Abstract
We report a clinical case of African tick-bite fever in a Brazilian traveler right after his return from South Africa. Definitive diagnosis was supported by seroconversion between acute-phase and convalescent-phase serum samples, detection of rickettsial DNA in skin lesions, and in vitro culture of Rickettsia africae from the patient’s skin. Most of the previous reported cases of African tick-bite fever were confirmed solely by serological or/and molecular methods. Through this first confirmed case of African tick-bite fever in Brazil, it is quite possible that other cases are occurring unnoticed by the health authorities, requiring a greater vigilance in traveler’s medicine in South America.
Keywords: Rickettsia africae, spotted fever, rickettsiosis, Brazil
Introduction
African tick-bite fever (ATBF), caused by Rickettsia africae, is the most common tick-borne bacterial zoonosis in sub-Saharan Africa,1 where two of its main vectors, the ticks Amblyomma variegatum (central Mozambique northwards) and Amblyomma hebraeum (mostly in South Africa), are usually found to have high infection rates in nature (>50%) and have significant aggressiveness to bite humans.1
The clinical profile of ATBF consists of an abrupt onset of fever, fatigue, headache, and myalgia, approximately 5–7 days following a bite by an infected tick. Inoculation eschars are identified in 50%−100% of cases. Other common features include regional lymphadenopathy, and generalized maculopapular or papulovesicular rash. Fatal cases have not been reported.1
ATBF has been reported in a number of European travelers returning from Africa2–5 and was the second most frequently identified etiology, after malaria, among febrile travelers returning from sub-Saharan Africa.1,6 On the other hand, ATBF has never been reported in South American travelers. Herein, we describe a clinical case of ATBF in a Brazilian traveler returning from South Africa.
Case report
A 32-year-old Brazilian white man was admitted to the emergency department of the Hospital of Clinics of the University of Campinas, Campinas City, Brazil, presenting an one-day history of fever (without chills), headache, myalgia, asthenia, diarrhea (one episode) and tenderness in right inguinal region. He had returned 2 days earlier from a 2-week trip to South Africa, when he did not recall a tick bite but did remove a tick from his back-pack after a day-trip to Hluhluwe-iMfolozi Park, a natural reserve in South Africa, 280 kilometers north of Durban. No repellent or malaria chemoprophylaxis was used. On physical examination, he presented well, alert; axillar temperature was 37.2°C, blood pressure 110 x 70 mmHg, respiratory frequency rate of 20 ipm, and cardiac pulse rate of 70 beats/min. Jaundice and conjunctival changes were not present. Lungs were clear and cardiac examination was normal. Abdomen was soft and non-tender; there was no hepatomegaly or splenomegaly. He presented non-pruritic maculopapular skin rash on trunk and arms, and additionally, a 2.5 cm erythematous papular lesion with a red halo and central purpuric necrotic eschar on the right iliac crest region (Figure 1). An enlarged, tenderness, not fluctuating lymph node was palpated on the right inguinal region.
Figure 1.
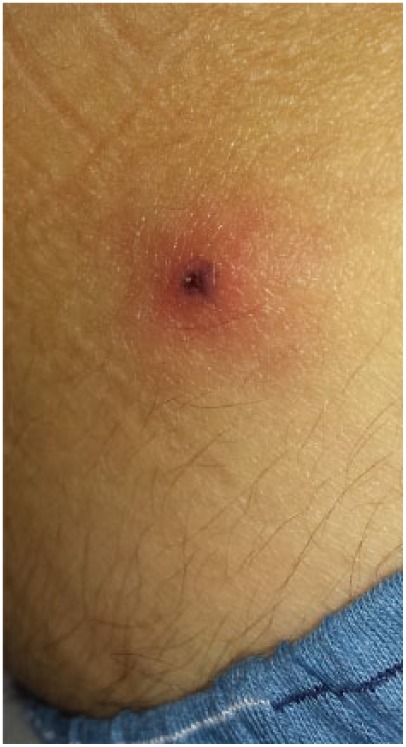
Eschar lesion on the right iliac crest region of an African tick-bite fever patient.
Initial routine laboratory findings included alanine aminotransferase 17 IU/L, aspartate aminotransferase 20 IU/L, creatine kinase 84 IU/L, lactate dehydrogenase 183 IU/L, bilirubin 0.6 mg/dL, blood urea nitrogen 24 mg/dL, creatinine 0.79 mg/dL, hemoglobin 14.8 g/L, hematocrit 44.3%, white blood cell count 6390 cells/mm3 (62% polymorphonuclear, 21.4% lymphocytes, 13.6% monocytes, 2.7% eosinophils, 0.3% basophils), platelets 142,000/mm3.
Aseptically skin punch biopsy of the eschar was performed in the first visit of the patient at the emergency department, and two fragments were collected: one fragment was put in brain–heart infusion (BHI) and was frozen at −70°C for microbiological procedures, and the other was fixed in formalin solution for routine histology. Histopathological analysis of the eschar revealed perivascular superficial and deeper unspecific inflammatory process with swelling and focal necrosis of endothelial cells of small dermal vessels blood cell extravasation. Thick and thin blood smears were negative for parasites. Whole blood and blood clot were collected (in BHI solution and frozen at −70°C) for microbiological procedures and serum samples for serological tests (spotted fever group rickettsiae, dengue, yellow fever, leptospirosis), malaria and dengue-NS1 point-of-care rapid tests.
An empiric outpatient antimicrobial 7-days course therapy with doxycycline PO 200 mg/day was prescribed. He became afebrile on second day of antibiotic treatment; the other symptoms resolved in the following days or weeks.
Serologic evaluation of paired (acute and convalescent) serum samples to detected anti-Rickettsia spp. IgG antibodies was performed by indirect immunofluorescence assay (IFA) by using antigens of six Rickettsia isolates from Brazil.7 The first serum sample of acute phase (day 2 of disease) showed no reactivity for any rickettsial antigen at the 1:64 serum dilution. The second serum sample—collected at convalescence phase (18 days after the first serum sample)—demonstrated seroconversion with the following endpoint titers for rickettsial antigens: R. rickettsii 512, R. parkeri 512, R. amblyommatis 1024, R. felis < 64, R. rhipicephali 256, and R. bellii 1024. All the other serological tests, and antigen detection (for malaria and dengue-NS1) and routine blood cultures were negative.
The frozen skin biopsied fragment was thawed in BHI, macerated, and processed by the shell vial technique for isolation of rickettsiae, as described.8,9 Briefly, cultures of Vero cells were inoculated with 200 µL of the eschar homogenate, and incubated at 28°C. The percentage of Vero cells infected with rickettsiae was monitored by the use of Giménez staining of cells scraped from each inoculated monolayer. After the establishment of the isolate in the laboratory (i.e., at least three cell passages, with the prevalence of infected cells exceeding 90%), rickettsial DNA was extracted from the infected cells by using the DNeasy Blood and Tissue kit (Qiagen, Chatsworth, CA, USA). The extracted DNA was tested in a battery of different polymerase chain reaction (PCR) protocols to amplify fragments of the rickettsial genes citrate synthase (gltA) (primers CS-78, CS-323, and primers CS-239, CS-1069), 17-kDa membrane protein (htrA) (primers 17k-5 and 17k-3), and the outer membrane proteins ompA (primers Rr190.70p, Rr190.701) and ompB (primers 120-M59, 120–807), as described.9–11 PCR products were purified using ExoSAP-IT (USB Corp., Cleveland, OH, USA) and underwent DNA sequencing in an ABI automated sequencer (Applied Biosystems/Perkin Elmer, model ABI Prism 3500 Genetic, Foster City, CA, USA), and the resultant sequences were compared with GenBank data by BLAST analysis (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Part of the eschar homogenate was submitted to DNA extraction by the DNeasy Tissue Kit, and tested by the above PCR protocols.
Viable rickettsiae were successfully isolated from the eschar, and established in the laboratory with several passages, each one reaching > 90% infection of the cells. The isolate, designated as strain PELE, has been cryopreserved and deposited at the Rickettsial Collection of our laboratory. PCR amplicons were generated by the four PCR protocols. The Vero cell isolate and the eschar remnants yielded identical DNA sequences for each ricketsial gene. Their gltA (1061-bp), htrA (482-bp), ompA (590-bp), and ompB (818-bp) gene fragments were 99.8%−100% equal to corresponding sequences of R. africae strain ESF-5 (GenBank accession number CP001612). DNA sequences generated in the present study have been submitted to GenBank under the following accession numbers: MG515012 (gltA), MG515013 (htrA), MG515014 (ompA), and MG515015 (ompB).
Discussion
The present report, to our knowledge, is the first confirmed case of ATBF in a South American traveler that returned from sub-Saharan Africa. Despite the fact that R. africae has been identified in at least 22 sub-Saharan countries,1 the well-reported relevance of ATBF among febrile travelers, and the increasing number of people from all over the world traveling for different purposes (including ecotourism and outdoor activities) to potential endemic areas for ATBF,2,6 the real number of cases, incidence, morbidity of this rickettsial disease among travelers worldwide is not known, and certainly underestimated. This probably results from a somatization of elements: a usually mild disease, not rarely self-limited; a poor clinician awareness with a possible misdiagnosis; and a limited access to laboratorial tools for diagnosis. Interestingly, some years ago, four cases of spotted fever illness were reported in Argentinean travelers returning from South Africa.12 While the clinical profiles of the cases were compatible with ATBF, there was no confirmatory diagnosis for R. africae or any other spotted fever-specific agent.
In the present report, some factors contributed to the early suspicion, opportune treatment—early and empiric therapy with the first line antimicrobial, doxycycline—and appropriated laboratorial investigation. Interestingly, we have previously reported an imported and fatal case of Mediterranean spotted fever in a Portuguese traveler that arrived at Brazil, which was also confirmed by rickettsial isolation and molecular detection.13 The recognition that eschar (tache noire) as an important clue for diagnosis of some rickettsial diseases was essential for the suspicion of the case presented here. Because of the cross-reactivity of antibody responses to spotted fever group Rickettsia species,1 clinicians must be aware of the importance of submitting appropriate samples for molecular detection or isolation techniques when available, as serology alone is not sufficient to identify the species responsible for infection. Thanks to the appropriated collection of samples in this report, we could isolate and identified R. africae as the etiological agent.
Conclusion
Through this first confirmed case of ATBF, it is quite possible that other cases are occurring unnoticed by the health authorities, requiring a greater vigilance in traveler’s medicine in South America.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors received financial support from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/PROEX 1841/2016).
Informed consent: Written informed consent was obtained from the patient for his anonymized information to be published in this article.
ORCID iD: Marcelo B Labruna  https://orcid.org/0000-0002-9675-3132
https://orcid.org/0000-0002-9675-3132
References
- 1. Parola P, Paddock CD, Socolovschi C, et al. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev 2013; 26: 657–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jensenius M, Fournier PE, Vene S, et al. African tick bite fever in travelers to rural sub-equatorial Africa.Clin Infect Dis 2003; 36: 1411–1417. [DOI] [PubMed] [Google Scholar]
- 3. Althaus F, Greub G, Raoult D, et al. African tick-bite fever: a new entity in the differential diagnosis of multiple eschars in travelers: description of five cases imported from South Africa to Switzerland. Int J Infect Dis 2010; 14(Suppl. 3): e274–126. [DOI] [PubMed] [Google Scholar]
- 4. Oteo JA, Portillo A, Blanco JR, et al. Rickettsia africae infection: three cases confirmed by PCR. Med Clin (Barc) 2004; 122: 786–788. [DOI] [PubMed] [Google Scholar]
- 5. Raoult D, Fournier PE, Fenollar F, et al. Rickettsia africae, a tick-borne pathogen in travelers to sub-Saharan Africa. N Engl J Med 2001; 344: 1504–1510. [DOI] [PubMed] [Google Scholar]
- 6. Freedman DO, Weld LH, Kozarsky PE, et al. Spectrum of disease and relation to place of exposure among ill returned travelers. N Engl J Med 2006; 354: 119–130. [DOI] [PubMed] [Google Scholar]
- 7. Barbieri AR, Filho JM, Nieri-Bastos FA, et al. Epidemiology of Rickettsia sp: strain Atlantic rainforest in a spotted fever-endemic area of southern Brazil. Ticks Tick-Borne Dis 2014; 5: 848–853. [DOI] [PubMed] [Google Scholar]
- 8. Marrero M, Raoult D. Centrifugation-shell vial technique for rapid detection of Mediterranean spotted fever rickettsia in blood culture. Am J Trop Med Hyg 1989; 40: 197–199. [DOI] [PubMed] [Google Scholar]
- 9. Labruna MB, Whitworth T, Horta MC, et al. Rickettsia species infecting Amblyomma cooperi ticks from an area in the State of São Paulo, Brazil, where Brazilian spotted fever is endemic. J Clin Microbiol 2004; 42: 90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eremeeva M, Yu X, Raoult D. Differentiation among spotted fever group rickettsiae species by analysis of restriction fragment length polymorphism of PCR-amplified DNA. J Clin Microbiol 1994; 32: 803–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roux V, Raoult D. Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer membrane protein rOmpB (ompB). Int J Syst Evol Micr 2000; 50: 1449–1455. [DOI] [PubMed] [Google Scholar]
- 12. Martino O, Orduna T, Lourtau L, et al. Rickettsioses do grupo das febres maculosas em viajantes argentinos. Rev Soc Bras Med Tro 2001; 34: 559–562. [DOI] [PubMed] [Google Scholar]
- 13. Gehrke FS, Angerami RN, Marrelli MT, et al. Molecular characterization of Mediterranean spotted fever rickettsia isolated from a European traveler in the state of São Paulo, Brazil. J Travel Med 2013; 20: 54–56. [DOI] [PubMed] [Google Scholar]


