Abstract
Introduction
Studies have reported on the use of frailty as a prognostic indicator in patients undergoing elective surgery. Similar data do not exist for patients undergoing emergency surgery. The aim of this study was to evaluate the effect of preoperative sarcopenia measured by computed tomography (CT) on outcome following emergency laparotomy.
Materials and methods
Data from the National Emergency Laparotomy Audit database were retrieved for patients who had undergone an emergency laparotomy over 12 months at York NHS Foundation Trust. Sarcopenia was assessed by psoas density and area on preoperative CT. Mortality rates at 30 days and 1 year were recorded. Secondary outcomes included discharge rates to non-independent living.
Results
A total of 259 patients were included. Overall cohort 30-day and 1-year mortality was 13.9% (36/259) and 28.2% (73/259), respectively. Sarcopenia measured by psoas density was associated with increased mortality compared with patients who did not develop sarcopenia at 30 days (29.7%, 19/64, vs. 8.7%, 17/195; P < 0.001; odds ratio, OR, 4.42; 95% confidence interval, CI 2.13–9.26) and at 1 year (57.8%, 37/64, vs. 18.5%, (36/195; P < 0.001; OR 6.05; 95%CI 3.28–11.18). An increase in mortality was seen in patients with sarcopenia measured by psoas area at 30 days (21.3%, 13/61, vs. 9.1%, 17/187; OR 2.71; 95%CI 1.23–5.96, P = 0.013) and at 1 year (42.6%, 26/61, vs. 20.9%, 39/187; OR 2.82; 95% CI 1.52–5.23, P < 0.001).
Conclusions
Sarcopenia assessed by measurement of psoas density and area on CT is associated with increased mortality following emergency laparotomy. The use of sarcopenia as a predictive tool merits further attention and may be useful in patients undergoing emergency surgery.
Keywords: Laparotomy, Sarcopenia, Emergency, Frail elderly
Introduction
The concept of frailty has received little attention in the emergency surgical patient. Frailty represents a state of decreased physiological reserve that results from the cumulative decline of multiple systems, reducing a patient’s ability to respond to stressors. Frailty has been shown to predict adverse events and outcome, particularly in elderly populations.1–4 In the setting of a medical geriatric admission, comprehensive geriatric assessment is often quoted as the gold standard of frailty assessment5 and has been shown to be of relevance to the perioperative care of surgical patients.6 Unfortunately this and other frailty assessments can be cumbersome and are not suited to surgery in an emergency setting.1–4 An alternative approach is to measure sarcopenia as a surrogate measure of frailty. The term sarcopenia was first described by Rosenberg in 1989 to describe the phenomenon of reduced muscle mass and weakness in older adults.7 Sarcopenia has been shown to be a mediator of frailty affecting the physical, social and metabolic domains.8–10 Evidence suggests that sarcopenia, measured using various parameters from computed tomography (CT) in elective surgical patients, may be a reliable indicator of frailty.
It is now routine clinical practice for most patients about to undergo non-resuscitative emergency laparotomy to undergo CT imaging of the abdomen. These data are now routinely collected as part of the National Emergency Laparotomy Audit (NELA), which commenced in the UK in 2015. In 2016, all 187 hospitals in England and Wales that perform emergency laparotomies submitted data to this audit.11
This study reports the results of a consecutive series of patients who underwent emergency surgery in one NHS trust and for whom data was entered into the NELA database. Our aim was to evaluate sarcopenia assessed from preoperative CT imaging and to relate this measure of frailty to postoperative outcomes.
Materials and methods
This study included a consecutive series of patients who required emergency laparotomy over a 12-month period from October 2014 to October 2015 across York NHS Foundation Trust hospitals. Patients and their data were identified from the NELA database, where data is prospectively entered. Patients were excluded from analysis if no abdominal CT had been performed within 30 days of surgery. The primary outcome was mortality at 30 days and 1 year. A secondary outcome measure was discharge to non-independent living, which was defined as a patient being admitted from their own residence and being discharged to a non-acute institution such as a rehabilitation unit or residential care. For this secondary outcome, patients were excluded from analysis if they did not survive to discharge or were admitted from dependant living.
In this study, sarcopenia was assessed at the L3 level on the first slice in which both transverse processes were visible. Sarcopenia was assessed in two ways, using average psoas density and area at this level. Psoas density was calculated by the creation of a ‘region of interest’ around each psoas muscle at L3 level. The imbedded software then calculated an average density for each psoas muscle in Hounsfield units. The average of these values was taken to give the psoas density value, as previously described.12,13 Psoas area was measured after standardisation for body surface area, which requires the documentation of anthropometric data. Patients for whom this anthropometric data were not available were excluded from this assessment.
As no cohort-specific cut off values for sarcopenia exist, we defined sarcopenia as the bottom quartile for psoas density analysis and the bottom sex-specific quartile for psoas area analysis, in line with previous studies. Data were analysed using SPSS for Windows® version 24 software with demographic variables being analysed using Chi-squared or t-test calculation. OR were calculated using binary logistic regression and 95% CIs were reported. Results adjusted for age, body mass index (BMI), malignant diagnosis and sex were also reported. These variables were chosen as they represent ‘hard’ endpoints that are not co-linear. A P-value of less than 0.05 was taken to signify statistical significance. Kaplan–Meier survival analysis was performed and data were presented as uncensored survival curves for the period of follow-up of a minimum of 1 year.
Results
A total of 309 consecutive patients undergoing emergency non-resuscitative laparotomy were identified from the NELA database during the specified time period, of whom 259 underwent preoperative CT. A further 11 patients had no documented anthropometric data leaving a total of 248 for psoas area analysis. A further 40 patients from the psoas density group and 37 from the psoas area group were excluded from analysis of the secondary outcome as they were either admitted from non-independent living or did not survive to discharge. Overall cohort 30-day and 1-year mortality were 13.9% (36/259) and 28.2% (73/259), respectively. Diagnoses at operation are displayed in Table 1.
Table 1.
Patient diagnoses.
| Diagnosis | Patients (n) |
| Obstruction | 141 |
| Perforation | 83 |
| Ischaemia | 23 |
| Haemorrhage | 8 |
| Colitis | 4 |
Psoas density
Sarcopenia measured from psoas density was identified in 64 of 259 patients. There was a significant difference in the age of patients between the sarcopenic and non-sarcopenic group (75 years vs. 68 years, respectively, P < 0.001) and postoperative length of stay (17 days vs. 13 days, P = 0.001). Additional patient demographics are summarised in Table 2.
Table 2.
Patient demographics.
| Psoas densitya | Psoas areab | ||||||
| Sarcopenia | No sarcopenia | P-value | Sarcopenia | No sarcopenia | P-value | ||
| Patients (n) | 64 | 195 | 61 | 187 | |||
| Median age (years) | 75 (SD 13.9) | 68 (SD 16.2) | 0.001 | 72 (SD 15.7) | 70 (SD 16.0) | 0.180 | |
| Body mass index | 25 (SD 5.0) | 25 (SD 6.9) | 0.410 | 24.4 (SD 6.1) | 25.6 (SD 6.6) | 0.220 | |
| Malignant diagnosis (n) | 18 | 35 | 0.790 | 15 | 36 | 0.380 | |
| Sex ratio M : F | 37 : 28 | 88 : 106 | 0.110 | 30 : 31 | 90 : 97 | 0.880 | |
| Length of stay (days) | 17 (SD 29.1) | 13 (SD 16.2) | < 0.001 | 15 (SD 22.3) | 14 (SD 20.5) | 0.090 | |
| Preoperative P-POSSUM mortality | 15% | 7% | < 0.001 | 6.3% | 8.3% | 0.843 | |
*psoas density <26 Hounsfield Units
**psoas area <3.29cm2/m2 for men and <2.71cm2/m2 for women
There was a significant difference in 30-day mortality between sarcopenic and non-sarcopenic groups (29.7%, 19/64, vs. 8.7%, 17/195; OR 4.42; 95% CI 2.13–9.26, respectively, P < 0.001). OR adjusted for age, BMI, malignant diagnosis and sex was 5.24 (95% CI 2.20–12.47, P < 0.001). Median preoperative P-POSSUM predicted mortality in the two groups was 15% vs. 7%, respectively. The results for 1-year mortality were 57.8% (37/64) compared with 18.5% (36/195; OR 6.05, 95% CI 3.28–11.18, P < 0.0001). OR adjusted for age, BMI, malignant diagnosis and sex was 6.34 (95% CI 3.12–12.86, P < 0.001). Kaplan–Meier survival analysis for psoas density is shown in Figure 1. With respect to discharge to non-independent living, there was a significant difference between the sarcopenic and non-sarcopenic groups (31%, 13/42, vs. 10.1%, 18/177; OR 3.96; 95% CI 1.75–8.95; P = 0.001). OR adjusted for age, BMI, malignant diagnosis and sex was 2.715 (95% CI 1.12–6.56, P = 0.026).
Figure 1.
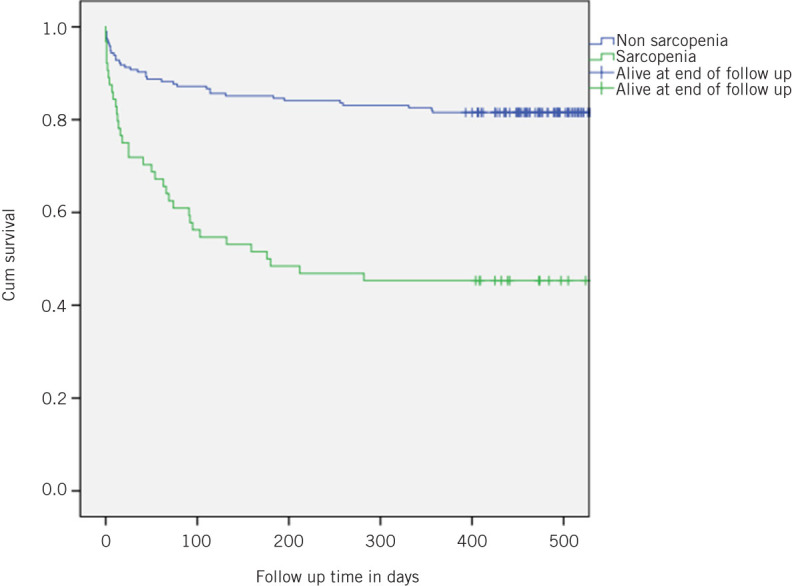
Kaplan–Meier survival plot of patients with sarcopenia compared with those without sarcopenia as measuredby psoas density.
Psoas area
Sarcopenia defined by psoas area was identified in 61 of 248 patients. There was no significant difference between the groups in any demographic variable, including age (72 years vs. 70 years, P = 0.18). These data are summarised in Table 2.
Mortality at 30 days in the sarcopenic group was 21.3% (13/61) compared with 9.1% (17/187) for the non-sarcopenic group (OR 2.71; 95% CI 1.23–5.96; P = 0.013). OR adjusted for age, BMI, malignant diagnosis and sex was 2.77 (95% CI 1.21–6.40; P = 0.016). The median preoperative P-POSSUM predicted mortality was 6.7% vs. 8.3%, respectively. Results for 1-year mortality were 42.6% (26/61) compared with 20.9% (39/187), respectively (OR 2.82; 95% CI 1.52–5.23; P < 0.001). OR adjusted for age, BMI, malignant diagnosis and sex was 2.84 (95% CI 1.45–5.59; P = 0.002). Kaplan–Meier survival analysis for this group is illustrated in Figure 2. There was no difference between the sarcopenic and non-sarcopenic groups assessed by psoas area regarding the numbers of patients discharged to non-independent living (15.6%, 7/45, vs. 14.5%, 24/166, respectively; P = 0.850).
Figure 2.
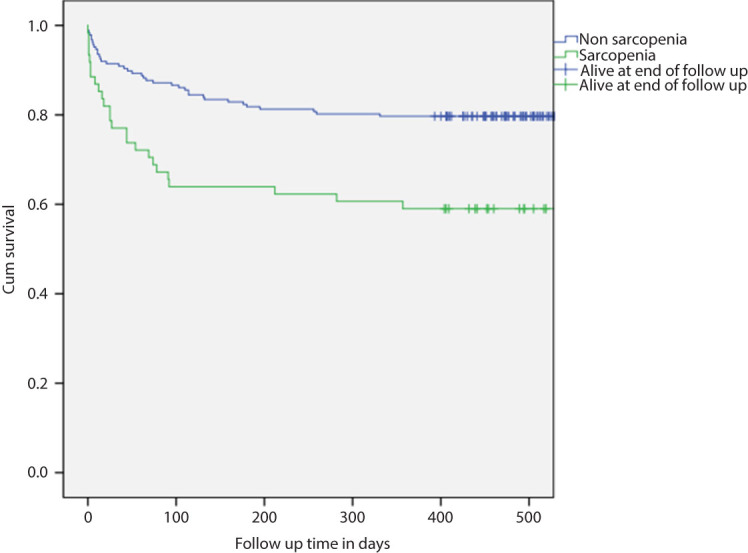
Kaplan-Meier survival plot of patients with sarcopenia compared with those without sarcopenia as measured by psoas area.
Discussion
The results of this study suggest that CT-defined sarcopenia is associated with an increased 30-day and one-year mortality rate following emergency laparotomy. Further, sarcopenic patients who survive surgery often fail to regain their preoperative level of function and frequently require admission to long-term residential care. P-POSSUM scores appear to significantly underestimate the mortality rate of this group of patients when compared to assessments of sarcopenia.
Preoperative assessment of patients scheduled for elective surgery is now standard practice. This is driven to some extent by the publication of surgeons’ outcome data. Not surprisingly, surgeons become reluctant to embark upon surgical procedures when the probability of adverse outcomes is high. Patients are now subjected to intensive investigation and assessment in the form of cardiopulmonary exercise testing, echocardiography and consultant anaesthetic evaluation. In some units,6 elderly patients undergo a formal comprehensive geriatric assessment, as there is increasing recognition that frailty syndrome is associated with a poor outcome but may be manipulated positively through the assessment process.
These assessment techniques, however, are often time consuming and may be inappropriate in the emergency situation, in which clinicians often rely on risk scoring formulae such as P-POSSUM to aid decision making. P-POSSUM and similar prognostic indices based on physiological parameters have been validated but suffer the common drawback that results are very variable depending on the state of physiological compromise and the efficacy of resuscitation received up until the time of measurement.
The measurement of sarcopenia using CT imaging as a measure of frailty is gaining increasing recognition and appears to positively correlate with postoperative outcome in elective patients.14 Previous studies have demonstrated a close concordance (> 90%) of psoas area at L3 with total lean body mass as measured by total body radiological imaging.15 There are many techniques described in the literature to measure sarcopenia but the method described in this study has the advantage that it does not require specialist radiological software and is rapid to perform. Some other ‘single slice methods’ include all muscles in the lumbar area but need expensive additional software or specialist input, rendering them unrealistic as a tool to aid decision making and consent in the emergency setting.15 Although there is no accepted definition of sarcopenia, the consensus statements suggest that it is best defined with a both a measure of muscle mass and function such as gait speed.16–18 Again, in an elective or research setting, this may be ideal. It is improbable that a measure of muscle function such as gait speed or hand-grip strength can be relied upon in a patient with sepsis or generalised peritonitis.
Whether or not to operate on elderly critically ill patients often with extensive comorbidity can pose significant ethical dilemmas for surgeons. Often, the decision to operate is easier than the decision not to operate and this defers difficult questions about quality of life. The reality is that many frail patients never regain social independence and may take many months to overcome the effects of an acute operation. The perturbing result from our data is that sarcopenia defined by psoas density is associated with a one-year mortality rate of 57%. In the new post-Montgomery world of informed consent,19 this information may help patients, families and surgeons to weigh up the risk–benefit ratio of emergency laparotomy.
One of the key NELA recommendations is that patients over 70 years of age would have routine input from physicians and other healthcare professionals to perform a comprehensive geriatric assessment. Unfortunately, this assessment is time consuming, expensive, multidisciplinary and rarely available out of hours. It is an unwieldy tool often ill suited to the rapidly changing physiology of patients awaiting emergency surgery. The reality is that only 10% of patients receive this care. If it is not possible to provide this service to all patients who undergo an emergency laparotomy, frailty assessment by CT sarcopenia may help to target this assessment to those who might potentially benefit the most.
A limitation to this study is that the adoption of the lower limit (25% confidence limit) as a cut-off point to define clinically significant sarcopenia was arbitrary. However, we justified this on the basis that no comparable data are available for this study population and we recognise that additional data are required to define ‘normality’ accurately. The reason why one measure of CT sarcopenia (psoas density) seems to predict higher rates of adverse outcome than another (psoas area) remains unclear, although both are associated with a significant increase in mortality. One suggestion may be that as an individual becomes sarcopenic, they lose ‘muscle quality’ not just muscle bulk, as lean muscle is replaced by adipose tissue. This has been previously described and termed myosteatosis.20 Psoas density gives an assessment of muscle quality, not just volume, and therefore may be a more sensitive instrument for sarcopenia and frailty detection than area alone. Finally, we accept the potential criticism that sarcopenia is nothing more than a surrogate marker for age. However, we consider this to be unlikely in our study. Our results demonstrate that although the patients deemed sarcopenic by psoas density were significantly older than those who were not, when adjusted for age and other factors sarcopenia remained an independent predictor for 30-day and 1-year morality. This suggests that the metabolic effects of sarcopenia exist independently of biological age.
A second limitation to this study is its retrospective nature. With this in mind, we have used variables and outcome measures that are definite, such as age and mortality. It is of clear interest to investigate the effect of sarcopenia in this cohort on morbidity rates and other outcomes or variables. We consider that, to accurately undertake this, a prospective study where validity of data can be controlled would be required and this is our intention.
Only one previous study has reported on the possible prognostic role of sarcopenia in patients about to undergo emergency surgery. This study used psoas area assessment and failed to confirm an association between sarcopenia and poor outcome on multivariate analysis, possibly because overall mortality was very high (almost 50% of all patients were American Society of Anesthesiologists score of III or IV).21 Further, as stated above, it is possible that the use of psoas area is less sensitive than psoas density.
Conclusions
In conclusion, sarcopenia defined by CT imaging appears to be a convenient and accurate prognostic indicator in the emergency surgical patient. The measurement of psoas density is technically simple and could easily be incorporated into routine clinical practice. Further work is required to determine threshold values of sarcopenia on a population basis and in different clinical scenarios.
References
- 1.Fried LP, Tangen CM, Walston J et al. Frailty in older adults: evidence for a phenotype. 2001; : M146–M156. [DOI] [PubMed] [Google Scholar]
- 2.Rockwood K, Howlett SE, MacKnight C et al. Prevalence, attributes, and outcomes of fitness and frailty in community-dwelling older adults: report from the Canadian study of health and aging. 2004; : 1,310–1,317. [DOI] [PubMed] [Google Scholar]
- 3.Bandeen-Roche K, Xue QL, Ferrucci L et al. Phenotype of frailty: characterization in the women’s health and aging studies. 2006; : 262–266. [DOI] [PubMed] [Google Scholar]
- 4.Ensrud KE, Ewing SK, Taylor BC et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. 2008; : 382–389. [DOI] [PubMed] [Google Scholar]
- 5.Ellis G, Whitehead MA, O’Neill et al. Comprehensive geriatric assessment for older adults admitted to hospital. 2011; (7): CD006211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Partridge JS, Harari D, Martin FC et al. Randomized clinical trial of comprehensive geriatric assessment and optimization in vascular surgery. 2017; (6): 679–687. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg I. Summary comments: epidemiological and methodological problems in determining nutritional status of older persons. 1989; : 1,231–1,233. [Google Scholar]
- 8.Beaudart C, McCloskey E, Bruyère O et al. Sarcopenia in daily practice: assessment and management. 2016; : 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janssen I, Heymsfield S, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. 2002; : 889–889. [DOI] [PubMed] [Google Scholar]
- 10.Cooper C, Dere W, Evans W et al. Frailty and sarcopenia: definitions and outcome parameters. 2012; : 1,839–1,848. [DOI] [PubMed] [Google Scholar]
- 11.National Emergency Laparotomy Audit Project Team . London: Royal College of Anaesthetists; 2017. [Google Scholar]
- 12.Buettner S, Wagner D, Kim Y et al. Inclusion of sarcopenia outperforms the modified frailty index in predicting 1-year mortality among 1,326 patients undergoing gastrointestinal surgery for a malignant indication. 2016; (4): 397–407. [DOI] [PubMed] [Google Scholar]
- 13.Mok M, Allende R, Leipsic J et al. Prognostic value of fat mass and skeletal muscle mass determined by computed tomography in patients who underwent transcatheter aortic valve implantation. 2016; (5): 828–823. [DOI] [PubMed] [Google Scholar]
- 14.Jones K, Doleman B, Scott S et al. Simple psoas cross-sectional area measurement is a quick and easy method to assess sarcopenia and predicts major surgical complications. 2015; (1): 20–26. [DOI] [PubMed] [Google Scholar]
- 15.Shen W, Punyanitya M, Wang Z et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. 2004; (6): 2,333–2,338. [DOI] [PubMed] [Google Scholar]
- 16.Fielding RA, Vellas B, Evans WJ et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia. 2011; (4): 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morley J, Abbatecola A, Argiles J et al. Sarcopenia with limited mobility: an international consensus. 2011; (6): 403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L, Liu L, Woo J et al. (2014) Sarcopenia in Asia: consensus report of the Asian working group for sarcopenia. 2014; : 95–101. [DOI] [PubMed] [Google Scholar]
- 19.Fryar C. Clarifying the Montgomery judgment. 2015; : h2217. [DOI] [PubMed] [Google Scholar]
- 20.Malietzis G, Currie AC, Athanasiou T. Influence of body composition profile on outcomes following colorectal cancer surgery. 2016; (5): 572–580. [DOI] [PubMed] [Google Scholar]
- 21.Dirks R, Edwards B, Tong E et al. Sarcopenia in emergency abdominal surgery. 2017; : 13–21. [DOI] [PubMed] [Google Scholar]


