Abstract
Purpose
To report the occurrence and the management of refractory interface haze that developed after epithelial ingrowth following small aperture inlay implantation.
Observations
A 52 year-old man with sub-clinical anterior basement membrane dystrophy (ABMD) underwent combined hyperopic laser in situ keratomileusis and KAMRA corneal inlay implantation to correct presbyopia. Post-operatively, epithelial ingrowth developed requiring debridement and KAMRA removal. Significant diffuse interface haze, ground-glass in texture, involving the central 6 mm of the cornea developed the next day, and was refractory to topical and systemic steroids, necessitating flap irrigation, gentle scraping, and MMC application to the residual stromal bed after 12 days. The interface haze gradually improved to near complete resolution over 12-months.
Conclusions and importance
Epithelial ingrowth can lead to flap interface haze refractory to medical therapy. Early surgical intervention is key to haze resolution.
Keywords: Refractory interface haze, KAMRA, Inlay implantation, Anterior basement membrane dystrophy, Epithelial ingrowth, Laser in situ keratomileusis
1. Introduction
Corneal inlays have recently emerged as a viable treatment of presbyopia.1 Here we discuss the possible causes and management of an unusual complication of refractory interface haze developing after inlay explantation and interface debridement to treat epithelial ingrowth in a patient with sub-clinical anterior basement membrane dystrophy (ABMD) who underwent simultaneous hyperopic LASIK and KAMRA inlay implantation. The haze gradually resolved over 6 months after interface irrigation and mitomycin c application to the residual stromal bed.
2. Case report
A 52 year-old man presented for elective KAMRA small aperture corneal inlay surgery. Preoperatively, the uncorrected distance visual acuity (UDVA) was 20/20 OU, the corrected distance visual acuity (CDVA) was 20/20 OU, and the uncorrected near visual acuity (UNVA) J10 OU. The preoperative cycloplegic refraction was +1.25–0.50 × 90 OD and +0.25D OS. Slit lamp examination revealed low-grade lissamine green staining of the nasal conjunctiva bilaterally, punctate staining of the epithelium, and very subtle map-like sub-epithelial changes, suggestive of mild dryness and mild anterior basement membrane dystrophy. The patient denied any history suggestive of recurrent erosions, and he was started on topical cyclosporine 0.05% and 0.2% preservative-free hyaluronic acid for 6 months until the lissamine green staining disappeared completely. LASIK on the right non-dominant eye, was performed using the Amaris excimer laser (Schwind eye-tech-solutions GmbH, Kleinostheim, Germany) and the LDV femtosecond laser (Ziemer, Port, Switzerland), with the corneal inlay implanted under the flap, centered between the first Purkinje reflex and the pupil centroid. The target refraction was −0.75D.
Surgery was uneventful, with UCVA of J1 and UDVA of 20/40 on the 3rd postoperative day (POD). On the 4th POD, small epithelial defects at the nasal and temporal margins of the flap were noted, and a bandage contact lens (BCL) was applied. On 7th POD the epithelial defects were healed, however epithelial ingrowth limited to the margins of the flap were noted at the site of the prior epithelial defects; a decision to follow-up the patient closely was taken.
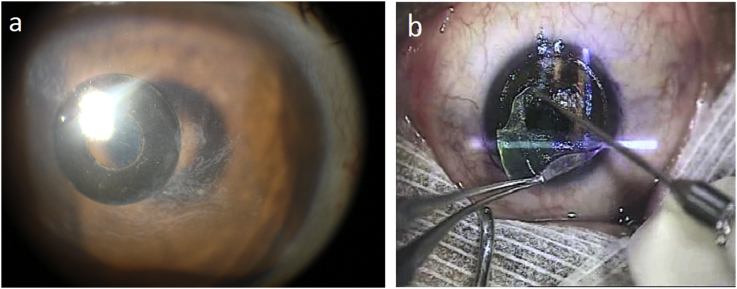
On 11th POD the ingrowth had encroached on the KAMRA (Fig. 1a). The flap was lifted, the inlay removed, and the interface was cleaned using a blunt spatula then thoroughly irrigated with balanced salt solution, followed by mechanical epithelial debridement of the epithelium around the flap edges to decrease the chance of recurrent ingrowth (Fig. 1b). A BCL was applied, and hourly prednisolone acetate ophthalmic suspension 1% together with moxifloxacin ophthalmic solution 0.5% four times a day was initiated.
Fig. 1.
(a) Slit-lamp photograph of the right eye showing the KAMRA corneal inlay with the epithelial ingrowth encroaching on it. (b) Intraoperative view of the epithelial ingrowth being peeled after flap lifting (arrow).
The next day, a diffuse “ground-glass” haze in the flap interface and over the overlying anterior stroma, more concentrated centrally than peripherally, was noted (Fig. 2a). Prednisolone acetate was kept hourly, and systemic prednisolone 60 mg/day was added. After 10 days of follow-up, the haze had not improved and UDVA and CDVA worsened to 20/200 and 20/60, respectively.
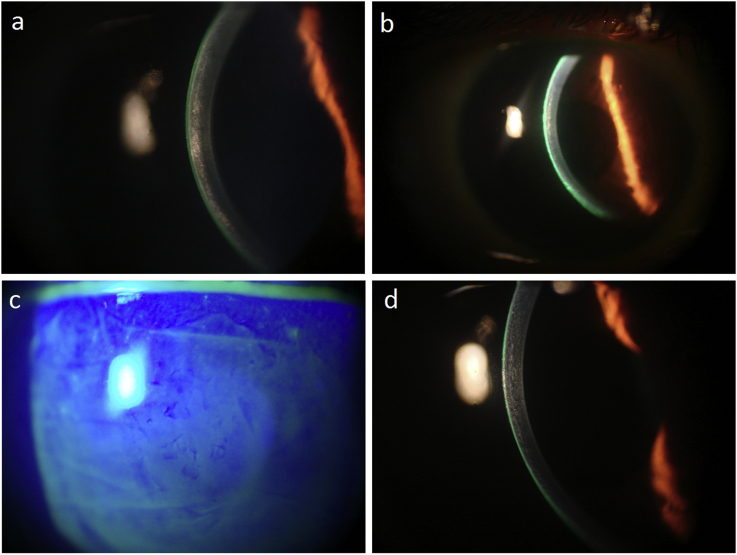
Fig. 2.
(a) Interface corneal haze developing the next day after KAMRA removal. (b) Decrease in the interface haze 2 weeks after irrigation and Mitomycin c application. (c) Slit-lamp photograph of the right eye stained with fluorescein showing the epithelial irregularity from the anterior basement membrane dystrophy 3month post-irrigation and application of mitomycin c. (d) Slit-lamp photograph of the right eye at 6 months showing near resolution of the corneal interface haze.
On day 12 post-inlay removal the flap was re-lifted and the haze was scraped, and preservative-free dexamethasone-impregnated weck-cells, then mitomycin c (MMC) 0.02% was applied on the interface for 1minute, then irrigated thoroughly. A drop of moxifloxacin 0.5% and dexamethasone 0.1% was instilled on the stromal bed, the flap repositioned, and BCL applied., UDVA improved to 20/70 the next day, with a subjective 60% decrease in haze. Topical preservative-free dexamethasone 0.1% Q1.5 hour and topical moxifloxacin 5 times per day were maintained over the next 10 days from the date of the flap re-lift. Haze density continued improving gradually over the 2-week (Fig.), 1, and 3-month postoperative follow-ups (CDVA = 20/40), while ABMD epithelial irregularity was gradually, albeit slowly, improving (Fig. 2c). By the 6-month follow-up, the haze was almost fully resolved (Fig. 2d) with CDVA of 20/30, and at 12 months, UDVA was 20/30, CDVA was 20/25, UNVA was J3, and the manifest refraction −0.75D. The rest of the vision deficit was attributed to the residual ABMD irregular epithelium.
3. Discussion
Our case does not fit a typical picture of diffuse lamellar keratitis (DLK). The diffuse central interface haze developing shortly after the KAMRA removal had a ground-glass texture, was confined to the central 6mm area of the cornea, had no signs of sterile infiltrates migrating from the limbus, and was not responsive to topical steroids. Instead, the overall picture resembles that of keratocyte activation and subsequent haze formation typically found after PRK.2,3
We postulate that activated keratocytes by the excimer and possibly the femtosecond lasers4 were further stimulated by a multitude of potential factors, including one or all of the following: epithelial and bowman's membrane injuries from ABMD, ingrown epithelial cells in the flap interface, and foreign material implanted in the interface.
Injury to the epithelial basement membrane and Bowman's layer could have leaked proinflammatory cytokines and chemokines to the stromal interface, which could have amplified the keratocyte activation. Those breaks were previously reported to happen after thin flap creation (<110μm) using femtosecond laser.2 However, in our case the flap was around 200μm deep and induced breaks by the femtosecond laser were less likely; the breaks were more likely to have happened due to ABMD.
The presence of epithelial ingrowth in the interface has been postulated to impair flap-adhesion, resulting in a low-resistance migration pathway for surface pro-inflammatory cytokines, and to be a local source of proteolytic enzymes and cytokines responsible for interface haze formation.5 Epithelial ingrowth in our patient can be attributed to the hyperopic treatment, older age, and ABMD.6 In addition, the angular anatomy of the side-cut created by the 1st generation LDV femtosecond laser allows easier access of epithelial ingrowth into the interface.7
Finally, the presence of a foreign material, such as the KAMRA inlay, can potentially stimulate an increased inflammatory reaction within the interface as demonstrated in rabbit eyes,8 possibly enhancing haze formation. The KAMRA inlay is however made of polyvinylidene fluoride (PVDF), an inert and biocompatible material with favorable long-term outcomes, and no reported complication other than occasional thin benign anterior stromal haze around its edges.1
Haze is characterized by an abnormal wound healing response, with myofibroblasts mainly responsible for collagen and extracellular matrix remodeling.5 A delicate balance exists between fibrosis and stromal regeneration, It has been shown that restoration of an intact basement membrane favors the non-fibrotic phenotype.3 Patients with ABMD may be at increased risk of fibrotic haze as a result of a constantly damaged basement membrane. We used MMC in our patient since it is an anti-metabolite with potent inhibitory effects on cell replication, preferentially affecting rapidly dividing cells.8 MMC might have inhibited the fibrotic arm of the healing process, tipping the balance towards stromal regeneration and haze resolution. MMC has also been shown not only to prevent corneal haze post-PRK, but also to reduce pre-existent corneal haze with success.9 Moreover, the successful use of MMC under a LASIK flap has been previously reported in Avelino corneal dystrophy by Jun RM et al.10
The gradual improvement of haze that occurred slowly over the 6month follow-up period might be due to the gradual time-dependent anti-proliferative effect of MMC on the corneal keratocytes,8 but could still purely be the effect of the interface irrigation and scraping, with slow, spontaneous resolution over time.
To conclude, interface haze refractory to medical treatment is a potential complication of epithelial ingrowth after LASIK. Surgical intervention needs to be early in the postoperative phase before haze maturation.
Patient consent
Patient unavailable for permission. Photographs and figures have no identifying features.
Funding
No funding or grant support.
Conflicts of interest
The following authors have no financial disclosures relating this topic (RA, NJ, MA, SA).
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
None.
References
- 1.Seyeddain O., Hohensinn M., Riha W. Small-aperture corneal inlay for the correction of presbyopia: 3-year follow-up. J Cataract Refract Surg. 2012;38:35–45. doi: 10.1016/j.jcrs.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 2.Rocha K.M., Kagan R., Smith S.D., Krueger R.R. Thresholds for interface haze formation after thin-flap femtosecond laser in situ keratomileusis for myopia. Am J Ophthalmol. 2009;147:966–972. doi: 10.1016/j.ajo.2009.01.010. 972 e961. [DOI] [PubMed] [Google Scholar]
- 3.Stramer B.M., Zieske J.D., Jung J.C., Austin J.S., Fini M.E. Molecular mechanisms controlling the fibrotic repair phenotype in cornea: implications for surgical outcomes. Invest Ophthalmol Vis Sci. 2003;44:4237–4246. doi: 10.1167/iovs.02-1188. [DOI] [PubMed] [Google Scholar]
- 4.Kitzmann A.S., Bourne W.M., Patel S.V. Confocal microscopy of a femtosecond laser LASIK flap before separation. Am J Ophthalmol. 2007;143:691–693. doi: 10.1016/j.ajo.2006.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dupps W.J., Jr., Wilson S.E. Biomechanics and wound healing in the cornea. Exp Eye Res. 2006;83:709–720. doi: 10.1016/j.exer.2006.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Letko E., Price M.O., Price F.W., Jr. Influence of original flap creation method on incidence of epithelial ingrowth after LASIK retreatment. J Refract Surg. 2009;25:1039–1041. doi: 10.3928/1081597X-20090617-13. [DOI] [PubMed] [Google Scholar]
- 7.Ahn H., Kim J.K., Kim C.K. Comparison of laser in situ keratomileusis flaps created by 3 femtosecond lasers and a microkeratome. J Cataract Refract Surg. 2011;37:349–357. doi: 10.1016/j.jcrs.2010.08.042. [DOI] [PubMed] [Google Scholar]
- 8.Santhiago M.R., Netto M.V., Wilson S.E. Mitomycin C: biological effects and use in refractive surgery. Cornea. 2012;31:311–321. doi: 10.1097/ICO.0b013e31821e429d. [DOI] [PubMed] [Google Scholar]
- 9.Majmudar P.A., Forstot S.L., Dennis R.F. Topical mitomycin-C for subepithelial fibrosis after refractive corneal surgery. Ophthalmology. 2000;107:89–94. doi: 10.1016/s0161-6420(99)00019-6. [DOI] [PubMed] [Google Scholar]
- 10.Jun R.M., Tchah H., Kim T.I. Avellino corneal dystrophy after LASIK. Ophthalmology. 2004;111:463–468. doi: 10.1016/j.ophtha.2003.06.026. [DOI] [PubMed] [Google Scholar]