Abstract
Purpose
We present the first detailed ophthalmic description of a child with Helsmoortel-Van der Aa Syndrome (HVDAS), including longitudinal follow-up and analysis.
Observations
After extensive workup, a young child with poor visual behavior, hypotonic cerebral palsy, intellectual disability, and global developmental delay was found to have a heterozygous de novo mutation in the ADNP gene and diagnosed with HVDAS. Ophthalmic findings were remarkable for progressive nystagmus, macular pigment mottling, mild foveal hypoplasia with abnormal macular laminations, persistent rod dysfunction with electronegative waveform, and progressive cone degeneration.
Conclusions and importance
Patients with HVDAS are known to have abnormal visual behavior due to refractive or cortical impairment. However, we present the first description, to our knowledge, of an association with retinal mal-development and degeneration. Thus, patients with HVDAS should be referred for ophthalmic genetics evaluation, and HVDAS should be on the differential diagnosis for young children with global developmental delay who present with nystagmus, rod and cone dysfunction with electronegative waveform, and relative lack of severe structural degeneration on optical coherence tomography.
Keywords: Helsmoortel-Van der Aa Syndrome, HVDAS, Activity-dependent neuroprotective protein, ADNP, Nystagmus, Retinal degeneration, Electronegative waveform, Optical coherence tomography
1. Introduction
Helsmoortel-Van der Aa Syndrome was a challenging diagnosis in a young child, who presented with poor visual behavior, global developmental delay, hypotonia, and plagiocephaly. The ophthalmic and systemic abnormalities are described in detail with longitudinal follow-up. Retinal dysfunction has not previously been associated with this condition, and we present the first evidence of abnormal retinal development and degeneration.
2. Case report
A 1.3 year old male with abnormal visual behavior, global developmental delay, mild hypotonia and plagiocephaly was referred to Oregon Health and Science University for additional evaluation and diagnostics in ophthalmic genetics and medical genetics.
Neurodevelopmental evaluation at 1.3 years of age revealed global delay in developmental skills equivalent to 7–9 months of age. He walked very late at 2.8 years old. At 4 years of age, he continued to have generalized mild hypotonia with delayed development of gross motor function and cognitive abilities (language and perceptual-motor skills) equivalent to 17–18 month and 14–18 months of age, respectively. Physical examination showed short stature with normal weight, brachycephaly, minor non-specific facial dysmorphism, and fifth finger brachydactyly. Diagnoses include hypotonic cerebral palsy and intellectual disability to a moderate or severe degree. Additional systemic evaluation revealed gastroesophageal reflux disease, constipation, presumed calcaneus osteomyelitis, and oropharyngeal dysphagia/feeding disorder.
Poor vision was suspected due to a history of poor eye contact and tracking beyond four months of age. The family history revealed only a paternal great grandfather with report of nyctalopia and poor vision, and two first-degree cousins with congenital stationary night blindness (CSNB). Both non-consanguineous parents and a younger sibling were healthy with no unexplained vision problems.
On presentation to ophthalmology at 1.3 years of age, the patient could fix and follow, but gaze was unsteady with small amplitude nystagmus. Retinoscopy showed mild hyperopia and astigmatism. Ophthalmic examination under anesthesia was notable for bilateral steep temporal sloping optic discs, mottled pigmentation of the macula and blonde fundus appearance. The fundus exam remained unchanged at 1.9, 2.9, and 3.9 years of age, however, the nystagmus eventually developed into a pendular type, which is typically seen in patients with low vision during childhood.
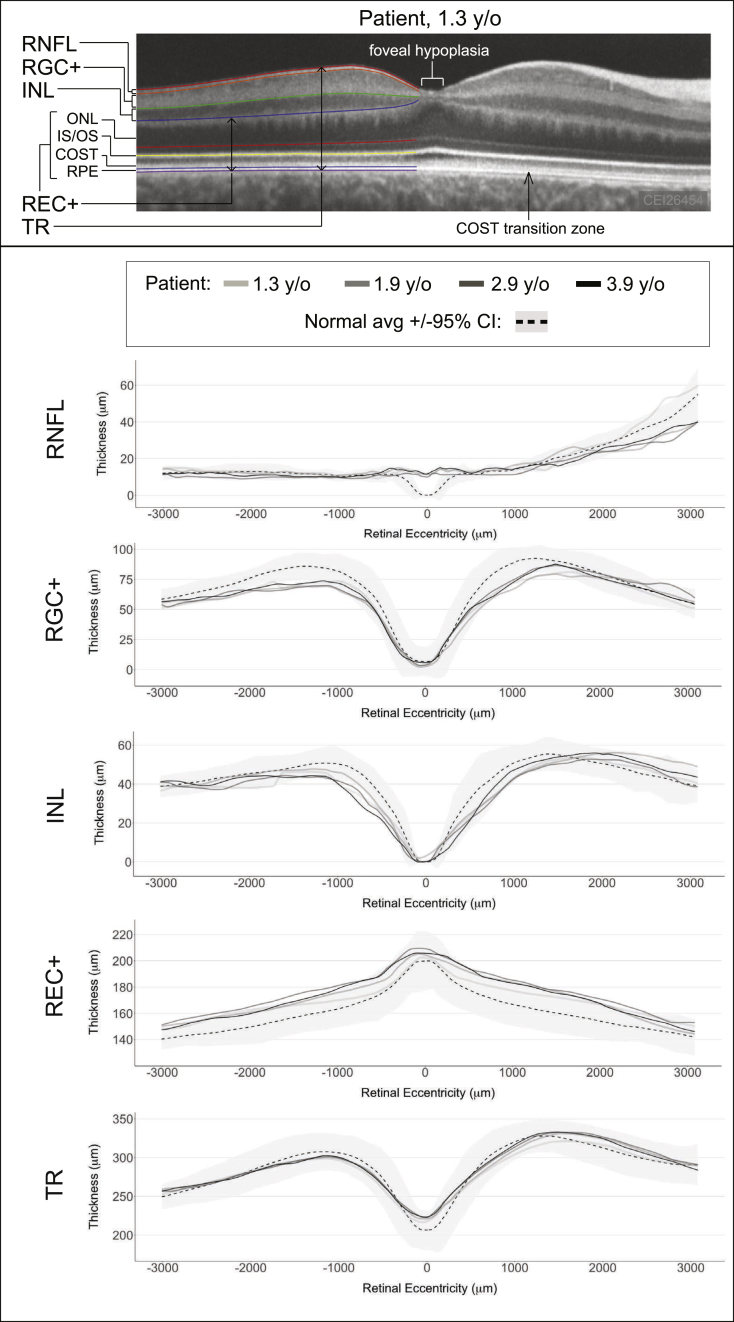
Sedated handheld spectral domain optical coherence tomography (OCT) was performed using the Envisu R2200-HR (Bioptigen, Durham, NC) as previously described,1 which showed retention of the inner retinal layers across the foveola, consistent with foveal hypoplasia in both eyes (Fig. 1, upper panel). The OCT also showed a transition in the normal cone outer-segment tips (COST) layer in the perifovea to abnormal COST with loss of discrete reflectivity in the fovea, which was thought to be indicative of abnormal foveal photoreceptor structure in both eyes. In addition, the OCT image from one eye was analyzed and segmented as previously described, and compared to a normal age-matched data set that has been previously published.1 Segmentation of the retinal layers showed that the patient's total retinal thickness (TR) was normal, except for retained retinal nerve fiber layer (RNFL) thickness at the foveola due to foveal hypoplasia (Fig. 1, lower panel). The outer retinal layer, which was defined as photoreceptor plus retinal pigment epithelial layer (REC+), was thicker than normal controls beyond the 95% confidence interval for most of the macular profile. The inner retinal layers include the retinal ganglion cell plus inner plexiform layer (RGC+) and inner nuclear layer (INL), which were mostly thinner than the average of normal controls with some regions of the macular profile below the 95% confidence interval. OCT was repeated multiple times between the ages of 1.3 and 3.9 years, however there was no trend towards thickening or thinning for each of the segmented layers. Thus, the OCT analysis showed that the patient had a mal-developed retina with the structural imbalance of a thick outer retina and thin inner retina.
Fig. 1.
Optical Coherence Tomography: Analysis and Segmentation Profiles.
Upper Panel: Image of patient's right macula at 1.3 years of age, with only the temporal macula segmented to illustrate the various retinal layers that were analyzed.
Lower Panel: Retinal layer thicknesses at each age plotted against the normal mean and 95% confidence interval.
y/o, years old; CI, confidence interval; RNFL, retinal nerve fiber layer; RGC+, retinal ganglion cell layer plus inner plexiform layer; INL, inner nuclear layer; ONL, outer nuclear layer; IS/OS, inner segment/outer segment junction; COST, cone outer segment tips; RPE, retinal pigment epithelium; REC+, photoreceptor layer from Bruch's membrane to the INL/outer plexiform layer interface; TR, total retina; negative and positive retinal eccentricity is temporal and nasal to the foveola, respectively.
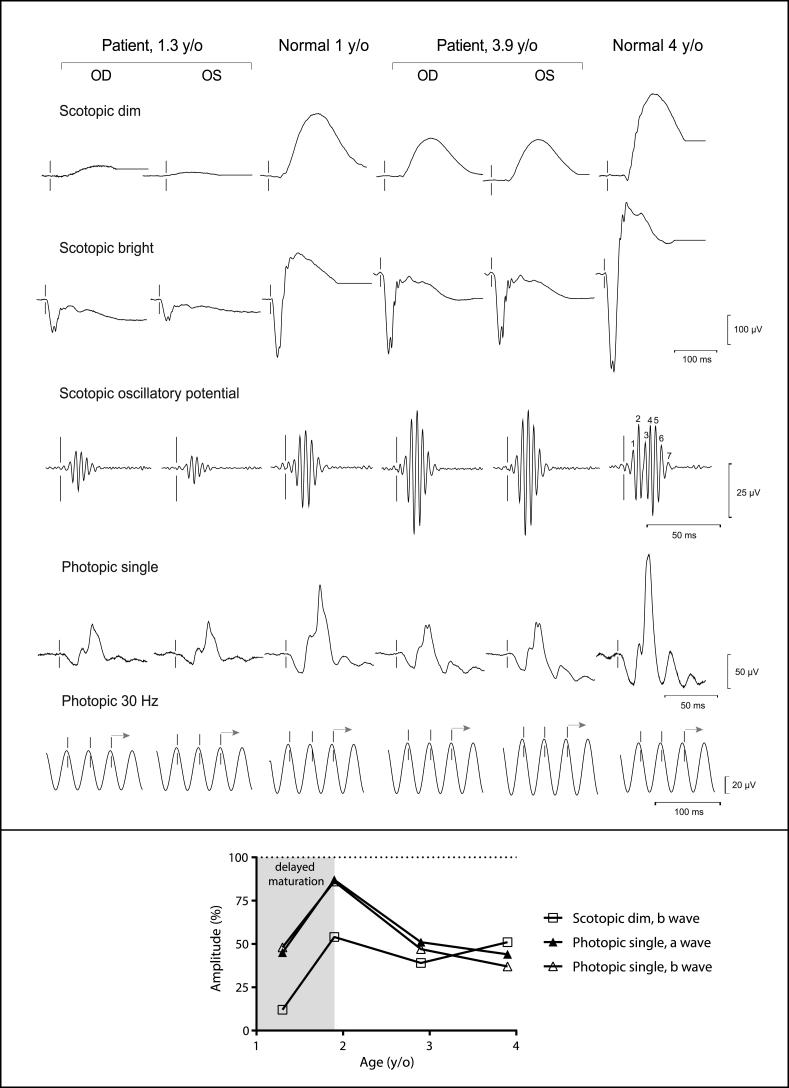
Sedated full-field electroretinogram (ERG) was performed as previously described and according to the International Society for Clinical Electrophysiology of Vision guidelines (Fig. 2, upper panel; Table 1).2,3 Compared to age-matched sedated controls, the rod-dependent (scotopic dim) responses were more severely attenuated than the cone-dependent responses (photopic single) in the patient at 1.3 years old. The spontaneous increase in all responses from 1.3 to 1.9 years of age was most likely due to delayed maturation of the visual system in a patient with global developmental delay, however, the rod-cone dysfunction remained (Fig. 2, lower panel). From age 1.9–3.9 years, there was a dramatic worsening of cone-dependent responses, while rod-dependent responses were relatively stable, such that both rod and cone function became equally subnormal. The year-to-year interval decline in cone-dependent responses for the a-wave amplitudes was 20–23%, while the b-wave amplitudes was 30%. Interestingly, implicit times for all responses were not prolonged at any age, which is atypical for retinal degenerations. At all time-points, the maximal mixed rod and cone response (scotopic bright) revealed a decreased b-to-a wave ratio, also known as an electronegative waveform, which is typically seen in disorders that affect the inner retina out of proportion to the outer retina (Fig. 2; Table 1). Inherited disorders classically associated with an electronegative waveform include neuronal ceroid lipofuscinoses (NCLs), CSNB, X-linked retinoschisis, and Goldmann-Favre syndrome, but can also be seen in Leber congenital amaurosis. None of these differential diagnoses were completely compatible with the patient's clinical presentation or the findings on OCT.
Fig. 2.
Full-Field Electroretinogram.
Upper Panel: The waveforms for the patient are shown for each stimulus at 1.3 and 3.9 years of age, along with the respective age-matched normal waveforms, to illustrate the persistence of the electronegative waveform and attenuation of all responses in both eyes.
Lower Panel: The a and b wave amplitudes, averaged from both eyes of the patient, are plotted over time and presented as a percentage of the mean of age-matched normal. With the exception of the initial rise in all amplitudes from 1.3 to 1.9 years of age due to delayed maturation, the scotopic dim amplitudes show stable dysfunction of the rod-dependent responses, while the photopic amplitudes show cone degeneration.
y/o, years old; OD, ocular dextrous; OS, ocular sinistrous; Hz, hertz.
Table 1.
Full-field electroretinogram.
| Patient Age | 1.3 yrs |
1.9 yrs |
2.9 yrs |
3.9 yrs |
||||
|---|---|---|---|---|---|---|---|---|
| Amplitude, μV (% nl) |
Implicit Time, ms |
Amplitude, μV (% nl) |
Implicit Time, ms |
Amplitude, μV (% nl) |
Implicit Time, ms |
Amplitude, μV (% nl) |
Implicit Time, ms |
|
| Scotopic dim | ||||||||
| b wave | 25 (12%) | 105 | 145 (54%) | 104 | 101 (39%) | 104 | 130 (51%) | 114 |
| Scotopic bright | ||||||||
| a wave | 92 (54%) | 17 | 258 (121%) | 16 | 187 (74%) | 16 | 254 (98%) | 16 |
| b wave | 72 (20%) | 56 | 270 (60%) | 61 | 196 (40%) | 58 | 246 (64%) | 64 |
| b:a ratio | 0.78 | 1.0 | 1.0 | 0.97 | ||||
| OP | 40 | 120 | 98 | 161 | ||||
| Photopic single | ||||||||
| a wave | 16 (45%) | 16 | 38 (87%) | 15 | 29 (51%) | 15 | 23 (44%) | 16 |
| b wave | 61 (48%) | 31 | 132 (86%) | 29 | 93 (47%) | 31 | 65 (37%) | 27 |
| Photopic 30-Hz | 48 (75%) | 30 | 64 (90%) | 29 | 52 (52%) | 29 | 63 (67%) | 29 |
Amplitudes were the average of the right- and left-eye responses for all measurements and are also presented in parentheses as a percentage of the mean of age-matched normal response.
ms, millisecond; nl, normal; OP, oscillatory potential; yrs, years.
Prior work-up included negative or normal results for fragile X syndrome, chromosome micro-array, MRI brain, electroencephalogram, and audiometry. Serum chemistries included comprehensive metabolic panel, magnesium, lead, very long chain fatty acids, ammonia, bilirubin, carnitine (including acylcarnitine esters), and thyroid stimulating hormone, which were all normal. There were mild elevations of creatine kinase (244; reference range 55–170) and lactate (3.6; reference range 0.5–1.9), but serum amino acids and urine organic acids were unremarkable. Additional work-up was negative for NCL types 1 and 2 (palmitoyl-protein thioesterase 1, and tripeptidyl-peptidase 1), and congenital disorders of glycosylation (serum carbohydrate deficient transferrin analysis and N-glycan profile). Specific mutation analysis showed no mutations in TRPM1 that were the cause of CSNB in his first-degree cousins. Finally, whole exome sequencing (Baylor Miraca Genetics Laboratories, Houston, TX) revealed a heterozygous de novo mutation (c.2157C > A, p. Y719X) in the ADNP gene, which has previously been shown to be associated with a recently described syndrome called Helsmoortel-van der Aa syndrome (HVDAS; OMIM 615873).
3. Discussion
Activity-dependent neuroprotective protein (ADNP)-related intellectual disability and autism spectrum disorder, also known as HVDAS, is characterized by the presence of both intellectual disability and some degree of autism spectrum disorder. In addition, dysmorphic features that may include prominent forehead, high anterior hairline, notched eyelids, ptosis, up- or down-slanted palpebral fissures, wide nasal bridge, thin vermilion border of the upper lip, and ear abnormalities may be present. Other clinical findings may include behavioral problems, delayed developmental milestones, premature primary tooth eruption, sleep disturbance, hypotonia, seizures, feeding difficulties, cardiac defects, and brain abnormality on MRI.4,5 HVDAS is typically associated with de novo heterozygous mutations in the ADNP gene,4,6 which encodes a transcription factor that is crucial for the regulation of genes involved in the control of organogenesis and neurogenesis.7,8 A knockout of the Adnp gene in mice results in neural tube defects and embryonic lethality.9 The de novo p. Y719X identified in our patient results in a truncated protein with a loss of 384 amino acids at the C-terminus of ADNP, including the DNA binding homeobox domain, and has been characterized as pathogenic. This mutation has previously been observed in several other patients with HVDAS according to two published studies and ClinVar, which includes a description of a case with non-refractive ophthalmic issues as discussed below.4,10
Visual impairment in the form of refractive issues (hypermetropia and astigmatism) was reported in approximately half of the original cohort of children with HVDAS.6 In addition, one case report also described exotropia, mild amblyopia, and cortical visual impairment.10 While abnormal visual behavior has indeed been described, to our knowledge, we present the first detailed characterization of retinal mal-development and degeneration in a child with HVDAS. The test results from 1.9 to 3.9 years of age that support abnormal development of the retina include the stability and persistence of the foveal hypoplasia, the decreased ratio of inner-to-outer retinal thickness, the electronegative waveform, and the rod dysfunction with normal implicit times. The electronegative waveform was correlated with the unique OCT findings of abnormally thin inner retina and thick outer retina in this patient. We would like to emphasize that the increase in ERG amplitudes from age 1.3–1.9 years, was due to delayed maturation of the visual system; while the ERG response is normally developed by 1 year of age in healthy infants, it may not fully develop until 2 years of age or older in a child with severe developmental delay. Thus, we regard the ERG results at 1.9 years of age in this patient to be the true baseline response. During the subsequent period of follow-up, a progressive degeneration of the cone-dependent responses was apparent on ERG, which showed a 30% annual rate of decline in inner retinal function (b-wave) out of proportion to a 20–23% annual rate of decline in outer retinal function (a-wave). These ERG findings are even more compelling given that amplitudes are expected to slowly increase until 5 years of age in normal children. Futhermore, the decline in retinal function is correlated with a dramatic worsening of nystagmus from a subtle small amplitude movementat 1.3 years to an obvious pendular type at 3.9 years of age. There was no associated structural decline of the foveal cone photoreceptors that was perceivable on exam or OCT imaging, but it is known that structural degeneration often lags behind functional decline in inherited retinal degenerations. Moreover, retinal degenerations that have a predilection for the inner retina may not produce obvious retinal pigment changes, especially at an early age. For example, there are no obvious changes on OCT or fundus photography in CSNB.
Continued longitudinal follow-up will be needed to determine the long-term consequences of these findings, and detailed ophthalmic testing of other HVDAS patients will be required to determine if retinal dysfunction is a common feature of HVDAS. We theorize that mutations in the ADNP gene may cause neural dysgenesis of the retina in patients with HVDAS, which results in stable rod photoreceptor and inner retinal dysfunction, and progressive cone degeneration.
In summary, we have shown that the abnormal visual behavior in children with HVDAS may not only be due to refractive or cortical impairment, but can also be due to retinal dysfunction. We recommend that patients with HVDAS be referred for ophthalmic evaluation with ERG and OCT, which can provide objective information on visual prognosis to better anticipate low-vision needs. Genetic testing panels for developmental delay, hypotonia, and retinal degeneration should include the ADNP gene. HVDAS should be in the differential diagnosis for young children with global developmental delay who present with nystagmus, rod and cone dysfunction with electronegative waveform on ERG, and relative lack of severe structural degeneration on OCT.
Patient consent
This research was approved by the Institutional Review Board of the Oregon Health & Science University and adhered to the tenets of the Declaration of Helsinki. The patient's legal guardian signed an informed written consent to publish personal information such as age, gender, and ethnicity.
Acknowledgments and disclosures
Funding
National Institute of Health: 1K08EY026650-01(Paul Yang).
Foundation Fighting Blindness: CD-NMT-0714-0648 (Paul Yang); C-CL-0711-0534-OHSU01 (Casey Eye Institute).
Research to Prevent Blindness: unrestricted departmental funding to Casey Eye Institute.
Conflicts of interest
The following authors have no financial disclosures: MG, HT, GH, TA, AD, JW, DMK, EF, PB, PWC, DJK, PY.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
None.
References
- 1.Yang P. Retinal morphology of patients with achromatopsia during early childhood: implications for gene therapy. JAMA Ophthalmol. 2014;132:823–831. doi: 10.1001/jamaophthalmol.2014.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oh K.T. Electroretinographic findings in patients with Stargardt disease and fundus flavimaculatus. Retina. 2004;24:920–928. doi: 10.1097/00006982-200412000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Marmor M.F. ISCEV Standard for full-field clinical electroretinography (2008 update) Doc Ophthalmol. 2009;118:69–77. doi: 10.1007/s10633-008-9155-4. [DOI] [PubMed] [Google Scholar]
- 4.Helsmoortel C. A SWI/SNF-related autism syndrome caused by de novo mutations in ADNP. Nat Genet. 2014;46:380–384. doi: 10.1038/ng.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gozes I. Premature primary tooth eruption in cognitive/motor-delayed ADNP-mutated children. Transl Psychiatry. 2017;7:e1043. doi: 10.1038/tp.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vandeweyer G. The transcriptional regulator ADNP links the BAF (SWI/SNF) complexes with autism. Am J Med Genet C Semin Med Genet. 2014;166C:315–326. doi: 10.1002/ajmg.c.31413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gozes I. Activity-dependent neuroprotective protein: from gene to drug candidate. Pharmacol Ther. 2007;114:146–154. doi: 10.1016/j.pharmthera.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Mandel S., Rechavi G., Gozes I. Activity-dependent neuroprotective protein (ADNP) differentially interacts with chromatin to regulate genes essential for embryogenesis. Dev Biol. 2007;303:814–824. doi: 10.1016/j.ydbio.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 9.Pinhasov A. Activity-dependent neuroprotective protein: a novel gene essential for brain formation. Brain Res Dev Brain Res. 2003;144:83–90. doi: 10.1016/s0165-3806(03)00162-7. [DOI] [PubMed] [Google Scholar]
- 10.Pescosolido M.F. Expansion of the clinical phenotype associated with mutations in activity-dependent neuroprotective protein. J Med Genet. 2014;51:587–589. doi: 10.1136/jmedgenet-2014-102444. [DOI] [PMC free article] [PubMed] [Google Scholar]