Short abstract
The distribution of copper (Cu) in the biological system is regulated by Cu transporters and chaperones. It has been known for a long time that myocardial ischemia is accompanied by the loss of Cu from the heart, but the mechanism by which this occurs remains unknown. The present study was undertaken to understand the relationship between Cu loss and alterations in Cu transporters during the pathogenesis of myocardial ischemia. Male mice (C57 BL/6J) were subjected to left anterior descending (LAD) coronary artery ligation to induce myocardial ischemia. Changes in Cu concentrations in serum and hearts were determined from blood and tissue samples harvested at different time points for a total of 28 days after the operation. Cu concentrations in the ischemic myocardium were continuously decreased starting at the fourth day after LAD artery ligation, gradually depleted by more than 80% of the normal level at the 10th day, and remained at the lowest level (about 20% of normal levels) thereafter. Serum Cu concentrations were correspondingly increased starting at the fourth day, reached to the highest level between day 7 and 10, and gradually recovered to the normal level until 21st day after the operation. Along with the same time course, the intracellular Cu exporter copper metabolism MURR domain 1 (COMMD1) was significantly and sustainably increased, but ATP7A and ATP7B were not significantly changed in the ischemic myocardium. These results suggest that during the pathogenesis of myocardial ischemia, COMMD1 would play a critical role in exporting Cu from the ischemic myocardium to the blood.
Impact statement
In this work, we found that copper efflux from the ischemic heart leads to the elevation of serum copper concentrations, addressing a long-term question related to serum copper elevation in myocardial ischemia patients. The efflux of copper from the ischemic heart results at least in part from the upregulation of copper metabolism MURR domain 1 (COMMD1) in the heart upon ischemic insult. This work provides a novel insight into copper homeostasis and alteration in cardiovascular system.
Keywords: Myocardial ischemia, copper, copper transporting ATPases, ATP7A, ATP7B, copper metabolism MURR domain 1
Introduction
The role of copper (Cu) in cardiac structure and function has been a focused topic in cardiovascular medicine.1–4 Dietary Cu deficiency leads to myocardial structural and functional alterations, resulting in cardiac hypertrophy transitioning to heart failure.5 Our previous work showed that Cu supplementation improved cardiac structure and function in not only dietary Cu deficiency-induced,6 but also ascending aortic constriction-induced cardiac hypertrophy along with repletion of Cu in the heart.7 Many studies demonstrated the relationship between Cu deficiency and ischemic heart disease.8–11 Myocardial ischemia is accompanied by the loss of cardiac Cu.12–14 It remains elusive how does this happen?
The homeostasis of Cu is tightly regulated by a series of proteins,15–20 including proteins responsible for Cu uptake, inter- and intra-cellular trafficking, and efflux. Cu efflux related proteins, such as Cu transporting ATPases21,22 (ATP7A and ATP7B) and COMMD1.23 It was well known that mutations in ATP7A and ATP7B led to Menkes disease and Wilson’s disease, due to Cu accumulation in the intestine and in the liver, respectively.24,16 Overexpression of ATP7A or ATP7B caused a significant increase in Cu efflux.25,26 COMMD1 is a Cu transport chaperone, and it directly interacts with ATP7B and is involved in the defined pathway of hepatic biliary Cu excretion.27 Furthermore, liver-specific knockdown of COMMD1 in mice led to hepatic accumulation of Cu.28 It is, however, unknown whether or not these Cu transporters are related to cardiac Cu efflux under ischemic conditions.
The present study was undertaken to use a mouse model of myocardial ischemia to determine the dynamic changes in Cu concentrations in the heart during the pathogenesis of myocardial ischemia and explore the relationship between Cu loss from the heart and Cu efflux related transporters.
Materials and methods
Animal
Male C57BL/6J mice (DaShuo, China), aged 8–10 weeks old and weighed 20–22 g, were fed standard rodent chow (5C02, LabDiet, USA) and tap water ad libitum. All animal procedures were approved by the institution animal care and use committee at the Sichuan University Western China Hospital, following the guidelines of the US National Institutes of Health.
Induction of myocardial ischemia
As described in a previous study,29 all animals were randomly divided into two groups: sham control (n = 52) and myocardial ischemia (n = 78). Myocardial ischemia was induced by the left anterior descending (LAD) coronary artery ligation. The anesthesia of the animals was done by 4% isoflurane with 100% O2. The LAD artery was exposed and permanently ligated in the anesthetized mice. The sham controls were subjected to the same surgical procedure with the exception of LAD ligation.
Tissue preparation
After LAD ligation surgery at different time points (1, 4, 5, 6, 7, 10, 14, 21, 28, 35 days), the blood was obtained for serum preparation, and then the mice were sacrificed to obtain heart tissue. The serum was stored at −80°C for the detection of Cu concentrations. For the Cu concentration determination and Western blotting, heart tissues were collected during the process of animal sacrifice and separated into two parts: ischemic area (IA) and remote area (RA). The IA part had a pale appearance compared with the right ventricular and base of the heart, which was located in the left ventricle under the silk suture ligation, including the apex, anterior wall, lateral wall, and part of posterior wall. The myocardium located 1 mm away from ischemic area was determined as RA part. Other heart samples were used for pathological examination.
Measurement of Cu concentrations
As described in our previous study,30 Cu concentrations were determined by graphite furnace atomic absorption spectrometry (AAS) (ICE3500, Thermo, USA). The quantified heart and serum samples were digested with nitric acid (HNO3, Sigma, USA) at 60°C overnight. Cu concentration was normalized by the dry weight of heart sample or serum volume.
Western blot
The total proteins were extracted by TRIzol reagent (Thermo Fisher Scientific, USA), following the instruction provided. The protein levels of ATP7A, ATP7B, and COMMD1 were determined by Western blot. Equal amounts of total proteins were separated by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membrane, which was incubated with primary antibodies: monoclonal mouse anti-mouse ATP7A (163 kDa, ab131400, Abcam, UK), polyclonal rabbit anti-mouse ATP7B (165 kDa, NB100-360, Novus biologicals, USA), and monoclonal mouse anti-mouse COMMD1 (21 kDa, sc-166248, Santa Cruz, USA), followed by hybridization with appropriate secondary antibodies. The protein bands were visualized by using DAB reagent (Millipore, USA), images were captured using a Vilber Fusion (VILBER LOURMAT Fusion FX, France) and analyzed by IPP 6.0 (Image-Pro Plus 6.0, Media Cybernetics, USA).
Statistical analysis
GraphPad prism 7.0 (GraphPad Software, USA) was applied to perform the statistical analysis. All data were presented as mean ± SD. Two-way ANOVA and Tukey’s multiple comparisons test were used for analysis of Cu concentrations and the Western blot intensity at different time points. P < 0.01 was considered to be statistically significant.
Results
Pathologic changes of the heart after LAD ligation
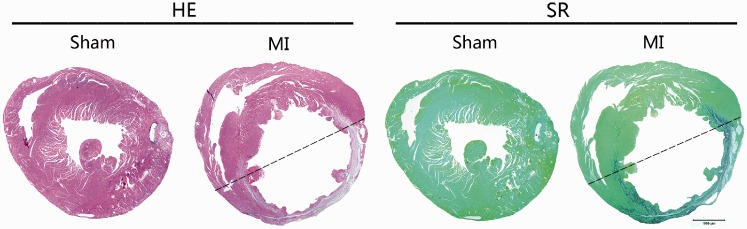
Pathological examination identified that the ischemic area was located in the left ventricular anterior wall after LAD ligation (Figure 1). The ischemic area was full of collagen deposition and the left ventricular anterior wall became thinner at the 35th day after LAD ligation relative to the sham-operated control mice (Figure 1).
Figure 1.
Histopathological observation of the heart after LAD ligation. Overview of the ischemic heart with HE and Sirius red (SR) staining at the 35th day after LAD ligation. Ischemic area was located in the left ventricular anterior wall. Bar = 1000 μm. (A color version of this figure is available in the online journal.)
Decreased Cu concentrations in the heart after LAD ligation
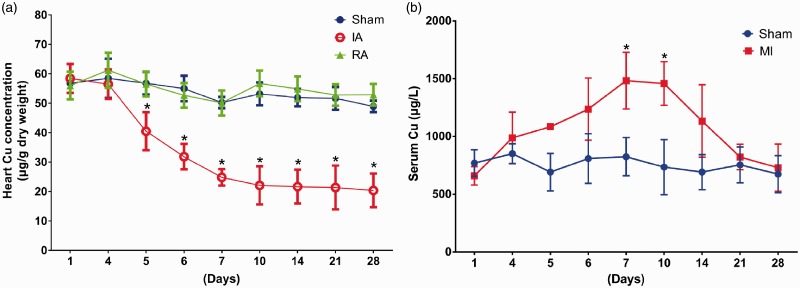
Cu concentrations in the ischemic area were continuously decreased, starting at the fourth day after the LAD ligation and reaching to the lowest depletion (more than 80% of the normal level) at the 14th day, and retained at the lowest level thereafter (Figure 2(a)). There was no significant difference in Cu concentrations between the remote area and the sham-operated controls (Figure 2(a)). Serum Cu levels began to increase at the fourth day after ischemia, and rose to the peak at the seventh day, and retained at the peak until the 10th day, and then gradually recovered and reached to the normal level at the 21st day after the operation (Figure 2(b)). However, Cu concentrations in the major storage organ, liver, were not significantly changed during the process of myocardial ischemia (Figure S1).
Figure 2.
The changes of Cu concentrations in the heart and serum. AAS was used to determine Cu concentrations at different time points after LAD ligation. (a) Cu concentrations decreased in the ischemic myocardium at the fourth day after ischemia, and further gradually decreased and reached to the lowest level at the 14th day, which level was sustained to the end of the observation (a total of 28 days). (b) Serum Cu levels were increased at the fourth day after myocardial ischemia, and rose to the peak between seventh and 10th day, and then gradually declined and returned to the normal level at the 21st day. Data were presented as mean ± SD. *P < 0.01, versus sham control, n = 3–5. IA: ischemic area; RA: remote area. (A color version of this figure is available in the online journal.)
Increased protein levels of COMMD1 after LAD ligation
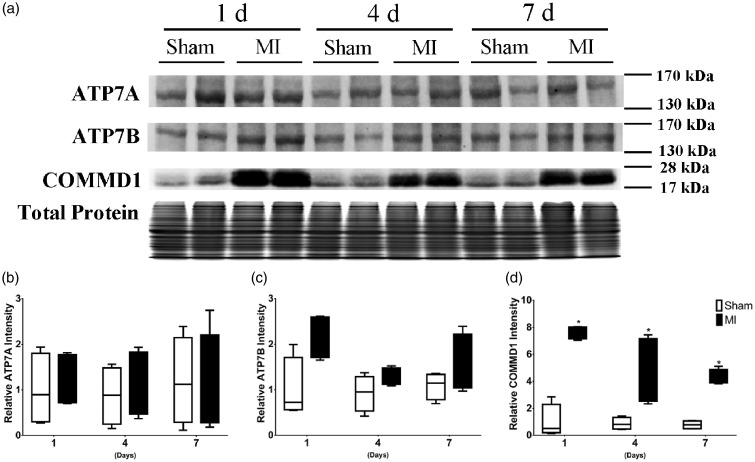
The changes in Cu efflux transporters were determined by their protein levels. In the ischemic area, ATP7A was not significantly changed in comparison to the normal myocardium (Figure 3(a) and (b)). ATP7B was slightly increased only at the first day (Figure 3(a) and (c)) after LAD ligation in the ischemic area. On the other hand, the COMMD1 protein levels were significantly increased (Figure 3(a) and (d)) in the ischemic area at all the time points (day 1, 4, and 7), prior to Cu loss from the heart.
Figure 3.
The changes of ATP7A, ATP7B, and COMMD1 protein levels in the ischemic myocardium. (a) Western blot analysis was performed to detect the protein levels at first, fourth, and seventh day after LAD ligation, ATP7A was not significantly changed, ATP7B was slightly increased only at the first day, and COMMD1 protein levels were significantly increased at the first, fourth, and seventh day. Total protein levels were assayed as a loading control. Data were presented as mean ± SD. *P < 0.01 versus sham control, n = 4 (independent samples for each group). IA: ischemic area.
Discussion
Myocardial Cu loss in response to ischemia is a unique response of myocytes to hypoxia, which has been observed in human studies,8,9,31,32 animal studies,30,33 and in vitro studies.12,13 It was also reported that Cu transporters are changed under ischemic conditions.34,35 The relationship between Cu loss and changes in Cu transporters in the pathogenesis of myocardial ischemia is thus an interesting topic for further understanding the role of Cu in the heart. The results obtained here suggest that the loss of Cu from the ischemic area of the heart is likely ascribed to the increase in the COMMD1. This provides a novel insight into Cu homeostasis in the cardiac system.
Serum Cu level elevation in patients with acute myocardial infarction has been reported previously,36 although there were differences in the extent of this increase due to varying experimental subjects.37,38 However, it remains unanswered where the elevated Cu in the serum comes from? There are some clues, such as in patients died from acute myocardial infarction, Cu concentrations in their heart were lower than in the hearts from subjects without cardiac events.9 Is the Cu loss from the heart related to the Cu elevation in the blood? This question was specifically addressed in the present study. The mice were only subjected to LAD artery ligation leading to myocardial ischemia and Cu levels in the ischemic heart were significantly decreased as a function of elapsed time. This gradual decrease in myocardial Cu concentrations was well correlated with the gradual increase in serum Cu concentrations. Under this condition, there were no other organ system injuries in these myocardial ischemic mice, and the major Cu storage organ, liver, in the body did not show changes in Cu concentrations during the process of myocardial ischemia. Furthermore, our previous studies showed Cu efflux from hypertrophic cardiomyocytes into the culture medium.39,40 Therefore, the elevated levels of Cu in the blood were ascribed to those released from the heart. In addition, there was a further evidence to link the myocardial Cu loss to the serum Cu elevation, as it was noticed that after Cu efflux from the heart ceased, serum Cu levels were equilibrated to normal level.
Cu efflux from the intestine and from the liver has been extensively studied, and Cu transporting ATPases (ATP7A and ATP7B) play a critical role in this process. Both ATP7A and ATP7B are P1B-type ATPases41 and their expressions are tissue-specific: ATP7A is mostly in the intestine and vasculature, and also found in almost all other organs except for liver.42 ATP7B is mainly in the liver, but also found in heart, kidney, brain, and lung.42 Therefore, both ATP7A and ATP7B are present in the heart, but their function in the heart has not been fully defined. In the present study, we observed that ATP7A was not changed and ATP7B was only slightly increased at the first day after the LAD ligation, suggesting that neither ATP7A nor ATP7B would be responsible for the observed Cu efflux from the heart.
COMMD1 is a member of the COMMD (copper metabolism MURR domain) protein family,43 and it contains 188 amino acid sequences and is involved in Cu homeostasis.23,44 Previous studies found that increased COMMD1 protein level was associated with a decrease in hepatic Cu concentrations in lipopolysaccharide-induced liver injury.45 We observed here that COMMD1 protein levels were significantly elevated in the ischemic myocardium, and this increase occurred prior to the extensive loss of Cu and was in parallel with the time course of Cu loss from the ischemic myocardium. Therefore, it is likely that the elevated COMMD1 is at least in part responsible for the observed Cu loss from the ischemic myocardium.
In summary, the present study addressed the cause-and-effect relationship between Cu loss from the heart and Cu elevation in the blood. Cu loss from the heart was not caused by ATP7A or ATP7B, but most likely ascribed to the increase in COMMD1 in the ischemic myocardium. How does ischemia cause the increase in COMMD1 in the heart remains to be further investigated.
Acknowledgments
The authors thank Qin Sheng, Xiaorong Sun, Ning Wang and Lily Zheng for technical support.
Author contributions
All authors participated in the design, interpretation of the results, and review of the manuscript; KL, CL, and YX were involved in the experimentation; TW and KL were involved in the analysis of the data; YJK and KL wrote the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: National Science Foundation of China (grant number 81230004 to YJ Kang).
References
- 1.Goodman JR, Warshaw JB, Dallman PR. Cardiac hypertrophy in rats with iron and copper deficiency: quantitative contribution of mitochondrial enlargement. Pediatr Res 1970; 4:244–56 [DOI] [PubMed] [Google Scholar]
- 2.Medeiros DM, Bagby D, Ovecka G, McCormick R. Myofibrillar, mitochondrial and valvular morphological alterations in cardiac hypertrophy among copper-deficient rats. J Nutr 1991; 121:815–24 [DOI] [PubMed] [Google Scholar]
- 3.Davidson J, Medeiros DM, Hamlin RL. Cardiac ultrastructural and electrophysiological abnormalities in postweanling copper-restricted and copper-repleted rats in the absence of hypertrophy. J Nutr 1992; 122:1566–75 [DOI] [PubMed] [Google Scholar]
- 4.Wildman RE, Medeiros DM, Jenkins J. Comparative aspects of cardiac ultrastructure, morphometry, and electrocardiography of hearts from rats fed restricted dietary copper and selenium. Biol Trace Elem Res 1994; 46:51–66 [DOI] [PubMed] [Google Scholar]
- 5.Elsherif L, Ortines RV, Saari JT, Kang YJ. Congestive heart failure in copper-deficient mice. Exp Biol Med 2003; 228:811–7 [DOI] [PubMed] [Google Scholar]
- 6.Elsherif L, Wang L, Saari JT, Kang YJ. Regression of dietary copper restriction-induced cardiomyopathy by copper repletion in mice. J Nutr 2004; 134:855–60 [DOI] [PubMed] [Google Scholar]
- 7.Jiang Y, Reynolds C, Xiao C, Feng W, Zhou Z, Rodriguez W, Tyagi SC, Eaton JW, Saari JT, Kang YJ. Dietary copper supplementation reverses hypertrophic cardiomyopathy induced by chronic pressure overload in mice. J Exp Med 2007; 204:657–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wester PO. Trace elements in human myocardial infarction determined by neutron activation analysis. Acta Med Scand 1965; 178:765–88 [DOI] [PubMed] [Google Scholar]
- 9.Chipperfield B, Chipperfield JR. Differences in metal content of the heart muscle in death from ischemic heart disease. Am Heart J 1978; 95:732–7 [DOI] [PubMed] [Google Scholar]
- 10.Klevay LM. Ischemic heart disease as copper deficiency. Adv Exp Med Biol 1989; 258:197–208 [DOI] [PubMed] [Google Scholar]
- 11.Klevay LM. Copper and ischemic heart disease. Biol Trace Elem Res 1983; 5:245–55 [DOI] [PubMed] [Google Scholar]
- 12.Chevion M, Jiang Y, Har-El R, Berenshtein E, Uretzky G, Kitrossky N. Copper and iron are mobilized following myocardial ischemia: possible predictive criteria for tissue injury. Proc Natl Acad Sci U S A 1993; 90:1102–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berenshtein E, Mayer B, Goldberg C, Kitrossky N, Chevion M. Patterns of mobilization of copper and iron following myocardial ischemia: possible predictive criteria for tissue injury. J Mol Cell Cardiol 1997; 29:3025–34 [DOI] [PubMed] [Google Scholar]
- 14.He W, James Kang Y. Ischemia-induced copper loss and suppression of angiogenesis in the pathogenesis of myocardial infarction. Cardiovasc Toxicol 2013; 13:1–8 [DOI] [PubMed] [Google Scholar]
- 15.Herd S, Camakaris J, Christofferson R, Wookey P, Danks D. Uptake and efflux of copper-64 in Menkes'-disease and normal continuous lymphoid cell lines. Biochem J 1987; 247:341–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bull PC, Thomas GR, Rommens JM, Forbes JR, Cox DW. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet 1993; 5:327–37 [DOI] [PubMed] [Google Scholar]
- 17.Zhou B, Gitschier J. hCTR1: a human gene for copper uptake identified by complementation in yeast. Proc Natl Acad Sci 1997; 94:7481–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison MD, Jones CE, Dameron CT. Copper chaperones: function, structure and copper-binding properties. J Biol Inorg Chem 1999; 4:145–53 [DOI] [PubMed] [Google Scholar]
- 19.Rae T, Schmidt P, Pufahl R, Culotta V, O'halloran T. Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science 1999; 284:805–8 [DOI] [PubMed] [Google Scholar]
- 20.Arredondo M, Muñoz P, Mura CV, Núñez MT. DMT1, a physiologically relevant apical Cu1+ transporter of intestinal cells. Am J Physiol Cell Physiol 2003; 284:C1525C30. [DOI] [PubMed] [Google Scholar]
- 21.Lutsenko S, Petris MJ. Function and regulation of the mammalian copper-transporting ATPases: insights from biochemical and cell biological approaches. J Membr Biol 2003; 191:1–12 [DOI] [PubMed] [Google Scholar]
- 22.Telianidis J, Hung YH, Materia S, Fontaine SL. Role of the P-Type ATPases, ATP7A and ATP7B in brain copper homeostasis. Front Aging Neurosci 2013; 5:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van De Sluis B, Rothuizen J, Pearson PL, van OBA, Wijmenga C. Identification of a new copper metabolism gene by positional cloning in a purebred dog population. Hum Mol Genet 2002; 11:165–73 [DOI] [PubMed] [Google Scholar]
- 24.Kodama H, Murata Y. Molecular genetics and pathophysiology of Menkes disease. Pediatr Int 1999; 41:430–5 [DOI] [PubMed] [Google Scholar]
- 25.Wadwa J, Chu YH, Nguyen N, Henson T, Figueroa A, Llanos R, Ackland ML, Michalczyk A, Fullriede H, Brennan G. Effects of ATP7A overexpression in mice on copper transport and metabolism in lactation and gestation. Physiol Rep 2014; 2:e00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michalcsyk A, Bastow E, Greenough M, Camakaris J, Linder MC, Mercer JF, Ackland L. Copper secretion from human breast epithelial cells is mediated by ATP7B and lactational hormones. FASEB J 2008; 22:443–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tao TY, Liu F, Klomp L, Wijmenga C, Gitlin JD. The copper toxicosis gene product Murr1 directly interacts with the Wilson disease protein. J Biol Chem 2003; 278:41593–6 [DOI] [PubMed] [Google Scholar]
- 28.Favier RP, Spee B, Schotanus BA, van den Ingh TS, Fieten H, Brinkhof B, Viebahn CS, Penning LC, Rothuizen J. COMMD1-deficient dogs accumulate copper in hepatocytes and provide a good model for chronic hepatitis and fibrosis. PLoS One 2012; 7:e42158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng X, Hou J, Liu J, Sun X, Sheng Q, Han P, Kang YJ. Safety evaluation of sevoflurane as anesthetic agent in mouse model of myocardial ischemic infarction. Cardiovasc Toxicol 2017; 17:150–6 [DOI] [PubMed] [Google Scholar]
- 30.Zhang W, Zhao X, Xiao Y, Chen J, Han P, Zhang J, Fu H, James Kang Y. The association of depressed angiogenic factors with reduced capillary density in the Rhesus monkey model of myocardial ischemia. Metallomics 2016; 8:654–62 [DOI] [PubMed] [Google Scholar]
- 31.Zama N, Towns RL. Cardiac copper, magnesium, and zinc in recent and old myocardial infarction. Biol Trace Elem Res 1986; 10:201–8 [DOI] [PubMed] [Google Scholar]
- 32.Anderson TW, Neri LC, Schreiber GB, Talbot FD, Zdrojewski A. Letter: ischemic heart disease, water hardness and myocardial magnesium. Can Med Assoc J 1975; 113:199–203 [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao Y, Nie X, Han P, Fu H, James Kang Y. Decreased copper concentrations but increased lysyl oxidase activity in ischemic hearts of rhesus monkeys. Metallomics 2016; 8:973–80 [DOI] [PubMed] [Google Scholar]
- 34.Zimnicka AM, Tang H, Guo Q, Kuhr FK, Oh MJ, Wan J, Chen J, Smith KA, Fraidenburg DR, Choudhury MS, Levitan I, Machado RF, Kaplan JH, Yuan JX. Upregulated copper transporters in hypoxia-induced pulmonary hypertension. PLoS One 2014; 9:e90544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goss JA, Barshes NR, Karpen SJ, Gao FQ, Wyllie S. Liver ischemia and ischemia-reperfusion induces and traffics the multi-specific metal transporter Atp7b to bile duct canaliculi: possible preferential transport of iron into bile. Biol Trace Elem Res 2008; 122:26–41 [DOI] [PubMed] [Google Scholar]
- 36.Singh MM, Singh R, Khare A, Gupta MC, Patney NL, Jain VK, Goyal SP, Prakash V, Pandey DN. Serum copper in myocardial infarction–diagnostic and prognostic significance. Angiology 1985; 36:504–10 [DOI] [PubMed] [Google Scholar]
- 37.Jain VK, Mohan G. Serum zinc and copper in myocardial infarction with particular reference to prognosis. Biol Trace Elem Res 1991; 31:317–22 [DOI] [PubMed] [Google Scholar]
- 38.Arnaud J, Faure H, Bourlard P, Denis B, Favier AE. Longitudinal changes in serum zinc concentration and distribution after acute myocardial infarction. Clin Chim Acta 1994; 230:147–56 [DOI] [PubMed] [Google Scholar]
- 39.Zuo X, Dong D, Sun M, Xie H, Kang YJ. Homocysteine restricts copper availability leading to suppression of cytochrome C oxidase activity in phenylephrine-treated cardiomyocytes. PLoS ONE 2013; 8:e67549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun M, Zuo X, Li R, Wang T, Kang YJ. Vascular endothelial growth factor recovers suppressed cytochrome c oxidase activity by restoring copper availability in hypertrophic cardiomyocytes. Exp Biol Med 2014; 239:1671–7 [DOI] [PubMed] [Google Scholar]
- 41.La Fontaine S, Mercer JF. Trafficking of the copper-ATPases, ATP7A and ATP7B: role in copper homeostasis. Arch Biochem Biophys 2007; 463:149–67 [DOI] [PubMed] [Google Scholar]
- 42.Chelly J, Tümer Z, Tønnesen T, Petterson A, Ishikawa-Brush Y, Tommerup N, Horn N, Monaco AP. Isolation of a candidate gene for Menkes disease that encodes a potential heavy metal binding protein. Nat Genet 1993; 3:14–9 [DOI] [PubMed] [Google Scholar]
- 43.Burstein E, Hoberg JE, Wilkinson AS, Rumble JM, Csomos RA, Komarck CM, Maine GN, Wilkinson JC, Mayo MW, Duckett CS. COMMD proteins, a novel family of structural and functional homologs of MURR1. J Biol Chem 2005; 280:22222–32 [DOI] [PubMed] [Google Scholar]
- 44.Narindrasorasak S, Kulkarni P, Deschamps P, She YM, Sarkar B. Characterization and copper binding properties of human COMMD1 (MURR1). Biochemistry 2007; 46:3116–28 [DOI] [PubMed] [Google Scholar]
- 45.Han M, Lin Z, Zhang Y. The alteration of copper homeostasis in inflammation induced by lipopolysaccharides. Biol Trace Elem Res 2013; 154:268–74 [DOI] [PubMed] [Google Scholar]