–579. [DOI] [PubMed] [Google Scholar]
- 32.Song EK, Seon JK, Yim JH, et al. Roboticassisted TKA reduces postoperative alignment outliers and improves gap balance compared to conventional TKA. Clin Orthop Relat Res 2013; : 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siebert W, Mai S, Kober R, Heeckt PF. Technique and first clinical results of robotassisted total knee replacement. Knee 2002; : 173–180. [DOI] [PubMed] [Google Scholar]
- 34.Bellemans J, Vandenneucker H, Vanlauwe J. Robot-assisted total knee arthroplasty. Clin Orthop Relat Res 2007; : 111–116. [DOI] [PubMed] [Google Scholar]
- 35.Kim SM, Park YS, Ha CW, et al. Robotassisted implantation improves the precision of component position in minimally invasive TKA. Orthopedics 2012; : 1,334–1,339. [DOI] [PubMed] [Google Scholar]
- 36.Marchand RC, Sodhi N, Khlopas A, et al. Coronal correction for severe deformity using robotic–assisted total knee arthroplasty. J Knee Surg 2018; (1): 2–5. [DOI] [PubMed] [Google Scholar]
- 37.Kim KI, Kim DK, Juh HS, et al. Robot-assisted total knee arthroplasty in haemophilic arthropathy. Haemophilia 2016; (3): 446–452. [DOI] [PubMed] [Google Scholar]
- 38.Marchand RC, Khlopas A, Sodhi N, et al. Difficult cases in robotic arm-assisted total knee arthroplasty: a case series. J Knee Surg 2018; (1): 27–37. [DOI] [PubMed] [Google Scholar]
- 39.Paul HA, Bargar WL, Mittlestadt B, et al. Development of a surgical robot for cementless total hip arthroplasty. Clin Orthop Relat Res 1992; : 57–66. [PubMed] [Google Scholar]
- 40.Bargar WL, Parise CA, Hankins A, et al. Fourteen year follow-up of randomized clinical trials of active robotic-assisted total hip arthroplasty. J Arthroplasty 2017; : 1–5. [DOI] [PubMed] [Google Scholar]
- 41.Bukowski BR, Anderson P, Khlopas A, et al. Improved functional outcomes with robotic compared with manual total hip arthroplasty. Surg Technol Int 2016; : 303–308. [PubMed] [Google Scholar]
- 42.Nd Illgen RL, Bukowski BR, Abiola R, et al. Robotic-assisted total hip arthroplasty: outcomes at minimum two-year follow-up. Surg Technol Int 2017; : 365–372. [PubMed] [Google Scholar]
- 43.Kamara E, Robinson J, Bas MA, et al. Adoption of robotic vs fluoroscopic guidance in total hip arthroplasty: is acetabular positioning improved in the learning curve? J Arthroplasty 2017; (1): 125–130. [DOI] [PubMed] [Google Scholar]
- 44.Tsai TY, Dimitriou D, Li JS Kwon YM. Does haptic robot-assisted total hip arthroplasty better restore native acetabular and femoral anatomy? Int J Med Robot 2016; (2): 288–295. [DOI] [PubMed] [Google Scholar]
- 45.Elmallah RK, Cherian JJ, Jauregui JJ, Padden DA, Harwin SF, Mont MA. Robotic-arm assisted surgery in total hip arthroplasty. Surg Technol Int 2015; : 283–8. [PubMed] [Google Scholar]
- 46.Domb BG, El Bitar YF, Sadik AY, et al. Comparison of robotic-assisted and conventional acetabular cup placement in THA: a matched-pair controlled study. Clin Orthop Rel Res 2014; : 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lewinnek GE, Lewis JL, Tarr R, et al. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am 1978; : 217–220. [PubMed] [Google Scholar]
- 48.Gupta A, Redmond JM, Hammarstedt JE, et al. Does robotic–assisted computer navigation affect acetabular cup positioning in total hip arthroplasty in the obese patient? a comparison Study. J Arthroplasty 2015; (12): 2,204–2,207. [DOI] [PubMed] [Google Scholar]
- 49.Domb BG, Chandrasekaran S, Gui C, et al. Can stem version consistently correct native femoral version using robotic guidance in total hip arthroplasty? Surg Technol Int 2017; : 891. [PubMed] [Google Scholar]
- 50.Bargar WL, Bauer A, Börner M. Primary and revision total hip replacement using the Robodoc system. Clin Orthop Relat Res 1998; : 82–91. [DOI] [PubMed] [Google Scholar]
- 51.Honl M, Dierk O, Gauck C, et al. Comparison of robotic-assisted and manual implantation of a primary total hip replacement. A prospective study. J Bone Joint Surg [Am] 2003; : 1,470–1,478. [DOI] [PubMed] [Google Scholar]
- 52.Nishihara S, Sugano N, Nishii T, et al. Comparison between hand rasping and robotic milling for stem implantation in cementless total hip arthroplasty. J Arthroplasty 2006; : 957–966. [DOI] [PubMed] [Google Scholar]
- 53.Nakamura N, Sugano N, Nishii T, et al. A comparison between robotic-assisted and manual implantation of cementless total hip arthroplasty. Clin Orthop Relat Res 2010; : 1,072–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lim SJ, Kim SM, Lim BH, et al. Comparison of manual rasping and robotic milling for short metaphyseal-fitting stem implantation in total hip arthroplasty: a cadaveric study. Comput Aided Surg 2013; : 33–40. [DOI] [PubMed] [Google Scholar]
- 55.Lim SJ, Ko KR, Park CW, et al. Robot-assisted primary cementless total hip arthroplasty with a short femoral stem: a prospective randomized short-term outcome study. Comput Aided Surg 2015; (1): 41–46. [DOI] [PubMed] [Google Scholar]
- 56.Hananouchi T, Sugano N, Nishii T, et al. Effect of robotic milling on periprosthetic bone remodeling. J Orthop Res 2007; : 1,062–1,069. [DOI] [PubMed] [Google Scholar]
- 57.Nishihara S, Sugano N, Nishii T, et al. Clinical accuracy evaluation of femoral canal preparation using the ROBODOC system. J Orthop Sci 2004; : 452–461. [DOI] [PubMed] [Google Scholar]
- 58.Siebel T, Käfer W. Clinical outcome following robotic assisted vs conventional total hip arthroplasty: a controlled and prospective study of seventy-one patients. Z Orthop Ihre Grenzgeb 2005; : 391–398. [DOI] [PubMed] [Google Scholar]
- 59.El Bitar YF, Jackson TJ, Lindner D, et al. Predictive value of robotic-assisted total hip arthroplasty. Orthopedics 2015; (1): e31– 7. doi: 10.3928/01477447-20150105-57. [DOI] [PubMed] [Google Scholar]
- 60.Redmond JM, Gupta A, Hammarstedt JE, et al. Accuracy of component placement in robotic-assisted total hip arthroplasty. Orthopedics 2016; (3): 193–199. doi: 10.3928/01477447-20160404-06. [DOI] [PubMed] [Google Scholar]
- 61.Benjamin G, Domb MD, Youssef F. Comparison of robotic-assisted and conventional acetabular cup placement in THA: a matched-pair controlled study. Clin Orthop Relat Res 2014; (1): 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hagio K, Sugano N, Takashina M, et al. Effectiveness of the ROBODOC system in preventing intraoperative pulmonary embolism. Acta Orthop Scand 2003; : 264–269. [DOI] [PubMed] [Google Scholar]
- 63.Suarez–Ahedo C, Gui C, Martin TJ, et al. Robotic-arm assisted total hip arthroplasty results in smaller acetabular cup size in relation to the femoral head size: a matchedpair controlled study. Hip Int 2017; (2): 147–152. [DOI] [PubMed] [Google Scholar]
- 64.Içik C, Apaydin N, Açar HI, et al. Robotic hip arthroscopy: a cadaveric feasibility study. Acta Orthop Traumatol Turc 2014; (2): 207–211. [DOI] [PubMed] [Google Scholar]
- 65.Kather J, Hagen ME, Morel P, et al. Robotic hip arthroscopy in human anatomy. Int J Med Robot 2010; : 301–305. [DOI] [PubMed] [Google Scholar]
- 66.Masjedi M, Tan WL, Jaskaranjit S, et al. Use of robotic technology in cam femoroacetabular impingement corrective surgery. Int J Med Robot 2013; : 23–28. [DOI] [PubMed] [Google Scholar]
- 67.Park CN, Nawabi DH, Christopher J, et al. Robotic-assisted femoral osteochondroplasty is more precise than a freehand technique in a Sawbone model. J Hip Preserv Surg 2015; (2): 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee Z, Lee JY, Welch WC, Eun D. Technique and surgical outcomes of robot-assisted anterior lumbar interbody fusion. J Robot Surg 2013; : 177–185. [DOI] [PubMed] [Google Scholar]
- 69.Ponnusamy K, Chewning S, Mohr C. Robotic approaches to the posterior spine. Spine 2009; : 2,104–2, 109. [DOI] [PubMed] [Google Scholar]
- 70.Kim HJ, Jung WI, Chang BS, et al. A prospective, randomized, controlled trial of robot-assisted vs freehand pedicle screw fixation in spine surgery. Int J Med Robot 2017; (3): e1779. [DOI] [PubMed] [Google Scholar]
- 71.Hyun SJ, Kim KJ, Jahng TA, Kim HJ. Minimally invasive, robotic vs. open uoroscopic-guided spinal instrumented fusions – A randomized, controlled trial. Spine 2017; (6): 353–358. [DOI] [PubMed] [Google Scholar]
- 72.Sukovich W, Brink-Danan S, Hardenbrook M. Miniature robotic guidance for pedicle screw placement in posterior spinal fusion: early clinical experience with the SpineAssist. Int J Med Robot 2006; : 114–122. [DOI] [PubMed] [Google Scholar]
- 73.Devito DP, Kaplan L, Dietl R, et al. Clinical acceptance and accuracy assessment of spinal implants guided with Spine Assist surgical robot: retrospective study. Spine 2010; : 2,109–2,115. [DOI] [PubMed] [Google Scholar]
- 74.Barzilay Y, Liebergall M, Fridlander A, Knoller N. Miniature robotic guidance for spine surgery – Introduction of a novel system and analysis of challenges encountered during the clinical development phase at two spine centres. Int J Med Robot 2006; : 146–153. [DOI] [PubMed] [Google Scholar]
- 75.Togawa D, Kayanja MM, Reinhardt MK, et al. Bone-mounted miniature robotic guidance for pedicle screw and translaminar facet screw placement: part 2 –Evaluation of system accuracy. Neurosurgery 2007; :ONS129–ONS139. [DOI] [PubMed] [Google Scholar]
- 76.Lieberman IH, Hardenbrook MA, Wang JC, Guyer RD. Assessment of pedicle screw placement accuracy, procedure time, and radiation exposure using a miniature robotic guidance system. J Spinal Disord Tech 2012; : 241–248. [DOI] [PubMed] [Google Scholar]
- 77.Kantelhardt SR, Martinez R, Baerwinkel S, et al. Perioperative course and accuracy of screw positioning in conventional, open robotic-guided and percutaneous roboticguided, pedicle screw placement. Eur Spine J 2011; : 860–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hu X, Ohnmeiss DD, Lieberman IH. Roboticassisted pedicle screw placement: lessons learned from the first 102 patients. Eur Spine J 2013; : 661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pechlivanis I, Kiriyanthan G, Engelhardt M, et al. Percutaneous placement of pedicle screws in the lumbar spine using a bone mounted miniature robotic system: first experiences and accuracy of screw placement. Spine 2009; : 392–398. [DOI] [PubMed] [Google Scholar]
- 80.Molliqaj G, Schatlo B, Alaid A, et al. Accuracy of robot-guided vs freehand fluoroscopy-assisted pedicle screw insertion in thoracolumbar spinal surgery. Neurosurg Focus 2017; (5): E14. [DOI] [PubMed] [Google Scholar]
- 81.Schatlo B, Molliqaj G, Cuvinciuc V, et al. Safety and accuracy of robot-assisted vs fluoroscopy-guided pedicle screw insertion for degenerative diseases of the lumbar spine: a matched cohort comparison. J Neurosurg Spine 2014; (6): 636–643. [DOI] [PubMed] [Google Scholar]
- 82.Van Dijk JD, van den Ende RP, Stramigioli S, et al. Clinical pedicle screw accuracy and deviation from planning in robot-guided spine surgery: robot-guided pedicle screw accuracy. Spine 2015; (17): E986–91. [DOI] [PubMed] [Google Scholar]
- 83.Lonjon N, Chan-Seng E, Costalat V, et al. Robot-assisted spine surgery: feasibility study through a prospective case-matched analysis. Eur Spine J 2016; (3): 947–955. [DOI] [PubMed] [Google Scholar]
- 84.Solomiichuk V, Fleischhammer J, Molliqaj G, et al. Robotic vs fluoroscopy-guided pedicle screw insertion for metastatic spinal disease: a matched-cohort comparison. Neurosurg Focus 2017; (5): E13. [DOI] [PubMed] [Google Scholar]
- 85.Schroder ML, Staartjes VE. Revisions for screw malposition and clinical outcomes after robot-guided lumbar fusion for spondylolisthesis. Neurosurg Focus 2017; (5): E12. [DOI] [PubMed] [Google Scholar]
- 86.Bozkurt M, Apaydin N, I.ik C, et al. Robotic arthroscopic surgery: a new challenge in arthroscopic surgery Part I: Robotic shoulder arthroscopy; a cadaveric feasibility study. Int J Med Robot 2011; : 496–500. [DOI] [PubMed] [Google Scholar]
- 87.Wiewiorski M, Valderrabano V, Kretzschmar M, et al. CT-guided robotically assisted infiltration of foot and ankle joints. Minim Invasive Ther Allied Technol 2009; : 291–296. [DOI] [PubMed] [Google Scholar]
- 88.Garcia P, Rosen J, Kapoor C, et al. Trauma Pod: a semi-automated telerobotic surgical system. Int J Med Robot 2009; : 136–146. [DOI] [PubMed] [Google Scholar]
- 89.Hung SS, Lee MY. Functional assessment of a surgical robot for reduction of lower limb fractures. Int J Med Robot 2010; : 413–421. [DOI] [PubMed] [Google Scholar]
- 90.Dagnino G, Georgilas I, Ko.hler P, et al. Navigation system for robot-assisted intraarticular lower-limb fracture surgery. Int J CARS 2016; : 1,831–1,843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dagnino G, Georgilas I, Morad S, et al. Image-guided surgical robotic system for percutaneous reduction of joint fractures. Ann Biomed Eng 2017; (11): 2,648 2,662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oszwald M, Westphal R, Klepzig D, et al. Robotized access to the medullary cavity for intramedullary nailing of the femur. Technol Health Care 2010; : 173–180. [DOI] [PubMed] [Google Scholar]
- 93.Lei H, Sheng L, Manyi W, et al. A biplanar robot navigation system for the distal locking of intramedullary nails. Int J Med Robot 2010; : 61–65. [DOI] [PubMed] [Google Scholar]
- 94.Mantovani G, Liverneaux P, Garcia JC Jr, et al. Endoscopic exploration and repair of brachial plexus with telerobotic manipulation: a cadaver trial. J Neurosurg 2011; : 659–664. [DOI] [PubMed] [Google Scholar]
- 95.Garcia JC Jr, Lebailly F, Mantovani G, et al. Telerobotic manipulation of the brachial plexus. J Reconstr Microsurg 2012; : 491–494. [DOI] [PubMed] [Google Scholar]
- 96.Wang JQ, Wang Y, Feng Y, et al. Percutaneous sacroiliac screw placement: a prospective randomized comparison of robotassisted navigation procedures with a conventional technique. Chin Med J 2017; : 2,527–2,534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Davies B. Robotic surgery. A personal view of the past, present and future. Int J Adv Robot Syst 2015; : 54; 1–6. [Google Scholar]
- 98.Trehan A, Dunn TJ. The robotic surgery monopoly is a poor deal. BMJ 2013; : f7470. [DOI] [PubMed] [Google Scholar]
- 99.Swank ML, Alkire M, Conditt M, Lonner JH. Technology and cost-effectiveness in knee arthroplasty: computer navigation and robotics. Am J Orthop (Belle Mead NJ) 2009; : 32–36. [PubMed] [Google Scholar]
- 100.Moschetti WE, Konopka JF, Rubash HE, Genuario JW. Can robot-assisted unicompartmental knee arthroplasty be cost. effective? A Markov decision analysis. J Arthroplasty 2016; (4): 759–765. [DOI] [PubMed] [Google Scholar]
- 101.Park SE, Lee CT. Comparison of roboticassisted and conventional manual implantation of a primary total knee arthroplasty. J Arthroplasty 2007; : 1,054–1,059. [DOI] [PubMed] [Google Scholar]
- 102.Gao S, Lv Z, Fang H. Robot-assisted and conventional freehand pedicle screw placement: a systematic review and metaanalysis of randomized controlled trials. Eur Spine J 2017; . [DOI] [PubMed] [Google Scholar]
- 103.Jassim SS, Benjamin-Laing H, Douglas SL, Haddad FS. Robotic and navigation systems in orthopaedic surgery: How much do our patients understand? Clin Orthop Surg 2014; (4): 462–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Karthik K, Colegate-Stone T, Dasgupta P, et al. Robotic surgery in trauma and orthopaedics: a systematic review. Bone Joint J 2015; (3): 292–299. [DOI] [PubMed] [Google Scholar]


