Abstract
Purpose
To report a case of disseminated silicone granulomatosis presenting with ptosis, proptosis and vision loss.
Observations
A 56-year-old female presented with ptosis, proptosis, and vision loss and was noted to have palpable, erythematous masses involving the orbit, face, trunk, and body. She had a history of bilateral silicone breast implants and cosmetic facial filler injections. Orbital biopsy demonstrated non-caseating granulomas with foreign-body giant cells and vacuoles containing material consistent with silicone. Removal of the patient's breast implants and systemic immunosuppression led to dramatic granuloma regression.
Conclusions
Silicone can induce a severe, systemic inflammatory response and should be considered in the differential for facial and periorbital granulomas in patients with a history of silicone breast implants. Management of disseminated silicone granulomatosis is challenging and requires multimodal treatment with silicone removal and systemic immunomodulation.
Keywords: Silicone, Disseminated, Silicone granulomatosis, Orbit, Breast implants, Autoimmune/inflammatory syndrome
1. Introduction
Silicone granulomatosis is an inflammatory tissue response to exogenous silica particles, characterized by the formation of palpable granulomas.1 The diagnosis is confirmed by tissue biopsy. While local granuloma formation in response to implanted silicone is well documented, disseminated silicone granulomatosis resulting from a focal source is rare and poorly understood. Herein we present a disseminated case with robust periorbital and facial involvement in a patient with a history of silicone breast implants. To our knowledge, there is no previous report of orbital silicone granuloma coupled with widespread silicone migration throughout the body. Granulomas manifested in multiple locations including the eyelids, orbit, face, trunk, arms, and legs. We discuss the importance of systemic immunotherapy and removal of the silicone stimulus when managing disseminated disease. Informed Consent for this report was obtained from the patient and the report is in accordance with HIPAA regulations.
2. Report of case
A 56-year-old female presented to the oculoplastics clinic for evaluation of orbitofacial swelling and nodularity associated with ptosis, proptosis, and vision loss. She had a remote history of cosmetic facial injections of an unknown substance with no adverse reactions. She was subsequently diagnosed with breast cancer and underwent bilateral mastectomies with silicone implant reconstruction. Five years after implantation, she developed muscle and joint pains, stiffness, and fatigue as well as painful lumps throughout her breasts and arms, which progressed to her face, eyelids, and orbit. Orbital biopsy showed non-caseating granulomatous inflammation with scattered clear vacuole-like structures highly suggestive of foreign silicone (Fig. 1). She underwent four orbital and periorbital debulking procedures at an outside hospital, along with trials of oral prednisone and minocycline, and injections with steroids and 5-fluorouracil to control the periorbital inflammation.
Fig. 1.
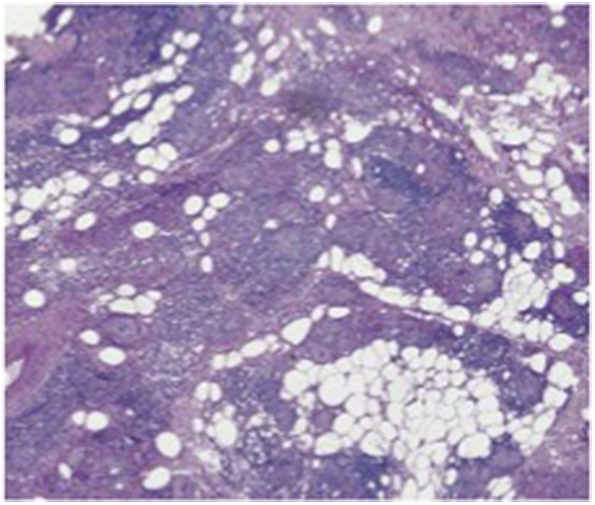
Histopathology of orbital granuloma biopsy. Biopsy specimen contains non-caseating granulomas and clear vacuole-like spaces which contain silicone (H&E stain, 10X).
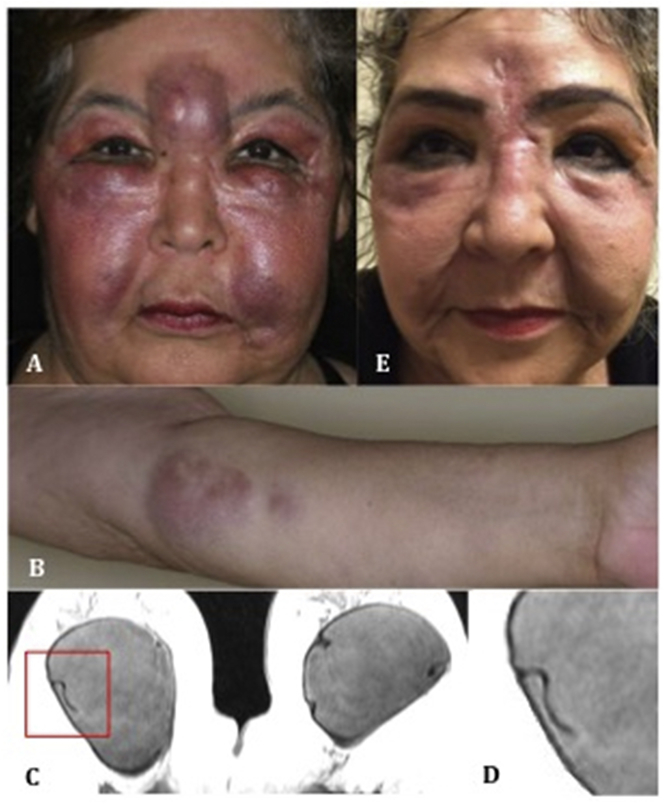
Upon presentation, examination revealed large, firm, erythematous periorbital and orbital masses (Fig. 2A). Similar lesions were noted throughout her arms and legs (Fig. 2B). Visual acuity was 20/40 right eye and 20/30 left eye. Humphrey visual fields showed bilateral peripheral defects. Adnexal exam was significant for ptosis and proptosis. Magnetic resonance imaging (MRI) showed diffuse, nodular soft tissue thickening of the face and periorbita as well as fat stranding within the orbit (Fig. 3). MRI of the breasts demonstrated grossly intact implants with a linguine sign – indicative of occult rupture (Fig. 2C–D). Infectious disease and endocrine workup was unremarkable.
Fig. 2.
External photographs and breast MRI. A) Facial swelling, erythema, and glabellar and periorbital masses, and (B) left upper extremity masses at the time of presentation, C) Breast MRI (T2-weighted) demonstrating the “linguine” sign, a low-signal-intensity curvilinear lines seen within the implants bilaterally, D) insert: magnified image of the “linguine” sign, E) Eight months after surgical removal of the implants, there is dramatic improvement in facial and periorbital masses and swelling.
Fig. 3.
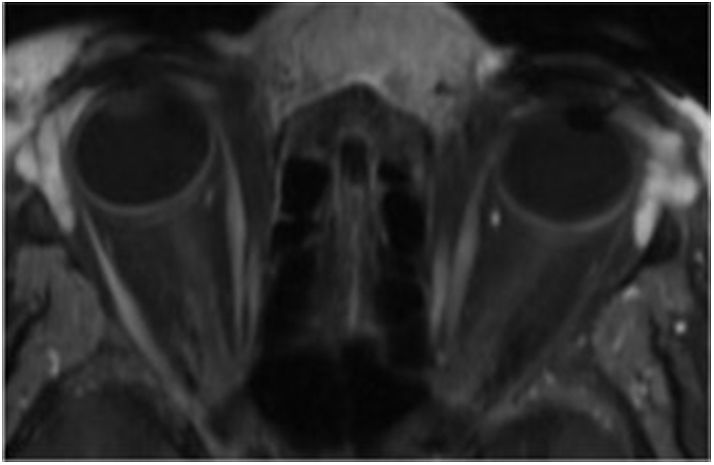
Orbital MRI (T1-weighted) at time of oculoplastics presentation. Diffuse eyelid soft tissue thickening, proptosis, and orbital fat stranding.
The patient's breast implants and facial injections were both considered as potential sources of orbitofacial silicone granulomatosis. Due to the systemic nature of her disease, recurrence after periorbital and orbital silicone excision, and suggestion of occult breast implant rupture on imaging, her silicone breast implants were deemed the likely source and removal was recommended. The patient was also treated with doxycycline and methotrexate for systemic immunosuppression. Eight months after breast implant removal, she had dramatic improvement of her periorbital and orbital disease (Fig. 2E) as well as significant regression of her forearm and leg granulomas. Visual acuity was restored to 20/20 in both eyes and visual fields normalized. The patient was subsequently tapered off methotrexate by dermatology with no recurrence of disease.
3. Discussion
We present the first case of disseminated silicone granulomatosis with robust orbitofacial involvement due to rupture of breast implants. Prior reports have found granulomas within joint synovium, skin, and locally within the implant capsules themselves.2 Disseminated silicone-induced lymphadenopathy was reported in women with ruptured or leaking breast implants, however no involvement of the face or orbit was found.3 Isolated eyelid edema and inflammation in conjunction with implant rupture has been described, but no other masses were detected throughout the body.4
Implant leakage and rupture are well-established safety concerns for silicone breast implants, with the risks increasing with implant age (up to 30–50% by 10 years). Rupture or extensive leakage may result in contour deformity, palpable mass-like lesions, pain, and focal inflammation. However, leakage is commonly “silent” with no obvious symptoms. Magnetic Resonance Imaging (MRI) is the imaging modality of choice for detection, with over 90% sensitivity and specificity.5 The linguine sign seen in our patient – indicative of occult leakage or rupture – is a low-signal-intensity curvilinear line adjacent to a thin layer of escaped high-signal-intensity silicone gel adjacent to an otherwise intact implant.5 This can occur through mechanical pressure or osmosis, which allows silicone gel to “bleed” through an intact shell due to the semi-permeable nature of the silicone elastomer membrane.6,7 Once escaped from the implant, it is thought that silicone can migrate through local soft tissue dispersion or via lymphatic and hematogenous routes.7, 8, 9
Regardless of the origin and mechanism of silicone dissemination, silicone granulomas can result from an inflammatory or autoimmune tissue response.10 A systemic manifestation of the inflammatory response to adjuvants, such as silicone, has been termed autoimmune/inflammatory syndrome induced by adjuvants (ASIA).11 An adjuvant is a substance that enhances the antigen-specific immune response, inducing a characteristic inflammatory cascade. The activation of the immune system by adjuvants, including silicone, could trigger manifestations often seen in de novo autoimmune disease, such as myalgias, arthralgias, and fatigue in addition to identifiable masses.11,12 ASIA has been described after silicone implant rupture, however these cases involved the lymph nodes, thorax, and lungs.13 Our patient demonstrated many of these symptoms, therefore systemic immunosuppression, in addition to silicone implant removal, was recommended.
Management of disseminated silicone granulomatosis is challenging. A thorough history is required to identify all potential sources of silicone in the body, followed by elimination of the inciting foreign material when possible. Even patients with intact silicone breast implants can demonstrate significant clinical improvement with removal.2 In addition, the systemic inflammatory and immune response must be controlled. Steroids have been used to temper the inflammatory reaction, but relapses are common when doses are tapered. Reports have also described silicone granulomas responding well to doxycycline (100 mg twice daily) and minocycline (100 mg daily) due to their immunomodulatory properties.14,15 Additionally, methotrexate may be used to treat inflammation through increased release of adenosine and immunosuppression through apoptosis and clonal deletion of activated T-cells.16,17 Finally, other modulators of the inflammatory cascade including etanercept (TNFα-inhibitor), imiquimod (Toll-like receptor activator with immune antiproliferative effects), and tacrolimus (macrolide immunosuppressant) have been successfully used against granulomatous diseases, and may be considered as additional options to treat silicone granulomatosis.18, 19, 20 Once disease control and regression is reached, patients may be slowly tapered off of systemic immunosuppression with careful monitoring for recurrence. Our patient had a dramatic improvement in periorbital and body granulomas after removal of her breast implants and treatment with methotrexate.
4. Conclusions
Disseminated silicone granulomatosis should be considered in the differential for patients with new facial or periorbital masses and a history of silicone breast implants. Successful management requires identifying and removing the inciting silicone material as well as controlling the granulomatous reaction through systemic immunosuppression.
Patient consent
The patient consented to publication of the case in writing/orally.
Acknowledgements/Disclosures
Funding
No funding or grant support.
Conflicts of Interest
There are no conflicts of interests for any author.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
None.
References
- 1.van Diest P.J., Beekman W.H., Hage J.J. Pathology of silicone leakage from breast implants. J Clin Pathol. 1998;51(7):493–497. doi: 10.1136/jcp.51.7.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silver R.M., Sahn E.E., Allen J.A. Demonstration of silicon in sites of connective-tissue disease in patients with silicone-gel breast implants. Arch Dermatol. 1993;129(1):63–68. [PubMed] [Google Scholar]
- 3.Bauer P.R., Krajicek B.J., Daniels C.E., Shah S.S., Ryu J.H. Silicone breast implant-induced lymphadenopathy: 18 cases. Respir Med CME. 2011;4(3):126–130. [Google Scholar]
- 4.Meyer D.R., Bui H.X., Carlson J.A. Silicon granulomas and dermatomyositis-like changes associated with chronic eyelid edema after silicone breast implant. Ophthalmic Plast Reconstr Surg. 1998;14(3):182–188. doi: 10.1097/00002341-199805000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Gorczyca D.P., Gorczyca S.M., Gorczyca K.L. The diagnosis of silicone breast implant rupture. Plast Reconstr Surg. 2007;120(7 Suppl 1):49S–61S. doi: 10.1097/01.prs.0000286569.45745.6a. [DOI] [PubMed] [Google Scholar]
- 6.Brody G.S. Fact and fiction about breast implant “bleed.”. Plast Reconstr Surg. 1977;60(4):615–616. doi: 10.1097/00006534-197710000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Travis W.D., Balogh K., Abraham J.L. Silicone granulomas: report of three cases and review of the literature. Hum Pathol. 1985;16(1):19–27. doi: 10.1016/s0046-8177(85)80209-4. [DOI] [PubMed] [Google Scholar]
- 8.Ahn C.Y., Shaw W.W. Regional silicone-gel migration in patients with ruptured implants. Ann Plast Surg. 1994;33(2):201–208. doi: 10.1097/00000637-199408000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Coble Y.D., Estes E.H., Head C.A. Silicone gel breast implants. Council on Scientific Affairs, American Medical Association. JAMA. 1993;270(21):2602–2606. [PubMed] [Google Scholar]
- 10.McDonald A.H., Schneider M., Gudenkauf L., Sanger J.R., Weir K. Silicone gel enhances the development of autoimmune disease in New Zealand black mice but fails to induce it in BALB/cAnPt Mice. Clin Immunol Immunopathol. 1998;87(3):248–255. doi: 10.1006/clin.1998.4532. [DOI] [PubMed] [Google Scholar]
- 11.Shoenfeld Y., Agmon-Levin N. 'ASIA' - autoimmune/inflammatory syndrome induced by adjuvants. J Autoimmun. 2011;36(1):4–8. doi: 10.1016/j.jaut.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Vera-Lastra O., Medina G., Cruz-Dominguez Mdel P., Jara L.J., Shoenfeld Y. Autoimmune/inflammatory syndrome induced by adjuvants (Shoenfeld's syndrome): clinical and immunological spectrum. Expet Rev Clin Immunol. 2013;9(4):361–373. doi: 10.1586/eci.13.2. [DOI] [PubMed] [Google Scholar]
- 13.Nesher G., Soriano A., Shlomai G. Severe ASIA syndrome associated with lymph node, thoracic, and pulmonary silicone infiltration following breast implant rupture: experience with four cases. Lupus. 2015;24(4-5):463–468. doi: 10.1177/0961203314562622. [DOI] [PubMed] [Google Scholar]
- 14.Paul S., Goyal A., Duncan L.M., Smith G.P. Granulomatous reaction to liquid injectable silicone for gluteal enhancement: review of management options and success of doxycycline. Dermatol Ther. 2015;28(2):98–101. doi: 10.1111/dth.12204. [DOI] [PubMed] [Google Scholar]
- 15.MJ1 Arin, Bäte J., Krieg T., Hunzelmann N. Silicone granuloma of the face treated with minocycline. J Am Acad Dermatol. 2005;52(2 Suppl 1):53–56. doi: 10.1016/j.jaad.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Cronstein B.N., Naime D., Ostad E. The antiinflammatory mechanism of methotrexate. Increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. J Clin Invest. 1993;92(6):2675–2682. doi: 10.1172/JCI116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Genestier L., Paillot R., Fournel S., Ferraro C., Miossec P., Revillard J.P. Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J Clin Invest. 1998;102(2):322–328. doi: 10.1172/JCI2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baumann L.S., Halem M.L. Lip silicone granulomatous foreign body reaction treated with aldara (imiquimod 5%) Dermatol Surg. 2003;29(4):429–432. doi: 10.1046/j.1524-4725.2003.29102.x. [DOI] [PubMed] [Google Scholar]
- 19.Rapaport M.J. Silicone granulomas treated with etanercept. Arch Dermatol. 2005;141(9):1171. doi: 10.1001/archderm.141.9.1171-a. [DOI] [PubMed] [Google Scholar]
- 20.Alijotas-Reig J, Garcia-Gimenez V, Vilardell-Tarres M. Tacrolimus in the treatment of chronic and refractory late-onset immune-mediated adverse effects related to silicone injections. Dermatol Surg. 38(1):38–47. [DOI] [PubMed]