Abstract
Background:
Gastrointestinal dysfunction plays a critical role in the prognosis of critically ill patients. Previous studies showed rhubarb, a traditional Chinese herb, can protect the intestinal barrier function, prevent intestinal bacterial translocation, and promote gastrointestinal peristalsis, but the clinical studies are less. The aim of this study was to evaluate the effects of rhubarb on gastrointestinal dysfunction in critically ill patients.
Methods:
From June 2015 to May 2017, a total of 368 critically ill patients with Grade I–III acute gastrointestinal injury (AGI) were enrolled in this study. Patients were divided into two groups according to the exposure factors (whether the patients received rhubarb treatment): the rhubarb group and the usual treatment group. Clinical data were collected within the first 24 h of the Intensive Care Unit (ICU) admission and 7 days after treatment. Survival data on day 28 after ICU admission and the durations of ICU and total hospitalization were also collected. Propensity score matching (PSM) was conducted to reduce confounding bias between the groups. The logistic regression was conducted to screen the influence factors.
Results:
The eligible patients were divided into rhubarb group (n = 219, 59.5%) and usual treatment group (n = 149, 40.5%). Before PSM, the remission rate of feeding intolerance in rhubarb group and usual treatment group were 59.8% and 39.6%, respectively. After PSM, the remission rate of feeding intolerance in rhubarb group and usual treatment group was 77.9% and 30.9%, respectively. The remission rates of feeding intolerance in rhubarb group were significantly higher than those in the usual treatment group (all P < 0.05). Compared with the usual treatment group, the rhubarb group had a higher rate of AGI improvement, lower level of C-reactive protein, shorter stay in ICU before and after PSM (P < 0.05). There was no significant difference in 28-day mortality between rhubarb and usual treatment groups before and after PSM (48 vs. 33, P = 0.959; and 16 vs. 21, P = 0.335). The logistic regression analysis showed that the single factor, whether receiving rhubarb therapy, affected the proportion of patients whose enteral nutrition needs ≥83.7 kJ·kg−1·d−1 after 7 days of treatment (odds ratio: 7.908, 95% confidence interval: 3.661–17.083, P < 0.001). No serious adverse effects were found in two groups.
Conclusions:
The rhubarb might significantly improve feeding tolerance and relieve gastrointestinal dysfunction in critically ill patients, without serious adverse reactions. It provided proof for the treatment of gastrointestinal dysfunction with rhubarb during clinical practice.
Keywords: Critically Ill Patients, Gastrointestinal Dysfunction, Propensity Score Matching, Rhubarb
摘要
背景:
胃肠功能障碍对危重症患者的预后起着至关重要的作用。前期研究表明大黄,作为一种传统中药,能够有效保护胃肠道屏障功能,阻止肠道细菌移位,促进胃肠道蠕动,但至今临床研究较少。本研究的目的在于探讨中药大黄对危重症患者胃肠功能障碍的治疗作用。
方法:
2015年6月至2017年5月期间共有368位患有I–III级急性胃肠道损伤(AGI)危重症患者纳入本次回顾性研究。根据暴露 因素(即胃肠功能障碍患者是否接受大黄治疗)将患者分为大黄组和常规治疗组。收集患者进入重症监护室(ICU)24小时内 及治疗7天后的临床资料,及患者28天死亡率、ICU入住时间和总住院时间。采用倾向性评分匹配法(PSM)控制两组间的混杂 偏倚,用Logistic回归分析模型筛选影响因素。
结果:
根据是否使用大黄治疗,入选患者被分为大黄组(219例,59.5%)和常规治疗组(149例,40.5%)。PSM匹配前大黄 组和常规治疗组喂养耐受率分别为59.8%、39.6%,PSM匹配后大黄组和常规治疗组喂养耐受率分别为77.9%、30.9%,大黄组 喂养耐受率均较常规治疗组高,差异有统计学意义(P<0.05)。PSM匹配前后大黄组患者AGI分级改善率均较常规治疗组高, ICU入住天数、CRP水平均较常规治疗组低,差异有统计学意义(P<0.05)。两组患者在PSM匹配前后28天死亡率均无统计学 差异(48 vs. 33, P=0.959; and 16 vs. 21, P=0.335)。Logistic回归分析显示,仅是否接受大黄治疗这一因素影响治疗7天后肠内 营养需求= 83.7 kJ·kg-1·d-1的患者的比例。两组患者均未发现严重不良反应。
结论:
中药大黄能够有效提高危重症患者肠内营养耐受性,缓解胃肠功能障碍,且无严重不良反应,为临床实践中应用大黄 治疗胃肠功能障碍提供了循证医学证据。
INTRODUCTION
The gastrointestinal tract is not only an organ of digestion and absorption but also carries out endocrine, immune, and barrier functions. The hypothesis of the gut as a motor of multiple organ failure has repeatedly been proposed in the past quarter-century.[1,2,3] With the deepening of research, it was found that all elements of the gut, including the epithelium, the immune system, and the microbiome, are closely associated with the progress of the critical disease.[4] Gastrointestinal dysfunction plays a critical role in the prognosis of critically ill patients. Once it occurs, the condition gets worse and the prognosis is fatal.[5,6,7] However, there are a few clinical data about gastrointestinal dysfunction in critically ill patients, which may be related to the various functions of gastrointestinal tract making its “normal” function fairly indefinable, even though the importance of gastrointestinal function has been recognized for years.[8] The previous study showed that rhubarb, a traditional Chinese herb, can protect the intestinal mucosal endothelial cells from damage in rats with sepsis and scald, prevent intestinal bacterial translocation, and promote gastrointestinal peristalsis and the remission of toxic enteroparalysis. We also found that rhubarb can improve the circulating von Willebrand factor, platelet (PLT) levels by the potential anticoagulant and anti-PLT aggregation property.[9,10] Rhubarb has been used to treat gastrointestinal dysfunction in critically ill patients for many years, but a few clinical studies have explored whether rhubarb has an association with the protection of gastrointestinal function. Therefore, this retrospective study was conducted to evaluate the effect of rhubarb on critically ill patients with gastrointestinal dysfunction.
METHODS
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Biomedicine Research of the Second Military Medical University. Informed consent was not required because of the retrospective and anonymous nature of this study.
Study population
This retrospective study was carried out in the Intensive Care Unit (ICU) of Shanghai Changzheng Hosptial, China, between June 2015 and May 2017. Patients were screened for eligibility within 24 h of ICU admission. Inclusion criteria were as follows: (1) 18–75 years of age; (2) acute gastrointestinal injury (AGI) I–III; and (3) complete anatomic structure of the gastrointestinal tract, including the ileum and colon stoma. The exclusion criteria were as follows: (1) pregnancy; (2) admitted to the ICU <7 d (including deaths); (3) cardiac function (New York Heart Association) III–IV; (4) Child-Pugh score >9; (5) abdominal compartment syndrome; (6) mechanical ileus; (7) gastrointestinal bleeding in active stage.
The patients were divided into two groups (rhubarb and usual treatment groups) according to the exposure factors (whether the patients receive rhubarb treatment for gastrointestinal dysfunction). For the rhubarb group, all patients received conventional medication plus rhubarb therapy. Rhubarb, commercially certified crude rhubarb, was provided by the hospital pharmacy. The dose of rhubarb was 9–18 g/d for 7 consecutive days and the route of administration included oral administration and nasal feeding. For the usual treatment group, all patients were treated with only conventional medication. Conventional drug therapy included primary disease treatment, anti-inflammatory, anti-infection, nutritional support, prokinetic agents treatment, and symptomatic treatment. After ICU admission, if the patient had stable hemodynamics or had no enteral nutrition contraindications, the patient was recommended to start enteral nutrition after 24–48 h from ICU admission.
Definitions for uniform data collection
The following definitions were used for uniform data collection:
The term “gastrointestinal dysfunction” in broader perspective describes all gastrointestinal symptoms frequently occurring in humans. All these aspects have been well considered by the Working Group on Abdominal Problems of the European Society of Intensive Care Medicine (ESICM) and resulted in definitions for AGI with four grades of severity.[11] The AGI grade was assessed daily according to the recommendation of the ESICM grading system during the 1st week of the subject's ICU stay.
Feeding intolerance is a sign of gastrointestinal dysfunction.[12] According to current clinical practice guidelines for nutritional support in critically ill patients, the enteral nutritional target was set for all patients at 83.7 kJ·kg−1·d−1 within the 1st week of ICU admission.[13,14,15] Therefore, the remission of feeding intolerance was considered if 83.7 kJ·kg−1·d−1 via the enteral route could be reached after 7 days of treatment.
Assessment of AGI grade: aggravation: the AGI grade is higher as compared with those before therapy; no improvement: AGI grade is the same as before; and improvement: the AGI grade was lower than before, no matter gastrointestinal function was recovered to normal.
The “28-day mortality” was defined as death from any cause occurring within the 28 days after ICU admission.
Data collection
Clinical data were collected within the first 24 h of ICU admission and 7 days after treatment. The data collected were as follows: (1) baseline demographic and clinical characteristics: age, gender, presence/absence of feeding, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, and Sequential Organ Failure Assessment (SOFA) score; (2) the patients' gastrointestinal function: vomiting, abnormal bowel sounds, diarrhea, bowel distention, gastrointestinal bleeding, nutritional support, and AGI grade; (3) other drugs for the improvement of gastrointestinal function: enemia glycerini, clostridium butyricum tablets, mosapride citrate tablets, and lactulose oral solution; (4) laboratory results: C-reactive protein (CRP), procalcitonin (PCT), endotoxin, creatinine, aspartate transaminase, and alanine transaminase. Survival data were collected on day 28 after ICU admission. In addition, the days in ICU and total hospitalization days were recorded. An electronic case report file was used for data collection.
Statistical analysis
Matchlt package for R software was used for propensity score matching (PSM) and SPSS version 21.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. Data are expressed as the mean ± standard deviation (SD) for normally distributed continuous variables and as the absolute number for categorical variables. Data are presented median (Q1, Q3) for nonnormal distributed variables. To compare groups, Student's t-test (for normal distribution) and nonparametric test (for nonnormal distribution) were used for continuous variables. The Chi-square test, Fisher's exact test, or nonparametric test were used to compare categorical variables. Fisher's exact test was applied if the minimal estimated expected value was <5. The PSM were applied to compare two groups of patients. First, the program performed a logistic regression to score all patients, parameters related to the endpoints of the study as covariates: age, gender, 1-day APACHE II score, 1-day SOFA score, whether feeding, the gastrointestinal symptoms within the first 24 h of ICU admission (vomiting, bowel sounds, diarrhea, abdominal distention, gastrointestinal bleeding, nutritional support, and AGI grade), the use of other drugs, and the laboratory data. Second, matching on the propensity score (1:1) was performed using a nearest neighbor-matching algorithm, with a maximum caliper distance of 0.25 of the SD of the propensity score.[16] Match adequacy was determined using standardized differences: a standard difference <10% indicates a negligible difference in the mean or prevalence of a covariate between two groups.[17] Analyses were then separately performed on the two matched groups. The logistic regression analysis was conducted to screen the influence factors for the remission rate of gastrointestinal dysfunction. All statistical analyses were two-sided, and a P < 0.05 was considered statistically significant.
RESULTS
Enrollment flowchart and the main diagnoses of patients
Between June 2015 and May 2017, 1147 patients from ICU were screened. Finally, a total of 368 critically ill patients met the criteria and were included in this study. The study flowchart is presented in Figure 1.
Figure 1.
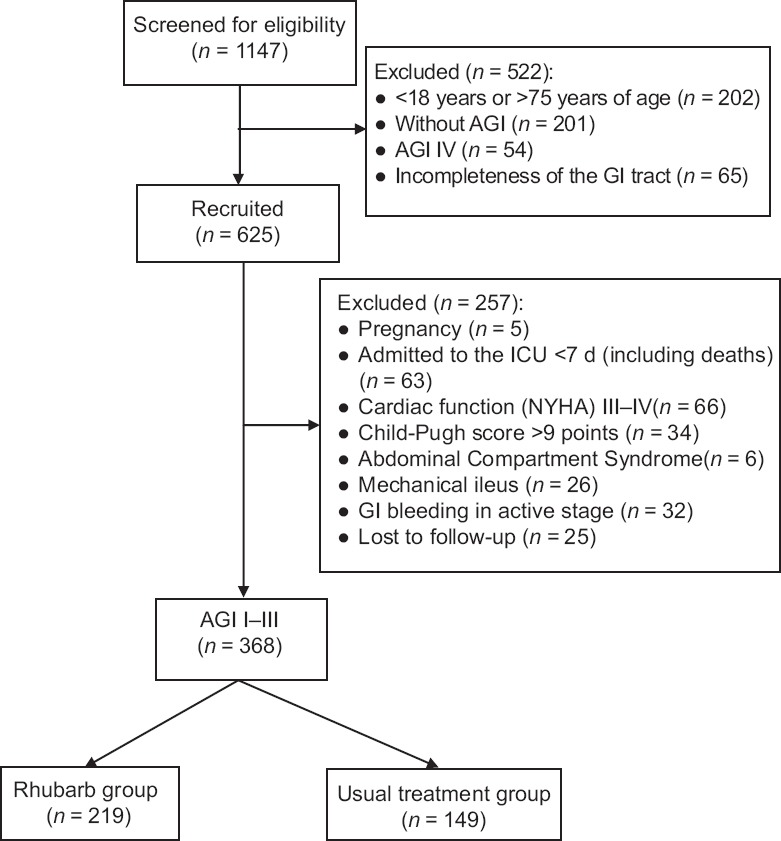
Enrollment flowchart of this study. AGI: Acute gastrointestinal injury; GI: Gastrointestinal; ICU: Intensive Care Unit; NYHA: New York Heart Association classification.
The main diagnoses of patients enrolled were as follows: multiple injuries in 124 cases, severe pneumonia in 73 cases, cardiopulmonary resuscitation in 25 cases, septic shock in 21 cases, acute cerebral infarction in 15 cases, severe craniocerebral trauma in 15 cases, bloodstream infection in 15 cases, acute exacerbation of chronic obstructive pulmonary disease with respiratory failure in 14 cases, hematencephalon in 13 cases, abdominal infection after gastrointestinal surgery in 13 cases, severe acute pancreatitis in 9 cases, acute heart failure in 9 cases, acute pyogenic cholangitis in 7 cases, central nervous system infections in 5 cases, acute intoxication in 3 cases, severe heat stroke in 3 cases, hemorrhagic shock in 3 cases, anaphylactic shock in 1 case.
Baseline characteristics of patients before propensity score matching
The eligible patients were then divided into rhubarb group (n = 219, 59.5%) and usual treatment group (n = 149, 40.5%). The baseline characteristics of the two groups are shown in Table 1. The degree of abdominal distention, the proportions of vomiting and AGI grade, and endotoxin level were higher in the rhubarb group than those in the usual treatment group (all P < 0.05). The APACHE II and SOFA scores in the rhubarb group were lower than those of the usual treatment group (all P < 0.05). Moreover, the lower proportion of enemia glycerini, clostridium butyricum tablets, prokinetic agents were used in the rhubarb group (all P < 0.05).
Table 1.
Baseline characteristics of all patients before propensity score matching
| Characteristics | Rhubarb group (n = 219) | Usual treatment group (n = 149) | Statistical values | P |
|---|---|---|---|---|
| Gender, n | ||||
| Male | 159 | 111 | 0.163* | 0.687 |
| Female | 60 | 38 | ||
| Age (years), mean ± SD | 58.6 ± 18.7 | 55.4 ± 17.6 | 1.639† | 0.102 |
| SOFA score, mean ± SD | 4.84 ± 3.09 | 6.44 ± 3.45 | −4.655† | <0.001 |
| APACHE II score, mean ± SD | 12.88 ± 6.14 | 14.98 ± 5.77 | −3.299† | 0.001 |
| Feeding, n | ||||
| Yes | 82 | 63 | 0.870* | 0.351 |
| No | 137 | 86 | ||
| Bowel sound, n | ||||
| Normal | 70 | 56 | −1.141‡ | 0.254 |
| Weakening | 145 | 91 | ||
| Disappearance | 4 | 2 | ||
| Abdominal distension, n | ||||
| No | 12 | 1 | −2.787‡ | 0.005 |
| Light | 97 | 94 | ||
| Medium | 78 | 52 | ||
| Heavy | 32 | 2 | ||
| Vomiting, n | 31 | 10 | 4.963* | 0.026 |
| Frequency of defecation, n | ||||
| 0 | 191 | 127 | −0.614‡ | 0.539 |
| 1 | 28 | 19 | ||
| 2 | 0 | 3 | ||
| GI bleeding, n | 3 | 1 | 0.015* | 0.903 |
| AGI grade, n | ||||
| I | 45 | 54 | −6.982‡ | <0.001 |
| II | 78 | 88 | ||
| III | 96 | 7 | ||
| Use of medications, n | ||||
| Enemia glycerini | 120 | 115 | 18.830* | <0.001 |
| Clostridium butyricum tablets | 77 | 72 | 6.375* | 0.012 |
| Prokinetic agents | 38 | 48 | 10.938* | 0.001 |
| Lactulose oral solution | 30 | 29 | 2.189* | 0.139 |
| Liver function injury, n | 63 | 33 | 2.015* | 0.156 |
| Renal function injury, n | 30 | 21 | 0.012* | 0.914 |
| CRP (mg/L), median (Q1, Q3) | 47.80 (21.00, 95.30) | 53.03 (20.85, 93.98) | −0.405‡ | 0.685 |
| PCT (ng/ml), median (Q1, Q3) | 0.50 (0.23, 1.37) | 0.58 (0.15, 1.72) | −0.508‡ | 0.612 |
| Endotoxin (EU/ml), median (Q1, Q3) | 0.10 (0.07, 0.16) | 0.06 (0.05, 0.10) | −5.520‡ | <0.001 |
*Chi-square test; †t-test; ‡Nonparametric test. SOFA: Sequential Organ Failure Assessment; APACHE II: Acute Physiology and Chronic Health Evaluation II; GI: Gastrointestinal; AGI: Acute gastrointestinal injury; SD: Standard deviation; CRP: C-reactive protein.
Baseline characteristics of patients after propensity score matching
Because of the differences in the baseline characteristics between the two groups, the 1:1 nearest-neighbor PSM was applied to reduce the confounding bias. The caliper value was 0.04. After PSM, 68 patients were matched from each group, and the baseline characteristics of the two groups after PSM are shown in Table 2. All baseline characteristics were well matched between two groups without statistical significance, except for endotoxin level. The endotoxin level was higher in the rhubarb group than that in the usual treatment group (P = 0.009).
Table 2.
Baseline characteristics of patients after propensity score matching
| Characteristics | Rhubarb group (n = 68) | Usual treatment group (n = 68) | Statistical values | P |
|---|---|---|---|---|
| Gender, n | ||||
| Male | 50 | 53 | 0.360* | 0.548 |
| Female | 18 | 15 | ||
| Age (years), mean ± SD | 55.7 ± 19.2 | 57.1 ± 18.0 | −0.419† | 0.676 |
| SOFA score, mean ± SD | 5.38 ± 3.08 | 6.13 ± 3.81 | −1.262† | 0.209 |
| APACHE II score, mean ± SD | 13.32 ± 5.44 | 13.50 ± 5.43 | −1.089† | 0.850 |
| Feeding, n | ||||
| Yes | 27 | 22 | 0.798* | 0.372 |
| No | 41 | 46 | ||
| Bowel sound, n | ||||
| Normal | 22 | 19 | −0.797‡ | 0.425 |
| Weakening | 46 | 47 | ||
| Disappearance | 0 | 2 | ||
| Abdominal distension, n | ||||
| No | 6 | 0 | −1.191‡ | 0.234 |
| Light | 35 | 37 | ||
| Medium | 25 | 29 | ||
| Heavy | 2 | 2 | ||
| Vomiting, n | 7 | 6 | 0.085* | 0.771 |
| Frequency of defecation, n | ||||
| 0 | 61 | 60 | −0.301‡ | 0.764 |
| 1 | 7 | 7 | ||
| 2 | 0 | 1 | ||
| GI bleeding, n | 1 | 1 | 0.000* | 1.000 |
| AGI grade, n | ||||
| I | 23 | 14 | −1.143‡ | 0.253 |
| II | 37 | 48 | ||
| III | 8 | 6 | ||
| Use of medications, n | ||||
| Enemia glycerini | 52 | 50 | 0.157* | 0.692 |
| Clostridium butyricum tablets | 27 | 33 | 1.074* | 0.300 |
| Prokinetic agents | 17 | 18 | 0.038* | 0.844 |
| Lactulose oral solution | 15 | 17 | 0.163* | 0.686 |
| Liver function injury, n | 0 | 2 | 2.030* | 0.154 |
| Renal function injury, n | 9 | 11 | 0.234* | 0.628 |
| CRP (mg/L), median (Q1, Q3) | 51.57 (18.34, 107.72) | 57.89 (25.68, 114.78) | −1.090‡ | 0.276 |
| PCT (ng/ml), median (Q1, Q3) | 0.50 (0.20, 1.25) | 0.62 (0.20, 1.72) | −1.121‡ | 0.262 |
| Endotoxin (EU/ml), median (Q1, Q3) | 0.09 (0.07, 0.12) | 0.06 (0.05, 0.12) | −2.620‡ | 0.009 |
*Chi-square test; †t-test; ‡Nonparametric test. SOFA: Sequential Organ Failure Assessment; APACHE II: Acute Physiology and Chronic Health Evaluation II; GI: gastrointestinal; AGI: Acute gastrointestinal injury; SD: Standard deviation; CRP: C-reactive protein.
Clinical outcomes after 7 days of treatment
The results of this study found that the remission rates of feeding intolerance in rhubarb group were 59.8% before PSM and 77.9% after PSM, which were significantly higher than those of usual treatment groups (39.6% and 30.9%, respectively; all P < 0.05).
The clinical characteristics of two groups after 7 days of treatment before and after PSM are shown in Tables 3 and 4. Compared with the usual treatment group, the rhubarb group had higher rates of improvement for AGI grade and bowel sound, shorter ICU duration, and lower level of CRP before and after PSM (all P < 0.05). Before PSM, patients in the rhubarb group had lower APACHE II and SOFA scores, a higher proportion of abdominal distension alleviation and longer duration of hospitalization, compared with usual treatment group (all P < 0.05); however, those showed no significant differences between two groups after PSM. There was no significant difference for the 28-day mortality between two groups before and after PSM.
Table 3.
Clinical characteristics of patients receiving 7-day treatment before propensity score matching
| Characteristics | Rhubarb group (n = 219) | Usual treatment group (n = 149) | Statistical values | P |
|---|---|---|---|---|
| SOFA score, mean ± SD | 4.23 ± 3.57 | 5.84 ± 3.69 | −4.186† | <0.001 |
| APACHE II score, mean ± SD | 11.92 ± 6.55 | 14.11 ± 6.30 | −3.191† | 0.002 |
| Bowel sound, n | ||||
| No improvement | 21 | 41 | 25.530* | <0.001 |
| Improvement | 198 | 108 | ||
| Abdominal distension, n | ||||
| No | 70 | 42 | −2.787‡ | 0.005 |
| Light | 107 | 78 | ||
| Medium | 32 | 28 | ||
| Heavy | 10 | 1 | ||
| Alleviation of abdominal distension, n | 148 | 78 | 8.680* | 0.003 |
| Frequency of defecation, median (Q1, Q3) | 1 (1, 2) | 1 (0, 1) | −4.947‡ | <0.001 |
| GI bleeding, n | 1 | 0 | 1.000§ | |
| Enteral nutrition ≥83.7 kJ·kg−1·d−1, n | 131 | 59 | 14.517* | <0.001 |
| AGI grade, n | ||||
| 0 | 71 | 24 | −6.982‡ | <0.001 |
| I | 71 | 44 | ||
| II | 48 | 64 | ||
| III | 29 | 17 | ||
| Improvement of AGI grade, n | ||||
| Aggravation | 8 | 19 | −7.584‡ | <0.001 |
| No improvement | 46 | 86 | ||
| Improvement | 165 | 44 | ||
| Aggravation of liver function injury, n | 7 | 5 | 0.000* | 1.000 |
| Aggravation of renal function injury, n | 3 | 0 | 0.712* | 0.399 |
| 28-day mortality, n | 48 | 33 | −0.003* | 0.959 |
| Duration of ICU (days), median (Q1, Q3) | 9.0 (6.5, 19.0) | 10.0 (7.0, 23.0) | 2.012‡ | 0.043 |
| Duration of hospitalization (days), median (Q1, Q3) | 22.0 (14.0, 39.0) | 20.0 (12.0, 27.0) | −1.992‡ | 0.046 |
| CRP (mg/L), median (Q1, Q3) | 23.21 (9.00, 52.97) | 47.08 (24.00, 92.86) | −5.292‡ | <0.001 |
| PCT (ng/ml), median (Q1, Q3) | 0.46 (0.13, 0.82) | 0.34 (0.12, 1.35) | −0.552‡ | 0.581 |
| Endotoxin (EU/ml), median (Q1, Q3) | 0.08 (0.05, 0.11) | 0.07 (0.05, 0.10) | −1.902‡ | 0.057 |
*Chi-square test; †t-test; ‡Nonparametric test; §Fisher exact test. SOFA: Sequential Organ Failure Assessment; APACHE II: Acute Physiology and Chronic Health Evaluation II; GI: Gastrointestinal; AGI: Acute gastrointestinal injury; ICU: Intensive Care Unit; CRP: C-reactive protein; PCT: Procalcitonin; SD: Standard deviation.
Table 4.
Clinical characteristics of patients receiving 7-day treatment after propensity score matching
| Characteristics | Rhubarb group (n = 68) | Usual treatment group (n = 68) | Statistical values | P |
|---|---|---|---|---|
| SOFA score, mean ± SD | 4.84 ± 3.61 | 5.53 ± 3.79 | −1.089† | 0.278 |
| APACHE II score, mean ± SD | 12.56 ± 6.03 | 12.74 ± 6.00 | −0.171† | 0.864 |
| Bowel sound, n | ||||
| No improvement | 6 | 24 | 14.170* | 0.001 |
| Improvement | 62 | 44 | ||
| Abdominal distension, n | ||||
| No | 30 | 22 | −1.789‡ | 0.074 |
| Light | 33 | 34 | ||
| Medium | 3 | 12 | ||
| Heavy | 2 | 0 | ||
| Alleviation of abdominal distension, n | 50 | 43 | 1.666* | 0.197 |
| Frequency of defecation, median (Q1, Q3) | 2 (1, 2) | 1 (0, 1) | −5.305‡ | <0.001 |
| GI bleeding, n | 0 | 0 | – | – |
| Enteral nutrition ≥83.7 kJ·kg−1·d−1, n | 53 | 21 | 30.354* | <0.001 |
| AGI grade, n | ||||
| 0 | 31 | 7 | −4.805‡ | <0.001 |
| I | 22 | 22 | ||
| II | 11 | 32 | ||
| III | 4 | 7 | ||
| Improvement of AGI grade, n | ||||
| Aggravation | 3 | 3 | −5.148‡ | <0.001 |
| No improvement | 13 | 42 | ||
| Improvement | 52 | 23 | ||
| Aggravation of liver function injury, n | 0 | 2 | 0.163§ | |
| Aggravation of renal function injury, n | 1 | 0 | 1.000§ | |
| 28-day mortality, n | 16 | 21 | −0.928* | 0.335 |
| Duration of ICU (days), median (Q1, Q3) | 9.0 (7.0, 11.5) | 9.0 (7.5, 12.5) | 2.003‡ | 0.045 |
| Duration of hospitalization (days), median (Q1, Q3) | 21.5 (12.0, 45.0) | 21.0 (14.3, 32.3) | −0.205‡ | 0.838 |
| CRP (mg/L), median (Q1, Q3) | 25.39 (12.03, 67.61) | 53.48 (28.19, 100.25) | −3.419‡ | 0.001 |
| PCT (ng/ml), median (Q1, Q3) | 0.30 (0.12, 1.01) | 0.38 (0.11, 1.41) | −1.171‡ | 0.242 |
| Endotoxin (EU/ml), median (Q1, Q3) | 0.07 (0.05, 0.10) | 0.07 (0.05, 0.10) | −0.538‡ | 0.590 |
*Chi-square test; †t-test; ‡Nonparametric test; §Fisher exact test. SOFA: Sequential Organ Failure Assessment; APACHE II: Acute Physiology and Chronic Health Evaluation II; GI: Gastrointestinal; AGI: Acute gastrointestinal injury; ICU: Intensive Care Unit; CRP: C-reactive protein; PCT: Procalcitonin; SD: Standard deviation; –: Not applicable.
Considering that the endotoxin level was not well balanced after PSM, a logistic regression model was carried out. Whether 83.7 kJ·kg−1·d−1 could be reached as the dependent variable, whether receiving rhubarb therapy, and endotoxin level were used as independent variables. The stepwise regression was conducted to screen the influence factors. The results showed that only the factor, whether receiving rhubarb therapy, affected the proportion of patients whose enteral nutrition needs ≥83.7 kJ·kg−1·d−1 after 7 days of treatment (odds ratio: 7.908, 95% confidence interval: 3.661–17.083, P < 0.001).
Clinical adverse effects
As shown in Tables 3 and 4, although the number of defecation in rhubarb group were more than that in the usual treatment group after 7 days of treatment (P < 0.001), no serious adverse effects including severe diarrhea (≥10 times/day or ≥1000 ml/day), frequent vomiting (≥5 times/day), and obvious aggravation of liver and kidney function injury were found in both groups.
DISCUSSION
Gastrointestinal dysfunction occurs frequently in critically ill patients.[18] A large number of previous studies confirmed the relationship between gastrointestinal dysfunction and the severity and the clinical outcome of the disease.[19,20] Therefore, gastrointestinal dysfunction has become an unnegligible problem for ICU patients.
Feeding intolerance is considered to be an important clinical sign of gastrointestinal dysfunction in critically ill patients, which indicates the hypofunction of gastrointestinal motility and absorption. Studies have reported that feeding intolerance was significantly associated with poor prognosis in critically ill patients. A multicenter retrospective study involving 1888 ICU patients found that patients with feeding intolerance had poorer nutritional status and longer stay in ICU and higher risk of death.[21] In this retrospective study, the remission of feeding intolerance was selected as the main research target. The results showed that rhubarb might significantly improve the feeding tolerance of critically ill patients. Western medicine uses gastrointestinal motility drugs as the most common intervention strategy to treat feeding intolerance. The use of gastrointestinal motility drugs, including metoclopramide, domperidone, and mosapride, was statistically analyzed in this study. The results showed that the proportion of gastrointestinal motility drugs used in rhubarb group before PSM was less than that in routine treatment group, and there was no significant difference between the two groups after PSM. The remission rates of feeding intolerance in rhubarb group were significantly higher than those in routine treatment group before and after PSM, further indicating that rhubarb had a positive effect on increasing the tolerance of enteral nutrition in critically ill patients. The mechanisms of rhubarb improving feeding tolerance are as follows: (1) Anthraquinone derivatives of rhubarb could stimulate intestinal submucous plexus, thus promoting intestinal peristalsis, inhibiting water absorption.[22] (2) Protection of intestinal mucosal epithelial barrier. Previous basic research had found that 5 of 21 monomers extracted from the rhubarb (emodin, 3,8-dihydroxy-1-methyl-anthraquinone-2-carboxylicacid, daucosterol linoleate, rhein and 1-O-caffeoyl-2-(4-hydroxyl-O-cinnamoyl)-β-D-glucose) could significantly prevent increased mucosal permeability and enhance the expression of tight junctional protein, thus maintaining the functional integrity of intestinal microvascular endothelial cells, improving intestinal tight junctions, reducing intestinal damage, and protecting the intestinal mucosal barrier in sepsis.[9] They also antagonized the matrix metalloproteinase-9 induced human umbilical vein vascular endothelial cell (HUVEC) monolayer permeability by promoting HUVEC proliferation and reducing extracellular vascular endothelial cadherin concentrations to protect capillary leakage.[23] (3) Protection of intestinal microbiological barrier. Animal studies showed that after the treatment of rhubarb, the number of commensal intestinal microflora, such as Escherichia coli and bifidobacteria and total anaerobes increased.[24] In addition, early adequate enteral nutrition in itself promoted the recovery of intestinal function, reversed the loss of gastrointestinal mucosal integrity, maintained intestinal blood perfusion, and improved the nutritional status of patients.[25,26,27]
The studies had confirmed the clinical feasibility of AGI grading system and showed that AGI grade was significantly associated with the severity and poor prognosis of the patients.[28,29] This retrospective study found that rhubarb treatment could significantly improve AGI grade in critically ill patients. The proportion of AGI III in rhubarb group was significantly lower than that in usual treatment group. This result confirmed that rhubarb has a significant effect on improving gastrointestinal dysfunction.
In this study, rhubarb could significantly shorten the duration of ICU in critically ill patients but had no significant effect on the total duration of hospitalization. To a certain extent, shortening the duration of ICU could reduce the economic burden of the patients, and the rhubarb is rich in sources and low in price, which makes the patients have good economic benefits.
This retrospective study showed that rhubarb could decrease the level of CRP in critically ill patients. CRP is an acute phase reactive protein synthesized by the liver. It is a sensitive index to reflect the tissue damage and infection. Under the pathological condition of severe trauma, shock and infection, it is the common case that edema, erosion and ulcer develop in the gastrointestinal mucosa because of intense stress reaction. The barrier of intestinal mucosa is destructed and the bacteria from the intestinal tract will invade the liver through the portal vein. The liver is the first physical barrier next to the gut. Kupffer's cells are the main effector cells in the liver, and their capacity of phagocytosis accounts for 95% of that over the body. Therefore, Kupffer's cells have a tremendous potential capability of inducing systemic inflammation reaction. On the one hand, Kupffer's cells can eliminate enterogenous bacteria and toxins. On the other hand, they will also be activated to release generous cytokines and inflammatory mediators leading to severe systemic inflammation reaction at the same time. Hepatic vein blood, which contains generous cytokines and inflammatory mediators, return to the lung, accompanied with lymph fluid from gastrointestinal tract. Consequently, the macrophages in the lung tissue are activated and participate in systemic inflammatory reaction. Gut-originated septic reaction may be further aggravated by lung, and the inflammatory reaction in the lung feeds back to the gut and liver through blood circulation. In the end, a pathway of gut-liver-lung cascade reaction, also named gut-liver-lung axis, is finally formed.[30] The decrease of CRP level in rhubarb group may be associated with inhibiting the activation of inflammatory effector cells in liver, reducing the synthesis and release of inflammatory cytokines in liver, and further inhibiting the inflammatory response in lung. Animal studies found that rhubarb could inhibit mitogen-activated protein kinase signal transduction and activation of transcriptional activators in severe acute pancreatitis (SAP) rats, thereby reducing the expression of inflammatory cytokines.[31] Rhubarb could also reduce the intestinal mucosa injury in rats with SAP, which was related to the reduction of mRNA expression of TLR2/4 in intestinal mucosa of SAP rats by rhubarb. TLRs are very important transmembrane receptors and signal transduction receptors of the innate immune system, able to activate a series of pro-inflammatory and anti-inflammatory cytokines as well as chemokines, and involve in the regulation of inflammatory responses.[32]
The barrier of intestinal mucosa is destructed and the endotoxin from the intestinal tract invades the liver, thus the level of endotoxin could indirectly reflect intestinal barrier function. The baseline level of endotoxin was significantly different between the two groups before and after PSM, and its baseline level in rhubarb group was higher than that in routine treatment group. To avoid its influence on the main results, the logistic regression model for all the gradually screening methods were used. Finally, it was proved that only rhubarb application affected the remission rate of feeding intolerance of the patients.
However, this retrospective study showed no significant difference in 28-day mortality, APACHE II score, SOFA score, and PCT level between the two groups. These results contradicted other evaluation indicators in this study. The causes of this phenomenon might include complicated bad conditions, multiple-organ involvement, failure in effectively controlling primary diseases, and other underlying confounding factors.
This study showed no serious adverse reactions during rhubarb treatment. In the rhubarb group, there were 63 cases of liver function injury and 30 cases of renal function injury at the time of inclusion. After 7 days of rhubarb treatment, there were no new cases of liver and kidney damages. Although 7 patients with liver function injury and 3 patients with kidney function injury became more serious after rhubarb treatment, there was no significant difference compared with the routine treatment group. Therefore, the aggravation of injury in these patients was related to the failure to control the primary condition, rather than the side effects of rhubarb.
In addition, the safety of rhubarb is also concerned with the dosage and frequency of administration. There is a dose-effect relationship in the use of rhubarb. Several studies had been conducted to evaluate the efficacy of different doses of rhubarb in the treatment of gastrointestinal dysfunction, and the results showed that the dosage of 0.05 g·kg−1·time−1, 3 time/day could significantly reduce the incidence of gastrointestinal failure and improve the prognosis.[33] In this study, the dosage of rhubarb as 3–6 g/time, 3 time/day was safe and applicable. Therefore, it is reasonable to determine the dosage according to the body weight. It could avoid not only the adverse reactions such as diarrhea, nausea and vomiting caused by excessive rhubarb but also the effect of dose deficiency.
PSM is a statistical method to deal with confounding bias in nonrandomized controlled studies in recent years. In this statistical method, all confounding factors (i.e., covariables) are represented by a propensity score, which reduces the dimension of the covariable, and then matches the different comparison groups according to the propensity score. After matching, the distribution of covariables reached equilibrium, which is equivalent to “randomization after the event.” Using PSM, all baseline characteristics of the two groups were balanced after matching, which made the results more reliable.
There are several limitations in this study. First, the evaluation of AGI grade is subjective and lacks objective and quantitative indicators, which limit the research in this field. Second, an inherent bias is present in the data collection of retrospective studies; several evaluation indicators, such as intra-abdominal pressure, residual gastric volume, were not included in this study. Third, the sample size was relatively small and the follow-up time was relatively short. In addition, PSM can only balance the observable variables but cannot help with the bias caused by potential unknown confounding factors. Therefore, multi-center double-blind randomized controlled studies with larger sample size are needed to explore the efficacy of rhubarb in critically ill patients with gastrointestinal dysfunction.
In conclusion, the results of this study suggest that rhubarb could significantly improve feeding tolerance and relieve gastrointestinal dysfunction in critically ill patients without serious adverse reactions. It provided proof for the treatment of gastrointestinal dysfunction with rhubarb during clinical practice.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Xin Chen
REFERENCES
- 1.Mittal R, Coopersmith CM. Redefining the gut as the motor of critical illness. Trends Mol Med. 2014;20:214–23. doi: 10.1016/j.molmed.2013.08.004. doi: 10.1016/j.molmed.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meakins JL, Marshall JC. The gastrointestinal tract: The “motor” of MOF. Arch Surg. 1986;121:197–201. [Google Scholar]
- 3.Lyons JD, Coopersmith CM. Pathophysiology of the gut and the microbiome in the host response. Pediatr Crit Care Med. 2017;18(3 Suppl 1):S46–9. doi: 10.1097/PCC.0000000000001046. doi: 10.1097/PCC.0000000000001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klingensmith NJ, Coopersmith CM. The gut as the motor of multiple organ dysfunction in critical illness. Crit Care Clin. 2016;32:203–12. doi: 10.1016/j.ccc.2015.11.004. doi: 10.1016/j.ccc.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montejo JC. Enteral nutrition-related gastrointestinal complications in critically ill patients: A multicenter study. The Nutritional and Metabolic Working Group of the Spanish Society of Intensive Care Medicine and Coronary Units. Crit Care Med. 1999;27:1447–53. doi: 10.1097/00003246-199908000-00006. doi: 10.1097/00003246-199908000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Reintam A, Parm P, Kitus R, Kern H, Starkopf J. Gastrointestinal symptoms in intensive care patients. Acta Anaesthesiol Scand. 2009;53:318–24. doi: 10.1111/j.1399-6576.2008.01860.x. doi: 10.1111/j.1399-6576.2008.01860.x. [DOI] [PubMed] [Google Scholar]
- 7.Lam SW, Nguyen NQ, Ching K, Chapman M, Fraser RJ, Holloway RH, et al. Gastric feed intolerance is not increased in critically ill patients with type II diabetes mellitus. Intensive Care Med. 2007;33:1740–5. doi: 10.1007/s00134-007-0712-1. doi: 10.1007/s00134-007-0712-1. [DOI] [PubMed] [Google Scholar]
- 8.Reintam Blaser A, Jakob SM, Starkopf J. Gastrointestinal failure in the ICU. Curr Opin Crit Care. 2016;22:128–41. doi: 10.1097/MCC.0000000000000286. doi: 10.1097/MCC.0000000000000286. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Cui YL, Zhang Z, Lin ZF, Chen DC. Rhubarb monomers protect intestinal mucosal barrier in sepsis via junction proteins. Chin Med J. 2017;130:1218–25. doi: 10.4103/0366-6999.205855. doi: 10.4103/0366-6999.205855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L, Chen J, Jiang D, Zhang P. Adjuvant treatment with crude Rhubarb for patients with systemic inflammation reaction syndrome/sepsis: A meta-analysis of randomized controlled trials. J Crit Care. 2015;30:282–9. doi: 10.1016/j.jcrc.2014.11.008. doi: 10.1016/j.jcrc.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Reintam Blaser A, Malbrain ML, Starkopf J, Fruhwald S, Jakob SM, De Waele J, et al. Gastrointestinal function in intensive care patients: Terminology, definitions and management. Recommendations of the ESICM Working Group on Abdominal Problems. Intensive Care Med. 2012;38:384–94. doi: 10.1007/s00134-011-2459-y. doi: 10.1007/s00134-011-2459-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reintam A, Parm P, Kitus R, Starkopf J, Kern H. Gastrointestinal failure score in critically ill patients: A prospective observational study. Crit Care. 2008;12:R90. doi: 10.1186/cc6958. doi: 10.1186/cc6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kreymann KG, Berger MM, Deutz NE, Hiesmayr M, Jolliet P, Kazandjiev G, et al. ESPEN guidelines on enteral nutrition: Intensive care. Clin Nutr. 2006;25:210–23. doi: 10.1016/j.clnu.2006.01.021. doi: 10.1016/j.clnu.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 14.McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) JPEN J Parenter Enteral Nutr. 2016;40:159–211. doi: 10.1177/0148607115621863. doi: 10.1177/0148607115621863. [DOI] [PubMed] [Google Scholar]
- 15.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45:486–552. doi: 10.1097/CCM.0000000000002255. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 16.Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39:33–8. doi: 10.1080/00031305.1985.10479383. [Google Scholar]
- 17.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reintam Blaser A, Poeze M, Malbrain ML, Björck M, Oudemans-van Straaten HM, Starkopf J, et al. Gastrointestinal symptoms during the first week of intensive care are associated with poor outcome: A prospective multicentre study. Intensive Care Med. 2013;39:899–909. doi: 10.1007/s00134-013-2831-1. doi: 10.1007/s00134-013-2831-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu B, Sun R, Wu A, Ni Y, Liu J, Guo F, et al. Severity of acute gastrointestinal injury grade is a predictor of all-cause mortality in critically ill patients: A multicenter, prospective, observational study. Crit Care. 2017;21:188. doi: 10.1186/s13054-017-1780-4. doi: 10.1186/s13054-017-1780-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reintam Blaser A, Starkopf J, Malbrain ML. Abdominal signs and symptoms in intensive care patients. Anaesthesiol Intensive Ther. 2015;47:379–87. doi: 10.5603/AIT.a2015.0022. doi: 10.5603/AIT.a2015.0022. [DOI] [PubMed] [Google Scholar]
- 21.Gungabissoon U, Hacquoil K, Bains C, Irizarry M, Dukes G, Williamson R, et al. Prevalence, risk factors, clinical consequences, and treatment of enteral feed intolerance during critical illness. JPEN J Parenter Enteral Nutr. 2015;39:441–8. doi: 10.1177/0148607114526450. doi: 10.1177/0148607195019006453. [DOI] [PubMed] [Google Scholar]
- 22.Zheng HZ, Dong ZH, Yu J. Beijing: Academy Press; 1997. Modern research and application of traditional Chinese Medicine; pp. 224–383. [Google Scholar]
- 23.Cui YL, Zhang S, Tian ZT, Lin ZF, Chen DC. Rhubarb antagonizes matrix metalloproteinase-9-induced vascular endothelial permeability. Chin Med J. 2016;129:1737–43. doi: 10.4103/0366-6999.185859. doi: 10.4103/0366-6999.185859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang GW, Ren AM, Zhang SW, Wang H, Wang C, Wang EB. Effects of Chinese rhubarb on intestinal flora disturbance of septic rats. J Clin Exp Med. 2012;11:897–8. doi: 10.1177/0148607195019006453. [Google Scholar]
- 25.Buchman AL, Moukarzel AA, Bhuta S, Belle M, Ament ME, Eckhert CD, et al. Parenteral nutrition is associated with intestinal morphologic and functional changes in humans. JPEN J Parenter Enteral Nutr. 1995;19:453–60. doi: 10.1177/0148607195019006453. doi: 10.1177/0148607195019006453. [DOI] [PubMed] [Google Scholar]
- 26.Hadfield RJ, Sinclair DG, Houldsworth PE, Evans TW. Effects of enteral and parenteral nutrition on gut mucosal permeability in the critically ill. Am J Respir Crit Care Med. 1995;152:1545–8. doi: 10.1164/ajrccm.152.5.7582291. doi: 10.1164/ajrccm.152.5.7582291. [DOI] [PubMed] [Google Scholar]
- 27.Niinikoski H, Stoll B, Guan X, Kansagra K, Lambert BD, Stephens J, et al. Onset of small intestinal atrophy is associated with reduced intestinal blood flow in TPN-fed neonatal piglets. J Nutr. 2004;134:1467–74. doi: 10.1093/jn/134.6.1467. doi: 10.1093/jn/134.6.1467. [DOI] [PubMed] [Google Scholar]
- 28.Reintam A, Parm P, Redlich U, Tooding LM, Starkopf J, Köhler F, et al. Gastrointestinal failure in intensive care: A retrospective clinical study in three different Intensive Care Units in Germany and Estonia. BMC Gastroenterol. 2006;6:1–7. doi: 10.1186/1471-230X-6-19. doi: 10.1186/1471-230X-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu BC, Sun RH, Wu AP, Ni Y, Liu JQ, Ying LJ, et al. Clinical application of acutegastrointestinal injury grading system assocaited with clinical severity outcome in critically ill patients: A multi-center prospective, observational study. Zhonghua Yi Xue Za Zhi. 2017;97:325–31. doi: 10.3760/cma.j.issn.0376-2491.2017.05.002. doi: 10.3760/cma.j.issn.0376-2491.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Chen DC, Wang L. Mechanisms of therapeutic effects of rhubarb on gut origin sepsis (in Chinese) Chin J Traumatol. 2009;12:365–9. doi: 10.3760/cma.j.issn.1008-1275.2009.06.008. [PubMed] [Google Scholar]
- 31.Feng Z, Fei J, Wenjian X, Jiachen J, Beina J, Zhonghua C, et al. Rhubarb attenuates the severity of acute necrotizing pancreatitis by inhibiting MAPKs in rats. Immunotherapy. 2012;4:1817–21. doi: 10.2217/imt.12.131. doi: 10.2217/imt.12.131. [DOI] [PubMed] [Google Scholar]
- 32.Yao P, Cui M, Li Y, Deng Y, Wu H. Effects of Rhubarb on intestinal flora and toll-like receptors of intestinal mucosa in rats with severe acute pancreatitis. Pancreas. 2015;44:799–804. doi: 10.1097/MPA.0000000000000339. doi: 10.1097/MPA.0000000000000339. [DOI] [PubMed] [Google Scholar]
- 33.Ling D, Huang LG, Wang YR. Clinical studies of different doses of rhubarb powder on gastrointestinal complications in critically ill patients. J Taishan Med Coll. 2014;5:367–69. doi: 10.3969/j.issn.1004-7115.2014.05.009. [Google Scholar]


