Abstract
Background:
Overweight and obesity are significant public health concerns that are prevalent in younger age cohorts. Preventive or therapeutic interventions are difficult to implement and maintain over time. On the other hand, the majority of adolescents in the United States have a smartphone, representing a huge potential for innovative digitized interventions, such as weight loss programs delivered via smartphone applications. Although the number of available smartphone applications is increasing, evidence for their effectiveness in weight loss is insufficient. Therefore, the proposed study aims to assess the efficacy of a gamification-based smartphone application for weight loss in overweight and obese adolescents. The trial is designed to be a phase II, single-centre, two-arm, triple-blinded, randomized controlled trial (RCT) with a duration of 6 months.
Method:
The intervention consists of a smartphone application that provides both tracking and gamification elements, while the control arm consists of an identically designed application solely with tracking features of health information. The proposed trial will be conducted in an urban primary care clinic of an academic centre in the United States of America, with expertise in the management of overweight and obese adolescents. Eligible adolescents will be followed for 6 months. Changes in body mass index z score from baseline to 6 months will be the primary outcome. Secondary objectives will explore the effects of the gamification-based application on adherence, as well as anthropometric, metabolic and behavioural changes. A required sample size of 108 participants (54 participants per group) was calculated.
Discussion:
The benefits of the proposed study include mid-term effects in weight reduction for overweight and obese adolescents. The current proposal will contribute to fill a gap in the literature on the mid-term effects of gamification-based interventions to control weight in adolescents. This trial is a well-designed RCT that is in line with the Consolidated Standards of Reporting Trials statement.
Keywords: adolescents, clinical trial, gamification, obesity, overweight, smartphone application, study protocol, weight loss
Introduction
Obesity in childhood and adolescence is a current public health challenge that is rising worldwide.1,2 Although prevalence in adolescents worldwide is uncertain and variable across regions and countries, among adolescents living in the United States (US), aged 12–19 years, obesity has increased from 5% in 1976 to over 18.4% in 2010. These growing rates in the US are even more profound in Black and Latino groups, exceeding 20%.3 According to the National Health and Nutrition Examination Survey 2009–10 (NHANES) overweight and obesity in this age (12–19) bears nearly 30% overall, showing also sex, geographic and socioeconomic discrepancies.4,5 Studies in paediatric populations have demonstrated the importance of lifestyle programs on the management of obesity, and its relation to self-learning of healthy habits, self-monitoring of food intake and physical activity, to improve health outcomes.6,7 However, treatments in this age group face difficulties such as high attrition, poor adherence, and complex programs, limiting the efficacy of weight control treatments.8,9
In the US, 78% of teenagers have a smartphone, demonstrating a huge potential for digitized cardiovascular prevention and health promotion in the youth.10 Studies show that technology-based interventions on weight loss and quality of life for patients suffering from overweight or obesity are more efficient than standard care in the short term.11 The use of mobile applications can be beneficial in monitoring patients’ health behaviour, chronic disease symptoms, promoting medication adherence and raising self-awareness.12 Mobile and multimedia technologies also show a promising opportunity to enhance healthcare knowledge, health promotion and disease prevention. Systematic reviews show that technology-based interventions might be valid tools for weight loss, leading to higher program adherence and improved monitoring.12–14 Furthermore, smartphone applications may represent an option to improve health habits, and to motivate for physical activity and weight loss using gamification.15,16 A systematic review of game-based interventions in rehabilitation of diabetic patients showed a significant effect on health-related quality of life, balance, and strength, although there was no effect on glycated haemoglobin.17
Although many mobile applications claim to improve health outcomes, such as supporting weight loss, efficacy evidence and gamification methodological quality is insufficient.12,14,17–19 Other groups underline the need for full-scale summative evaluation studies of existing games.20 Despite the increasing interest in studying technology-based tools to prevent or manage obesity and overweight in adolescent, a lack of trials, small sample sizes and mixed results are still hampering a more standardized and evidence-based approach to this target population.21 Additionally, trials using standardized evaluations are limited and mostly focus on adult populations.22
Therefore, this study aims to assess the efficacy of a gamification-based smartphone application for weight loss in overweight and obese adolescents using a single-centre, two-arm, triple-blinded, phase II randomized controlled trial (RCT). The application combines tracking (monitoring) and gamification to develop a healthy lifestyle and can be a tool in the management of obesity in children and teenagers. In this trial protocol, a hypothetical smartphone application is considered.
Methods
Trial design
The trial will be a phase II, single-centre, two-arm, triple-blinded, RCT with a duration of 6 months. The proposed trial protocol was developed to answer the following research question: does a 6-month intervention, based on the use of a gamification-based smartphone application, on top of standard care, help overweight and obese adolescents to lose weight, in comparison with the combination of a passive tracking application and standard care alone? Figure 1 illustrates the PICOT23 (population, intervention, comparison, outcome, time) details for the proposed study.
Figure 1.
PICOT.
BMI, body mass index; PICOT, population, intervention, comparison, outcome, time.
Figure 2.
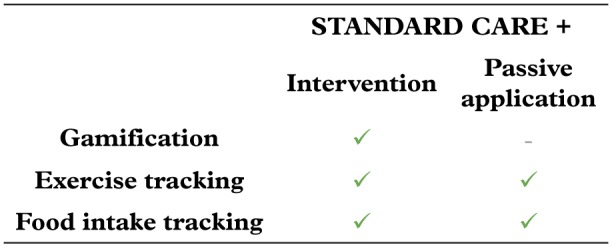
Features of the application in the intervention and control arm.
Figure 3.
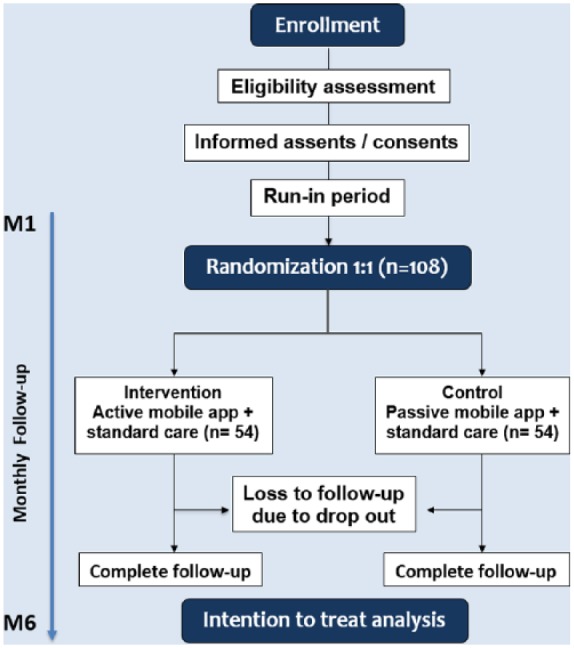
CONSORT flowchart of the suggested trial.
CONSORT, Consolidated Standards of Reporting Trials
Study setting
The trial will be conducted in an urban primary care clinic of an academic centre in the USA, with expertise in the management of overweight and obese adolescents.
Eligibility criteria
Patients who fulfill the following inclusion criteria will be eligible to be enrolled in the study:
12–18 years old
Fluent in English (due to study setting and language of the app)
Overweight [body mass index (BMI)-for-age > +1 standard deviation (SD)] or obese (BMI-for-age > +2 SD), according to the World Health Organization definition24,25
Daily access to a smartphone.
Patients fulfilling any of the following criteria will be excluded from the trial:
Secondary causes of obesity (e.g. endocrine abnormality)
Chronic diseases such as cardiovascular diseases, renal diseases, liver diseases, obstructive pulmonary diseases, classic symptoms of hyperglycaemia (polyuria, polyphagia, polydipsia and weight loss)
Severe acute illness, children receiving chemotherapy
Patients on pharmacologic intervention for weight reduction, children already participating in weight loss programs or already using lifestyle-targeting application
Patients that underwent any metabolic surgery
Obstructive sleep apnoea
Genetic syndromes
Neurodevelopmental abnormality, mental health disorders
Long-term use of medications
Neurological and physical disability.
Interventions
The intervention consists of a hypothetical smartphone application that provides both tracking and gamification elements, while the control arm consists of an identically designed application with solely tracking features of health information (Fig. 2).
Eligible patients will be randomized to use a specific smartphone application on top of standard care for a 6-month period. The hypothesis is that specific application features will improve engagement in lifestyle changes towards weight loss. The application uses a personalized approach and involves games and activities to educate children and adolescents about healthy eating and physical activity.
The application comprises tracking and monitoring functions using diaries of food intake (calories consumed each day in each meal), exercises (type and duration) and daily steps. Additionally, elements of gamification, such as challenges and points systems, are used in order to promote healthy nutrition choices and exercises.
Standard care will be left to the attending physician’s discretion in consonance with recommendations by 2017 Endocrine Society Clinical Practice Guideline on Paediatric Obesity,26 in which weight loss medications are not a part of initial management of obesity.
Standard care comprises monthly office visits as well as planned reinforcement for achieving targeted behaviour; a planned diet by a dietitian or a clinician, with balanced macronutrients in proportion with dietary reference intake recommendations; reduction of television and other screen time to less than an hour; supervised, planned, physical activity for 60 min per day.27
Adherence
To increase adherence, both the control and intervention arm will receive text messages one week prior to clinic appointments as well as phone contact the day before. Centers will provide an opportunity to reschedule missed appointments within 1 week.
A 1-month run-in period will be used to evaluate participants’ motivation and to educate participants on how to use the basic smartphone application (without gamification features), prior to randomization.
Process evaluation, as a part of the secondary analysis, will be measured to assess adherence of included participants. Interactions with the application (utilization) per week, time using the application and number of attendance to appointments will be recorded.
Timeline
After study enrollment, the eligibility criteria will be assessed for each participant according to the baseline clinical assessment in the first visit (Fig. 3). Written informed assent and consent will be taken from all participants and their parents or legal guardian, respectively.
Participants will be followed up for 6 months. Data for the primary outcome will be assessed at baseline and after 6 months while data for secondary outcomes will be collected monthly.
Randomization
Patients will be randomized to either the experimental or control arm with a 1:1 allocation ratio, through an online central randomization service. The randomization process will use permuted blocks of four, stratified according to school, in order to prevent covariate imbalances between groups. Allocation concealment will be ensured, as the service will not release the randomization code until the end of the trial. Access to the application will be given by independent access personnel. Throughout the study, the randomization will be conducted by the independent service account for blinding of data manager and statistician.
Blinding
The study will be designed as a triple-blinded trial. Participants, treating physicians and dietitians, as well as outcome evaluators and data analysts will be blinded.
Participants in both arms will receive standard care and access to a smartphone application that will be identical in terms of interface style and design. Care providers will participate in an intense training program before entering the study. Independent research personnel, not involved in patient care, will give access to the application to each participant. Blinding will be supported by the similarities of the smartphone application versions used for the intervention group and the control groups in terms of design and function.
To evaluate the robustness of blinding, participants will be asked after the trial ends to which group they anticipate they were initially assigned to.
Sample size calculation
In the light of observational study results, an expert panel of the US Preventive Services Task Force recommends a BMI z score reduction in the range of 0.20 to 0.25 to be a suitable threshold for clinically important changes.28 Considering a clinically meaningful difference of 0.25, power of 80%, α-level of 0.05, and a potential dropout rate of 20%, obtained from previous trials of similar methodology,29,30 a required sample size of 108 participants (54 participants per group) was calculated.
Recruitment
Recruitment will be based on two parallel strategies. Advertising in schools belonging to the district of the relevant primary care center will be conducted using posters, leaflets and a webpage. Additionally, teachers from the schools situated in the relevant districts will be involved.
In addition to school-based advertisements, the primary care center itself will be involved using posters, leaflets and a webpage. Practitioners and health staff will be motivated to approach patients during routine appointments.
Primary objective
The primary objective will be to determine if the gamification-based intervention delivered via a smartphone application plus standard care is superior to standard care combined with a tracking application in reducing BMI z score (as a measure adjusted for age and sex) from baseline to 6 months.
Secondary objectives
Measurements for secondary objectives will explore the effects of the gamification-based application on adherence, as well as on anthropometric, metabolic and behavioural changes. Including aspects derived from the Health Action Process Approach by Schwarzer and Fuchs will increase the understanding of the behaviour change process.31
In addition to the primary measurement, BMI z score will also be evaluated monthly to support understanding of behaviour change. Monthly anthropometric changes in the body shape index (ABSI, waist circumference normalized to height and weight) will also be verified. To assess cardiovascular risk factors, blood pressure, lipids and glycaemic status (fasting plasma glucose, glycated haemoglobin, insulin) will be evaluated at baseline and after 6 months.
On top of that, steps will be counted by a built-in feature of the application and a physical activity questionnaire for adolescents (PAQ-A) will be applied. This information will allow for classifying adolescents into different physical activity clusters and will help to investigate the connection between physical activity, health behaviour and health-related outcomes.32 Measurements of service utilization will be performed at baseline as well as after 3 and 6 months to support the understanding of the behaviour change process.
Readiness to change lifestyle, self-efficacy (‘generalized self-efficacy scale’) and quality of life [‘impact of weight on quality of life-kids’ (IWQOL-Kids)] will be measured. The IWQOL is a 27-item, self-administered, brief (taking around 8 min) and weight-related measure to assess quality of life in adolescents.33 Self-efficacy is seen as an indicator for coping strategies and confidence in weight loss treatments.34 The generalized self-efficacy scale developed by Schwarzer and colleagues will be applied.35 It will be enriched by using dimensions of accurate measurements appropriate for the target group in terms of age, sex and ethnic differences to cover specific elements of physical exercise self-efficacy.36,37
Adherence will also be analyzed by evaluating the self-reported frequency of application utilization and its most relevant features using a structured questionnaire.
Statistical analysis
The primary and secondary outcomes will be analyzed and compared between the two study groups. Distribution of data will be analyzed by visual (histograms and SD) and statistical normality tests (Shapiro–Wilk).
Changes in BMI z score from baseline to 6 months will be compared using an unpaired t test (Table 1). In the analysis of secondary outcomes, continuous variables will be analyzed with an unpaired t test, ordinal data will be analyzed using a Mann–Whitney U test and, for categorical variables, Fisher’s exact test or Chi-square test will be applied (Table 1).
Table 1.
Statistical analysis of outcomes.
| Outcome | Statistical test |
|---|---|
| Primary | |
| Change in BMI z score at 6 months | Unpaired t test |
| Secondary | |
| Monthly BMI z score | Repeated measures ANOVA |
| ABSI | Unpaired t test |
| Blood pressure | Unpaired t test |
| Glucose, lipidsa | Unpaired t test |
| IWQOL-Kids | Mann–Whitney U test |
| PAQ-A | Mann–Whitney U test |
| Self-efficacy | Unpaired t test |
| Step counts | Unpaired t test |
| Self-reported frequency of application utilization and most relevant features | Repeated measures ANOVA |
ABSI, A body shape index (WC normalized to height and weight); ANOVA, analysis of variance; BMI, body mass index; IWQOL-Kids, impact of weight on quality of life-kids; PAQ-A, physical activity questionnaire for adolescents; WC, waist circumference.
Triglycerides will be log-transformed to improve normalization of the distribution.
Analysis and reporting will be in line with the Consolidated Standards of Reporting Trials (CONSORT) guidelines for reporting parallel group RCTs.38 No interim analysis will be performed.
Missing data
Data will be analyzed on an intention to treat basis. Missing data will be handled through multiple imputation. A sensitivity analysis will be carried out considering a worst-case scenario (i.e. patients did not lose any weight during the trial).
Data management
Patient data and profiles will be protected with an individual code only the principal investigator will have access to. The patient’s profiles and collected data will be stored on secure servers using only password-protected computers, only available for personnel approved by the Institutional Review Board (IRB). No formal data monitoring committee will be installed as there is no safety concern.
Withdrawal and stopping rules
Patients will be free to withdraw from the study whenever they want. If they decide to withdraw, they will be asked to attend the follow-up measurement after month 6 and provide data. No further data will be collected if participants refuse to attend the follow-up measurement or refuse to provide data.
No stopping rules will be defined, as the intervention will not ask adolescents to do anything beyond standard care and using a smartphone application providing tracking (physical activity and diet) and gamification. Nevertheless, all adverse events will be documented and evaluated in terms of causality.
Discussion
The main aim of the proposed RCT is to assess the efficacy of a smartphone application with a gamification approach for healthy lifestyle promotion, to help overweight and obese adolescents to reduce weight. The hypothetical smartphone health application is designed specifically for children and teenagers, to help in self-monitoring of food habits and physical activity in order to develop a healthy lifestyle. Benefits of the proposed study include the evaluation of mid-term effects in weight reduction for overweight and obese adolescents, which is also relevant for future trial protocols.
A recent systematic review on the evaluation of smartphone applications for health behaviour change summarized that no single best practice approach can be suggested to evaluate mobile health apps due to insufficient data, leading to potentially incomplete and inaccurate guidance.39 As reported by Neugebauer and colleagues there are specific barriers to the conduct of RCTs on medical devices including matters of trial design, such as randomization, acceptability, blinding, determining appropriate outcomes.40 Accordingly, the study group carefully designed the proposed trial aiming to minimize the risk of bias.
The current study will address multiple gaps in the literature, such as missing data on the mid-term effects of smartphone interventions. The efficacy of innovative treatment strategies, like gamification, will be analyzed due to their current heterogeneity in terms of contents and methodologies of interventions and studies.41 Due to their potential to improve the reach and access to target populations, the trial uses a smartphone application.42 However, to this day there is no clear understanding of the processes through which such applications work.43 It is a huge challenge for study designs to investigate these combined mechanisms singularly. In the proposed study protocol this challenge is addressed by the introduction of a passive application that enables a more specified investigation of the active treatment.
Due to the repetitive nature of games (e.g. video games), the potential of promoting health-related learning can be of special benefit for children.44,45 The suggested application combines multiple components including monitoring, tracking and gamification. The proposed protocol will provide further knowledge on the efficacy of combining functionalities within one intervention in overweight and obese children.
Although designed as a triple-blinded RCT, un-blinding will be a serious threat in this trial. Participants may guess that they are in the control arm when talking to other participants of the study. However, the study group addressed this challenge by introducing a sham-like application in the control arm that will have an identical design as the smartphone application used in the intervention group, in order to lower the risk of dropout and un-blinding. Similarly, low adherence may hamper the study feasibility. However, the study group will address this issue in multiple ways, such as establishing multiple application features, structured reminders for appointments and the fact that the intervention is accessible on a 24-hour basis. Maintenance of blinding and concealment is also supported by separating randomization from the treating professionals and restricting the access to the randomization code until the end of the project.
A 6-month follow-up protocol will be chosen, assuming this is a reasonable period to detect meaningful changes in BMI z score. Indeed, this is the duration frequently used in studies investigating behaviour changes.46,47 However, the effects of the intervention may not be sustained over time. In this regard, monthly measurements, as well as information on application usage and self-reported feedback by the participants will allow developing a deeper understanding of progressive effects of the intervention up to 6 months.
A recent review on the effect of smartphone applications on glycaemic control improvement summarized that the functionality and application of the technology require standardization. The authors also call for policy and guidance.48 The developed trial protocol has the potential to provide such guidance by presenting a high-quality approach to test technological solutions for specific target groups, such as overweight and obese adolescents, which allows gaining a deeper understanding for these emerging technologies and their possible benefits for specific populations.
The developed framework of this protocol is in line with the CONSORT statement38 and provides new strategies of dealing with smartphone applications in clinical research. The proposed study protocol can be used by other groups that aim to generate efficacy data evaluating smartphone applications for the management of obesity and other chronic metabolic conditions in adolescents.
In conclusion, this protocol intends to evaluate an innovative, cheap, increasingly popular intervention (smartphone application) aimed to help control a relevant condition, that is, obesity in the young, focussing on a specific, novel aspect of mobile interventions (gamification). The proposal is a well-designed RCT based on standardized, robust procedures and following the most rigorous requirements in scientific investigation.
Trial status
At the time of submission, the trial was at the planning stage and had not yet started recruiting patients. The protocol will be registered in an online clinical trial database before starting with the trial. The protocol will be presented to an IRB for approval.
The study protocol was developed based on intense discussions of international young scientists taking part in a distance learning program in the field of clinical research. Due to their affiliations in heterogeneous disciplines, the developed study protocol is intended to be a methodological contribution rather than a protocol of a trial that will be effectively conducted by the present group of authors. The results of this proposed trial may provide guidance for clinical practice and this protocol can be used by other groups.
Acknowledgments
The authors wish to thank the staff of the Clinical Programme on Principles and Practice of Clinical Research, especially Felipe Fregni and Ingeborg Friehs for their support and critical appraisal.
Additionally, we would like to thank the colleagues Gerhard da Paz Lauterbach, Alexandra Halalau and Walter Marrou Pautraut for their feedback during the development of the study protocol.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Patrick Timpel, Prevention and Care of Diabetes Mellitus, Department of Medicine III, Faculty of Medicine Carl Gustav Carus, Technische Universität Dresden, Fetscherstrasse 74, Dresden 01307, Germany.
Fernando Henpin Yue Cesena, Hospital Israelita Albert Einstein, São Paulo, SP, Brazil.
Christiane da Silva Costa, Brazilian Health Regulatory Agency, Anvisa, Brazil.
Matheus Dorigatti Soldatelli, Department of Radiology, Hospital das Clínicas de Porto Alegre, Faculdade de Medicina, Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brazil.
Emanuel Gois, Jr, Department of Surgery, State University of Londrina, Brazil; Pontifical University of Parana, Brazil.
Eduardo Castrillon, Departamento de Clínicas Médicas, Pontificia Universidad Javeriana de Cali, Cali, Colombia.
Lina Johana Jaime Díaz, Clínica Universidad de la Sabana, Bogotá, Colombia.
Gabriela M. Repetto, Center for Genetics and Genomics, Facultad de Medicina, Clínica Alemana Universidad del Desarrollo, Santiago, Chile
Fanah Hagos, Department of Palliative and Hospice Care Liaison for CD at Emerson Hospital, Concord, MA, USA.
Raul E. Castillo Yermenos, Centro de Investigaciones Biomédicas y Clínicas ‘Dr Sergio Bencosme’ (CINBIOCLI), Santiago de Los Caballeros, República Dominicana
Kevin Pacheco-Barrios, Neuroscience and Behavior Laboratory, Universidad Peruana Cayetano Heredia, Lima, Peru.
Wafaa Musallam, Family Medicine Specialist, Primary Health Care Corporation, Doha, Qatar.
Zilda Braid, Departament of Pediatrics, Ribeirão Preto Medical School – University of São Paulo, Ribeirão Preto, São Paulo, SP, Brazil.
Nesreen Khidir, Bariatric and Metabolic Surgery, Hamad Medical Corporation, Doha, Qatar.
Marcela Romo Guardado, Mental Health Hospital of Tijuana, Tijuana, Mexico.
Roberta Muriel Longo Roepke, Disciplina de Emergencias Clínicas, Hospital das Clínicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, São Paulo, SP, Brasil.
References
- 1. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128∙9 million children, adolescents, and adults. Lancet 2017; 390: 2627–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017; 377: 13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ogden CL, Carroll MD, Lawman HG, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA 2016; 315: 2292–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whittemore R, Chao A, Jang M, et al. Implementation of a school-based internet obesity prevention program for adolescents. J Nutr Educ Behav 2013; 45: 586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bibiloni Mdel M, Pons A, Tur JA. Prevalence of overweight and obesity in adolescents: a systematic review. ISRN Obesity 2013; 2013: ArticleID 392747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kalarchian MA, Levine MD, Arslanian SA, et al. Family-based treatment of severe pediatric obesity: randomized, controlled trial. Pediatrics 2009; 124: 1060–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Theim KR, Sinton MM, Goldschmidt AB, et al. Adherence to behavioral targets and treatment attendance during a pediatric weight control trial. Obesity (Silver Spring) 2013; 21: 394–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Idelson PI, Scalfi L, Valerio G. Adherence to the Mediterranean Diet in children and adolescents: a systematic review. Nutr Metab Cardiovasc Dis 2017; 27: 283–299. [DOI] [PubMed] [Google Scholar]
- 9. Berkowitz RI, Marcus MD, Anderson BJ, et al. Adherence to a lifestyle program for youth with type 2 diabetes and its association with treatment outcome in the TODAY clinical trial. Pediatric Diabetes. Epub ahead of print 30 June 2017. DOI: 10.1111/pedi.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Common Sense Media Incorporated. The common sense census: media use by tweens and teens. Common Sense Media Incorporated, 2017. Available at: www.commonsense.org/research (accessed 26 March 2018). [Google Scholar]
- 11. Chen JL, Wilkosz ME. Efficacy of technology-based interventions for obesity prevention in adolescents: a systematic review. Adolesc Health Med Ther 2014; 5: 159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raaijmakers LC, Pouwels S, Berghuis KA, et al. Technology-based interventions in the treatment of overweight and obesity: a systematic review. Appetite 2015; 95(Suppl. C): 138–151. [DOI] [PubMed] [Google Scholar]
- 13. Dute DJ, Bemelmans WJ, Breda J. Using mobile apps to promote a healthy lifestyle among adolescents and students: a review of the theoretical basis and lessons learned. JMIR MHealth UHealth 2016; 4: e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Turner T, Spruijt-Metz D, Wen C, et al. Prevention and treatment of pediatric obesity using mobile and wireless technologies: a systematic review. Pediatric Obes 2015; 10: 403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schoech D, Boyas JF, Black BM, et al. Gamification for behavior change: lessons from developing a social, multiuser, web-tablet based prevention game for youths. J Technol Hum Serv 2013; 31: 197–217. [Google Scholar]
- 16. Patel MS, Benjamin EJ, Volpp KG, et al. Effect of a game-based intervention designed to enhance social incentives to increase physical activity among families: The BE FIT randomized clinical trial. JAMA Intern Med 2017; 177: 1586–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Christensen J, Valentiner LS, Petersen RJ, et al. The effect of game-based interventions in rehabilitation of diabetics: a systematic review and meta-analysis. Telemed J E Health 2016; 22: 789–797. [DOI] [PubMed] [Google Scholar]
- 18. Whitehead L, Seaton P. The effectiveness of self-management mobile phone and tablet apps in long-term condition management: a systematic review. J Med Internet Res 2016; 18: e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fu H, McMahon SK, Gross CR, et al. Usability and clinical efficacy of diabetes mobile applications for adults with type 2 diabetes: a systematic review. Diabetes Res Clin Pract 2017; 131: 70–81. [DOI] [PubMed] [Google Scholar]
- 20. Boulos MN, Gammon S, Dixon MC, et al. Digital games for type 1 and type 2 diabetes: underpinning theory with three illustrative examples. JMIR Serious Games 2015; 3: e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Majeed-Ariss R, Baildam E, Campbell M, et al. Apps and adolescents: a systematic review of adolescents’ use of mobile phone and tablet apps that support personal management of their chronic or long-term physical conditions. J Med Internet Res 2015; 17: e287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kirwan M, Vandelanotte C, Fenning A, et al. Diabetes self-management smartphone application for adults with type 1 diabetes: randomized controlled trial. J Med Internet Res 2013; 15: e235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brian Haynes R. Forming research questions. In: Haynes BR, Sackett DL, Guyatt GH, et al. (eds) Clinical Epidemiology: How to Do Clinical Practice Research, 3rd edn Philadelphia, PA: Lippincott Williams & Wilkins; 2006, pp. 3–14. [Google Scholar]
- 24. World Health Organization. Obesity and overweight [Fact Sheet], http://www.who.int/mediacentre/factsheets/fs311/en/ (2017, accessed December 2017).
- 25. Onis MD, Onyango AW, Borghi E, et al. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Org 2007; 85: 660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Styne DM, Arslanian SA, Connor EL, et al. Pediatric obesity—assessment, treatment, and prevention: an Endocrine Society Clinical Practice guideline. J Clin Endocrinol Metab 2017; 102: 709–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barlow SE. Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 2007; 120(Suppl. 4): S164–S192. [DOI] [PubMed] [Google Scholar]
- 28. US Preventive Services Task Force. Screening for obesity in children and adolescents: Us preventive services task force recommendation statement. JAMA 2017; 317: 2417–2426. [DOI] [PubMed] [Google Scholar]
- 29. van der Baan-Slootweg O, Benninga MA, Beelen A, et al. Inpatient treatment of children and adolescents with severe obesity in the Netherlands: a randomized clinical trial. JAMA Pediatr 2014; 168: 807–814. [DOI] [PubMed] [Google Scholar]
- 30. Smith JJ, Morgan PJ, Plotnikoff RC, et al. Smartphone obesity prevention trial for adolescent boys in low-income communities: the ATLAS RCT. Pediatrics 2014; 134: e723–e731. [DOI] [PubMed] [Google Scholar]
- 31. Schwarzer R, Fuchs R. Changing risk behaviors and adopting health behaviors: the role of self-efficacy beliefs. In: Bandura A. (ed.) Self-efficacy in changing societies. 1995, New York: Cambridge University Press, 1995, pp.259–288. [Google Scholar]
- 32. Kowalski KC, Crocker PR, Kowalski NP. Convergent validity of the physical activity questionnaire for adolescents. Pediatr Exerc Sci 1997; 9: 342–352. [Google Scholar]
- 33. Kolotkin RL, Zeller M, Modi AC, et al. Assessing weight-related quality of life in adolescents. Obesity (Silver Spring) 2006; 14: 448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Theim KR, Sinton MM, Stein RI, et al. Preadolescents’ and parents’ dietary coping efficacy during behavioral family-based weight control treatment. J Youth Adolesc 2012; 41: 86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schwarzer R, Jerusalem M. Self-efficacy measurement: Generalized Self-Efficacy Scale (GSES). In: Weinman SWJ, Johnston M. (eds) Measures in health psychology: a user’s portfolio. Causal and control beliefs. Windsor: NFER-NELSON; 1995, pp.35–37. [Google Scholar]
- 36. Minter A, Pritzker S. Measuring adolescent social and academic self-efficacy: cross-ethnic validity of the SEQ-C. Res Soc Work Pract 2015; 27: 818–826. [Google Scholar]
- 37. Schwarzer R, Fuchs R. Self-efficacy and health behaviors. In: Conner M, Norman P. (eds) Predicting health behavior: research and practice with social cognition models. Buckingham: Open University Press; 1996, pp.163–196. [Google Scholar]
- 38. Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. BMC Med 2010; 8: 18. [PMC free article] [PubMed] [Google Scholar]
- 39. McKay FH, Cheng C, Wright A, et al. Evaluating mobile phone applications for health behaviour change: a systematic review. J Telemed Telecare. Epub ahead of print 18 October 2016. DOI: 10.1177/1357633X16673538. [DOI] [PubMed] [Google Scholar]
- 40. Neugebauer EA, Rath A, Antoine SL, et al. Specific barriers to the conduct of randomized clinical trials on medical devices. Trials 2017; 18: 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Soares NM, Leão AS, Santos JR, et al. Systematic review shows only few reliable studies of physical activity intervention in adolescents. Sci World J 2014; 2014: 206478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Greenwood DA, Gee PM, Fatkin KJ, et al. A systematic review of reviews evaluating technology-enabled diabetes self-management education and support. J Diabetes Sci Technol 2017; 11: 1015–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yasmin F, Banu B, Zakir SM, et al. Positive influence of short message service and voice call interventions on adherence and health outcomes in case of chronic disease care: a systematic review. BMC Med Inform Decis Mak 2016; 16: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kato PM. Video games in health care: closing the gap. Rev Gen Psychol 2010; 14: 113–121. [Google Scholar]
- 45. Rosas R, Nussbaum M, Cumsille P, et al. Beyond Nintendo: design and assessment of educational video games for first and second grade students. Comput Educ 2003; 40: 71–94. [Google Scholar]
- 46. Prince SA, Reid RD, Pipe AL, et al. An evaluation of CardioPrevent: a technology-enabled, health-behavior change program for the global reduction of cardiovascular risk. Curr Opin Cardiol 2017; 32: 580–589. [DOI] [PubMed] [Google Scholar]
- 47. Hadjiconstantinou M, Byrne J, Bodicoat DH, et al. Do web-based interventions improve well-being in type 2 diabetes? A systematic review and meta-analysis. J Med Internet Res 2016; 18: e270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hou C, Carter B, Hewitt J, et al. Do mobile phone applications improve glycemic control (HbA1c) in the self-management of diabetes? A systematic review, meta-analysis, and GRADE of 14 randomized trials. Diabetes Care 2016; 39: 2089–2095. [DOI] [PubMed] [Google Scholar]



